Abstract
The objective of the present study was to determine the effects that hemolysis, lipemia, bilirubinemia, and anticoagulants might have on the most commonly used assays for C-reactive protein and serum amyloid A, and determination of ceruloplasmin values in dogs. Solutions of hemoglobin, lipid, and bilirubin were added to serum aliquots. Additionally, serum and plasma samples with different anticoagulants (heparin, EDTA, and citrate) were obtained from healthy dogs. Hemolysis, lipemia, and hyperbilirubinemia interfered significantly with the C-reactive protein and ceruloplasmin results, but not with those for the serum amyloid A assay. The use of anticoagulants produced significant changes in the results for the assays tested. However, the magnitude of the differences caused by the interfering substances does not appear to have an important impact on the clinical interpretation of the tests.
Résumé
Effets de l’hémolyse, de la lipémie, de l’hyperbilirubinémie et des anti-coagulants sur le dosage de protéine C-réactine canine, d’amyloïde A sérique et de céruloplasmine. L’objectif de cette étude était de déterminer les effets que l’hémolyse, la lipémie, la bilirubinémie et les anticoagulants peuvent avoir sur les tests les plus couramment utilisés pour mesurer la protéine C-réactine, l’amyloïde A sérique et la céruloplasmine chez le chien. Des solutions d’hémoglobine, de lipides et de bilirubine ont été ajoutées à des aliquots de sérum. De plus, des échantillons de sérum et de plasma auxquels on a ajouté différents anticoagulants (héparine, EDTA et citrate) ont été obtenus à partir de chiens en santé. L’hémolyse, la lipémie et l’hyperbilirubinémie modifiaient significativement les résultats de protéine C-réactine et de céruloplasmine, mais pas ceux d’amyloïde A sérique. L’utilisation d’anticoagulants a modifié significativement le résultats des tests. Cependant, l’ampleur des modifications apportées par les substances d’interférence ne semble pas avoir d’impact majeur sur l’interprétation clinique des tests.
(Traduit par Docteur André Blouin)
Introduction
The term acute phase response refers to the nonspecific inflammatory reaction of the host that occurs shortly after any tissue injury. Its cause can be infections, immunologic, neoplastic, traumatic, parasitic, or other (1,2). During this response, the concentration of some plasma proteins, collectively known as acute phase proteins (APPs), changes. Studies in veterinary medicine have shown that the quantification of these proteins provides valuable clinical information for the diagnosis, prognosis, and treatment monitoring of different pathologic processes (3,4), and it has been suggested that, in the future, APP determinations should be included in any health check regime for companion animals (5).
Serum samples that are hemolyzed, hyperbilirubinemic, or lipemic are sometimes submitted for routine biochemical testing at veterinary clinical pathology laboratories, but results from these samples may be altered by the presence of hemolysis, or excess bilirubin or lipid (6). Specific information about the effect that the interfering substances could have on the methods employed for testing would be very valuable. Assays for determination of canine APPs, such as haptoglobin, C-reactive protein (CRP), and serum amyloid A (SAA), are now commercially available, and an automated method for the determination of canine ceruloplasmin (Cp) values has been developed and validated (7); however, the possible effects of hemolysis, lipemia, hyperbilirubinemia have been determined only for the haptoglobin assay (8).
Most veterinary clinical pathology laboratories and reagent suppliers recommend the use of serum or heparinized plasma for clinical biochemical determinations. However, there are other anticoagulants, such as ethylenediamine tetraacetic acid (EDTA) or citrate, that can be used for certain assays, so that, in some cases, samples with these anticoagulants are submitted to veterinary laboratories for testing of different biochemical parameters, including APP determinations (9).
Since the knowledge of all these possible effects is important for the correct interpretation of APP results, the aim of the present work was to evaluate the effects that hemolysis, lipemia, hyperbilirubinemia, and anticoagulants could have on commonly used assays for CRP and SAA, and for the determination of Cp in dogs.
Material and methods
Acute phase protein determinations
A commercial solid phase sandwich immunoassay (Tridelta Phase Range Canine C-reactive Protein Assay; Tridelta Development, Bray, Ireland) was used for determination of CRP values. The assay was performed according to the manufacturer’s instructions.
A commercial solid phase sandwich enzyme linked immunosorbent assay (Tridelta Phase Range Serum Amyloid A assay; Tridelta Development) was used for determination of SAA values. The assay was performed according to the manufacturer’s instructions.
The final absorbance of the samples was measured in a microplate spectrophotometer (PowerWave XS; Bio-tek, Winooski, Vermont, USA) at 450 nm by using 630 nm as the reference, and the difference in the absorbance at the 2 wavelengths was used to calculate the concentration.
A method based on that described by Sunderman and Nomoto (10), with the modifications of Cerón and Martínez-Subiela (7) was used for determination of Cp values. This protein catalyzes the oxidation of P-phenylenediamine to yield a purple product whose rate of formation is proportional to the concentration of serum Cp present in the specimen (10).
These assays were validated in the authors’ laboratory (7,11,12). Within-run coefficients of variation (CVs), based on the mean of 5 repetitive assays of 6 serum samples, and between-run CVs, based on the mean of the assay of 6 samples analyzed on 5 different days, were lower than 10%, with the exception of between-run CVs for CRP (26.1%) and SAA (16.4%). Therefore, in order to avoid the influence of the high between-run CVs found, all samples were stored at −20ºC and analyzed in the same run. Accuracy was tested indirectly by investigating linearity under dilution; it resulted in a linear regression equation with a correlation coefficient of 0.99 in all cases, showing good accuracy. The detection limit estimated as the mean plus 2 standard deviations of 10 replicate zero samples was 0.02 g/L for haptoglobin, 7.0 mg/L for Cp, 0.15 mg/L for CRP, and 0.79 mg/L for SAA.
Effects of hemolysis, lipemia, and hyperbilirubinemia
For the interference studies, a normal serum pool was prepared by centrifuging clotted blood samples obtained from 10, clinically healthy, beagle dogs. The pool had a CRP concentration of 4.47 mg/L, an SAA concentration of 3.37 mg/L, and a Cp concentration of 30.2 mg/L.
Fresh hemolysate (with hemoglobin concentration of 100 g/L) was prepared by the addition of distilled water to packed, washed canine red blood cells from healthy dogs, followed by centrifugation to remove cell debris. Dilutions of the hemolysate with hemoglobin concentrations of 50, 25, 12.5, and 6.25 g/L were prepared, and 10 μL of each dilution was added to 90 mL of an aliquot of the normal serum pool. The final concentrations of hemoglobin in the samples were 10, 5, 2.5, 1.25, 0.62, and 0 g/L (l0 μL of distilled water was added to 90 mL of normal serum pool to give 0 g/L concentration). These hemoglobin concentrations could correspond to slight hemolysis, 0.62g/L; moderate hemolysis, 1.25 and 2.5 g/L; and marked hemolysis, 5 and 10 g/L.
To investigate the effects of lipids, lipemia was simulated by adding a commercial fat emulsion (Lipofundina 20%; Braun Medical S.A., Barcelona, Spain) to aliquots of a normal serum pool. The emulsion was serially diluted in the diluent buffer of the assays (sample diluent provided in the kit for CRP and SAA, and sodium acetate buffer prepared by dissolving 40.75 g of sodium acetate [trihydrate] in distilled water and adjusting the pH to 5.2 with glacial acetic acid and the volume to 500 mL for Cp), giving a series of triglyceride solutions with concentrations of 20, 10, 5, 2.5, and 1.25 g/L. Then 30 μL of each solution (and distilled water as a zero control) were mixed with 90 μL of the normal pooled serum to produce test samples with final triglyceride concentration of 5, 2.5, 1.25, 0.62, 0.31, and 0 g/L. These triglyceride concentrations could correspond to slight lipemia, 0.312 g/L; moderate lipemia, 0.62 and 1.25 g/L; and marked lipemia 2.5 and 5 g/L.
To study the effects of hyperbilirubinemia, 6 mg of bilirubin (Sigma Chemical Company, St. Louis, Missouri, USA) were suspended in 10 mL of the sample diluent provided in the commercial kit for the CRP and the SAA assays, and with acetate buffer for the determination of Cp, and then mixed with serum aliquots to make samples with added bilirubin concentrations of 0.15, 0.075, 0.037, 0.018, 0.009, and 0 g/L.
Lipid and bilirubin concentrations of the diluted series were conf irmed by analysis, using an automated chemistry analyzer (Cobas Mira Plus; ABX Diagnostic, Montpellier, France). Commercially available kits: Triglycerides CP (GPO/PAP method; ABX Diagnostics) and total bilirubin (DMSO method; Spinreact, Sant Esteve de Bas, Spain) were used as directed by the manufacturer. Hemoglobin concentrations in hemolysate were conf irmed by analysis on an automated hematological analyzer (Vet Abc; ABX Diagnostic).
All diluted series were assayed in duplicate for CRP, SAA, and Cp, and the mean was calculated.
Effects of anticoagulants
Blood samples were obtained from 8 dogs, with no abnormal findings on physical examination, and routine hematological and biochemical results within reference ranges established by the Clinical Pathology Laboratory at Murcia Veterinary School. Aliquots of the blood samples were added to different types of tubes with this sequence:
Lithium heparin at a concentration of 17 IU/mL, 4 mL tube (BD Vacutainer, LH PST; Becton, Dickinson, Madrid, Spain).
Tripotasium ethylenediamine tetraacetic acid (EDTA K3), 0.072 mL at 7.5%, 3 mL tube (BD Vacutainer; Becton, Dickinson).
Trisodium citrate, 0.3 mL at 3.8%, 3 mL tube (Venoject Na3 citrate buffered; Terumo Europe, NV, Leuven, Belgium).
Serum (Tapval; Aquisel, Barcelona, Spain).
All samples were assayed for CRP, SAA, and Cp, and the mean and standard deviation for each anticoagulant were calculated.
Statistical analysis
Data from interference studies (hemolysis, lipemia, and bilirubinemia) are presented in interferograms made according to previously reported protocols (13,14). The graphs show on the x-axes, increasing concentrations of hemoglobin, lipids, or bilirubin, and on the y-axes, the mean percentage change of each protein expressed as final value/original value × 100 (final value = value obtained for enriched serum; original value = value obtained for the same volume of serum without the interferents).
Results from original serum and serum enriched by either hemoglobin, lipid, or bilirubin were compared via paired Student’s t-test. Mean values obtained for each anticoagulant were compared with mean serum values by the same test. All statistical significances were evaluated at the 5% probability level by using a statistical program (SPSS, version 9.0; SPSS, Chicago, Illinois, USA).
Results
Effects of hemolysis
The results obtained are presented in Figure 1. The addition of hemoglobin significantly interfered with the results for CRP and Cp values, but not with SAA values. A rise in CRP values was observed when hemoglobin concentrations were increased. Ceruloplasmin values increased until concentrations of hemoglobin reached 2.5 g/L; however, greater concentrations of hemoglobin caused a decrease in the results for this protein.
Figure 1.
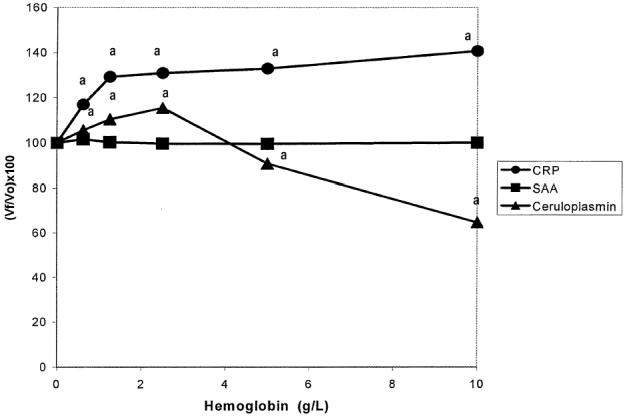
Interferogram for hemoglobin. Vf: Final value; Vo: Original value.^: C-reactive protein (CRP), ▪: serum amyloid A (SAA), ▴: Ceruloplasmin (Cp). Each data point is the mean of duplicate determination.
aP < 0.05.
Effects of triglycerides
The results obtained are presented in Figure 2. Triglycerides significantly interfered with CRP and Cp values, but not with SAA values. Although the changes for CRP were statistically significant, they were not directly proportional to the lipid concentration. Ceruloplasmin levels showed an increase, reaching the maximum value when lipid concentration was 2.50 g/L.
Figure 2.
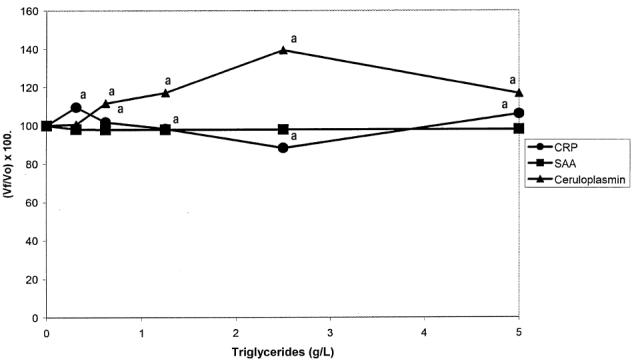
Interferogram for triglycerides. Vf: Final value; Vo: Original value. ^: C-reactive protein (CRP), ▪: serum amyloid A (SAA), ▴: Ceruloplasmin (Cp). Each data point is the mean of duplicate determination.
aP < 0.05.
Effects of bilirubin
The results obtained are presented in Figure 3. Increased bilirubin concentrations caused a significant decrease in CRP values and a significant increase in Cp values; however, it did not cause any significant change on SAA values.
Figure 3.
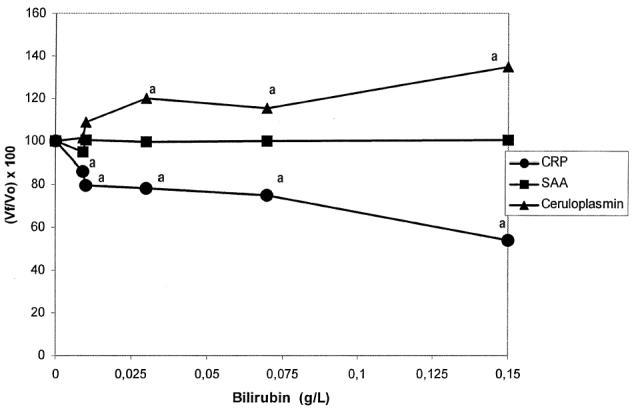
Interferogram for bilirubin. Vf: Final value; Vo: Original value. ^: C-reactive protein (CRP), ▪: serum amyloid A (SAA), ▴: Ceruloplasmin (Cp). Each data point is the mean of duplicate determination.
aP < 0.05.
Effects of anticoagulants
Values for CRP were significantly lower in samples with citrate than with serum (Table 1). The SAA values were significantly higher in all plasma samples than in serum samples. Ceruloplasmin values showed a significant increase when heparin was used as the anticoagulant, and a significant decrease when EDTA was employed.
Table 1.
C-reactive protein (CRP), serum amyloid A (SAA), and ceruloplasmin (Cp) values in samples (n = 8) of canine serum and plasma obtained with different anticoagulants.
| CRP (mg/L)
|
SAA (mg/L)
|
Cp (mg/L)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| χ̄ | s | P-value | χ̄ | s | P-value | χ̄ | s | P-value | |
| EDTA | 1.56 | 0.86 | 0.31 | 1.33 | 0.85 | 0.02a | 23.7 | 2.8 | < 0.001a |
| Heparin | 1.82 | 1.30 | 0.13 | 1.15 | 0.62 | 0.04a | 52.2 | 19.9 | 0.01a |
| Citrate | 1.09 | 0.81 | 0.002a | 1.10 | 0.42 | 0.01a | 28.5 | 9.5 | 0.38 |
| Serum | 1.44 | 0.90 | 0.71 | 0.55 | 31.3 | 2.8 | — | ||
— Mean; s — Standard deviation
P < 0.05
Ethylenediamine tetraacetic acid (EDTA) (0.072 mL at 7.5%, 3 mL tube), heparin (17 IU/mL, 4 mL tube), citrate (0.3 mL at 3.8%, 3 mL tube)
Discussion
Despite some literature concerning the interferences caused by different substances, such as hemoglobin, lipids, bilirubin, and anticoagulants, in hematologic or biochemical analyses (6,13,15–17), and besides the general advice included by all manufacturers on proper sample collection, little is know about the potential effect of these substances on commonly determined APP in canine serum samples.
Hemolysis is an important interference factor that must be considered when making laboratory measurements. It is usually attributable to in vitro damage to erythrocytes from different factors, such as prolonged storage of the blood before separation of the serum, rapidly forcing blood through small needles, excessive agitation when mixing, or the physical act of centrifugation and separation of serum (18). In vivo hemolysis occurs less frequently, but it has a similar effect on laboratory tests (6). The main mechanisms by which hemolysis interferes with the testing procedures are as follows (6): Leakage of analytes from damaged erythrocytes, decrease in levels of analytes present in plasma at higher concentrations than in erythrocytes, and color interference and chemical interactions between red blood cell components and analytes. In the present study, hemolysis produced significant changes in CRP and Cp values. It would be very difficult to identify precisely which mechanisms of the 3 described above were responsible for the variations in the results that appeared in our study. However, a similar effect to that identified for the Cp assay (an increase in Cp values when hemoglobin was lower than 2.5 g/L, and a decrease with greater hemoglobin concentrations) has been described for lipase and serum alkaline phosphatase assays and could be attributable to the color interference caused by hemoglobin (6). The presence of free hemoglobin usually increases absorbance of tests run at wavelengths within the absorbance range of hemoglobin (500 to 600 nm); however, if the level of interference from hemoglobin is high enough to increase the initial absorbance above the linear range of the reaction, it could produce falsely lower analyte concentrations (6,19). Additionally, in the present study, the effect of hemolysis was tested by adding hemolysate rather than purified hemoglobin to the specimens. This implies the presence of debris (cell membranes), proteins, and other potential interferents that can hamper immunoassays (20). Therefore, it could explain the changes observed in CRP concentrations.
The term “lipemia” is used to describe samples of blood, serum, or plasma that have a cloudy or milky appearance (21). It is due to an increased lipid content, the result of both physiological and pathological events in the metabolism of plasma lipid. The presence of lipemia can interfere with many clinical chemistry tests by different mechanisms, the most frequent mechanism is due to the scattering of light rays by the lipids (mainly chylomicrons and very low density lipoproteins) (6,21). In our study, Cp levels increased with added triglycerides to a certain point and then fell. This effect could be explained, since lipemia produces an increase in Cp values due to light scattering, as occurs with hemolysis, but when the interference is great enough to raise the absorbance above the range of linearity, the effect is the opposite. Lipemia also modified CRP levels significantly. No data concerning the influence of lipids on canine CRP immunoassays could be found, but a hypothesis may be that triglycerides could alter the binding between the protein and the antibodies used for the assay, causing the observed interferences. Interference produced by lipemia has been described in other immunoassays (15,22,23).
Bilirubin is a yellow pigment produced by enzymatic degradation of hemoglobin and the presence of elevated concentration of this substance in a canine serum sample could be due to hemolytic anemia or liver disease, such as hepatitis or hepatocellular swelling, and bile duct obstruction (24,25). No previous reports regarding the effects of hyperbilirubinemia on CRP, Cp, or SAA determinations could be found, but in the present study, bilirubin produced a significant increase in Cp values and a decrease in CRP values, so these effects should be considered when submitting hyperbilirubinemic specimens for CRP or Cp assays.
Although it has been shown that hemolysis, lipemia, and hyperbilirubinemia caused different statistically significant changes in the results obtained for CRP and Cp assays, the magnitude of the differences observed were relatively small and unlikely to be clinically relevant. The increase in these acute phase proteins observed in dogs in relation to inflammation is greater than the changes resulting from interferences. For example, the maximum value for CRP in hemolytic samples was 5.72 mg/L, while mean CRP values observed in dogs with different inflammatory processes were 65.03 mg/L for leishmaniasis (26), 61.4 mg/L for pyometra, and 169.3 mg/L for bacterial enteritis (27).
A number of different anticoagulants are used in routine practice, depending on the purpose of the analysis. Usually, only serum is used and recommended for routine acute phase proteins analysis; however, sometimes samples with anticoagulants, such as heparin, EDTA, or sodium citrate, are submitted to veterinary clinical pathology laboratories for APP determinations. The use of those anticoagulants produced significant changes in the results obtained for the APP assays tested; however, these results should be interpreted with caution, since the levels of acute phase proteins in samples used for the anticoagulant study were very low and near the limit of detection of the assays, so the results could have been influenced by the possible inaccuracy of the standard curve at this point.
Our study has demonstrated that hemolysis, lipemia, hyperbilirubinemia, and anticoagulants can interfere with acute phase protein assays. However, since the results were obtained with specific commercial kits or methods and analyzers, it is possible that important variation would be found if other reagents or instruments were used. Furthermore, these results were derived from healthy animals, so more studies are required to determine if the same interferences are present in samples obtained from diseased animals with high serum acute phase protein concentrations. CVJ
References
- 1.Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- 2.Martínez Subiela S, Tecles F, Parra MD, Cerón JJ. Proteínas de fase aguda: conceptos básicos y principales aplicaciones en medicina veterinaria. An Vet (Murcia) 2001;17:99–116. [Google Scholar]
- 3.Solter PF, Hoffmann WE, Hungerford LL, Siegel JP, St Denis SH, Dorner JL. Haptoglobin and Cp as determinants of inflammation in dogs. Am J Vet Res. 1991;52:1738–1742. [PubMed] [Google Scholar]
- 4.Martínez-Subiela S, Bernal LJ, Cerón JJ. Serum concentrations of acute-phase proteins in dogs with leishmaniosis during short-term treatment. Am J Vet Res. 2003;64:1021–1026. doi: 10.2460/ajvr.2003.64.1021. [DOI] [PubMed] [Google Scholar]
- 5.Eckersall PD. The time is right for acute phase protein assays. Vet J. 2004;168:3–5. doi: 10.1016/j.tvjl.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Alleman AR. The effects of hemolysis and lipemia on serum biochemical constituents. Vet Med. 1990;85:1272–1284. [Google Scholar]
- 7.Cerón JJ, Martínez-Subiela S. An automated spectrophotometric method for measuring canine ceruloplasmin in serum.Vet Res 2004; In press. [DOI] [PubMed]
- 8.Eckersall PD, Duthie S, Safi S, et al. An automated biochemical assay for haptoglobin: prevention of the interference from albumin. Comp Hematol Int. 1999;9:117–121. [Google Scholar]
- 9.Ceron JJ, Martínez-Subiela S, Hennemann C, Tecles F. The effects of different anticoagulants on routine canine plasma biochemistry. Vet J. 2004;167:294–301. doi: 10.1016/j.tvjl.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Sunderman FW, Jr, Nomoto S. Measurement of human serum ceruloplasmin by its p-phenylenediamine oxidase activity. Clin Chem. 1970;16:903–910. [PubMed] [Google Scholar]
- 11.Martínez-Subiela S, Tecles F, Parra MD, Cerón JJ. Analytical validation of different methods for determination of main acute phase proteins in canine serum samples [Abstract] Proc 4th Eur Colloq Acute Phase Proteins 2003:142.
- 12.Martínez Subiela S, Ginel PJ, Ceron JJ. Effects of different glucocorticoid treatments on serum acute phase proteins in dogs. Vet Rec 2004; In Press. [DOI] [PubMed]
- 13.Glick MR, Ryder KW, Jackson SA. Graphical comparisons of interferences in clinical chemistry instrumentation. Clin Chem. 1986;32:470–475. [PubMed] [Google Scholar]
- 14.Jacobs RM, Lumsden JH, Grift E. Effects of bilirubinemia, hemolysis, and lipemia on clinical chemistry analytes in bovine, canine, equine, and feline sera. Can Vet J. 1992;33:605–608. [PMC free article] [PubMed] [Google Scholar]
- 15.Lucena R, Moreno P, Perez-Rico A, Ginel PJ. Effects of hemolysis, lipemia and bilirubinemia on an enzyme-linked inmunosorbent assay for cortisol and free thyroxine in serum samples from dogs. Vet J. 1998;156:127–131. doi: 10.1016/s1090-0233(05)80038-3. [DOI] [PubMed] [Google Scholar]
- 16.Moreno P, Ginel PJ. Effects of hemolysis, lipemia and bilirubinemia on prothrombin time, activated partial thromboplastin time and thrombin time in plasma samples from healthy dogs. Res Vet Sci. 1999;67:273–276. doi: 10.1053/rvsc.1999.0321. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Subiela S, Tecles F, Montes A, Gutierrez C, Ceron JJ. Effects of hemolysis, lipemia, bilirubinemia and fibrinogen on protein electropherogram of canine samples analysed by capillary zone electrophoresis. Vet J. 2002;164:261–268. doi: 10.1053/tvjl.2001.0672. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill SL, Feldman BF. Hemolysis as a factor in clinical chemistry and hematology of the dog. Vet Clin Pathol. 1989;18:58–68. doi: 10.1111/j.1939-165x.1989.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 19.Dorner JL, Hoffman WE, Filipov MM. Effect of in vitro hemolysis on values for certain porcine serum constituents. Vet Clin Pathol. 1983;12:15–19. doi: 10.1111/j.1939-165x.1983.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 20.Wenk RE. Mechanism of interference by hemolysis in immunoassays and requirements for sample quality. Clin Chem. 1998;44:2554. [PubMed] [Google Scholar]
- 21.Watson TDG. Why is this sample lipemic? Canine Pract. 1993;18:26–31. [Google Scholar]
- 22.Parra MD, Bernal LJ, Ceron JJ. Cortisol and free thyroxine determination by time-resolved fluorometry in canine serum. Can J Vet Res. 2004;68:98–104. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DE, Lamb SV, Reimers TJ. Effects of hyperlipemia on radioimmunoassays for progesterona, testosterona, thyroxine and cortisol in serum and plasma simples from dogs. Am J Vet Res. 1991;52:1489–1491. [PubMed] [Google Scholar]
- 24.Tennant BC. Hepatic function. In: Kaneko JJ, Harvey JW, Bruss ML, eds. Clinical Biochemistry of Domestic Animals. 5th ed. New York: Academic Pr, 1997:327–352.
- 25.Weiss DJ. Test for evaluation liver disease. In: Cowell RL, ed. Veterinary Clinical Pathology Secrets. 1st ed. St Louis: Elsevier, 2004:168–173.
- 26.Martínez-Subiela S, Tecles F, Eckersall PD, Cerón JJ. Serum concentrations of acute phase proteins in dogs with leishmaniasis. Vet Rec. 2002;150:241–244. doi: 10.1136/vr.150.8.241. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S, Shida T, Miyaji S, et al. Changes in serum C-reactive protein levels in dogs with various disorders and surgical traumas. Vet Res Comm. 1993;17:85–93. doi: 10.1007/BF01839236. [DOI] [PubMed] [Google Scholar]


