Abstract
The fission yeast Schizosaccharomyces pombe grows in a single-celled form or can mate and undergo meiosis and sporulation. Here we show that wild-type S. pombe can also differentiate to form elaborately branched hyphae which invade deep into solid medium. Branches appear in the hyphae adjacent to unseparated septa. Electron microscopy reveals unusual multivesicular structures within the hyphae. Nitrogen deprivation appears to be the main stimulus for hyphal growth. No mitogen-activated protein kinase is necessary for the response. Inhibition of cyclic AMP (cAMP) production or signaling prevents the response, and exogenous cAMP promotes it, suggesting that detection of a good carbon source is required for hyphal growth but not for mating.
Yeasts are widely used as model eukaryotic cells since their genetic tractability allows detailed analyses of many aspects of a simple unicellular life cycle. However, many yeasts are, in addition, capable of differentiating into a variety of filamentous or hyphal growth forms. In the budding yeast Saccharomyces cerevisiae, limited or poor nitrogen sources can trigger such a growth switch (reviewed in references 4 and 5), which may be considered as an adaptive, foraging response to nutrient deprivation. In pathogenic yeasts such as Candida, hyphal growth can be induced by serum and has been implicated in the process of infection (15). Thus, the study of these growth transitions can provide both a system to study eukaryotic differentiation and polarized growth in molecular detail and an important insight into pathogenicity.
The fission yeast Schizosaccharomyces pombe provides a model eukaryote which differs greatly from budding yeasts. In several respects, it may be a better model for metazoans, having apparently diverged less from their common ancestor than have the budding yeasts (17). Control of the fission yeast cell cycle has been particularly well studied. In contrast to budding yeasts, S. pombe is a rod-shaped cell, growing at each end at different stages of the cell cycle, and much is known of the cytoskeleton-dependent events which underlie these growth transitions (see, e.g., reference 16). Unlike S. cerevisiae, S. pombe mates in response to starvation; cyclic AMP (cAMP) and mitogen-activated protein (MAP) kinase pathways involved in this response are similar to those required by S. cerevisiae for invasive growth (5, 11, 21). Thus, as a system to analyze changes in growth polarity and signaling pathways, S. pombe would be an attractive organism in which to study hyphal differentiation, but such a growth pattern has apparently not so far been reported for this organism.
However, Sipiczki and colleagues (19, 20) have shown that another fission yeast, Schizosaccharomyces japonicus, can be induced to form invasive filamentous structures, showing that not all fission yeasts lack this ability. In addition, the original description of S. pombe (7) (reproduced at http://www-rcf.usc.edu/∼forsburg/history/lindner2.html) shows distinctly elongated and branching structures, very different from the uniform single cells of current laboratory strains. We therefore investigated whether conditions could be found to induce hyphal growth in S. pombe. Here we describe such conditions for two different isolates and the resulting structures. We show that the signaling pathways required to induce the response differ from those operating in filamentous growth of either the budding yeast S. cerevisiae or the fission yeast S. japonicus.
MATERIALS AND METHODS
Strains used in this study are described in Table 1.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source | Hyphal growth |
|---|---|---|---|
| NCYC132 | h90 wild type | National Collection of Yeast Cultures, Norwich, United Kingdom | Yes |
| 972 | h− wild type | Our stock | Yes |
| 975 | h+ wild type | Our stock | Yes |
| 557 | h+ade6-210, ura4-D18, leu1-32 | Our stock | Yes |
| 611 | h+/h−ade6-M210/ade6-M216, ura4-D18/ura4-D18, leu1-32/leu1-32 | Our stock | Yes |
| CB55 | h90 spk1::ura4+, leu1, ura4-D18 | D. Hughes | Yes |
| JM1160 | h+sty1::ura4, ade6-m20, leu1-32, ura4-D18 | J. Millar | Yes |
| TP319-31A | h−pmk1::ura4+, leu1, ura4 | T. Toda | Yes |
| JZ633 | h90 pka1::ura4, ade6-m216, leu1-32, ura4-D18 | A. Carr | No |
| No. 10 | h+cyr1::leu2, leu1-32, ura4-D18 | A. Carr | No |
| JZ666 | h90 cgs2::ura4, ura4-D18, leu1-32, ade6-M216 | C. Hoffman | Yes |
| RWP38 | h−cgs1::ura4+, Ura4::fbp1-lacZ, leu1-32 | C. Hoffman | Yes |
| SLP47 | h+git11::kan, ura4::fbp1-lacZ, leu1-32, his7-366 | C. Hoffman | No |
| RWP29 | h+git3::kan, ura4::fbp1-lacZ, leu1-32 | C. Hoffman | No |
| CHP460 | h−gpa2::ura4 +, ura4::fbp1-lacZ, leu1-32, his7-366 | C. Hoffman | No |
| CHP463 | h−git5-1::his7+, ura4::fbp1-lacZ, leu1-32, his7-366 | C. Hoffman | No |
Rich (yeast extract with supplements), standard minimal (Edinburgh minimal medium [EMM]), and nitrogen-free minimal (EMM-N) media were as previously described (12). Unless otherwise stated, hyphae were formed on LNB medium, consisting of 0.067 g/liter yeast nitrogen base without amino acids (Bacto), 20 g/liter glucose, 20 g/liter agar, and salts and vitamins as for EMM. Variations to these media were as described in the Results section.
To test the effect of cAMP analogues on hyphal growth, a 2.5-cm filter disk was soaked in 40 μl of 0.1 M 8-Br-cAMP (8-bromoadenosine 3′,5′-cyclic monophosphate; Sigma) and placed on an LNB plate. S. pombe cells were then inoculated approximately 1 cm from the disk, and growth was observed.
For microscopy of hyphal colonies, a column approximately 2 mm square was cut from the agar at the edge of the colony, and the top 2 mm was excised, flattened between a slide and coverslip, and observed using a Zeiss LiveCell imaging system, equipped with Nomarski optics and objectives of 10×, 20×, or 40× (1). z-axis stacks of images were collected from unflattened blocks at 5-μm intervals through a total depth of 1 mm. Images were displayed using Zeiss Axiovision software and were further processed with Adobe Photoshop.
For labeling of septa, agar blocks were cut as above and then labeled with 1 mM centrifuged calcofluor (Fluorescent Brightener 28; Sigma) (13). The blocks were flattened under a coverslip to allow detection of fluorescence and observed by fluorescence and transmission microscopy with a Zeiss LiveCell microscope.
For electron microscopy, unflattened agar blocks were cut as above, fixed overnight in 4% formaldehyde-2.5% glutaraldehyde, and then postfixed in 2% osmium tetroxide for 4 h. The samples were dehydrated in an ethanol series and embedded in Spurr resin. Thin sections were cut by microtome and, optionally, poststained with 0.5% uranyl acetate for 1 h. Sections were examined in a Hitachi-7100 transmission electron microscope at 100 kV, and images were acquired with an axially mounted Gatan Ultrascan 1000 CCD camera.
RESULTS
Elongated and branched growth in S. pombe NCYC132.
Most current laboratory stocks of S. pombe derive from strain 972, as originally described by Leupold (6). This strain is heterothallic; that is, it does not switch mating types readily. However, earlier work described other isolates, including NCYC132, which is homothallic (9). After prolonged storage, this strain developed elongated structures on the surface of the agar that were quite distinct from normal vegetatively growing cells (H. Levin, personal communication).
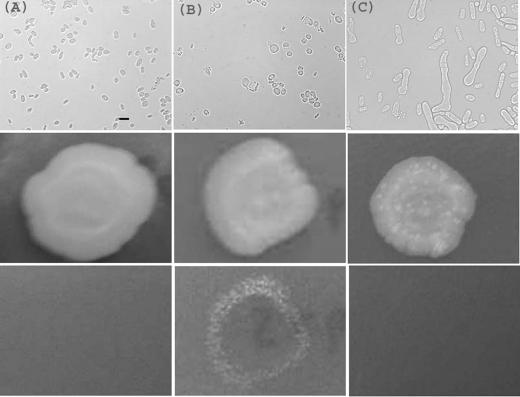
Since hyphal growth in other yeasts, including S. japonicus (19), is stimulated by starvation, we investigated the effects of growing S. pombe NCYC132 on various media. On agar containing only 20 g/liter glucose and 1% of the standard concentration of yeast nitrogen base, after 14 days at 37°C, this strain developed highly extended and branched structures which we term pseudohyphae (see references 19 and 20 and Discussion for an explanation of this usage) (Fig. 1C, top frame). At 27°C, no elongation occurred (Fig. 1A), but mating and meiosis were occasionally observed. At 30°C, no elongated growth or mating was apparent on the surface of the agar (Fig. 1B). However, invasive growth into the agar took place. This was demonstrated by resistance of some of the colony to washing (Fig. 1, middle and bottom panels). The resistant cells took the form of a ring at the edge of the colony, indicating that only in these regions does invasion occur. Microscopic imaging (Fig. 2) revealed elaborately branched three-dimensional structures, which we term hyphae.
FIG. 1.
Pseudohyphal and invasive growth of S. pombe NCYC132. Yeast were grown at 27 (A), 30 (B) or 37°C (C) for 14 days on agar containing 20 g/liter glucose and 1% of the standard level of yeast nitrogen base. Cells were imaged microscopically by transmission optics (top frames; bar, 10 μm), or the whole colony (approximately 1-cm diameter) was photographed before (middle frames) or after (bottom frames) the plate was washed. At 37°C, branched pseudohyphae are visible (C, top frame). At 30°C, resistance to washing (B, bottom frame) indicates invasion of the medium.
FIG. 2.
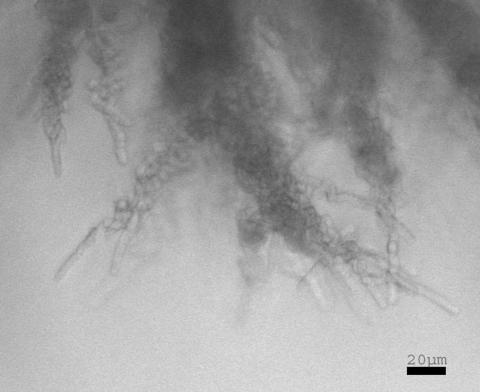
Hyphae of strain NCYC132. Cells within flattened agar blocks were imaged by Nomarski optics. Bar, 10 μm.
Various other media were tested. The same medium supplemented with salts and additional vitamins (LNB medium; see Materials and Methods) was also found to promote hyphal growth and was used for subsequent experiments.
Thus, under appropriate conditions S. pombe NCYC132 can form extensively branched and invasive hyphae. The medium that promoted this growth contained an abundant supply of carbon and most other obvious nutrients but was limited in nitrogen; thus, lack of nitrogen might trigger this alternative mode of growth.
Hyphal growth in S. pombe 972.
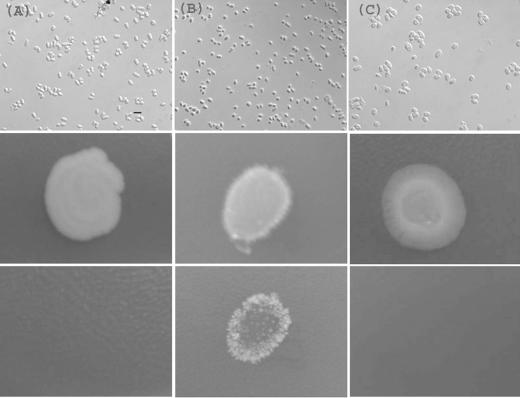
Having identified conditions for hyphal growth in the rarely studied strain NCYC132, we investigated the effect of these conditions on the common laboratory strain 972. At 27°C, this strain, which is heterothallic, showed slow unicellular growth (Fig. 3A). At 37°C, the strain also grew slowly but showed signs of cell death (Fig. 3C). At 30°C, however, as with strain 132, this strain developed branched and invasive hyphae after 2 weeks (Fig. 3B).
FIG. 3.
Invasive growth of the common S. pombe laboratory strain 972. Cells were grown for 14 days on LNB medium at 27°C (A), 30°C (B) or 37°C (C) and imaged as described in the legend of Fig. 1. Invasive growth (B, bottom frame) occurred under the same conditions as for strain NCYC132, but pseudohyphae were not observed.
As strain 972 is of h− mating type, we tested the response of strain 975, which is h+ but otherwise thought to be isogenic (3). Strain 975 behaved indistinguishably from strain 972 (not shown), indicating that mating type is not a determinant of hyphal growth.
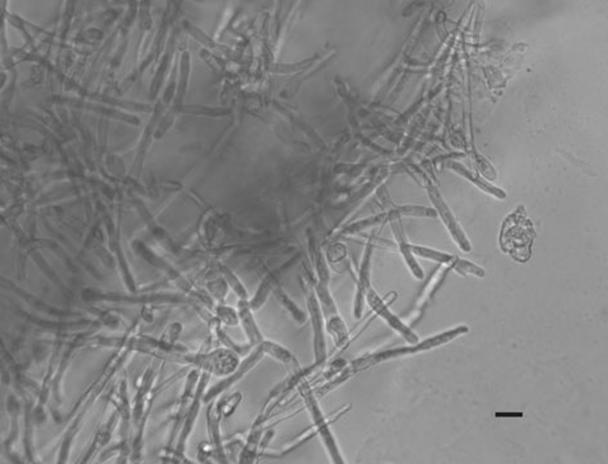
To gain a clearer picture of the three-dimensional structure of S. pombe hyphae, transmission images were collected from unflattened agar blocks in a series of focal planes through a depth of 1 mm, or approximately 100 normal cell lengths. A selection of these images at low and high magnification (Fig. 4 and 5; see also supplemental material) emphasized the elaborate branching structures at different levels through the medium.
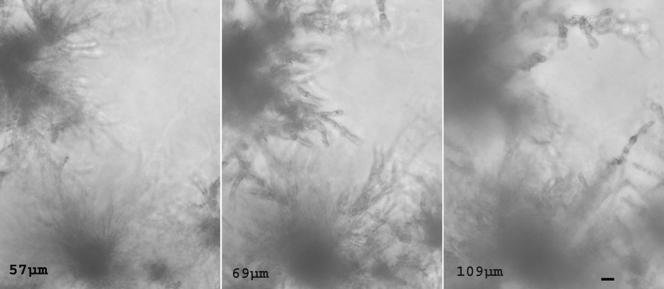
FIG. 4.
Three-dimensional organization of hyphae in strain 972. Images were collected at various distances below the surface of the agar as indicated (in μm). Bar, 10 μm.
FIG. 5.
Detailed view of the growing tip regions of hyphae, from approximately 0.7 mm below the surface. Bar, 20 μm.
Conditions for hyphal growth versus mating.
The results above suggest that lack of nitrogen is a primary stimulus for hyphal growth. However, this is also a stimulus for mating, raising the question of how these responses are distinguished. This was investigated in two ways. First, equal numbers of cells of strains 972 and 975 were plated on LNB medium and incubated at 30°C. These formed hyphae indistinguishably from the individual strains and showed no sign of mating (not shown). Second, the possibility of hyphal formation in conditions usually used to promote mating was tested. A typical medium for mating of S. pombe is the standard minimal agar but with the nitrogen source removed (EMM-N). Unlike LNB, this medium is buffered and contains a phosphate source. Mixtures of the two strains of opposite mating type, 972 and 975, mate on this medium at 27°C and, less efficiently, at 30°C. Strain 972 alone at 27°C showed little growth or other response on EMM-N. However, at 30°C after 4 weeks, invasive hyphae had begun to form (not shown), albeit much less efficiently than on LNB medium. Thus, under some conditions the same strain may either mate or form hyphae to some extent, depending only on the presence of a mating partner. Neither phosphate starvation nor lack of buffering in the medium is absolutely required for hyphal growth.
Two other potential stimuli of hyphal growth were investigated. First, cells were plated on EMM containing 1% of the normal level of sulfate. Second, glucose was replaced with glycerol in the medium. Neither of these changes was observed to produce hyphae. Thus, the only conditions so far identified to result in hyphal growth are a combination of a limited source of nitrogen and a rich source of carbon and energy, within a temperature range around 30°C.
Auxotrophy and hyphal formation.
Many laboratory strains of S. pombe, while derived from strain 972, carry one or more auxotrophies, for example, in adenine, leucine, or uracil. We tested mutants in ade6, leu1, and ura4 for hyphal growth on LNB medium as described above. No growth of any sort occurred, presumably due to the lack of appropriate nutritional supplements. When these were added, unicellular growth occurred, but there was no indication of hyphal formation (not shown). We then tested the prototrophic strain 972 on LNB medium but with the addition of the supplements adenine, leucine, or uracil. Only single-celled growth occurred, with no indication of hyphal formation (not shown). Since each of these nutritional supplements represents a significant source of nitrogen, the minimum level of each to allow growth of auxotrophs may also provide a general nitrogen source. Thus, these results are consistent with the idea that nitrogen depletion is a stimulus for hyphal growth.
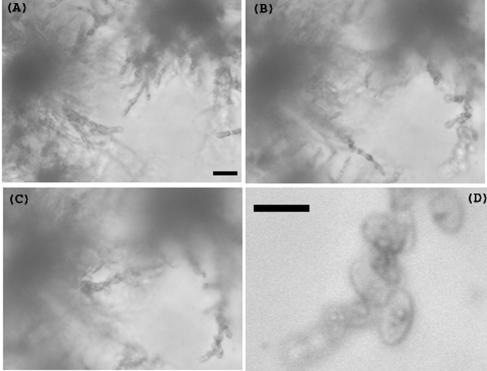
If deprivation of a nutrient such as nitrogen triggers hyphal formation, the response could be interpreted as a means to explore the cell's surroundings for better sources of nutrient. To investigate this possibility, we tested the response of S. pombe to growth on LNB agar with an underlying layer of rich medium (yeast extract with supplements; see Materials and Methods). This medium supported hyphal growth well. Under these conditions, several different auxotrophic strains formed hyphae (Fig. 6), showing that auxotrophy per se does not prevent hyphal growth and suggesting that the necessary nutrient could diffuse through the agar to allow growth but not sufficiently to provide a good overall supply of nitrogen.
FIG. 6.
Hyphal formation by the multiply auxotrophic strain 557 (Table 1). Cells were grown for 14 days on double-layered agar, as described in Materials and Methods, and images were collected at depths as marked (in μm). Bar, 10 μm.
This two-layered medium allowed us to investigate hyphal growth in a diploid strain. Diploids of S. pombe are generally unstable and occur principally as intermediates between mating and sporulation. However, such strains can be propagated by the use of interallelic complementation of alleles in the ade6 gene (12). One of these, strain 611 (Table 1), was inoculated on the two-layered medium and found to form hyphae which appeared indistinguishable from those formed by haploids (not shown). A very few spores were visible on the surface of the colony, but no more than could be observed with this strain on any growth medium, and this result was quite distinct from the efficient sporulation undergone on mating medium. Thus, the hyphal growth medium promotes neither mating nor meiosis.
Reversibility of hyphal differentiation.
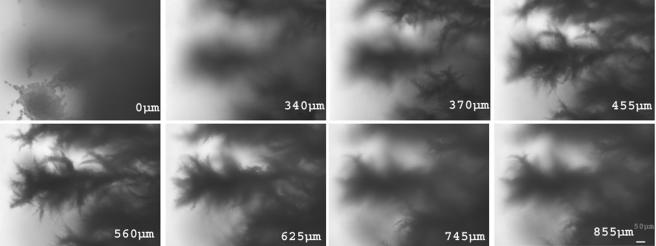
The two-layered growth medium described above should result in hyphae growing into regions in which the nitrogen source is relatively rich, raising the question of whether the growth pattern could reverse. Mycelia were examined from deep within the two-layered plates (Fig. 7). This showed structures that appeared to resemble single cells, although they were still attached to the hyphal structure. Thus, when hyphae extend into regions of rich medium, the growth pattern reverts to one more reminiscent of single cells.
FIG. 7.
Reversibility of hyphal differentiation. Hyphae were formed on double-layered agar as described, and images were taken at 335 (A), 545 (B), and 625 (C and D) μm below the top of the agar block, moving toward the layer of rich medium. (D) Higher magnification of the lower left corner of panel C showing structures resembling single yeast cells. Bar, 20 μm.
Morphology of hyphal septa.
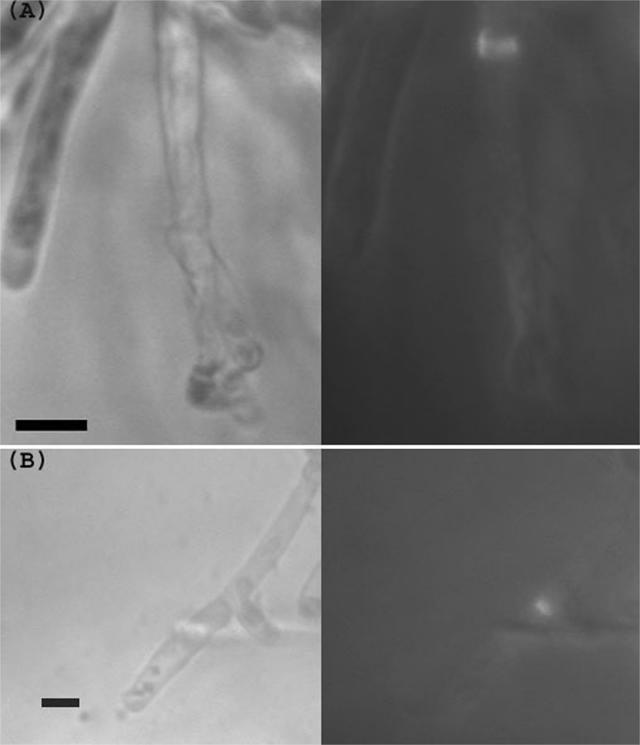
Hyphae and pseudohyphae in different ascomycetes may contain septa, which can contribute to formation of a variety of multicellular structures (14). Septa in S. pombe hyphae were observed by transmission and fluorescence optics. Sections were cut from the agar and labeled with calcofluor. Under these conditions calcofluor did not appear to permeate and label the cells efficiently. However, labeled septa were sometimes evident within the filaments; usually a septum was also apparent by transmission optics (Fig. 8A). Occasionally a labeled septum was detected immediately adjacent to a branch point (Fig. 8B), similar to the branch points described in hyphae of S. japonicus (19).
FIG. 8.
Septa in S. pombe hyphae. Hyphae were excised from the agar, labeled with calcofluor, and imaged by transmission optics (left) or UV fluorescence (right). (A) A labeled septum within a filament. (B) A septum apparently adjacent to the point at which a branch has formed. Bar, 10 μm.
Electron microscopy of hyphae.
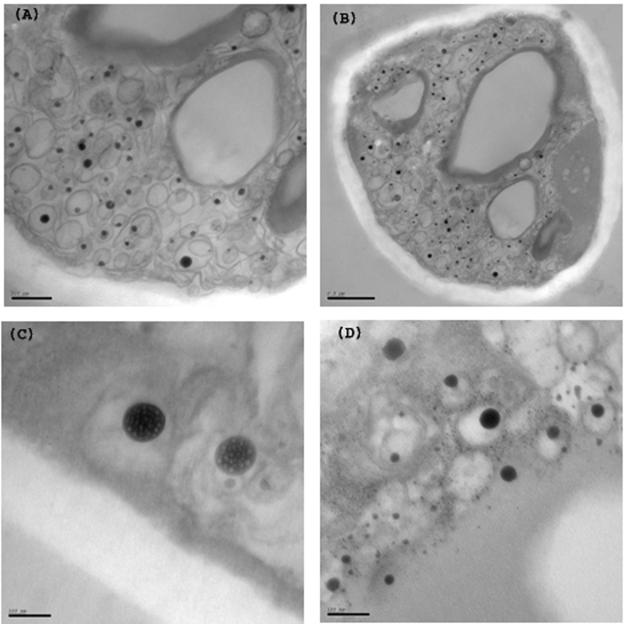
The ultrastructure of S. pombe hyphae was investigated by electron microscopy, using aldehyde fixation and osmium tetroxide staining (Fig. 9). This revealed various structures not observed in single-celled yeast. First, large irregular vacuoles were apparent with thickened walls. Second, there was a massive accumulation of distinctive vesicular structures within the hyphae. These had complex multivesicular morphologies. Within an outer membrane were heavily labeled spherical structures of diameters up to 100 nm, each surrounded by a membrane. Within these, lighter-stained granules 5 to 10 nm in size were visible (Fig. 9C and D; see also supplemental material).
FIG. 9.
Electron microscopy of S. pombe hyphae. Aldehyde-fixed and osmium tetroxide-treated agar blocks containing hyphae were sectioned and examined. (A and B) Sections showing irregular vacuolar structures with thickened edges and multivesicular membrane compartments containing heavily stained internal vesicles. (C and D) Higher-magnification images showing small granules within the vesicles. Panel D is additionally poststained with uranyl acetate. Bars = 500 μm (A), 200 μm (B), and 100 μm (C and D). See also supplemental material.
Genetic analysis of hyphal growth.
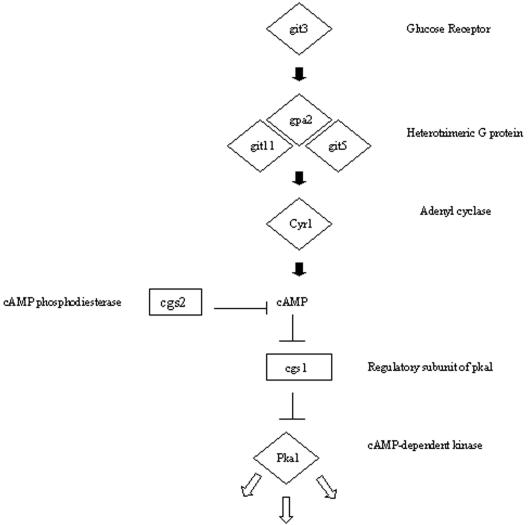
Invasive growth in the budding yeast S. cerevisiae requires a MAP kinase signaling pathway, most of whose components are shared by the pathway required for pheromone response in mating (5). S. pombe has a similar signaling pathway for mating. However, unlike in S. cerevisiae, mating is initiated by starvation for nitrogen or carbon, which leads to a fall in cAMP and induction of pheromone synthesis and of the MAP kinase pathway (5, 11), raising two particular questions. First, how do the signaling requirements for invasive growth compare between the two divergent yeasts? Second, if nitrogen limitation promotes both mating and hyphal growth, how are the two responses differentiated? To begin to answer these questions, various mutants defective in these and two other signaling pathways were tested for hyphal growth (Table 1 and Fig. 10). The results show that neither the MAP kinase involved in the pheromone response, Spk1, nor the MAP kinases acting in stress and cell integrity responses, Sty1 and Pmk1, respectively, are required for hyphal growth. Deletion of components of the cAMP pathway, however, gave differing results. Hyphal growth was prevented by loss of any one of the following: the receptor Git3, the trimeric G-protein γ subunit Git11, the adenylyl cyclase Cyr1, or the cAMP-dependent protein kinase A Pka1. In contrast, loss of either the Pka1 inhibitory subunit Cgs1 or the cAMP phosphodiesterase Cgs2 did not prevent hyphal growth. Thus, in contrast to S. cerevisiae, invasive growth in S. pombe does not appear to require the pheromone-responsive MAP kinase pathway. However, it does depend on several components of the cAMP pathway (Fig. 10).
FIG. 10.
Outline of components in the S. pombe cAMP pathway. Diamond, deletion of the gene prevents hyphal growth and causes constitutive mating without starvation; rectangle, deletion of the gene does not prevent hyphal growth but causes sterility.
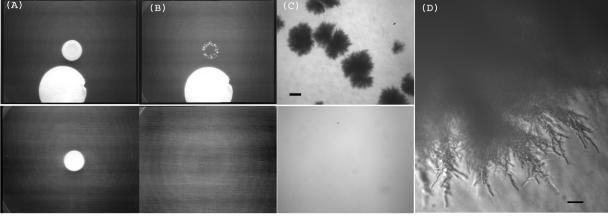
The mutants in Cgs1 and Cgs2 formed hyphae efficiently, and in addition we observed a tendency for them to form more rapidly than in wild-type cells (not shown). This raised the possibility that externally applied cAMP might influence hyphal growth. To test this, hyphae were formed in patches adjacent to a filter soaked in the hydrolysis-resistant and membrane-permeant analogue 8-Br-cAMP. After 1 week, the surface cells were removed by washing (Fig. 11A and B). Control cells showed no significant hyphal invasion by this time. In contrast, hyphae had clearly formed from the cells exposed to the cAMP analogue. These appeared similar to hyphae formed naturally (Fig. 11C and D). Thus, an exogenous source of cAMP can stimulate hyphal growth to beyond the normal rate of formation.
FIG. 11.
Stimulation of hyphal growth by cAMP. An aliquot of 2.5 × 107 cells was plated on LNB medium next to a 2.5.-cm filter disk soaked in 8-Br-cAMP (A and B, top frames) or with no disk (A and B, bottom frames). The cultures were incubated for 7 days, photographed (A), and washed and photographed again (B). Invasion is clearly visible after the addition of 8-Br-cAMP. (C and D) Higher-power images of the same hyphae. Bars = 400 μm (C) and 20 μm (D).
DISCUSSION
Historically, one of the main attractions of studying the fission yeast S. pombe has been its simple, uniform unicellular appearance and growth pattern. We have shown here that this organism is in fact capable of a significantly greater degree of variation in growth and can differentiate in response to environmental circumstances to make complex and elaborate three-dimensional hyphal assemblies within the growth substrate. In this respect S. pombe is therefore comparable to many other dimorphic ascomycetes.
In its simplest form, growth as filaments could result from a defect in the process of cell separation. Such mutants of S. pombe have been described (18), although their phenotypes are quite distinct from the hyphae described here, particularly in the lack of substrate invasion. More usually, filamentous growth is considered to be an adaptive response to environmental change, for example, nutrient deprivation as described here and for the fission yeast S. japonicus (19, 20), or as a growth form facilitating attachment to surfaces, as appears to be the case in Candida infections (15). Hyphae can be defined as “discrete, tubular, polarized elements exhibiting a pronounced longitudinal growth pattern” (14). Initially, we observed surface structures in the S. pombe strain NCYC 132 which show a limited degree of elongation and branching (Fig. 1C), reminiscent of diagrams from the first description of S. pombe (7), and which we term pseudohyphae. In contrast, the elaborate invasive growth form we have described in both NCYC 132 and the common laboratory strain 972 appears to satisfy the definition of hyphae. These structures grow slowly as a consequence of the nutritional conditions but have no intrinsic limit that we have yet observed to their breadth or depth. The edges of the mycelia comprise long and apparently septate filaments; within the structure, however, other cell shapes may occur.
The main stimulus for S. pombe to differentiate into hyphae appears to be lack of nitrogen, as long as the cells are within a fairly narrow temperature range around 30°C and a good carbon source is present. We found no evidence that depletion of other nutrients, such as phosphorus or sulfur, could act as a stimulus. Nitrogen starvation, however, also promotes mating, raising the question of how the distinction is made between the two responses. Our results indicate possible answers to this. First, mating is most efficient at lower temperatures, typically below 30°C. Second, mating can proceed in the absence of any significant nitrogen source in the medium, whereas hyphal growth requires a small but measurable amount. This could reflect the fact that mating in S. pombe in principle only requires sufficient nutrients to reorient the cells, form the zygote, and undergo meiosis and sporulation, whereas hyphal differentiation requires net growth. Third, glucose depletion can also promote mating (2, 7), in contrast to its effect on hyphal growth, which underscores the interpretation that mating is a more general response to severe nutrient deprivation. However, the distinction between the two responses may not be absolute, as under some conditions either may occur inefficiently. In addition, since an early stage of both responses is presumably a change in the polarity of growth, some mechanistic aspects may be common to both.
What are the signaling mechanisms that result in hyphal differentiation? Work in the budding yeasts S. cerevisiae and Candida albicans has implicated several pathways and also shown the complexity and diversity of the responses. C. albicans can differentiate into hyphae in response to serum in liquid medium or on certain solid media, phenomena which appear to be distinct both mechanistically and morphologically (10). S. cerevisiae can switch to invasive growth as a haploid or to pseudohyphal growth as a diploid; for the latter alone, at least four parallel signaling pathways have been implicated (3).
One of these pathways involves the MAP kinase Kss1p, which shares several components of its activation pathway with the MAP kinase involved in response to pheromone, Fus3p (5). The S. pombe genome appears to encode only a single MAP kinase corresponding to these molecules, Spk1, which is involved in the pheromone response but not required for hyphal switching (Table 1). This difference is understandable, given that in S. pombe the Spk1 pathway, including pheromone and receptor, is not produced constitutively but is induced by starvation; it therefore cannot be involved in a primary response to starvation (5).
A second signaling pathway repeatedly implicated in hyphal signaling in other yeasts involves a G-protein-coupled receptor and cyclic AMP (4, 5, 8). In S. pombe this pathway is involved in the early stages of mating. Starvation leads to a drop in cAMP levels, which in turn results in induction of the pheromone pathway (5, 11). We found that removal of any of several components of the cAMP pathway, including adenylyl cyclase, prevented the development of hyphae. The two components which were not required for the response, cAMP phosphodiesterase and the protein kinase A inhibitory factor Cgs1, are “negative” regulators of the pathway (Fig. 10). There is a striking complementarity in the ability of these mutants to mate or differentiate into hyphae: those unable to form hyphae can mate without requiring starvation, suggesting a role for this pathway in discriminating between the two responses.
Since the conditions for hyphal growth, like mating, involve nitrogen depletion, it seems likely that cAMP levels would fall transiently, although this has not yet been measured directly. Even if this is the case, however, it does not seem to be a necessary element of the response, since deletion of cAMP phosphodiesterase or addition of a cAMP analogue appears to accelerate hyphal growth. This enigmatic role of the cAMP pathway might be clarified by work indicating that its primary role in mating is to respond to a lack of glucose rather than nitrogen, which is detected by the stress-activated Sty1 MAP kinase pathway (2, 22). However, neither Sty1 nor the third MAP kinase identified from the S. pombe genome, Pmk1, is required to form hyphae (Table 1). Thus, the cAMP pathway may be required to detect high levels of glucose for hyphal growth, but the sensor for nitrogen depletion is yet to be established.
Comparing S. pombe with the different types of hyphal growth in budding yeasts, the general requirements of high glucose, low nitrogen, and high cAMP levels are reminiscent of both pseudohyphal growth of diploid S. cerevisiae and invasive growth of C. albicans, although the signaling pathways differ (2, 10). However, there is a striking comparison with hyphal growth in another fission yeast, S. japonicus as described by Sipiczki and colleagues (19, 20). These hyphae contain unseparated septa, and branches appear adjacent to the point where growth would occur if separation had taken place. Our images so far suggest that the same is true in S. pombe (Fig. 8), and mutants defective in separation appear to branch in just this way (18). In contrast a notable difference between the species may be the role of cAMP, high levels of which applied extracellularly inhibit and reverse hyphal differentiation in S. japonicus, suggesting that intracellular cAMP must be lowered for hyphal growth. This may indicate a major difference between the two species. Alternatively, fission yeasts, like budding yeasts, may be capable of more than one type of hyphal response. The rather narrow range of conditions which we have so far identified for hyphal growth in S. pombe might suggest that other stimuli for differentiation remain to be discovered.
Our initial investigations of the morphology of hyphae in S. pombe by electron microscopy have revealed remarkable multivesicular structures and inclusions. The origin and composition of these remain to be determined, as does their development in space and time and their contribution, if any, to hyphal differentiation. Likewise, many basic questions remain concerning the behavior of nuclei, septa, and molecules implicated in directing polarized growth. An answer to several of these questions would be greatly facilitated by a method to visualize fluorescent molecules within the growing hyphae, which is currently under investigation. An investigation of the changes in gene expression which accompany hyphal switching is also under way.
Supplementary Material
Acknowledgments
We thank Henry Levin for informing us of the behavior of strain NCYC 132, James Dodgson for help with microscopic imaging, Julian Thorpe for electron microscopy, and Antony Carr, Charles Hoffman, David Hughes, Jonathan Millar, and Takashi Toda for strains. Imaging was carried out in the Sussex Centre for Advanced Microscopy.
This work was supported by a studentship from the Biotechnology and Biological Sciences Research Council to E.A.-B.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Bone, N., J. B. A. Millar, T. Toda, and J. Armstrong. 1998. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr. Biol. 8:135-144. [DOI] [PubMed] [Google Scholar]
- 2.Byrne, S. M., and C. S. Hoffman. 1993. Six git genes encode a glucose-induced adenylate cyclase activation pathway in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 105:1095-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutler, N. S., X. Pan, J. Heitman, and M. E. Cardenas. 2001. The TOR signal transduction cascade controls cellular differentiation in response to nutrients. Mol. Biol. Cell 12:4103-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagiano, M., F. F. Bauer, and I. S. Pretorius. 2002. The sensing of nutritional status and the relationship to filamentous growth in Saccharomyces cerevisiae. FEMS Yeast Res. 2:433-470. [DOI] [PubMed] [Google Scholar]
- 5.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W.-C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leupold, U. 1993. The origins of Schizosaccharomyces pombe genetics. p. 125-128. In M. N. Hall and P. Linder (ed.), The early days of yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 7.Lindner, P. 1893. Schizosaccharomyces pombe n. sp., ein neuer Gährungserreger. Wochenschr. Brauerei 10:1298-1300. [Google Scholar]
- 8.Medoff, J., E. Jacobson, and G. Medoff. 1981. Regulation of dimorphism in Histoplasma capsulatum by cyclic adenosine 3′ 5′-monophosphate. J. Bacteriol. 145:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchison, J. M., and P. Nurse. 1985. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 75:57-376. [DOI] [PubMed] [Google Scholar]
- 10.Miwa, T., Y. Takagi, M. Shinozaki, C. W. Yun, W. A. Schell, J. R. Perfect, H. Kumagai, and H. Tamaki. 2004. Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot. Cell 3:919-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochizuki, N., and M. Yamamoto. 1992. Reduction in the intracellular cAMP level triggers initiation of sexual development in fission yeast. Mol. Gen. Genet. 233:17-24. [DOI] [PubMed] [Google Scholar]
- 12.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 13.Pringle, J. R. 1991. Staining of bud scars and other cell wall chitin with calcofluor. Methods Enzymol. 194:732-735. [DOI] [PubMed] [Google Scholar]
- 14.Read, N. D. 1994. Cellular nature and multicellular morphogenesis of higher fungi. p. 251-269. In D. S. Ingram and A. Hudson (ed.), Shape and form in plants and fungi. Academic Press, London, United Kingdom.
- 15.Saville, S. P., A. L. Lazzell, C. Monteagudo, and J. L. Lopez-Ribot. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaroyt. Cell 2:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawin, K. E., and H. A. Snaith. 2004. Role of microtubules and tea1p in establishment and maintenance of fission yeast cell polarity. J. Cell Sci. 117:689-700. [DOI] [PubMed] [Google Scholar]
- 17.Sipiczki, M. 2000. Where does fission yeast sit on the tree of life? Genome Biol. 1:1011.1-1011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sipiczki, M., B. Grallert, and I. Miklos. 1993. Mycelial and syncytial growth in Schizosaccharomyces pombe induced by novel septation mutations. J. Cell Sci. 104:485-493. [DOI] [PubMed] [Google Scholar]
- 19.Sipiczki, M., K. Takeo, M. Yamaguchi, S. Yoshida, and I. Miklos, I. 1998. Environmentally controlled dimorphic cycle in a fission yeast. Microbiology 144:1319-1330. [DOI] [PubMed] [Google Scholar]
- 20.Sipiczki, M., K. Takeo, and A. Grallert. 1998. Growth polarity transitions in a dimorphic fission yeast. Microbiology 144:3474-3485. [DOI] [PubMed] [Google Scholar]
- 21.Stettler, S., E. Warbrick, S. Prochnik, S. Mackie, and P. Fantes. 1996. The wis1 signal transduction pathway is required for expression of cAMP-repressed genes in fission yeast. J. Cell Sci. 109:1927-1935. [DOI] [PubMed] [Google Scholar]
- 22.Welton, R. M., and C. S. Hoffman. 2000. Glucose monitoring in fission yeast via the gpa2Gα, git5 Gβ and git3 putative glucose receptor. Genetics 156:513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.