Abstract
In natural populations of the pea aphid Acyrthosiphon pisum, a facultative bacterial symbiont of the genus Rickettsia has been detected at considerable infection frequencies worldwide. We investigated the effects of the Rickettsia symbiont on the host aphid and also on the coexisting essential symbiont Buchnera. In situ hybridization revealed that the Rickettsia symbiont was specifically localized in two types of host cells specialized for endosymbiosis: secondary mycetocytes and sheath cells. Electron microscopy identified bacterial rods, about 2 μm long and 0.5 μm thick, in sheath cells of Rickettsia-infected aphids. Virus-like particles were sometimes observed in association with the bacterial cells. By an antibiotic treatment, we generated Rickettsia-infected and Rickettsia-eliminated aphid strains with an identical genetic background. Comparison of these strains revealed that Rickettsia infection negatively affected some components of the host fitness. Quantitative PCR analysis of the bacterial population dynamics identified a remarkable interaction between the coexisting symbionts: Buchnera population was significantly suppressed in the presence of Rickettsia, particularly at the young adult stage, when the aphid most actively reproduces. On the basis of these results, we discussed the possible mechanisms that enable the prevalence of Rickettsia infection in natural host populations in spite of the negative fitness effects observed in the laboratory.
The genus Rickettsia is a phylogenetically distinct bacterial group that belongs to the order Rickettsiales in the α-subdivision of the class Proteobacteria (33, 37). All members of the genus are gram-negative bacteria in obligate association with eukaryotic cells (9, 44). This bacterial group includes notorious human and animal pathogens vectored by blood-sucking arthropods: R. prowazekii, vectored by lice and causing epidemic typhus; R. typhi, vectored by fleas and causing murine typhus; and R. rickettsii and allied species, vectored by ticks and causing spotted fever, etc. (9).
Although some Rickettsia species occasionally infect vertebrates with pathology, and a few species are obligatorily associated with vertebrates, it is believed that the primary hosts of most Rickettsia species are arthropods and other invertebrates (9, 21, 44). In these cases, the Rickettsia bacterium is inherited by ovarial transmission through host generations with a high fidelity, is detected in host populations at a considerable frequency, and can be regarded as a facultative endosymbiotic associate (9, 44). Thus far, such Rickettsia symbionts have been identified from a wide variety of invertebrates such as ladybird beetles (19, 43, 46), an aphid (6), a leafhopper (10), a bruchid beetle (13), ticks (2, 30), leeches (21), and others.
Many obligate insect endosymbionts, like Buchnera in aphids and Wigglesworthia in tsetse flies, are harbored by specialized cells for endosymbiosis called mycetocytes (or bacteriocytes) (1, 3, 4). Some facultative insect endosymbionts, such as γ-proteobacterial secondary symbionts in aphids, are housed in different types of specialized cells called secondary mycetocytes and sheath cells (4, 14, 16, 22, 40). On the other hand, no such specific cellular localization has been reported for facultative symbionts that are widely found across diverse insect taxa, such as Wolbachia, Spiroplasma, and Rickettsia (8, 11, 15, 20, 21, 23), although concentrated distributions in ovary and germ plasm were recognized in some cases (8, 18, 20).
In addition to the essential symbiont Buchnera, five facultative symbionts have been identified from natural populations of the pea aphid Acyrthosiphon pisum: PASS (pea aphid secondary symbiont or R-type symbiont), PAUS (pea aphid U-type symbiont or U-type symbiont), PABS (pea aphid Bemisia-type symbiont or T-type symbiont), Rickettsia symbiont (or PAR, after pea aphid Rickettsia), and Spiroplasma symbiont (35, 36, 39). In the host body, the three γ-proteobacterial symbionts PASS, PAUS, and PABS were reported to exhibit a remarkable cellular localization; they were detected in secondary mycetocytes and sheath cells and also in hemolymph (16, 22, 35, 40). By contrast, no such cellular tropism has been reported for the Rickettsia symbiont of A. pisum. Previous studies indicated that the Rickettsia symbiont is present in hemolymph (6) and confers negative fitness effects on the host aphid, while the intensity of the effects was dependent on environmental factors such as temperature and host plant species (5, 7, 28).
In this study, the Rickettsia symbiont of A. pisum was analyzed in detail by using molecular phylogenetic analysis, in situ hybridization, light and electron microscopy, a selective elimination technique, and quantitative PCR, which revealed previously unknown biological aspects of the Rickettsia symbiont, including highly specific cellular localization and interaction with the essential symbiont Buchnera.
MATERIALS AND METHODS
Materials.
The following isofemale strains of A. pisum were used in this study. The Rickettsia-infected strain OY was collected at Tochigi, Japan, in 2000 and has been maintained in the laboratory with stable Rickettsia infection (39). The strain AIST, which is free of facultative symbionts, was collected at Tsukuba, Japan, in 1999 (16). Aphids were reared on seedlings of the broad bean Vicia faba at 20°C in a long-day regimen (16 h of light and 8 h of dark).
DNA extraction, cloning, and sequencing.
Aphids of the strain OY were subjected to DNA extraction by using a QIAamp mini kit (QIAGEN). Genes of the Rickettsia symbiont for 16S rRNA and citrate synthase (gltA) were PCR amplified by using primers 16SA1 (5′-AGAGTTTGATCMTGGCTCAG-3′) and 16SB1 (5′-TACGGYTACCTTGTTACGACTT-3′) (16) and primers CS1 (5′-GGGGGCCTGCTCACGGCGG-3′) and CS2 (5′-ATTGCAAAAAGTACAGTGAACA-3′) (32), respectively. The PCR products were cloned and sequenced as described previously (16). More than three clones for each of the genes were sequenced to eliminate possible PCR errors.
Molecular phylogenetic analysis.
Multiple alignments of nucleotide sequences were generated by using the program package Clustal W (38). The final alignments were inspected and corrected manually. Neighbor-joining trees, with Kimura's two-parameter distance and 1,000 bootstrap resamplings, were constructed by using the program package MEGA, version 2.1 (26).
PCR detection of symbionts.
PCR detection of Rickettsia was performed by using primers Apis-Ric16SF1 (5′-TGGCTGTCGTCAGCTCGT-3′) and Apis-Ric16SR1 (5′-TCCACGTCACCGTCTTGC-3′) for the 16S rRNA gene and RicCS-AF (5′-TTTAGGCTAATGGGTTTTGGTCAT-3′) and RicCS-AR (5′-TGCCCAAGTTCTTTTAACACCTC-3′) for the gltA gene. Buchnera was detected by using primers Buch16S1F (5′-GAGCTTGCTCTCTTTGTCGGCAA-3′) and Buch16S1R (5′-CTTCTGCGGGTAACGTCACGAA-3′) for the 16S rRNA gene (39). The absence of other facultative symbionts, including PASS, PAUS, PABS, and Spiroplasma, was confirmed by using the primer sets described previously by Tsuchida et al. (39). The temperature profile for PCR amplification was 40 cycles consisting of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min. To confirm the specificity of the detection, the diagnostic PCR experiments included the positive- and negative-control DNA samples from a Rickettsia-infected bruchid beetle, Kytorhinus sharpianus (13); a Rickettsia-infected leech, Torix tagoi (21); and a Rickettsia-free aphid strain, AIST (16).
In situ hybridization.
Aphid embryos were dissected from adult aphids and fixed overnight in Carnoy's solution (chloroform-ethanol-acetic acid [6:3:1]). The samples were decolorized with 6% H2O2 in ethanol for 2 h and subjected to whole-mount fluorescence in situ hybridization. A probe, ApisP2-Cy5 (5′-Cy5-CCTCTTTTGGGTAGATCC-3′), targeted 16S rRNA of Buchnera spp. (22), and a probe, Apis-Ric16R1-Cy3 (5′-Cy3-TCCACGTCACCGTCTTGC-3′), targeted 16S rRNA of Rickettsia spp. The embryos were incubated in a hybridization buffer (20 mM Tris-HCl [pH 8.0], 0.9 M NaCl, 0.01% sodium dodecyl sulfate, 30% formamide) containing 10 pmol/ml each of the probes and 0.5 μM SYTOX green. After an overnight incubation, the samples were thoroughly washed in a washing buffer (0.3 M NaCl, 0.03 M sodium citrate, 0.01% sodium dodecyl sulfate) and were observed under a laser confocal microscope (PASCAL5; Carl Zeiss). To confirm the specificity of the detection, the following control experiments were conducted: no-probe control experiment, RNase digestion control experiment, and competitive suppression control experiment with excess unlabeled probe (15, 16, 22). Nuclei of the host cells were counterstained with SYTOX Green.
Electron microscopy.
Adult aphids were dissected in 0.1 M phosphate buffer (pH 7.4) containing 2.5% glutaraldehyde. The dissected embryos were prefixed in the fixative at 4°C overnight, postfixed in the phosphate buffer containing 2% osmium tetroxide at 4°C for 3 h, and subjected to block staining with uranyl acetate for 1 h. The embryos were dehydrated through an ethanol series and embedded in Spurr resin. Ultrathin sections were made with an ultramicrotome (Ultracat-N; Leichert-Nissei), mounted on collodion-coated copper meshes, stained with uranyl acetate and lead citrate, and observed with a transmission electron microscope (model H-7000; Hitachi).
Antibiotic treatment.
Adult aphids (12 days old) were injected with ampicillin solution at a dose of about 1 μg/mg body weight as described previously (22). The injected insects were reared individually to establish isofemale lines, and their nymphs were born between 48 and 60 h after injection and were collected. The nymphs were defined as G1 of each isofemale line, and five aphids from each line per generation were subjected to diagnostic PCR detection as described above to confirm the elimination of Rickettsia. For a control treatment, adult aphids were injected with distilled water instead of the antibiotic solution.
Fitness measurement.
Adult aphids (12 days old) were allowed to deposit nymphs for 12 h. The newborn nymphs were defined as 0 days old and were reared on the broad bean at 20°C in the long-day regimen. Fresh body weight, age of first reproduction, number of offspring, and longevity were monitored every day. To eliminate possible side effects of the antibiotic treatment, fitness measurements were conducted after 15 generations of the treatment.
Quantitative PCR.
Individual aphids were subjected to DNA extraction by using a QIAamp mini kit (QIAGEN). Bacterial titers in the DNA samples were measured by a quantitative PCR technique by using the TaqMan PCR and the ABI7700 system (Applied Biosystems) essentially as described previously (22, 24). Rickettsia was quantified in terms of gltA gene copies by using primers RicCS-AF and RicCS-AR and a probe, RicCS-ATQ (5′-6-carboxyfluorescein (FAM)-CGGGCTGCAGTACTTAAAGAAACCTGCA-6-carboxytetramethylrhodamine (TAMRA)-3′). Buchnera was quantified in terms of groEL gene copies by using primers BuchGroEL-1824F (5′-CGTTTCAGATCCATTGGATTCA-3′) and BuchGroEL-1967R (5′-AGCTCAAATGGTAAAAGAAGTTGCA-3′) and a probe, BuchGroEL-1914TQ (5′-FAM-CCATCACCTGCTGCATCGTTTGCT-TAMRA-3′). For normalization, a host nuclear gene for elongation factor 1α (ef1α) was quantified by using primers ApisEF1-422F (5′-CTCTGGATGGAATGGAGACAACA-3′) and ApisEF1-522R (5′-ATTTACCGTCGGCCTTTCCT-3′) and a probe, ApsiEF1-463TQ (5′-FAM-AAATGTCGTGGTTCAAGGGATGGGC-TAMRA-3′).
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA gene and the gltA gene of the Rickettsia symbiont from A. pisum described in this paper have been deposited in the DDBJ nucleotide sequence database under accession numbers AB196668 and AY744287, respectively.
RESULTS
Detection and characterization of Rickettsia symbiont in the aphid strain OY.
The aphid strain OY has been maintained in the laboratory through 80 to 90 parthenogenetic generations since being collected in 2000. Periodical PCR checks always indicated that all individuals examined harbored Rickettsia symbiont in addition to the essential symbiont Buchnera. Other facultative symbionts, such as PASS, PAUS, PABS, and Spiroplasma, were not detected from the aphid strain (data not shown).
16S rRNA gene and gltA gene of Rickettsia symbiont.
A 1.5-kb segment of the eubacterial 16S rRNA gene was amplified by PCR from the DNA of the strain OY and was subjected to cloning and restriction fragment length polymorphism typing. Two restriction fragment length polymorphism types were identified among 25 clones examined, of which four clones from each of the types were sequenced. The sequences of one type, 1,470 bp in length, were identical to each other and exhibited 100% similarity to the sequences of Buchnera sp. from A. pisum (DDBJ accession number AB033774). The sequences of the other type, 1,422 bp in length, were also identical to each other and exhibited 99.8%, 98.9%, and 99.4% similarity to the sequence of a Rickettsia sp. from A. pisum collected in California (accession number U42084), the sequence of a Rickettsia sp. from papaya leafhopper Empoasca papayae (accession number U76910), and the sequence of Rickettsia bellii (accession number U11014), respectively.
A 0.38-kb segment of the rickettsial gltA gene was also amplified by PCR from the DNA of the strain OY and was subjected to cloning and sequencing. The nucleotide sequences of six clones determined were completely identical (382 bp) and exhibited 95.5% and 91.9% similarity to the sequence of a Rickettsia sp. from papaya leafhopper (accession number U76908) and the sequence of R. bellii (accession number AY375161), respectively.
Molecular phylogenetic analysis of Rickettsia symbiont.
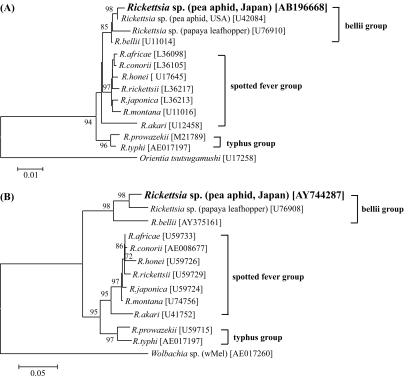
Molecular phylogenetic analysis on the basis of 16S rRNA gene sequences from Rickettsia spp. revealed that our Rickettsia sequence belonged to neither the “spotted fever group” nor the “typhus group” but was placed within the clade consisting of R. bellii and Rickettsia spp. from aphid and leafhopper (Fig. 1A). Molecular phylogenetic analysis on the basis of gltA gene sequences gave substantially the same result: our Rickettsia sequence was placed in the clade containing R. bellii and the Rickettsia sp. from leafhopper (Fig. 1B).
FIG. 1.
Molecular phylogenetic analysis of the Rickettsia symbiont from A. pisum strain OY. (A) Neighbor-joining tree on the basis of 16S rRNA gene sequences. A total of 1,417 unambiguously aligned nucleotide sites were subjected to the analysis. (B) Neighbor-joining tree on the basis of gltA gene sequences. A total of 353 unambiguously aligned nucleotide sites were subjected to the analysis. The percent bootstrap values are shown at the nodes, although values less than 70% are not indicated. The numbers in brackets are accession numbers. Three major groups in the genus Rickettsia, “spotted fever group,” “typhus group,” and “bellii group,” are indicated on the right side.
In situ hybridization of Rickettsia symbiont.
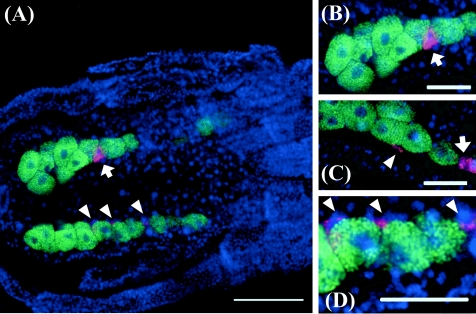
Whole-mount fluorescence in situ hybridization revealed a remarkable cellular tropism of the Rickettsia symbiont (Fig. 2). In aphid embryos of the strain OY, strong signals of Rickettsia-specific probe were found in two types of cells: secondary mycetocytes (Fig. 2, arrows) and sheath cells (Fig. 2, arrowheads). The secondary mycetocytes were relatively large and intercalated between the primary mycetocytes harboring Buchnera (Fig. 2A and B). The sheath cells were small and flat, located on the periphery of the primary mycetocytes (Fig. 2A, C, and D). The primary mycetocytes, secondary mycetocytes, and sheath cells constituted a large symbiotic organ, mycetome, in the body cavity of the aphids. No-probe control, RNase digestion control, and competitive suppression control confirmed the specificity of the detected signals (data not shown). In situ hybridization of the Rickettsia-free aphid strain AIST detected signals of Buchnera but not those of Rickettsia (data not shown).
FIG. 2.
Whole-mount in situ hybridization of aphid embryos targeting Rickettsia (red) and Buchnera (green). (A) Mycetomes of an aphid embryo in which secondary mycetocytes and sheath cells harboring Rickettsia are seen in addition to a number of primary mycetocytes harboring Buchnera. (B to D) Magnified images of mycetome. Arrows, secondary mycetocytes; arrowheads, sheath cells; bars, 50 μm.
Electron microscopy of Rickettsia symbiont.
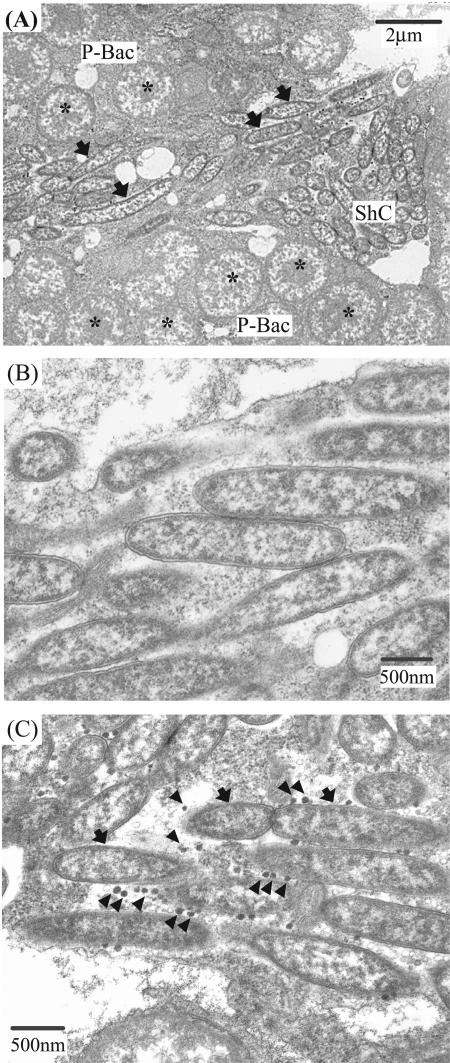
Electron microscopy of embryos of the strain OY identified a dense population of rod-shaped bacteria in the cytoplasm of the sheath cells (Fig. 3A). The bacterial cells were slender rods, 1.84 ± 0.43 μm long and 0.48 ± 0.05 μm thick (mean ± standard deviation [n = 20]) and exhibited cell wall structure (Fig. 3B). The rod-shaped bacteria were not observed in the Rickettsia-free aphid strain AIST (data not shown). Virus-like particles were sometimes observed in association with the bacterial cells (Fig. 3C).
FIG. 3.
Electron microscopy of the Rickettsia symbiont. (A) A sheath cell harboring rod-shaped cells of the Rickettsia symbiont (arrows) surrounded by primary mycetocytes harboring Buchnera (asterisks). (B) A magnified image of the Rickettsia cells in a sheath cell. (C) Virus-like particles (arrowheads) in association with the Rickettsia cells. Abbreviations: ShC, sheath cell; PM, primary mycetocyte.
Selective elimination of Rickettsia symbiont by antibiotic treatment.
Fifteen adult aphids of the Rickettsia-infected strain OY were injected with ampicillin solution. In the G1 offspring, both Buchnera and Rickettsia were detected by diagnostic PCR. In the G2 offspring, Rickettsia infection disappeared in all the lines, while Buchnera infection was maintained. After maintenance for 10 generations and on, Rickettsia infection did not recover in the treated lines (data not shown), whereby we established a Rickettsia-eliminated aphid strain, OYamp. A control aphid strain, OYdw, was generated in the same way, but distilled water was injected instead of the antibiotic.
Fitness effects of Rickettsia symbiont.
Fitness parameters were compared between the Rickettsia-infected aphid strain OYdw and the Rickettsia-eliminated aphid strain OYamp with identical genetic backgrounds. The Rickettsia-infected aphids exhibited lower fresh body weight and total number of offspring than the Rickettsia-eliminated aphids, whereas there were no significant differences with regard to age of first reproduction and longevity between the aphid strains (Table 1).
TABLE 1.
Effect of Rickettsia infection on fitness parameters of the host aphida
| Characteristic | OYdw (+Rickettsia) | OYamp (−Rickettsia) |
|---|---|---|
| No. of aphids measured | 17 | 20 |
| Body wt* (mg) at 12 days old | 3.78 ± 0.48 | 4.38 ± 0.40 |
| Total no. of offspring* | 113 ± 19.5 | 134 ± 12.3 |
| Age at first reproduction (days after birth) | 8.35 ± 0.49 | 8.41 ± 0.50 |
| Longevity (days) | 28 ± 4.0 | 28 ± 3.8 |
Means ± standard deviations are shown. Asterisks indicate the fitness parameters that exhibited statistically significant differences (Mann-Whitney U test, P < 0.001). The measurements were conducted 15 generations after the injection treatments.
Population dynamics of Rickettsia and Buchnera in the developmental course of the host aphid.
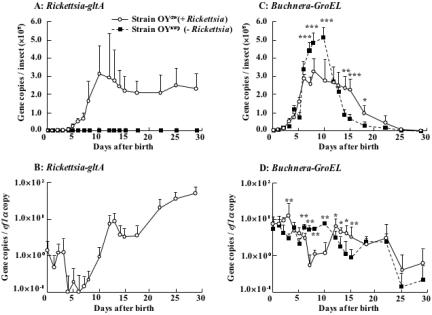
By using a quantitative PCR technique, we investigated the population dynamics of the symbiotic bacteria in the Rickettsia-infected aphids and the Rickettsia-eliminated aphids with identical genetic backgrounds. The population of Rickettsia increased during nymphal growth, reaching a plateau in 10-day-old adults, and the infection level was maintained in older insects (Fig. 4A). When normalized by the titers of a host gene, the density of Rickettsia exhibited similar dynamics but a transient drop around the 5-day-old stage and a resurgence at the 15-day-old and later stages (Fig. 4B).
FIG. 4.
Infection dynamics of Rickettsia and Buchnera in the developmental course of the host aphid. (A) Rickettsia titers in terms of gltA gene copies per insect. (B) Rickettsia densities in terms of gltA gene copies per ef1α gene copy. (C) Buchnera titers in terms of groEL gene copies per insect. (D) Buchnera densities in terms of groEL gene copies per ef1α gene copy. Open circles, the Rickettsia-infected aphid strain OYdw; closed squares, the Rickettsia-eliminated aphid strain OYamp. Means and standard deviations are shown (n = 12, respectively). Asterisks indicate statistically significant differences (Mann-Whitney U test; *, P < 0.05; **, P < 0.01; ***, P < 0.001). The measurements were conducted at 18 to 20 generations after the injection treatments.
The population dynamics of Buchnera were different between the Rickettsia-infected aphids and the Rickettsia-eliminated aphids. In the absence of Rickettsia, the population of Buchnera increased during nymphal growth, reaching a peak in actively reproducing young adults, and declined in older insects. Notably, the titers of Buchnera were significantly lower in the presence of Rickettsia (at 50 to 60% of those in the absence of Rickettsia) during the young adult period, when the aphids most actively reproduce. At later stages, in contrast, the titers of Buchnera became lower in the absence of Rickettsia (Fig. 4C). Even when normalized by the titers of a host gene, the density of Buchnera exhibited qualitatively similar dynamics: significantly higher densities in the absence of Rickettsia during the young adult period but lower densities at later stages (Fig. 4D).
DISCUSSION
Members of the genus Rickettsia are found from a wide variety of arthropods and are obligatorily associated with the host cells (9, 44). However, no specific cells for harboring Rickettsia have been reported, although concentrated infections in ovaries for vertical transmission and/or in salivary glands for horizontal transmission might be presumed implicitly (9, 30). Previous studies suggested that the Rickettsia symbiont of A. pisum is present in the hemolymph, on the grounds that hemolymph transfer from infected aphids to uninfected ones established Rickettsia infection in the recipients (5), and rod-shaped bacteria were observed in the hemolymph of Rickettsia-infected aphids (6). In addition to the hemolymph infection, we identified the localization of the Rickettsia symbiont in two types of cells specialized for endosymbiosis: secondary mycetocytes and sheath cells (Fig. 2). To our knowledge, this study provides the first report of specific host cells that harbor Rickettsia. The cellular tropism suggests that elaborate molecular and cellular interactions are involved in the infection and maintenance of the Rickettsia symbiont in the host aphid.
The in vivo localization of the Rickettsia symbiont in secondary mycetocytes, sheath cells, and hemolymph was quite similar to the localization of other secondary symbionts of A. pisum such as PASS, PAUS, and PABS (16, 22, 35, 40). It should be noted that these facultative symbionts belong to phylogenetically distinct bacterial lineages: the Rickettsia symbiont is a member of the α-Proteobacteria, whereas PASS, PAUS, and PABS constitute distinct clades in the γ-Proteobacteria (34, 35). The similar localization patterns of PASS, PAUS, PABS, and the Rickettsia raise the possibility that although speculative, these facultative symbionts might share some common molecular and cellular mechanisms for their infection and maintenance in the host aphid.
To evaluate the biological effects of the Rickettsia symbiont, it was pivotal to generate aphid strains that are genetically identical and differ only in Rickettsia infection. Recently, a novel selective elimination technique was developed for the γ-proteobacterial facultative symbionts PASS and PAUS: microinjection of ampicillin, an antibiotic that inhibits bacterial cell wall synthesis, resulted in complete elimination of these symbionts in the offspring of injected aphids, while the coexisting essential symbiont Buchnera was not affected (22, 41). The technique was also effective for selective elimination of the α-proteobacterial Rickettsia symbiont, suggesting potential applicability of the technique to a wider variety of bacterial symbionts. Previous therapeutic studies showed that penicillin and allied antibiotics, including ampicillin, are generally not effective in Rickettsia-infected patients (9, 44). However, in vitro studies demonstrated that penicillin is potentially able to eradicate Rickettsia, suggesting that inefficient permeation of the antibiotic to the intracellular location of the bacterium may be responsible for the apparent in vivo resistance (47, 48). It should be noted that Buchnera is always intracellular, whereas PASS, PAUS, and Rickettsia have an extracellular phase in the hemolymph (6, 22, 40). The susceptibility of the facultative symbionts to the antibiotic could be understood in this context.
By using a quantitative PCR technique, we monitored the dynamics of population and density of Buchnera and Rickettsia during the host development (Fig. 4), which provided some insights into the nature of the symbiotic associations. The proliferation of Buchnera was coincident with the reproductively active stage of the host aphid (Fig. 4C and D), which probably reflects the principal biological role of the symbiont: the provision of essential amino acids and other nutrients to support the rapid development of a large number of embryos (12). The proliferation pattern also suggests that the host aphid may have a control mechanism over the proliferation of Buchnera, which must have evolved during the long history of the host-symbiont coevolution (29). By contrast, the population of Rickettsia exhibited simple growth-and-plateau dynamics (Fig. 4A). The density of Rickettsia exhibited similar dynamics but with a transient drop around the 5-day-old stage and a resurgence in old insects (Fig. 4B). The transient drop probably reflects the condition that the proliferation of Rickettsia cannot catch up with the rapid growth of young aphids. The resurgence might be related to the normalization by the titers of a host gene: young aphids produce embryos very actively, but the activity drastically declines in old insects, which results in reduced titers of the host gene and consequently overestimated densities of the symbiont. These patterns are suggestive of less strict control over the proliferation of Rickettsia in the host aphid, which may be relevant to the facultative nature of the symbiont.
The secondary mycetocytes and sheath cells harboring Rickettsia are in close association with the primary mycetocytes harboring Buchnera, constituting a symbiotic organ called the mycetome (Fig. 2). The spatial proximity suggests that various biological interactions may take place between the facultative and essential symbionts. The dynamics of Buchnera in the presence and absence of Rickettsia revealed an aspect of such symbiont-symbiont interactions: coinfection with Rickettsia significantly suppressed the population density of Buchnera (Fig. 4C and D). In the endosymbiotic ecosystem of the host body, the facultative symbiont Rickettsia negatively affected the essential symbiont Buchnera, probably via competition for limited resources, energy, and/or an endocellular niche. It was reported that the presence of PASS similarly suppressed the population of Buchnera (22).
In previous studies, fitness effects of Rickettsia infection were investigated by using Rickettsia-infected and uninfected aphids with identical genetic backgrounds that were generated by hemolymph transfer. These studies indicated that Rickettsia infection generally showed negative effects on the host aphids, while the intensity of the effects was dependent on environmental factors such as temperature and host plant species (5, 7, 28). In this study, by using the antibiotic-treated aphids, we identified reduced body weight and fecundity of the Rickettsia-infected host aphids (Table 1), reinforcing the notion that Rickettsia infection generally has negative effects on A. pisum, at least under the laboratory rearing conditions.
In this study, we demonstrated that Rickettsia infection negatively affects both the population density of Buchnera (Fig. 4C and D) and the host fitness (Table 1). The following hypotheses are conceivable to account for the action of the Rickettsia symbiont: (i) Rickettsia negatively affects the host aphid, and as a result, the population density of Buchnera is indirectly suppressed; and (ii) Rickettsia suppresses the essential symbiont Buchnera, and this leads to reduced performance of the host aphid. Of course, these hypotheses are not necessarily mutually exclusive and should be tested in future studies. Even the possibility that a third party is involved cannot be ruled out; the ampicillin treatment might have suppressed unidentified microbial associates of the aphid and thus led to the improved fitness of the host aphid and the increased population density of Buchnera.
Electron microscopy detected the occurrence of virus-like particles associated with the Rickettsia cells (Fig. 2C). At present, without molecular data, the nature of the particles is obscure. Even the possibility that the particle is merely an artifact cannot be excluded. It should be noted, however, that a bacteriophage of the Podoviridae, referred to as APSE-1, has been identified from A. pisum and other aphids, whose occurrence was associated with PABS and allied secondary symbionts (35, 42). Although speculative, such elements might be involved in the negative effects of the facultative symbionts on Buchnera and/or host aphids. These elements might also contribute to evolutionary processes such as symbiont-symbiont and host-symbiont horizontal gene transfer and genetic recombination (25, 45).
Rickettsia infection has been detected at considerable frequencies in natural populations of A. pisum: 48% (59/122) in California (6); 22% (17/77) in another survey in California (27); 4% (35/858) in Japan (39); 30 to 37% on pea and 0 to 10% on alfalfa in France (36), etc. The prevalence in natural populations appears contradictory to the negative fitness effects of Rickettsia infection observed in the laboratory. At present, we do not understand what mechanisms maintain the Rickettsia symbiont in the aphid populations. In a simple model, the infection frequency of an endosymbiont in a host population is determined mainly by three parameters: (i) fidelity of vertical transmission, (ii) fitness effect on the host, and (iii) frequency of horizontal transmission (16, 17). Therefore, for example, sufficiently frequent horizontal transfers between aphid individuals may be able to counter the negative fitness effects for maintaining Rickettsia infection in host populations. More plausibly, the negative fitness effects of Rickettsia infection may be cancelled out by positive fitness effects on some aspects of the physiology and ecology of the host aphid. Previous studies have demonstrated that other facultative symbionts of A. pisum conferred context-dependent positive fitness effects on the host aphid. PASS infection conferred tolerance of the aphid to high temperature (28). PABS infection induced resistance of the aphid against parasitoid wasps (31). PAUS infection improved fecundity of the aphid on an unsuitable host plant (41). PASS infection could support reproduction of the aphid deprived of the essential symbiont Buchnera (22). In this context, it is of great interest to investigate the effects of Rickettsia infection on various life history traits of A. pisum under different environmental conditions. In Japan, the Rickettsia symbiont tended to be detected in southwestern regions (39). In France, the Rickettsia symbiont was frequently found on pea but not on red clover (36). These observations might be relevant to the biological significance of the facultative symbiont for the host aphid.
Acknowledgments
We thank E. Zchori-Fein for reading the manuscript and S. Koike, J. Makino, K. Nomura, and H. Ohta for technical and secretarial assistance.
This research was supported by the Program for Promotion of Basic Research Activities for Innovation Biosciences (ProBRAIN) of the Bio-Oriented Technology Research Advancement Institution.
REFERENCES
- 1.Aksoy, S. 1995. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. Int. J. Syst. Bactoriol. 45:848-851. [DOI] [PubMed] [Google Scholar]
- 2.Benson, M. J., J. D. Gawronski, D. E. Eveleigh, and D. R. Benson. 2004. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Appl. Environ. Microbiol. 70:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braendle, C., T. Miura, R. Bickel, A. W. Shingleton, S. Kambhampati, and D. L. Stern. 2003. Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis. PLoS Biol. 1:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, N.Y.
- 5.Chen, D. Q., and A. H. Purcell. 1997. Occurrence and transmission of facultative endosymbionts in aphids. Curr. Microbiol. 34:220-225. [DOI] [PubMed] [Google Scholar]
- 6.Chen, D. Q., B. C. Campbell, and A. H. Purcell. 1996. A new Rickettsia from a herbivorous insect, the pea aphid Acyrthosiphon pisum (Harris). Curr. Microbiol. 33:123-128. [DOI] [PubMed] [Google Scholar]
- 7.Chen, D. Q., C. B. Montllor, and A. H. Purcell. 2000. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid, Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol. Exp. Appl. 95:315-323. [Google Scholar]
- 8.Cheng, Q., T. D. Ruel, W. Zhou, S. K. Moloo, P. Majiwa, S. L. O'Neill, and S. Aksoy. 2000. Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med. Vet. Entomol. 14:44-50. [DOI] [PubMed] [Google Scholar]
- 9.Dasch, G. A., and E. Weiss. 1992. The genera Rickettsia, Rochalimaea, Ehrlichia, Cowdaria, and Neorickettsia, p. 2407-2470. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, vol. 3. Springer-Verlag, New York, N.Y. [Google Scholar]
- 10.Davis, M. J., Z. Ying, B. R. Brunner, A. Pantoja, and F. H. Ferwerda. 1998. Rickettsial relative associated with papaya bunchy top disease. Curr. Microbiol. 36:80-84. [DOI] [PubMed] [Google Scholar]
- 11.Dobson, S. L., K. Bourtzis, H. R. Braig, B. F. Jones, W. Zhou, F. Rousset, and S. L. O'Neill. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29:153-160. [DOI] [PubMed] [Google Scholar]
- 12.Douglas, A. E. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43:17-37. [DOI] [PubMed] [Google Scholar]
- 13.Fukatsu, T., and M. Shimada. 1999. Molecular characterization of Rickettsia sp. in a bruchid beetle Kytorhinus sharpianus (Coleoptera: Bruchidae). Appl. Entomol. Zool. 34:391-397. [Google Scholar]
- 14.Fukatsu, T. 2001. Secondary intracellular symbiotic bacteria in aphids of the genus Yamatocallis (Homoptera: Aphididae: Drepanosiphinae). Appl. Environ. Microbiol. 67:5315-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukatsu, T., and N. Nikoh. 2000. Endosymbiotic microbiota of the bamboo pseudococcid Antonina crawii (Insecta, Homoptera). Appl. Environ. Microbiol. 66:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukatsu, T., N. Nikoh, R. Kawai, and R. Koga. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukatsu, T., T. Tsuchida, N. Nikoh, and R. Koga. 2001. Spiroplasma symbiont of the pea aphid Acyrthosiphon pism (Insecta: Homoptera). Appl. Environ. Microbiol. 67:1284-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadfield, S. J., and J. M. Axton. 1999. Reproduction—germ cells colonized by endosymbiotic bacteria. Nature 402:482. [DOI] [PubMed] [Google Scholar]
- 19.Hurst, G. D. D., T. C. Hammarton, J. J. Obrycki, T. M. O. Majerus, L. E. Walker, D. Bertrand, and M. E. N. Majerus. 1996. Male-killing bacterium in a fifth ladybird beetle, Coleomegilla maculata (Coleoptera: Coccinellidae). Heredity 77:177-185. [DOI] [PubMed] [Google Scholar]
- 20.Ijichi, N., N. Kondo, R. Matsumoto, M. Shimada, H. Ishikawa, and T. Fukatsu. 2002. Internal spatio-temporal population dynamics of infection with three Wolbachia strains in the adzuki bean beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). Appl. Environ. Microbiol. 68:4074-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi, Y., S. Sameshima, O. Kitade, J. Kojima, and T. Fukatsu. 2002. Novel clade of Rickettsia spp. from leeches. Appl. Environ. Microbiol. 68:999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koga, R., T. Tsuchida, and T. Fukatsu. 2003. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. R. Soc. Lond. B 270:2543-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo, N., M. Shimada, and T. Fukatsu. 1999. High prevalence of Wolbachia in the azuki bean beetle Callosobruchus chinensis (Coleoptera, Bruchidae). Zool. Sci. 16:955-962. [Google Scholar]
- 24.Kondo, N., N. Ijichi, M. Shimada, and T. Fukatsu. 2002. Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae). Mol. Ecol. 11:167-180. [DOI] [PubMed] [Google Scholar]
- 25.Kondo, N., N. Nikoh, N. Ijichi, M. Shimada, and T. Fukatsu. 2002. Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc. Natl. Acad. Sci. USA 99:14280-14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: Molecular Evolutionary Genetics Analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 27.Leonardo, T. E., and G. T. Muiru. 2003. Facultative symbionts are associated with host plant specialization in pea aphid populations. Proc. R. Soc. Lond. B 270:S209-S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montllor, C. B., A. Maxman, and A. H. Purcell. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27:189-195. [Google Scholar]
- 29.Moran, N. A., M. A. Munson, P. Baumann, and H. Ishikawa. 1993. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc. R. Soc. Lond. B 253:167-171. [Google Scholar]
- 30.Noda, H., U. G. Munderloh, and T. J. Kurrti. 1997. Endosymbionts of ticks and their relationships to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 63:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver, K. M., J. A. Russell, N. A. Moran, and M. S. Hunter. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 10:1803-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regnery, R. L., C. L. Spruill, and B. D. Plikaytis. 1991. Genotypic identification of rickettsiae and estimation of intraspecific sequence divergence for proteins of two rickettsial genes. J. Bacteriol. 173:1576-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roux, V., and D. Raoult. 1995. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res. Microbiol. 146:385-396. [DOI] [PubMed] [Google Scholar]
- 34.Russell, J. A., A. Latorre, B. Sabater-Munoz, A. Moya, and N. A. Moran. 2003. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 12:1061-1075. [DOI] [PubMed] [Google Scholar]
- 35.Sandström, J. P., J. A. Russell, J. P. White, and N. A. Moran. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217-228. [DOI] [PubMed] [Google Scholar]
- 36.Simon, J. C., S. Carre, M. Boutin, N. Prunier-Leterme, B. Sabater-Munoz, A. Latorre, and R. Bournoville. 2003. Host-based divergence in populations of the pea aphid: insights from nuclear markers and the prevalence of facultative symbionts. Proc. R. Soc. Lond. B 270:1703-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stothard, D. R., and P. A. Fuerst. 1995. Evolutionary analysis of the spotted fever and typhus groups of Rickettsia using 16S rRNA gene sequences. Syst. Appl. Microbiol. 18:52-61. [Google Scholar]
- 38.Thompson, J. D., D. G. Higgins, and J. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuchida, T., R. Koga, H. Shibao, T. Matsumoto, and T. Fukatsu. 2002. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 11:2123-2135. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchida, T., R. Koga, X.-Y. Meng, T. Matsumoto, and T. Fukatsu. 2005. Characterization of a facultative endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum. Microb. Ecol. 49:126-133. [DOI] [PubMed]
- 41.Tsuchida, T., R. Koga, and T. Fukatsu. 2004. Host plant specialization governed by facultative symbiont. Science 303:1989. [DOI] [PubMed] [Google Scholar]
- 42.van der Wilk, F., A. M. Dullemans, M. Verbeek, and J. F. J. M. van den Heuvel. 1999. Isolation and characterization of APSE-1, a bacteriophage infecting the secondary endosymbiont of Acyrthosiphon pisum. Virology 262:104-113. [DOI] [PubMed] [Google Scholar]
- 43.von der Schulenburg, J. H. G., M. Habig, J. J. Sloggett, K. M. Webberley, D. Bertrand, G. D. D. Hurst, and M. E. N. Majerus. 2001. Incidence of male-killing Rickettsia spp. (α-proteobacteria) in the ten-spot ladybird beetle Adalia decempunctata L. (Coleoptera: Coccinellidae). Appl. Environ. Microbiol. 67:270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss, E., and J. W. Moulder. 1984. Order I. Rickettsiales Gieszczykiewicz 1939, p. 687-704. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 45.Werren, J. H., and J. D. Bartos. 2001. Recombination in Wolbachia. Curr. Biol. 11:431-435. [DOI] [PubMed] [Google Scholar]
- 46.Werren, J. H., G. D. D. Hurst, W. Zheng, J. A. J. Breeuwer, R. Stouthamer, and M. E. N. Majerus. 1994. Rickettsial relative associated with male killing in the ladybird beetle (Adalia dipunctata). J. Bacteriol. 176:388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wisseman, C. L., Jr., D. J. Silverman, A. Waddell, and D. T. Brown. 1982. Penicillin-induced unstable intracellular formation of spheroplasts by rickettsiae. J. Infect. Dis. 146:147-158. [DOI] [PubMed] [Google Scholar]
- 48.Wisseman, C. L., Jr., A. D. Waddell, and W. T. Walsh. 1974. In vitro studies of action of antibiotics on Rickettsia prowazeki by two basic methods of cell culture. J. Infect. Dis. 130:564-574. [DOI] [PubMed] [Google Scholar]