Abstract
Candida albicans is a pathogenic fungus able to change morphology in response to variations in its growth environment. Simple inoculation of stationary cells into fresh medium at 37°C, without any other manipulations, appears to be a powerful but transient inducer of hyphal formation; this process also plays a significant role in classical serum induction of hyphal formation. The mechanism appears to involve the release of hyphal repression caused by quorum-sensing molecules in the growth medium of stationary-phase cells, and farnesol has a strong but incomplete role in this process. We used DNA microarray technology to study both the resumption of growth of Candida albicans cells and molecular regulation involving farnesol. Maintaining farnesol in the culture medium during the resumption of growth both delays and reduces the induction of hypha-related genes yet triggers expression of genes encoding drug efflux components. The persistence of farnesol also prevents the repression of histone genes during hyphal growth and affects the expression of putative or demonstrated morphogenesis-regulating cyclin genes, such as HGC1, CLN3, and PCL2. The results suggest a model explaining the triggering of hyphae in the host based on quorum-sensing molecules.
Candida albicans is an opportunistic fungal pathogen found in the normal gastrointestinal flora of most healthy humans. It can cause disorders ranging from localized infections to death, the latter especially in immunocompromised patients. As is the case with many fungal pathogens, this yeast is able to use different morphological forms during host invasion (14). Although the link between virulence and morphogenesis is circumstantial, the changes in morphology are predicted to increase the efficiency of dissemination in the host (14). The wide range of morphologies of C. albicans is one of its distinguishing features: it can exist in three common forms (yeast cells, pseudohyphae, and true hyphal cells) as well as in some less common shapes, like chlamydospores or opaque cells (6, 12, 23, 26, 32, 33, 35). The yeast-to-hyphal transition is the best-documented morphological transition. Hyphae appear as microscopic tubes with or without branches; to generate hyphae, budding cells change their growth mode to a continuous apical extension followed by septation. Pseudohyphae are created by unipolar budding, with the cells remaining attached to the mother cell. Both pseudohyphae and true hyphae produce chains of cells. The difference between the two can appear minor; however, it is commonly accepted that true hyphae have no constrictions at their septa, and they have parallel cell walls with branches forming perpendicular to these walls (34).
Wide ranges of both chemicals and environmental conditions have been identified based on their ability to induce the yeast-to-hyphal switch (12, 26, 28). Addition of serum appears to be a powerful trigger with a dependence on low cell density and elevated temperature. Despite the widespread use of this technique to induce hyphae, the inherent mechanism is still poorly understood. Hudson et al. (16) recently suggested that the active role of serum is mainly the result of its nutrient properties (nitrogen and carbon). Another poorly understood method of hyphal induction consists of inoculating fresh medium with stationary cells at 37°C (4, 5, 22). Despite its simplicity, this technique is not commonly used (9, 10), although the process may have influenced recent observations (17).
Quorum-sensing molecules allow bacteria to monitor their growth and to control cell density-dependent phenomena. Similar regulatory systems have been recently identified in the eukaryotic pathogen Candida albicans. Hornby et al. (15) identified farnesol as a quorum-sensing molecule responsible for inhibiting hyphal formation in stationary-phase cells. As well, Chen et al. (10) characterized tyrosol as a candidate quorum-sensing molecule involved in abolishing the lag phase preceding the resumption of growth of stationary-phase cells.
In this paper, we reinvestigate the capacity of C. albicans cells to form hyphae when initiating growth from stationary phase and suggest that classical serum-based induction is significantly dependent on this process. The basis of this process appears to be a release from the inhibition caused by quorum-sensing molecules, including farnesol, present in the medium of stationary cells. We performed transcriptional profiling of C. albicans initiating hyphal development due to this trigger both in the presence and in the absence of farnesol in the fresh medium. The results provide information about the transcriptional changes involved in the resumption of growth, in the hyphal development process, and in the mechanism of response to farnesol.
MATERIALS AND METHODS
Growth medium.
Cultures of C. albicans strain SC5314 (13) were grown in buffered yeast extract-peptone-dextrose (YPD) (2% glucose, 2% Bacto peptone, 1% yeast extract)-based medium; the required pH is obtained by adding the correct proportion of an Na2HPO4-NaH2PO4 mix to a 100 mM final concentration. The buffering ensures pH stability throughout the experiments. Fetal bovine serum was purchased from Gibco (Grand Island, N.Y.); trans,trans-farnesol was purchased from Sigma-Aldrich (Oakville, Ontario, Canada).
Culture conditions.
Cells were cultured in a shaking incubator (180 rpm) at 30°C or 37°C. Absorbance was read at 600 nm. Serial dilutions were achieved by lightly inoculating a 4-ml YPD tube from a YPD plate culture no older than 2 weeks. One milliliter of this tube was transferred to a second 4-ml YPD tube; the latter is vortexed and 1 ml taken to inoculate a third tube and so on to generate 10 cultures. After overnight growth, this approach generates a range of growth states from early-exponential- to stationary-phase cells.
The cells were counted under a microscope (40× lens), and about 200 cells were categorized as budded (less than three joined cells), true hyphae (no constriction at the septa, walls remaining parallel throughout the hyphae, and branching perpendicular to the cell walls), or pseudohyphal. Several positions per slide were examined to ensure a representative selection of cells was characterized. Hyphal lengths were measured with the software Openlab (Improvision).
RNA extraction, mRNA purification, cDNA labeling, and microarray analysis.
An overnight culture (optical density at 600 nm [OD600] > 18) was used to initiate the resumption of growth in a volume of 300 ml of buffered YPD at an OD600 of 0.5. Aliquots (50 ml) were removed and centrifuged before being quick-frozen and stored at −80°C at 0, 10, 30, 60, and 180 min following the inoculation. The experiments were done at 30°C or at 37°C; the experiments at 37°C with farnesol were the same as those at 37°C except for the addition of 30 μM farnesol in the medium prior to inoculation. We used slight variations of the methods and microarrays described by Enjalbert et al. (11); results presented consist of the average of four completely independent experiments (two Cy3/Cy5 and two Cy5/Cy3 labelings) except for the 37°C plus farnesol condition (two Cy3/Cy5 and one Cy5/Cy3 labeling). For each experiment and replicate, the control corresponds to the time zero of the same replicate. Detailed protocols and full results can be obtained from the supplementary material (http://candida.bri.nrc.ca/papers/Enjalbert2005/index.cfm).
RESULTS
Dilution of stationary cells is a potent and transient trigger of hyphal induction.
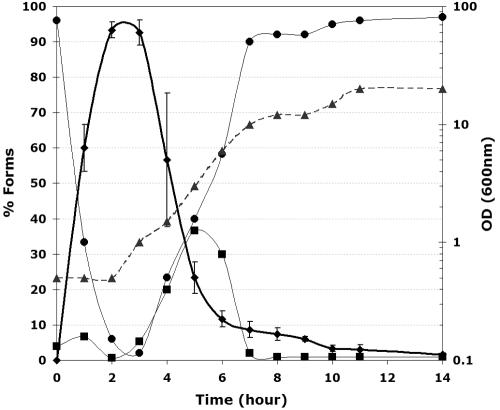
We reinvestigated previous results that demonstrated the potential of inducing hyphae in Candida albicans during release from stationary phase (4, 5, 21, 22, 31). We confirmed that dilution of cells grown to stationary phase at 37°C into fresh YPD triggers induction of hyphae, generating as much as 90% synchronized hyphal cells after 3 h (Fig. 1). However, this induction is transient: the hyphae start to produce budded cells after 3 h, so that buds rapidly become the major growth form (Fig. 1). The initial hyphal structures remain stable through the 15-h experimental period. We observed no hyphal formation at 37°C other than those arising from the initial dilution of the stationary-phase cells.
FIG. 1.
Hyphal triggering by dilution of stationary cells. Cells were grown overnight at 37°C in buffered YPD, pH 7.5, in order to obtain stationary cells (OD600 ≥ 20). This culture was diluted to an OD600 of 0.5 in the same medium, same temperature, and same pH. At each hour following the initiation, the cell forms were counted (⧫, hyphae; ▪, pseudohyphae; •, yeast cells), and the OD600 was measured (▴). The figure represents the average of two independent experiments, and the standard deviations of the hyphal points only are shown for clarity.
Connection with serum induction.
Classical serum induction consists of diluting stationary cells at 30°C into fresh medium at 37°C containing serum (24). As this method includes the dilution of stationary-phase cells and has the same pH and temperature requirements as the preceding protocol, we investigated the role of stationary-phase conditioning during serum induction. We measured the capacity of cells to produce serum-induced hyphae after growth for various times in fresh YPD at 30°C before the switch to 37°C plus serum. Figure 2 demonstrates that the cells lose most of their capacity to produce hyphae with increasing lengths of time at 30°C. Ultimately, transfer of cells growing exponentially for 24 h to 37°C plus serum triggered hyphal development in less than 20% of the cells. No hyphae were observed in the absence of serum with these long-term exponential-phase cells (data not shown). Thus, the classical serum induction appears linked to outgrowth from stationary-phase cells even if the serum possesses genuine inducer properties.
FIG. 2.
Serum induction of stationary cells with increased time of growth resumption at 30°C. Cells were grown overnight at 30°C in YPD in order to obtain stationary cells (OD600 ≥ 20). These cultures were diluted to an OD600 of 0.01 in the same medium, same temperature, and same pH. At each hour from 0 to 6 at 30°C, 10% of final serum was added to a sample, and the tube was transferred at 37°C for 3 h. The 24-h time point corresponds to an overnight exponentially growing culture at 30°C with a final OD600 of 0.01 treated the same way. Then, the cell forms were counted (⧫, hyphae; ▪, pseudohyphae; •, yeast cells). These results are from three independent experiments.
Acquisition of hyphal competence increases with entry into stationary phase.
We investigated at which stage of growth the cells become competent to produce hyphae (Fig. 3). Diluting different samples at different phases of growth of a culture results in a range of morphological behaviors. As seen in Fig. 1, only cells that have exited the exponential phase of growth can produce hyphae after the reinoculation. The closer the cells are to stationary phase, the higher is the percentage of hyphae 3 h after the dilution. Finally, completely stationary-phase samples are the only ones that generate an extensive production of true hyphae.
FIG. 3.
Influence of the growth phase on hyphal formation after dilution in fresh medium. Cells were grown overnight in serial dilution at 37°C in buffered YPD, pH 7.5, in order to obtain a range of concentrations. These cultures were diluted to an OD600 of 0.01 in fresh medium at the same temperature and same pH. At 3 h following the initiation, the cell forms were counted. The data are the results from three independent experiments, and the standard deviations of the hyphae percentages only are shown for the clarity of the graph. ⧫, hyphae; ▪, pseudohyphae; •, yeast cells.
Identification of the inherent mechanism.
There could be several explanations for a requirement of stationary-phase cells to permit the induction of hyphae: (i) the release of the frequent cellular contacts found in an environment with high cell concentration, (ii) the sudden availability of nutrients, (iii) the exit from stationary phase, or (iv) the release from inhibitory molecules. To distinguish among these hypotheses, we transferred stationary-phase cells at high concentration (OD600 = 20) into fresh medium (inoculation to OD600 = 1 to 20) or into water, always at 37°C (Fig. 4a). All of these conditions triggered germ tube formation, and the germ tube length was proportional to the cell concentration (Fig. 4b). The initiation of hyphal development after transfer of the cells to the fresh medium without dilution (thus maintaining the same OD) suggests that cellular contacts are not preventing hyphal formation. Moreover, the induction of hyphae after the transfer to distilled water makes it unlikely that hyphal induction requires the nutrients available in the new medium. This result correlates with and supports the observation of Bell and Chaffin (4) that established that 54% of stationary cells diluted in a minimal medium without a carbon source were able to form germ tubes. Therefore, a plausible explanation for the induction of hyphae during the resumption of growth of stationary-phase cells is a release from the repression generated by inhibitory molecules present in the conditioned medium.
FIG. 4.
Dilution of stationary-phase cells at different final concentrations. Cells were grown overnight at 37°C in buffered YPD, pH 7.5, in order to obtain stationary cells (OD600 = 20). The cells were centrifuged and resuspended in fresh medium at the same temperature and same pH at an OD600 of 1 (1), an OD600 of 5 (2), an OD600 of 10 (3), an OD600 of 15 (4), or an OD600 of 20 (5) or in water at an OD600 of 0.5 (6). After 3 h, a sample of cells was photographed (a). Hyphal lengths were measured, and the averages and standard deviations are reported in the graph (b).
Release from quorum-sensing molecules triggers hyphal formation.
We studied the impact of the initial medium on hyphal formation using serial dilutions: we diluted stationary-phase cells from the first tube into the conditioned media of the other cultures. As shown in Fig. 5a, there is a clear step between an OD600 of 5 and an OD600 of 10 where the medium becomes effective at inhibiting hyphal development. This observation suggests the presence of inhibitory quorum-sensing molecules released in the spent medium by stationary cells. Of the two quorum-sensing molecules described so far for C. albicans, only farnesol inhibits hyphal formation (10, 15). A 30 μM concentration of this molecule has been reported to inhibit, by 50%, the hyphal formation triggered by serum induction (15). We also found that addition of 30 μM farnesol to the new medium prior to inoculation inhibited hyphal formation by 61% ± 18% (Fig. 5b). Unlike the spent medium, increasing concentrations of farnesol were not able to completely suppress hyphal formation. In addition, while the spent medium blocked cells in the yeast form, farnesol treatment generated a mix of yeast, pseudohyphal, and hyphal cells. Finally, we found that conditioning exponential-phase cells with farnesol followed by farnesol removal was not sufficient to trigger hyphal formation (data not shown). The inability of farnesol addition to completely mimic the properties of spent medium suggests the existence of other inhibitor molecules.
FIG. 5.
Release from quorum-sensing molecules triggers hyphal formation. Cells were grown overnight at 37°C in buffered YPD, pH 7.5, in serial dilution in order to obtain a range of cultures from early-exponential- to stationary-phase cells (OD600 ≥ 20). All of the samples but the more grown were centrifuged, and their medium was sterilized by filtration. Then, the more grown culture was used to inoculate the range of spent media to an OD600 of 0.5 at the same temperature (a) or diluted to an OD600 of 0.5 in fresh medium at the same temperature plus a range of farnesol concentrations (b). After 3 h, the cell forms were counted. The data are the results from at least three independent experiments, and the standard deviations of the hyphal percentages only are shown for the clarity of the graph. ⧫, hyphae; ▪, pseudohyphae; •, yeast cells.
Microarray analysis of the resumption of growth.
We monitored the transcriptional reprogramming caused by the resumption of growth of stationary cells transferred to fresh medium at 37°C in the presence of farnesol. We took advantage of the similarity of concentration between the maximum impact of farnesol on hyphal growth (30 μM) and the in vitro maximum accumulation of farnesol during stationary phase (10 to 50 μM [15]). We hypothesized that applying 30 μM farnesol in the new medium would likely result in a minimal change of farnesol concentration from that of the spent medium. As the cells leave stationary phase and resume growth, transcriptional profiling highlights the induction of ribosomal protein genes and RNA production components as well as the repression of stationary-phase genes, like the stress-related genes (supplementary web data). To bypass the complexity caused by the metabolic changes and to identify farnesol-dependent components, we compared the responses of cells resuming growth at 37°C in the presence or absence of farnesol at different times (Fig. 6, top panel). We examined whether the same patterns of expression were obtained when we compared the hyphal form resumption of growth at 37°C to the yeast form resumption of growth at 30°C. We hypothesized that the presence of genes in the two comparisons would be the consequence of the partial hyphal deficiency created by farnesol. This pattern was observed for the functional groups related to biogenesis, to organization and properties of the cell wall, and to ergosterol metabolism as well as the groups described as “chromatin assembly and disassembly” and “DNA replication.” Beside the morphological consequences, the comparison exposed the gene sets whose expression was modulated by farnesol independently of morphology. The quorum-sensing molecule decreases the expression of some fatty acid oxidation genes after 30 min and creates cell cycle changes (from G2/M transition at 60 min to cell separation at 180 min). The continued presence of farnesol appears to generate a greater expression of genes implicated in the response to stress, as well as to drug and DNA damage stimuli. We observed also an increase in the expression of genes involved in various metabolic processes, such as alcohol metabolism, iron ion transport, and lipid metabolism. Recently, Cao et al. (8) performed a genomic investigation of farnesol addition to Candida albicans biofilms. Despite the use of a dissimilar experimental model and a semicomplete genome with a different annotation, they identified equivalent functional categories (i.e., iron transport, cell wall, drug resistance, and cell cycle) but with very few overlaps for specific genes (see supplementary web data). This enforces the idea that the specific presence of farnesol, rather than variations in concentration, is directly related to the expression of these genes. Maintaining a similar concentration of farnesol throughout the experiment leads to the expected variations based on the morphology of the cells but also causes specific adaptations.
FIG. 6.
Classification of the functional groups with divergent responses. For each time point ratio (time 10, 30, 60, or 180 min/time zero), the log2 value at 37°C was subtracted from the log2 at 37°C plus farnesol. Genes with a score greater than 0.8 or lower than −0.8 were selected (both with about 150 genes). The list was refined to keep only genes with an S. cerevisiae homologue in order to use the GO resources (http://www.yeastgenome.org/GOContents.shtml). The lists of function were simplified to remove redundant categories and weak probability groups with no clear significance in the C. albicans response. The upper part of the table indicates the functional categories overexpressed at 37°C without farnesol, while the lower part indicates the functional categories with an increased expression in the presence of farnesol. The finding of functional categories at a time point is represented by a square. The same analysis has been done with the 37°C experiment versus the 30°C study in order to distinguish between the consequences of hyphal growth and the consequences of continued farnesol treatment. Functional categories found in both analyses are represented by a gray square (░⃞), while functional categories specific to the presence of farnesol are represented by black squares (▪). The best probability for each group occurrence for the 37°C plus farnesol versus 37°C analysis is presented on the right. Illustrations of the predominant growth forms for each time point of the 37°C and 37°C plus farnesol experiment are displayed on top of the figure.
Expression profiles of farnesol-responsive genes.
In order to unravel the quorum-sensing-dependent mechanisms governing the choice of the growth form, we focused on the functional categories identified after 10 min of growth. We reasoned that transcriptional variations observed after 10 min imply that the cells have already sensed the new conditions and selected their next growth mode. Figure 7 presents the responses of genes with farnesol-modulated early variation during the resumption of growth at 37°C with farnesol, at 30°C, and at 37°C. Farnesol has a major and expected impact on the hypha-related genes (Fig. 7A). We observed a delay in the induction of HWP1, ECE1, and RBT1 as well as a reduction in their level of induction. The mix of cellular forms in the presence of farnesol (Fig. 4b) does not explain the reduction of the level of induction as simply an intermediary result between the extremes of 30°C (yeast cells) and 37°C (hyphal cells), because farnesol results in a total absence of induction for HWP1, ECE1, and RBT1 10 min after the inoculation, and PHR1 remains noninduced through the whole time course. One hypothesis could be a direct impact of farnesol on these genes, with a partial transcriptional inhibition impairing hyphal growth. However, none of these hypha-related genes have yet been demonstrated as essential for hyphal development (7). So far, the only hypha-specific gene needed for hyphal induction is HGC1, which encodes a G1 cyclin (36). The early induction of HGC1 during hyphal growth, which is blocked by the presence of farnesol, supports the importance of this gene in the morphogenesis control (Fig. 7B). Other cyclin-related genes present an early specific profile in the presence of farnesol: the G1/S cyclin gene CLN3 is repressed, while the G1/S cyclin gene PCL2 no longer displays the strong repression observed during hyphal growth. Similarly, the repression of the histone genes during hyphal growth is blocked in the presence of farnesol in fresh medium or at 30°C (Fig. 7C). This tendency is amplified after 60 min, with the induction of the histone genes dramatically opposed to their repression at 37°C without farnesol.
FIG. 7.
A selection of genes with different profiles due to farnesol. Genes from some of the functional categories described in Fig. 6 at time 10 min were selected, and their profiles in response to the 37°C plus farnesol, 30°C, and 37°C experiments are presented. The data correspond to the ratio between the times 10, 30, 60, or 180 min after the dilution versus time zero. A, cell wall/hyphal genes; B, cell cycle genes; C, chromatin/histone genes; D, β-oxidation/glyoxylate cycle genes; and E, drug response genes.
The continued presence of farnesol can trigger specific early responses (Fig. 7D and E). The glyoxylate and β-oxidation pathways are induced by starvation or during macrophage internalization (19). Following exit from stationary phase, we observed a reduction of expression of some of these genes (see supplementary web data); this repression is enhanced by the presence of farnesol at the earlier time points (Fig. 7D). We also observed specific induction of the drug response genes CDR1 and CDR2, as well as the putative aryl-alcohol dehydrogenase YPL88 that is regulated by the multidrug transcriptional factors Yrm1 and Yrr1 in Saccharomyces cerevisiae (20).
DISCUSSION
This study investigates the mechanism of hyphal induction triggered by the resumption of growth of Candida albicans cells held in stationary phase. Changing the medium of stationary cells is a strong but transient way to generate hyphae by releasing the inhibition caused by the conditioned medium. This phenomenon represents a major component of the process of serum-induced hyphal development (24). The quorum-sensing molecule farnesol is implicated in this process, but its inability to completely inhibit hyphal development suggests the possible existence of other unknown inhibitory molecules. We studied the transcriptional changes due to growth resumption in the presence of farnesol in fresh medium at 37°C to mimic a continuous farnesol concentration throughout the experiment. We identified the classes of genes whose expression was influenced by the presence of farnesol through comparisons among experiments at 37°C, at 30°C, and at 37°C in presence of farnesol. This strategy allowed us to distinguish between the classes identified because of the morphological differences created by the farnesol (cell adhesion, cell wall formation, chromatin, DNA replication, and cell cycle) and those due specifically to the continued presence of farnesol (drug response and fatty acid oxidation).
Several genes of interest that were influenced by farnesol were identified. The hypha-specific genes RBT1, ECE1, and HWP1 show delayed and reduced induction in the presence of farnesol. However, as these genes are not necessary for hyphal formation, their inhibition likely reflects an effect of the block in hyphal development rather than a cause. Drug response genes are quickly and significantly induced by the continued presence of farnesol in the fresh medium, a response similar to that generated by antimycotic drugs (25, 27, 30). This induction of the Candida drug resistance genes was surprising because 30 μM farnesol should be similar to the farnesol concentration in the conditioned medium of stationary cells (15). The appearance of stress response genes in a specific response to farnesol may indicate that the presence of the quorum-sensing molecule in the fresh medium is creating damage in the cells. However, this farnesol concentration does not reduce growth (15, 29; our unpublished data). Moreover, the glyoxylate and β-oxidation pathways are not induced as they are in other stress conditions (19; supplementary web data) but in fact are repressed (Fig. 7D). We hypothesize that this response could reflect a misinterpretation of the situation by the cells, due to the artificial presence of the stationary-related quorum-sensing molecule in the new medium.
The presence of cell cycle and histone genes under the influence of farnesol can be more easily related to the choice of the growth form. The set of histone genes is repressed under the hyphal growth conditions and up-regulated at 30°C or in the presence of farnesol. It is likely that their induction reflects the growth requirement of the cells. However, this does not explain the need to reduce the amount of histones during hyphal growth. This suggests other possible functions for histone regulation, especially in morphogenesis. In mammalian cells, hydroxyurea creates a rapid decrease of the histone mRNA pool (3), while in Candida albicans, hydroxyurea is able to induce filament formation (1). It is therefore possible that a decrease in the amount of histone message plays a role in hyphal development. The presence of farnesol in the fresh medium affects the expression profiles of the cell cycle genes PCL2, HGC1, and CLN3. These three genes possess putative or demonstrated function in morphogenesis. In S. cerevisiae, pcl1/2 family mutants display an abnormal cell morphology upon starvation and random budding in diploids (18). The cyclin HGC1 is the only reported hypha-induced gene necessary for hyphal growth (36). Repression of the cyclin gene CLN3 causes yeast cells to develop into hyphae and pseudohyphae (2). The presence of farnesol prevents the PCL2 repression and the HGC1 induction and represses CLN3. Therefore, these cyclins are attractive as potential links between farnesol and morphogenesis.
The triggering of hyphal development through growth resumption of stationary-phase cells appears to be a major component of the standard serum induction process: even if serum brings robustness to this process, its role in initiation may be modest. This could explain some of the difficulties encountered in understanding the mechanism of serum induction (16). Dilution of stationary cells provides a clear bias in the interpretation of results related to the C. albicans morphology switch; true exponential cells should always be used to measure the influence of molecules on hyphal growth. This could help resolve discrepancies in the literature about the effect of molecules that are described as both inducers and repressors of hyphal formation (26).
There have been many investigations aiming to demonstrate which of the yeast or hyphal states represents the virulent form of C. albicans in the host. Odds (26) concluded that both forms are implicated in virulence. One clue is the presence of both yeast cells and hyphae in the majority of established candidosis cases. The suggestion is that each form possesses its own function: hyphae would carry the yeast infection through the cell walls, and yeast would efficiently spread in the bloodstream. Based on this work, we propose that the first step of infection by isolated cells would involve yeast form growth. Yeast cells surrounded by others will be in a high concentration of farnesol and other inhibitors, while cells in contact with the host cells or otherwise isolated will be relieved of this inhibition. Thus, the latter cells would be able to induce hyphae that would allow them to adhere better to the host cell walls and to progress through in order to carry the infection elsewhere (26). This hypothesis could explain the presence of the different types of C. albicans morphologies and the mechanism of their creation.
Acknowledgments
This work was supported by the Genomics and Health Initiative of the National Research Council of Canada, by grant CRP004 from the British Council/NRC Collaborative Research Program, and by CIHR grant MOP-42516 to M.W. B.E. received an NSERC visiting fellow award through the GHI.
We are grateful to Alistair J. P. Brown and Neil A. R. Gow for comments, and we thank members of the Genetics group for their support.
Footnotes
This is National Research Council publication number 47468.
REFERENCES
- 1.Bachewich, C., D. Y. Thomas, and M. Whiteway. 2003. Depletion of a Polo-like kinase in Candida albicans activates cyclase-dependent hyphal-like growth. Mol. Biol. Cell 14:2163-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachewich, C., and M. Whiteway. 2005. Cyclin Cln3p links G1 progression to hyphal and pseudohyphal development in Candida albicans. Eukaryot. Cell 4:95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumbach, L. L., G. S. Stein, and J. L. Stein. 1987. Regulation of human histone gene expression: transcriptional and posttranscriptional control in the coupling of histone messenger RNA stability with DNA replication. Biochemistry 26:6178-6187. [DOI] [PubMed] [Google Scholar]
- 4.Bell, W. M., and W. L. Chaffin. 1983. Effect of yeast growth conditions on yeast-mycelial transition in Candida albicans. Mycopathologia 84:41-44. [DOI] [PubMed] [Google Scholar]
- 5.Bell, W. M., and W. L. Chaffin. 1980. Nutrient-limited yeast growth in Candida albicans: effect on yeast-mycelial transition. Can. J. Microbiol. 26:102-105. [DOI] [PubMed] [Google Scholar]
- 6.Berman, J., and P. E. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918-930. [DOI] [PubMed] [Google Scholar]
- 7.Brown, A. J. P. 2002. Expression of growth form-specific factors during morphogenesis in Candida albicans, p. 87-93. In R. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 8.Cao, Y. Y., Y. B. Cao, Z. Xu, K. Ying, Y. Li, Y. Xie, Z. Y. Zhu, W. S. Chen, and Y. Y. Jiang. 2005. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob. Agents Chemother. 49:584-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapa y Lazo, B., S. Bates, and P. Sudbery. 2005. The G1 cyclin Cln3 regulates morphogenesis in Candida albicans. Eukaryot. Cell 4:90-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, H., M. Fujita, Q. Feng, J. Clardy, and G. R. Fink. 2004. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. USA 101:5048-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enjalbert, B., A. Nantel, and M. Whiteway. 2003. Stress-induced gene expression in Candida albicans: absence of a general stress response. Mol. Biol. Cell 14:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst, J. F. 2000. Transcription factors in Candida albicans environmental control of morphogenesis. Microbiology 146:1763-1774. [DOI] [PubMed] [Google Scholar]
- 13.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 14.Gow, N. A. R., A. J. P. Brown, and F. D. Odds. 2002. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5:366-371. [DOI] [PubMed] [Google Scholar]
- 15.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson, D. A., Q. L. Sciascia, R. J. Sanders, G. E. Norris, P. J. B. Edwards, P. A. Sullivan, and P. C. Farley. 2004. Identification of the dialysable serum inducer of germ-tube formation in Candida albicans. Microbiology 150:3041-3049. [DOI] [PubMed] [Google Scholar]
- 17.Kadosh, D., and A. D. Johnson. 2005. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Mol. Biol. Cell 16:2903-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenburg, M. E., and E. K. O'Shea. 2001. Genetic evidence for a morphogenetic function of the Saccharomyces cerevisiae Pho85 cyclin-dependent kinase. Genetics 157:39-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenz, M. C., J. A. Bender, and G. R. Fink. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 3:1076-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucau-Danila, A., T. Delaveau, G. Lelandais, G. Devaux, and C. Jacq. 2003. Competitive promoter occupancy by two yeast paralogous transcription factors controlling the multidrug resistance phenomenon. J. Biol. Chem. 278:52641-52650. [DOI] [PubMed] [Google Scholar]
- 21.Mattia, E., and A. Cassone. 1979. Inducibility of germ tube formation in Candida albicans at different phases of yeast growth. J. Gen. Microbiol. 133:439-442. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell, L. H., and D. R. Soll. 1979. Commitment to germ tube or bud formation during release from stationary phase in Candida albicans. Exp. Cell Res. 120:167-179. [DOI] [PubMed] [Google Scholar]
- 23.Montazeri, M., and H. G. Hedrick. 1984. Factors affecting spore formation in a Candida albicans strain. Appl. Environ. Microbiol. 47:1341-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nantel, A., D. Dignard, C. Bachewich, D. Harcus, A. Marcil, A.-P. Bouin, C. W. Sensen, H. Hogues, M. van het Hoog, P. Gordon, T. Rigby, F. Benoit, D. C. Tessier, D. Y. Thomas, and M. Whiteway. 2002. Transcription profiling of C. albicans cells undergoing the yeast to hyphal transition. Mol. Biol. Cell 13:3452-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niewerth, M., D. Kunze, M. Seibold, M. Schaller, H. C. Korting, and B. Hube. 2003. Ciclopirox olamine treatment affects the expression pattern of Candida albicans genes encoding virulence factors, iron metabolism proteins, and drug resistance factors. Antimicrob. Agents Chemother. 47:1805-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Bailliere Tindall, London, United Kingdom.
- 27.Rogers, P. D., and K. S. Barker. 2003. Genome-wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Antimicrob. Agents Chemother. 47:1220-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd, M. G., C. Y. Yin, S. P. Ram, and P. A. Sullivan. 1980. Germ tube induction in Candida albicans. Can. J. Microbiol. 26:21-26. [DOI] [PubMed] [Google Scholar]
- 29.Smith, D. A., S. Nicholls, B. A. Morgan, A. J. P. Brown, and J. Quinn. 2004. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol. Biol. Cell 15:4179-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, W. L., and T. D. Edlind. 2002. Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: correlation with reduction in CDR and ERG upregulation. Antimicrob. Agents Chemother. 46:3532-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soll, D. R., and G. W. Bedell. 1978. Bud formation and the inducibility of pseudo-mycelium outgrowth during release from stationary phase in Candida albicans. J. Gen. Microbiol. 108:173-180. [Google Scholar]
- 32.Soll, D. R., B. Morrow, and T. Srikantha. 1993. High-frequency phenotypic switching in Candida albicans. Trends Genet. 9:61-65. [DOI] [PubMed] [Google Scholar]
- 33.Soll, D. R. 1997. Gene regulation during high-frequency switching in Candida albicans. Microbiology 143:279-288. [DOI] [PubMed] [Google Scholar]
- 34.Sudbery, P., N. Gow, and J. Berman. 2004. The distinct morphogenic states of Candida albicans. Trends Microbiol. 12:317-324. [DOI] [PubMed] [Google Scholar]
- 35.Whiteway, M., and U. Oberholzer. 2004. Candida morphogenesis and host-pathogen interactions. Curr. Opin. Microbiol. 7:350-357. [DOI] [PubMed] [Google Scholar]
- 36.Zheng, X. D., Y. M. Wang, and Y. Wang. 2004. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 23:1845-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]