Manganese represents an essential trace element that is accumulated and utilized by virtually all forms of life. This redox active metal is a key cofactor for a wide range of metalloenzymes, including oxidases and dehydrogenases, DNA and RNA polymerases, kinases, decarboxylases, and sugar transferases (11, 25). In addition to serving as an essential nutrient, manganese can also be toxic. In humans, exposure to manganese can cause severe neurological damage, leading to a Parkinsonian-like disorder known as “manganism” (3, 4, 46, 59, 69). While the biological importance of manganese has long been recognized, there is scarce understanding regarding the mechanisms of manganese homeostasis.
Virtually every compartment of the cell contains one or more enzymes that require manganese for activity, and in order for manganese to reach said targets, the ion must overcome a number of obstacles. Lipid bilayer membranes must be crossed at both the cell surface and at organelles. In addition, the metal must evade the detoxification factors designed to sequester or eliminate manganese from the cell. Because manganese is potentially toxic, the ion is not likely to diffuse in the cell unattended, but, rather, is handled in a carefully controlled fashion by manganese homeostasis proteins. Such homeostasis factors include cell surface and intracellular manganese transporters and putative manganese chaperones that collectively guide the metal down a designated trafficking pathway, ultimately culminating in the activation of manganese enzymes. To date, only a few of the players involved in the network of manganese trafficking have been identified, and these have largely been revealed through molecular genetic studies of the baker's yeast, Saccharomyces cerevisiae.
This review shall highlight pathways of manganese homeostasis and trafficking that are operative under three distinct metabolic conditions: (i) manganese replete or “physiological” conditions when manganese is amply available for activation of manganese-requiring enzymes; (ii) manganese starvation stress, including the cellular response to compensate for low manganese availability; and (iii) manganese surplus or manganese toxicity, including the transport pathways and mechanisms of detoxification. Where applicable, references shall be made to analogous pathways described for humans, including potential implications for disease. (Physiological, as used in item i above, means nonstressed laboratory growth conditions, i.e., when manganese is amply available to activate manganese enzymes but is not at surplus levels at which toxicity might ensue. However, “physiological” conditions or the natural growth state for S. cerevisiae in the wild might be more akin to manganese starvation conditions.)
TRANSPORTING MANGANESE UNDER PHYSIOLOGICAL CONDITIONS: A ROLE FOR THE YEAST Nramp TRANSPORTERS
In S. cerevisiae, two members of the Nramp family of metal transporters (Smf1p and Smf2p) (Fig. 1) act in the uptake and intracellular trafficking of manganese. Nramp (for natural resistance-associated macrophage protein) represents a large family of metal ion transporters that are widely conserved from bacteria to humans (7). These are metal-proton symporters that act on a broad range of divalent metals, including manganese, iron, cadmium, and copper. In eukaryotes, these transporters are localized at the cell surface or at intracellular sites, but in all cases, the metal is transported in the direction of the cytosol (1, 12, 19, 20, 43).
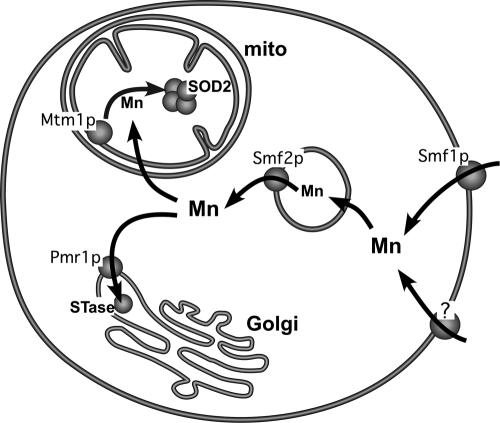
FIG. 1.
A model for intracellular trafficking of manganese under physiological growth conditions. When S. cerevisiae cells are grown under normal laboratory growth conditions (i.e., standard enriched or minimal medium containing ≈1 to 5 μM Mn), the metal is taken up via the Nramp metal transporter Smf1p and also by another, as-yet-unidentified metal transporter(s) (“?”) at the cell surface. The manganese then traffics through Smf2p-containing vesicles that may represent storage or transient passage stations for the metal. The Smf2p-transported manganese can then move to either the Golgi or mitochondria (mito). Pmr1p pumps manganese into the Golgi for activation of STase enzymes. Although the means by which mitochondria take up manganese is still unknown, Mtm1p in the inner membrane facilitates insertion of the metal into mitochondrial SOD2.
The fungal Nramp transporter Smf1p was first identified as one of two genes which, when overexpressed in yeast, would overcome a manganese-dependent protein processing defect in the mitochondria (67). Subsequently, Supek et al. demonstrated that Smf1p is a high-affinity transporter for manganese (56, 57). As with other members of the Nramp family, a range of divalent metals can act as substrates for Smf1p. When overexpressed in yeast, Smf1p can increase cellular accumulation of manganese, copper, cadmium, and iron (10, 34). And when expressed in oocytes, the metals manganese, copper, iron, zinc, and cadmium were all transported by Smf1p in a proton-dependent manner (9, 43).
Smf1p has been observed to localize to the plasma membrane of S. cerevisiae cells (32, 57). However, under physiological conditions, Smf1p appears to contribute little to cellular manganese. In measurements of whole-cell manganese, the levels accumulated by smf1Δ mutants were similar to those accumulated by wild-type cells (36, 57). Furthermore, smf1Δ cells showed no obvious defect in activity of manganese-dependent enzymes (36). Two enzymatic markers for manganese in S. cerevisiae include the manganese superoxide dismutase 2 (SOD2) of the mitochondrial matrix (66) and manganese-requiring sugar transferase (STase) enzymes of the Golgi (13, 36, 52). Both classes of manganese-dependent enzymes are fully active in smf1Δ mutants (36). Therefore, Smf1p cannot be the only source of cellular manganese, and other cell surface transporters for manganese must be operative under physiological conditions (Fig. 1). These might include high affinity transporters for other metals, such as iron, copper, or zinc, which can also transport manganese at low affinity. Alternatively, as discussed below, manganese can enter the cell via phosphate transporters (24), and one or more of the phosphate uptake systems in yeast (70) may contribute to manganese accumulation.
The second Nramp transporter for manganese is Smf2p (67). Smf2p shares nearly 50% identity with Smf1p at the amino acid level, but the two proteins are not functionally redundant. While yeast cells can easily tolerate loss of Smf1p, a defect in Smf2p has a profound impact on manganese accumulation and bioavailability, even under manganese-replete physiological conditions. S. cerevisiae strains containing a smf2Δ deletion accumulate very low levels of manganese (36), and there is a cell-wide deficiency in manganese that translates to widespread inactivation of manganese-dependent enzymes. STase enzyme activity in the Golgi is inhibited in smf2Δ mutant strains, as is activity of manganese containing SOD2 in the mitochondria (36).
With such a profound effect on manganese accumulation and distribution, one might postulate that Smf2p resides at the plasma membrane to facilitate uptake of manganese from the growth medium. However, to date, there is no evidence of Smf2p at the cell surface. Instead, Smf2p has been localized to intracellular Golgi-like vesicles (36, 49). These vesicles are not the product of Smf2p endocytosis from the cell surface, as there is no change in Smf2p localization in end4 mutants of yeast defective in endocytosis (36). Instead, we propose that these Smf2p-containing vesicles function as storage depots or as transient passage stations for manganese. Manganese coming into the cell may first converge at these vesicles; Smf2p then functions to release the manganese from these stores for utilization by the cell (Fig. 1).
The aforementioned model raises the question of how intracellular Smf2p would affect total cellular accumulation of the metal. A feedback mechanism may be at play in smf2Δ mutants, where absence of vesicular release of manganese signals back to the cell surface to down-regulate manganese uptake from the growth medium. Such a model is analogous to what has been reported for the mammalian Nramp transporter DMT1. In nonintestinal cells, DMT1 localizes to intracellular endocytic vesicles, and loss of DMT1 somehow signals to inhibit iron uptake at the cell surface (1, 14, 22). Alternatively, we cannot totally exclude the possibility that a small fraction of S. cerevisiae Smf2p directly transports manganese at the cell surface. Thus far, we have detected Smf2p only at intracellular locations and only under manganese starvation when the polypeptide readily accumulates (36, 49) (see “WHEN YEASTS ARE STARVED FOR MANGANESE” section below). Perhaps under manganese-replete conditions, a minute undetectable fraction of Smf2p also resides at the cell surface.
Regardless of the site of Smf2p action, the manganese transported by Smf2p is bioavailable. Some of the Smf2p-transported manganese reaches the Golgi and the mitochondria for activation of STase enzymes and SOD2, respectively. Precisely how the metal is delivered from Smf2p to the organelles is not understood but may involve fusion of Smf2p-containing vesicles with the organelles, as has been suggested for iron trafficking to mitochondria (48, 73). Alternatively, manganese chaperone molecules may carry the metal across the cytosol, as has been described for copper (44). Once at the organelle, manganese needs to be taken up by specific membrane transporters of the Golgi and of mitochondria.
Pmr1p SUPPLIES THE SECRETORY PATHWAY WITH MANGANESE
The Golgi apparatus in yeast acquires its manganese through the action of Pmr1p, a P-type Ca2+- and Mn2+-transporting ATPase (53). The calcium and manganese transported by Pmr1p facilitates the proper processing and trafficking of polypeptides that move through the secretory pathway (2, 13, 53, 55). Pmr1p is the prototype of a growing family of transporting ATPases known as SPCA (for secretory pathway Ca2+-ATPases), found in various fungi, Caenorhabditis elegans, Drosophila melanogaster, and mammals (e.g., human SPCA1 and SPCA2) (39, 58, 71). In fact, mutations in human SPCA1 have been associated with Hailey-Hailey disease, a severe skin blistering disorder consistent with defects in protein glycosylation (26, 58). The Golgi-localized SPCA molecules are members of a large superfamily of Ca2+-ATPases that also includes calcium pumps at the cell surface and sarco/endoplasmic reticulum calcium ATPase pumps at the sacroplasmic reticulum (39, 55); however, the SPCA transporters are unique in that they function in manganese and in calcium transport (39).
Two regions of Pmr1p have been implicated in ion binding and ion translocation. First, all SPCA transporters contain a calcium binding “EF hand” motif at the N terminus, and this region is necessary for ion binding in vitro and for manganese and calcium transport activity in vitro (65). By mutagenesis of the EF hand, Wei et al. have succeeded in altering the selectivity for manganese over calcium (65). In addition to the EF hand, the putative cation translocating regions have been localized to transmembrane helices 4 to 6. And specific mutations in these regions can alter the ion selectivity of Pmr1p. For example, a Q783A substitution near the cytoplasmic face of TM6 destroys manganese transport activity with no apparent effect on Pmr1p's capacity for calcium transport (38, 64). Such mutants are invaluable for segregating the roles of Pmr1p in donating manganese versus calcium to the secretory pathway.
Pmr1p is clearly critical for manganese activation of Golgi STase enzymes. Strains lacking pmr1 show a defect in STase activity that can be corrected by manganese supplements (13, 36, 53). However, pmr1 mutants show no loss in activity of manganese SOD2 in the mitochondria (36). The delivery of manganese to the mitochondria requires a separate pathway that is distinct from Pmr1p (Fig. 1).
ROLE FOR Mtm1p IN MANGANESE ACTIVATION OF MITOCHONDRIAL SOD2
In order for manganese to reach SOD2 in the mitochondrial matrix, the metal must cross both the outer and inner membranes of mitochondria. The pores in the outer membrane might permit translocation of small manganese complexes (e.g., manganese-phosphate or manganese-glutathione, although evidence for this is lacking). However, passage through the inner mitochondrial membrane would certainly require a membrane transporter.
In an attempt to identify mitochondrial transporters for manganese, we screened the yeast collection of the mitochondrial carrier family (MCF) of transport proteins. This large family of transporters which lie in the inner membrane of mitochondria has evolved to exchange solutes between the mitochondria and cytosol (reviewed in references 5, 41, and 47). S. cerevisiae expresses ≈35 distinct MCFs, and humans are estimated to produce at least 40 members (42, 47). Each member is thought to transport a distinct mitochondrial solute. The substrates for many MCFs have been identified, including compounds of the tricarboxylic acid cycle and nucleotides, while the substrates for other MCFs remain unknown. In our analysis of S. cerevisiae MCFs, we identified a single member that, when deleted, resulted in inactivation of SOD2 due to manganese deficiency in the enzyme. We designated this MCF Mtm1p, for manganese trafficking protein for mitochondrial SOD2 (35). We surmised that Mtm1p may be a mitochondrial transporter for manganese; however, mtm1Δ mutants show no deficiency of mitochondrial manganese. In fact, mtm1Δ mutants accumulate somewhat higher than normal levels of mitochondrial manganese and also very high levels of mitochondrial iron (35).
In our most recent studies, we observed a connection between the high mitochondrial iron of mtm1 mutants and loss of SOD2 activity. Iron is known to bind to, and inhibit activity of, the highly homologous bacterial forms of manganese SOD (6, 40, 50, 68). Mitochondrial SOD2 of eukaryotes is expected to show similar inactivation by iron. Recent studies suggest that Mtm1p ensures manganese occupancy in the active site of SOD2 over the more abundant metal iron (unpublished results). The precise substrate of transport by Mtm1p has not yet been elucidated, but may represent a small molecule(s) that interacts with metals and favors manganese insertion into the enzyme over iron.
WHEN YEASTS ARE STARVED FOR MANGANESE: UPREGULATING Smf1p AND Smf2p AT THE POSTTRANSLATIONAL LEVEL
Yeast cells respond to manganese depravation by increasing levels of the Smf1p and Smf2p transporters. The transporters then abundantly accumulate at the cell surface (Smf1p) and intracellular sites (Smf2p) to increase the uptake and dissemination of essential manganese (31, 32, 36, 49). Starving yeast cells for other metals, such as copper, iron and zinc, also results in enhanced expression of the corresponding metal transporters (e.g., Ctr1p for copper, Ftr1p/Fet3p for iron, and Ztr1p/Ztr2p for zinc) (reviewed in reference 54). Induction of the copper, iron, and zinc transporters occurs largely at the level of gene transcription (54). However, manganese starvation does not effect an increase in SMF1 and SMF2 mRNA (32, 49). In fact, our global transcription profiling analysis failed to reveal an obvious transcriptional regulon for manganese (E. Luk and V. C. Culotta, unpublished results). The response to manganese instead occurs at the posttranslational level. Manganese starvation leads to enhanced stability of the Smf1 and Smf2 polypeptides and a shift in the cellular localization of these transporters (31, 32, 49).
Under normal conditions, when manganese is amply available, much of the Smf1 and Smf2 polypeptides are directed to the lumen of the vacuole, where they are degraded by vacuolar proteases (31, 32, 49). Since these transporters can act on a variety of heavy metals, including toxic ions, this degradation of Smf1p and Smf2p is important to minimize cellular uptake of toxic metals. But under conditions of manganese starvation, the transporters fail to arrive at the vacuole and their localization is shifted to either the cell surface (Smf1p) or intracellular vesicles (Smf2p). This shift represents a change in the sorting of Smf1p and Smf2p in the secretory pathway and is mediated by S. cerevisiae Bsd2p (31, 32, 34, 49) (Fig. 2).
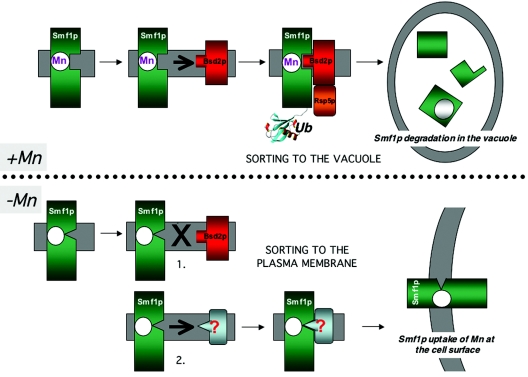
FIG. 2.
Manganese regulation of Smf1p at the level of protein sorting in the secretory pathway. Shown is a model depicting the way yeast cells may respond to changes in manganese availability by shifting localization of the Smf1p manganese transporter. (Top) When cells have ample manganese (e.g., grown in medium supplemented with >1 μM Mn [“+Mn”]), Smf1p in the secretory pathway is thought to interact with manganese and adopt a conformation that is recognized by membrane-bound Bsd2p. Bsd2p recruits the E3 ubiquitin ligase Rsp5, and Smf1p becomes tagged with ubiquitin (“Ub”), resulting in movement of Smf1p to the vacuole for degradation by vacuolar proteases. (Bottom) When cells are starved for manganese (extracellular manganese concentration of less than approximately 1.0 μM [“−Mn”]), Smf1p adopts a conformation that is not recognized by Bsd2p (step 1). This lack of recognition by Bsd2p is not sufficient to move Smf1p to the cell surface, and in a second step, manganese-free Smf1p may be recognized by another protein trafficking factor (as yet unknown; “?”) that helps direct Smf1p to the cell surface for the uptake of manganese from the growth medium.
We originally identified BSD2 as a gene that, when mutated, would suppress oxidative damage in yeast cells lacking Cu/Zn superoxide dimutase (SOD1), hence the name bsd2 for bypass SOD1 defect (33). Mutations in bsd2 are associated with increased manganese accumulation, which helps to neutralize reactive oxygen species (34). The increase in manganese was found to result from overexpression of Smf1p and Smf2p (32, 34). In bsd2Δ mutants, these transporters fail to arrive at the vacuole and are not degraded. Smf1p and Smf2p accrue to high levels in bsd2Δ cells, even when manganese is amply available (32). As a result, bsd2Δ mutants hyperaccumulate a variety of metals, including manganese, copper, cadmium, and cobalt, and become prone to metal toxicity (33, 34).
Bsd2p is a membrane protein of the secretory pathway found in the endoplasmic reticulum (34), prevacuolar compartments, and vacuole (23). Recent exciting work by H. Pelham has demonstrated that Bsd2p helps direct misfolded proteins to the vacuole for degradation. Although misfolded proteins are generally removed in the ER by a retrieval mechanism involving the proteasome (the unfolded protein response) (37), malfolded proteins can also be removed in the secretory pathway by ubiquitin tagging, which flags polypeptides for destruction in the vacuole. Bsd2p participates in such ubiquitin tagging by recruiting the E3 ubiquitin ligase Rsp5 to the site of the unfolded protein (23).
Bsd2p is believed to recognize certain transmembrane segments that are misfolded or contain polar amino acids. Targets that have been identified include aberrantly folded mutants of the proton ATPase Pma1p (8) or of the SNARE protein Pep12p (23), and polypeptides misfolded during heat shock or treatment with the arginine analogue canavanine (23).
In addition to clearing abnormally folded proteins, Bsd2p is responsible for the vacuolar targeting of certain polypeptides in their native state. These include the carboxypeptidase S Cps1p and the polyphosphatase Phm5p, both enzymatically active residents of the vacuolar lumen (23). And Bsd2p recognizes Smf1p and Smf2p for vacuolar targeting, leading to their destruction. Indeed, Eguez et al. have noted that Smf1p is subject to ubiquitination and that this modification is necessary for targeting Smf1p to the vacuole (15) (Fig. 2).
The Smf1p and Smf2p transporters appear unique in that they are a conditional substrate for Bsd2p-ubiquitination, i.e., only when cells have ample manganese. Bsd2p does not recognize these polypeptides during manganese starvation (31, 32, 34). We have proposed a model in which the binding of manganese to Smf1p and Smf2p in the secretory pathway induces a conformational change that is recognized by Bsd2p (Fig. 2). Indeed, we have found that mutations G190A and G424A in Smf1p that abolish transport activity also abolish recognition by Bsd2p, and these proteins accumulate to high steady-state levels in cells with ample manganese (31). Perhaps these mutants are unable to bind manganese and are therefore unable to adopt the conformation recognized by Bsd2p. We have also identified Smf1p mutants Q419A and E423A, which are constitutively targeted to the vacuole for degradation in a Bsd2p-dependent manner, even under manganese starvation conditions (31). Presumably, these mutants are always misfolded and mimic the conformer of manganese-activated wild-type Smf1p.
Although Bsd2p is a major player in dictating the trafficking of Smf1p and Smf2p, this is not the complete story. In cells lacking Bsd2p, Smf1p does not enter the vacuole, nor does it move to the cell surface; rather, the transporter accumulates in the Golgi and endoplasmic reticulum (32). Smf1p only reaches the plasma membrane when cells are starved for manganese (32). We therefore propose a two-step model for Smf1p movement to the cell surface (Fig. 2). First, in the absence of manganese, Smf1p adopts a conformation that escapes recognition by Bsd2p. Second, this manganese-free Smf1p is recognized by another regulator(s) of the secretory pathway that directs its movement to the plasma membrane (Fig. 2).
WHEN MANGANESE LEVELS BECOME TOXIC: BRINGING THE METAL IN AND MOVING IT OUT
At high doses, manganese is toxic. Even through Smf1p and Smf2p are largely absent under manganese-replete or manganese toxicity conditions, cells still accumulate the metal by other transport mechanisms. Our recent studies support a role for the phosphate transporter Pho84p in yeast uptake of toxic manganese.
PHO84 encodes a cell surface inorganic phosphate transporter that is a major source of phosphate for S. cerevisiae (70). Our studies show that Pho84p also acts as a low-affinity manganese transporter. When cells are exposed to high manganese concentrations (in excess of 10 μM), they accumulate the metal in a linear fashion, and a deletion in pho84 eliminates the uptake of excess manganese (24). As such, Pho84p appears to be the major source of toxic manganese for the cell.
Using reconstituted proteoliposomes, Fristedt and colleagues have shown that recombinant Pho84p can transport metal-phosphate (MeHPO4) complexes, with manganese-phosphate being a particularly good substrate (21). It is therefore conceivable that when yeast cells are exposed to very high levels of manganese, the manganese-phosphate complexes that form in the environment are readily taken up via the Pho84p transporter (Fig. 3). Unlike Smf1p and Smf2p, there is no known down-regulation of Pho84p in response to manganese; therefore, the cell becomes quite vulnerable to manganese toxicity. Fortunately, much of the accumulated manganese is eliminated from the cell via the action of Pmr1p.
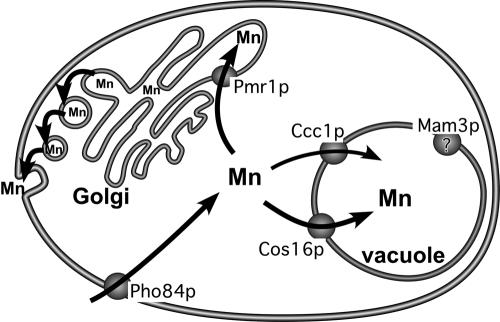
FIG. 3.
Manganese trafficking under manganese surplus or toxicity conditions. When S. cerevisiae cells are grown in the presence of surplus manganese (≈10 to 100 μM), the metal is taken up largely in the form of manganese-phosphate complexes via the Pho84p phosphate transporter. Two major manganese detoxification systems sequester the metal and help eliminate excess manganese from the cell. A bulk of the excess manganese is pumped into the Golgi via Pmr1p and the metal then exits the cell via the secretory pathway. Manganese is also delivered into the yeast vacuole by Ccc1p and perhaps by Cos16p as well. The action of vacuolar Mam3p is not known, but it helps contribute to manganese toxicity.
Pmr1p not only plays a role in supplying Golgi STase enzymes with manganese (as described above) (Fig. 1) but also is the major route for eliminating toxic manganese from the cell. Mutants lacking Pmr1p are exquisitely sensitive to manganese toxicity and accumulate very high levels of the metal, largely in the cytosol (27, 28, 58). Presumably, the excess manganese pumped into the Golgi by Pmr1p proceeds to exit the cell via secretory pathway vesicles that merge with the cell surface and release the manganese contents back into the extracellular environment (Fig. 3).
Humans express two Pmr1p homologues: SPCA1 functions in both calcium and manganese transport of diverse cell types (18, 39, 58), and SPCA2 is expressed specifically in the brain (71). Recent work by Xiang et al. strongly indicates that SPCA2 does not function in calcium transport but that it has specifically evolved for manganese homeostasis (71). The central nervous system is particularly vulnerable to manganese toxicity, and SPCA2 has been implicated as a major player in eliminating toxic manganese from the brain (71).
CONFISCATING TOXIC MANGANESE IN THE YEAST VACUOLE
In S. cerevisiae, the vacuole is somewhat analogous to the mammalian lysosome in that it plays an important role in turnover of macromolecules. Yet unlike the lysosome, the yeast vacuole is also a major site for ion homeostasis, including the storage and detoxification of heavy metals. Manganese is more concentrated in the vacuole than in the cytosol, and by sequestrating this metal, the vacuole helps protect other cellular compartments from manganese-related damage (51, 72). There is ample genetic evidence for the role of the vacuole in manganese detoxification. Manganese homeostasis is interrupted by numerous mutants affecting vacuole biogenesis and vacuolar acidification (16, 45, 51). Manganese is believed to enter the yeast vacuole via two transport pathways involving S. cerevisiae Cos16p (45) and Ccc1p (29, 30) (Fig. 3); mutations in either pathway increase cellular sensitivity towards manganese, presumably due to lack of vacuolar sequestration of the metal. An additional protein that affects manganese toxicity is the vacuolar membrane protein S. cerevisiae Mam3p.
Mam3p is a member of the ACDP (ancient conserved domain protein) family of membrane proteins (62, 63). Unlike vacuolar Cos16p and Ccc1p, which help to detoxify manganese, Mam3p seems to augment manganese toxicity (72). Deletion of MAM3 increases cellular resistance to manganese, while MAM3 overexpression lowers such resistance (72). MAM3 was originally identified as a yeast mutant with aberrant mitochondrial morphology (hence the name) (17); however, these results were not reproducible for other strain backgrounds and there is no obvious mitochondrial defect in mam3 mutants (72). Mam3p resides in the vacuolar membrane, and although total vacuolar accumulation of manganese in mam3Δ mutants is not affected, the bioavailability and/or reactivity of the metal may be altered such that manganese is more toxic in cells expressing vacuolar Mam3p (72).
It is noteworthy that one of the human homologues to Mam3p, namely ACDP1, has been associated with urofacial syndrome, a rare genetic disorder affecting facial expression and urinary tract functions (60, 61). While the precise function of ACDP1 is still unclear, our findings with the Mam3p homologue suggest a role in metal homeostasis.
SUMMARY
Manganese is a biologically important metal that is both a nutrient and a toxic element. Cells must therefore carefully control the uptake and trafficking of this ion. While the picture of manganese homeostasis is far from complete, many advances have been made with the baker's yeast, S. cerevisiae.
Under nonstressful conditions, when manganese is amply available, the uptake and intracellular dissemination of the metal rely heavily on Smf2p, a Nramp manganese transporter. Emerging downstream of Smf2p are at least two manganese trafficking pathways, one that escorts manganese to the Golgi, where the transporting ATPase Pmr1p delivers manganese to sugar transferases, and a separate pathway that delivers manganese to mitochondrial SOD2 (Fig. 1). The mitochondrial transporter for manganese is still unknown, yet the metal is made available to SOD2 through the action of Mtm1p, a member of the mitochondrial carrier family (Fig. 1).
When cells are faced with manganese starvation, they respond by increasing levels of both Nramp manganese transporters, Smf1p and Smf2p. Normally, Smf1p and Smf2p are largely degraded in the vacuole to minimize uptake of toxic metals. But with manganese starvation, the transporters fail to arrive at the vacuole and instead localize to the cell surface and intracellular vesicles to enhance uptake and distribution of the metal (Fig. 2). The mechanism by which cells sense manganese and respond by shifting localization of the Nramp transporters is an area of current investigation.
At the opposite end of the spectrum, i.e., when cells are exposed to toxic levels of manganese, the Smfp transporters are essentially nonexistent. However, cells still accumulate the metal by way of manganese-phosphate complexes taken up by the Pho84p phosphate transporter. The excess manganese is then either sequestered in the vacuole or eliminated from the cell by way of Pmr1p and the secretory pathway (Fig. 3). The role of the secretory pathway in manganese homeostasis and detoxification is likely to be conserved among eukaryotes and is of particular relevance in cases of manganese neurotoxicity in humans.
Acknowledgments
V.C.C. is supported by the JHU NIEHS center and by NIH grant ES 08996. M.Y. is supported by NIH fellowship F32 GM 074402 and by NIEHS training grant ES 07141. M.D.H. is the recipient of an award from the American Australian Association.
REFERENCES
- 1.Andrews, N. 2000. Iron homeostasis: insights from genetics and animal models. Nat. Genet. Rev. 1:208-217. [DOI] [PubMed] [Google Scholar]
- 2.Antebi, A., and G. R. Fink. 1992. The yeast Ca2+-ATPase homologue, PMR1, is responsible for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell 3:633-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbeau, A. 1984. Manganese and extrapyramidal disorders. Neurotoxicology 5:13-35. [PubMed] [Google Scholar]
- 4.Barceloux, D. G. 1999. Manganese. J. Toxicol. Clin. Toxicol. 37:293-307. [DOI] [PubMed] [Google Scholar]
- 5.Belenkiy, R., A. Haefele, M. B. Eisen, and H. Wohlrab. 2000. The yeast mitochondrial transport proteins: new sequences and consensus residues, lack of direct relation between consensus residues and transmembrane helices, expression patterns of the transport protein genes, and protein-protein interactions with other proteins. Biochim. Biophys. Acta 1467:207-218. [DOI] [PubMed] [Google Scholar]
- 6.Beyer, W. F., and I. Fridovich. 1991. In vivo competition between iron and manganese for occupancy of the active site region of the manganese-superoxide dismutase of Escherichia coli. J. Biol. Chem. 266:303-308. [PubMed] [Google Scholar]
- 7.Cellier, M., G. Prive, A. Belouchi, T. Kwan, V. Rodrigues, W. Chia, and P. Gros. 1995. Nramp defines a family of membrane proteins. Proc. Natl. Acad. Sci. USA 92:10089-10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, A., and G. R. Fink. 1995. Targeting of the yeast plasma membrane [H+]ATPase: a novel gene AST1 prevents mislocalization of mutant ATPase to the vacuole. J. Cell Biol. 128:39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, X.-Z., J.-B. Peng, A. Cohen, H. Nelson, N. Nelson, and M. A. Hediger. 1999. Yeast SMF1 mediates H+-coupled iron uptake with concomitant uncoupled cation currents. J. Biol. Chem. 274:35089-35094. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, A., H. Nelson, and N. Nelson. 2000. The family of SMF metal ion transporters in yeast cells. J. Biol. Chem. 275:33388-33394. [DOI] [PubMed] [Google Scholar]
- 11.Crowley, J. A., D. A. Traynor, and D. C. Weatherburn. 1999. Enzymes and proteins containing manganese: an overview, p. 209-257. In A. Sigel and H. Sigel (ed.), Manganese and its role in biological processes. Metal ions in biological systems, vol. 37. Marcel Dekker, New York, N.Y. [PubMed]
- 12.Culotta, V. C. 2000. Manganese transport in microorganisms, p. 35-56. In A. Sigel and H. Sigel (ed.), Manganese and its role in biological processes. Metal ions in biological systems, vol. 37. Marcel Dekker, New York, N.Y. [PubMed]
- 13.Durr, G., J. Strayle, R. Plemper, S. Elbs, S. K. Klee, P. Catty, D. H. Wolf, and H. K. Rudolph. 1998. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9:1149-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards, J. A., and J. E. Hoke. 1975. Red cell iron uptake in hereditary microcytic anemia. Blood 46:381-388. [PubMed] [Google Scholar]
- 15.Eguez, L., Y. S. Chung, A. Kuchibhatla, M. Paidhungat, and S. Garrett. 2004. Yeast Mn2+ transporter, Smf1p, is regulated by ubiquitin-dependent vacuolar protein sorting. Genetics 167:107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eide, D. J., J. T. Bridgham, Z. Zhao, and J. R. Mattoon. 1993. The vacuolar H+-ATPase of Saccharomyces cerevisiae is required for efficient copper detoxification, mitochondrial function, and iron metabolism. Mol. Gen. Genet. 241:447-456. [DOI] [PubMed] [Google Scholar]
- 17.Entian, K. D., T. Schuster, J. H. Hegemann, D. Becher, H. Feldmann, U. Guldener, R. Gotz, M. Hansen, C. P. Hollenberg, G. Jansen, W. Kramer, S. Klein, P. Kotter, J. Kricke, H. Launhardt, G. Mannhaupt, A. Maierl, P. Meyer, W. Mewes, T. Munder, R. K. Niedenthal, M. Ramezani Rad, A. Rohmer, A. Romer, A. Hinnen, et al. 1999. Functional analysis of 150 deletion mutants in Saccharomyces cerevisiae by a systematic approach. Mol. Gen. Genet. 262:683-702. [DOI] [PubMed] [Google Scholar]
- 18.Fairclough, R. J., L. Dode, J. Vanoevelen, J. P. Andersen, L. Missiaen, L. Raeymaekers, F. Wuytack, and A. Hovnanian. 2003. Effect of Hailey-Hailey disease mutations on the function of a new variant of human secretory pathway Ca2+/Mn2+-ATPase (hSPCA1). J. Biol. Chem. 278:24721-24730. [DOI] [PubMed] [Google Scholar]
- 19.Fleming, M. D., and N. C. Andrews. 1998. Mammalian iron transport: an unexpected link between metal homeostasis and host defense. J. Lab Clin. Med. 132:464-468. [DOI] [PubMed] [Google Scholar]
- 20.Forbes, J. R., and P. Gros. 2001. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9:397-403. [DOI] [PubMed] [Google Scholar]
- 21.Fristedt, U., M. van der Rest, B. Poolman, W. N. Konings, and B. L. Persson. 1999. Studies of cytochrome c oxidase-driven H+-coupled phosphate transport catalyzed by the Saccharomyces cerevisiae Pho84 permease in coreconstituted vesicles. Biochemistry 38:16010-16015. [DOI] [PubMed] [Google Scholar]
- 22.Gruenheid, S., F. Canonne-Hergaux, S. Gauthier, D. J. Hackam, S. Grinstein, and P. Gros. 1999. The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J. Exp. Med. 189:831-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hettema, E. H., J. Valdez-Taubas, and H. R. Pelham. 2004. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J. 23:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen, L. T., M. Ajua-Alemanji, and V. C. Culotta. 2003. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J. Biol. Chem. 278:42036-42040. [DOI] [PubMed] [Google Scholar]
- 25.Keen, C. L., J. L. Ensunsa, and M. S. Clegg. 1999. Manganese metabolism in animals and humans including the toxicity of manganese, p. 90-114. In A. Sigel and H. Sigel (ed.), Manganese and its role in biological processes. Metal ions in biological systems, vol. 37. Marcel Dekker, New York, N.Y. [PubMed]
- 26.Kellermayer, R. 2005. Hailey-Hailey disease as an orthodisease of PMR1 deficiency in Saccharomyces cerevisiae. FEBS Lett. 579:2021-2025. [DOI] [PubMed] [Google Scholar]
- 27.Lapinskas, P. 1995. Characterization of genes involved in the homeostasis of oxygen free radicals and metal ions in Saccharomyces cerevisiae. Ph. D. dissertation. Johns Hopkins University, Baltimore, Md.
- 28.Lapinskas, P. J., K. W. Cunningham, X. F. Liu, G. R. Fink, and V. C. Culotta. 1995. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 15:1382-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapinskas, P. J., S. J. Lin, and V. C. Culotta. 1996. The role of the Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol. Microbiol. 21:519-528. [DOI] [PubMed] [Google Scholar]
- 30.Li, L., O. S. Chen, D. M. Ward, and J. Kaplan. 2001. Ccc1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 276:29515-29519. [DOI] [PubMed] [Google Scholar]
- 31.Liu, X. F., and V. C. Culotta. 1999. Mutational analysis of Saccharomyces cerevisiae Smf1p, a member of the Nramp family of metal transporters. J. Mol. Biol. 289:885-891. [DOI] [PubMed] [Google Scholar]
- 32.Liu, X. F., and V. C. Culotta. 1999. Post-translational control of Nramp metal transport in yeast: role of metal ions and the BSD2 gene. J. Biol. Chem. 274:4863-4868. [DOI] [PubMed] [Google Scholar]
- 33.Liu, X. F., and V. C. Culotta. 1994. The requirement for yeast superoxide dismutase is bypassed through mutations in BSD2, a novel metal homeostasis gene. Mol. Cell. Biol. 14:7037-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, X. F., F. Supek, N. Nelson, and V. C. Culotta. 1997. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J. Biol. Chem. 272:11763-11769. [DOI] [PubMed] [Google Scholar]
- 35.Luk, E., M. Carroll, M. Baker, and V. C. Culotta. 2003. Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a member of the mitochondrial carrier family. Proc. Natl. Acad. Sci. USA 100:10353-10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luk, E., and V. C. Culotta. 2001. Manganese superoxide dismutase in S. cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transproter, Smf2p. J. Biol. Chem. 276:47556-47562. [DOI] [PubMed] [Google Scholar]
- 37.Ma, Y., and L. M. Hendershot. 2001. The unfolding tale of the unfolded protein response. Cell 107:827-830. [DOI] [PubMed] [Google Scholar]
- 38.Mandal, D., T. B. Woolf, and R. Rao. 2000. Manganese selectivity of Pmr1, the yeast secretory pathway ion pump, is defined by residue Gln783 in transmembrane segment 6. J. Biol. Chem. 31:23933-23938. [DOI] [PubMed] [Google Scholar]
- 39.Missiaen, L., L. Raeymaekers, L. Dode, J. Vanoevelen, K. Van Baelen, J. B. Parys, G. Callewaert, H. De Smedt, S. Segaert, and F. Wuytack. 2004. SPCA1 pumps and Hailey-Hailey disease. Biochem. Biophys. Res. Commun. 322:1204-1213. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno, K., M. M. Whittaker, H. P. Bachinger, and J. W. Whittaker. 2004. Calorimetric studies on the tight-binding metal interactions of Escherichia coli manganese superoxide dismutase. J. Biol. Chem. 279:27339-27344. [DOI] [PubMed] [Google Scholar]
- 41.Moualij, B. E., C. Duyckaerts, J. Lamotte-Brasseur, and F. E. Sluse. 1996. Phylogenetic classification of the mitochondrial carrier family of Saccharomyces cerevisiae. Yeast 13:573-581. [DOI] [PubMed] [Google Scholar]
- 42.Nelson, D. R., C. M. Felix, and J. M. Swanson. 1998. Highly conserved charge-pair networks in the mitochondrial carrier family. J. Mol. Biol. 277:285-308. [DOI] [PubMed] [Google Scholar]
- 43.Nelson, N. 1999. Metal ion transporters and homeostasis. EMBO J. 18:4361-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Halloran, T. V., and V. C. Culotta. 2000. Metallochaperones: an intracellular shuttle service for metal ions. J. Biol. Chem. 275:25057-25060. [DOI] [PubMed] [Google Scholar]
- 45.Paidhungat, M., and S. Garrett. 1998. Cdc1 and the vacuole coordinately regulate Mn2+ homeostasis in the yeast Saccharomyces cerevisiae. Genetics 148:1787-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal, P. K., A. Samii, and D. B. Calne. 1999. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology 20:227-238. [PubMed] [Google Scholar]
- 47.Palmieri, L., F. M. Lasorsa, A. Vozza, G. Agrimi, G. Fiermonte, M. J. Runswick, J. E. Walker, and F. Palmieri. 2000. Identification and functions of new transporters in yeast mitochondria. Biochim. Biophys. Acta 1459:363-369. [DOI] [PubMed] [Google Scholar]
- 48.Ponka, P., A. D. Sheftel, and A. S. Zhang. 2002. Iron targeting to mitochondria in erythroid cells. Biochem. Soc. Trans. 30:735-738. [DOI] [PubMed] [Google Scholar]
- 49.Portnoy, M. E., X. F. Liu, and V. C. Culotta. 2000. Saccharomyces cerevisiae expresses three functionally distinct homologues of the Nramp family of metal transporters. Mol. Cell. Biol. 20:7893-7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Privalle, C. T., and I. Fridovich. 1992. Transcriptional and maturation effects of manganese and iron on the biosynthesis of manganese-superoxide dismutase in Escherichia coli. J. Biol. Chem. 267:9140-9145. [PubMed] [Google Scholar]
- 51.Ramsay, L. M., and G. M. Gadd. 1997. Mutants of Saccharomyces cerevisiae defective in vacuolar function confirm a role for the vacuole in toxic metal ion detoxification. FEMS Microbiol. Lett. 152:293-298. [DOI] [PubMed] [Google Scholar]
- 52.Romero, P. A., M. Lussier, A. M. Sdicu, H. Bussey, and A. Herscovics. 1997. Ktr1p is an alpha-1,2-mannosyltransferase of Saccharomyces cerevisiae. Comparison of the enzymic properties of soluble recombinant Ktr1p and Kre2p/Mnt1p produced in Pichia pastoris. Biochem. J. 321:289-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudolph, H. K., A. Antebi, G. R. Fink, C. M. Buckley, T. E. Dorman, J. LeVitre, L. S. Davidow, J. I. Mao, and D. T. Moir. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+-ATPase family. Cell 58:133-145. [DOI] [PubMed] [Google Scholar]
- 54.Rutherford, J. C., and A. J. Bird. 2004. Metal-responsive transcription factors that regulate iron, zinc, and copper homeostasis in eukaryotic cells. Eukaryot. Cell 3:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sorin, A., G. Ross, and R. Rao. 1997. PMR1, a Ca2+ ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 272:9895-9901. [DOI] [PubMed] [Google Scholar]
- 56.Supek, F., L. Supekova, H. Nelson, and N. Nelson. 1997. Function of metal-ion homeostasis in the cell division cycle, mitochondrial protein processing, sensitivity to mycobacterial infection and brain function. J. Exp. Biol. 200:321-330. [DOI] [PubMed] [Google Scholar]
- 57.Supek, F., L. Supekova, H. Nelson, and N. Nelson. 1996. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc. Natl. Acad. Sci. USA 93:5105-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ton, V., D. Mandal, V. Cordelia, and R. Rao. 2002. Functional expression in yeast of the human secretory pathway Ca2+, Mn2+-ATPase defective in Hailey-Hailey disease. J. Biol. Chem. 277:6422-6427. [DOI] [PubMed] [Google Scholar]
- 59.Uversky, V. N., J. Li, and A. L. Fink. 2001. Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein. A possible molecular link between Parkinson's disease and heavy metal exposure. J. Biol. Chem. 276:44284-44296. [DOI] [PubMed] [Google Scholar]
- 60.Wang, C. Y., A. Davoodi-Semiromi, J. D. Shi, P. Yang, Y. Q. Huang, J. A. Agundez, J. M. Moran, B. Ochoa, B. Hawkins-Lee, and J. X. She. 2003. High resolution mapping and mutation analyses of candidate genes in the urofacial syndrome (UFS) critical region. Am. J. Med. Genet. A 119:9-14. [DOI] [PubMed] [Google Scholar]
- 61.Wang, C. Y., J. D. Shi, Y. Q. Huang, P. E. Cruz, B. Ochoa, B. Hawkins-Lee, A. Davoodi-Semiromi, and J. X. She. 1999. Construction of a physical and transcript map for a 1-Mb genomic region containing the urofacial (Ochoa) syndrome gene on 10q23-q24 and localization of the disease gene within two overlapping BAC clones (<360 kb). Genomics 60:12-19. [DOI] [PubMed] [Google Scholar]
- 62.Wang, C. Y., J. D. Shi, P. Yang, P. G. Kumar, Q. Z. Li, Q. G. Run, Y. C. Su, H. S. Scott, K. J. Kao, and J. X. She. 2003. Molecular cloning and characterization of a novel gene family of four ancient conserved domain proteins (ACDP). Gene 306:37-44. [DOI] [PubMed] [Google Scholar]
- 63.Wang, C. Y., P. Yang, J. D. Shi, S. Purohit, D. Guo, H. An, J. G. Gu, J. Ling, Z. Dong, and J. X. She. 2004. Molecular cloning and characterization of the mouse Acdp gene family. BMC Genomics 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei, Y., J. Chen, G. Rosas, D. A. Tompkins, P. A. Holt, and R. Rao. 2000. Phenotypic screening of mutations in Pmr1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport. J. Biol. Chem. 275:23927-23932. [DOI] [PubMed] [Google Scholar]
- 65.Wei, Y., V. Marchi, R. Wang, and R. Rao. 1999. An N-terminal EF hand-like motif modulates ion transport by Pmr1, the yeast Golgi Ca2+/Mn2+-ATPase. Biochemistry 38:14534-14541. [DOI] [PubMed] [Google Scholar]
- 66.Weisiger, R. A., and I. Fridovich. 1973. Mitochondrial superoxide dismutase. J. Biol. Chem. 248:4793-4796. [PubMed] [Google Scholar]
- 67.West, A. H., D. J. Clark, J. Martin, W. Neupert, F. U. Hart, and A. L. Horwich. 1992. Two related genes encoding extremely hydrophobic proteins suppress a lethal mutation in the yeast mitochondrial processing enhancing protein. J. Biol. Chem. 267:24625-24633. [PubMed] [Google Scholar]
- 68.Wintjens, R., C. Noel, A. C. May, D. Gerbod, F. Dufernez, M. Capron, E. Viscogliosi, and M. Rooman. 2004. Specificity and phenetic relationships of iron- and manganese-containing superoxide dismutases on the basis of structure and sequence comparisons. J. Biol. Chem. 279:9248-9254. [DOI] [PubMed] [Google Scholar]
- 69.Witholt, R., R. H. Gwiazda, and D. R. Smith. 2000. The neurobehavioral effects of subchronic manganese exposure in the presence and absence of pre-parkinsonism. Neurotoxicol. Teratol. 22:851-861. [DOI] [PubMed] [Google Scholar]
- 70.Wykoff, D. D., and E. K. O'Shea. 2001. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159:1491-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiang, M., D. Mohamalawari, and R. Rao. 2005. A novel isoform of the secretory pathway Ca2+,Mn2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J. Biol. Chem. 280:11608-11614. [DOI] [PubMed] [Google Scholar]
- 72.Yang, M., L. T. Jensen, A. J. Gardner, and V. C. Culotta. 2005. Manganese toxicity and Saccharomyces cerevisiae Mam3p, a member of the ACDP (ancient conserved domain protein) family. Biochem. J. 386:479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, A. S., A. D. Sheftel, and P. Ponka. 2005. Intracellular kinetics of iron in reticulocytes: evidence for endosome involvement in iron targeting to mitochondria. Blood 105:368-375. [DOI] [PubMed] [Google Scholar]