Abstract
We studied the presence of botulinum toxin-producing clostridia in 2,009 soil samples from five geographical regions of Argentina. The prevalence was 23.5%, and the distribution was not homogeneous among the regions. We observed a great multiplicity of serological types and a higher prevalence in nonvirgin soils than in virgin soils.
The geographical distribution of botulinum toxin-producing clostridia (BTPC) (7) has been extensively studied in Europe, Asia, and North America (1, 2, 4, 5, 8, 9, 10, 11, 12, 13, 15, 16, 18, 19, 20, 21, 23, 24, 25, 26). However, our knowledge concerning the presence of these anaerobes in South America is very restricted. In this study our principal aim was to examine the prevalence and distribution of BTPC in soils of Argentina.
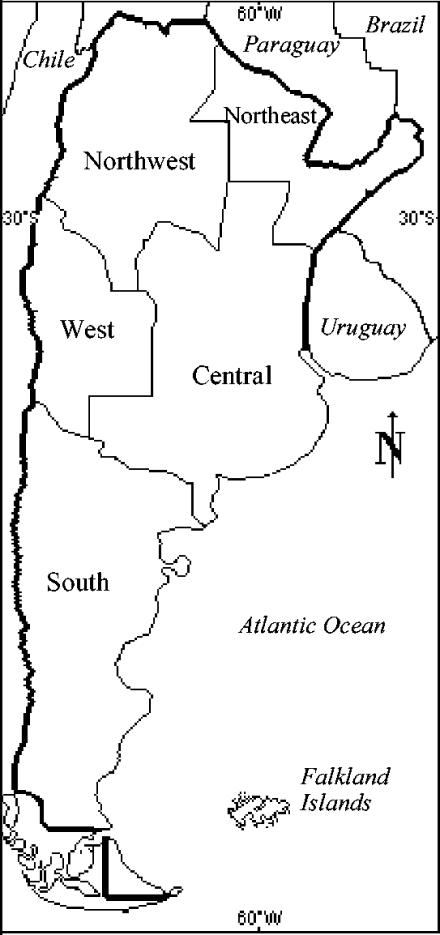
We examined 2,009 soil samples from Argentina. Because of the vast the territory and the climate and geographical variations, this country was divided into five regions (Fig. 1 and Table 1). Soil samples were obtained and processed from 1964 until 2002. In each case the time between collection and examination was less than 2 days. Each sample was collected from a 100-cm2 area by using a sterile metal spoon and then was transferred to a sterile receptacle and stored at room temperature until examination. Soils were classified into two categories: virgin and nonvirgin. Virgin soils were defined as soils that still were in their natural state and had not been used or changed by people. Nonvirgin soils were defined as soils that had been changed by people (cultivated, urbanized, and industrialized soils). Samples were processed by diluting 25 g of soil in 50 ml of a saline solution (0.15 M NaCl). Two aliquots were taken after 40 min of resting. One aliquot was immediately inoculated into chopped-meat medium (6). The other aliquot was subjected to a heat shock (80°C, 10 min) and later was inoculated into chopped-meat medium. After incubation for 5 days at 31°C, broth media were centrifuged at 12,000 × g for 10 min at 4°C, and 0.5 ml was inoculated in duplicate intraperitoneally into mice. Mice were observed for 96 h for characteristic botulinal signs and death (14). Cultures without signs of proteolysis were treated by mixing equal volumes of the culture supernatant and 1% trypsin (1:250; Difco), followed by incubation at 37°C for 1 h. Toxic cultures were cultivated in solid media with 1.5% and 4.0% agar (0.4% meat extract, 1.0% glucose, 4.0% Proteose Peptone, 0.5% NaCl, 1.5% or 4.0% agar [pH 7.2]) and egg yolk agar (3). They were incubated for up to 24, 48, and 72 h, respectively, at 34°C in BBL jars with an atmosphere containing 80% N2, 10% CO2, and 10% H2. Selected colonies were transferred to chopped-meat medium and incubated for 4 days at 31°C. The presence of botulinum toxin was investigated in each of these cultures, as described previously. Toxic broth media were cultivated in solid media to ensure that the cultures were pure. Genera were identified by using gram-positive, strictly anaerobic, and sporulated bacilli. Species were not identified. Strains were cultivated in chopped-meat medium and incubated at 31°C for 18 h. An aliquot was cultured by dialysis in a sack of cellophane immersed in toxin production medium (4.0% Trypticase peptone, 1.0% Proteose Peptone, 1.0% glucose, 1.0% yeast extract [pH 7.2] [22]) and was incubated for 6 days at 32°C. After incubation, the contents of the sacks of cellophane were centrifuged at 12,000 × g for 10 min at 4°C and serially diluted twofold in buffered solution, and 0.5 ml of each dilution was intraperitoneally inoculated into six mice. Deaths were recorded for 96 h, and the 50% lethal doses were calculated by the Reed-Muench method (17). Serologic typing was carried out by quantitative neutralization tests with positive samples by using a toxin titer of 2,000 50% lethal doses per ml and monovalent and polyvalent botulinum toxin antisera. Some positive samples with low toxin titers that could not be isolated in solid media were not typed by toxin neutralization tests and were designated nonspecific. Comparisons between the prevalence of BTPC in one of the five regions and the prevalence of BTPC in another region were performed using chi-square analysis.
FIG. 1.
Geographical regions of Argentina.
TABLE 1.
Characteristics of five geographical regions of Argentina
| Region | Localization | Area (km2) | Population | Climate | Annual precipitation (mm) | Annual mean temp (°C) |
|---|---|---|---|---|---|---|
| Central | 28°-41° S, 57°-68° W | 824,493 | 12,667,709 | Temperate | 500 | 15 |
| Northeast | 23°-30° S, 63°-53° W | 289,699 | 3,367,518 | Subtropical without dry season in east | 500-1,500 | 21 |
| Northwest | 22°-32° S, 69°-62° W | 559,864 | 4,458,470 | Arid; subtropical in northeast | 250 (1,500 in northeast) | 18-22 |
| South | 38°-55° S, 63°-73° W | 787,291 | 1,738,251 | Cold and arid | 250 in northwest and 1,500 in east | 0-10 |
| West | 28°-38° S, 65°-71° W | 315,226 | 2,567,607 | Arid | 300 | 14-16 |
A total of 2,009 Argentine soil samples were examined. BTPC were detected in 472 of these samples (23.5%) (Table 2). Thus, the prevalence was relatively high compared to the following values for other countries: 5.7% (10 of 174 samples) in the United Kingdom (18), 16.5% (44 of 266 samples) in Japan (26), 23.3% (7 of 30 samples) in Costa Rica (4), and 24.3% (375 of 1,538 samples) in the United States (12).
TABLE 2.
Prevalence of BTPC in soila
| Region | Soil samples (n = 2009)
|
||
|---|---|---|---|
| No. positive | Total no. | % | |
| Central | 120 | 485 | 24.7 |
| Northeast | 12 | 270 | 4.4 |
| Northwest | 85 | 283 | 30.0 |
| South | 72 | 490 | 14.7 |
| West | 183 | 481 | 38.0 |
| Total | 472 | 2,009 | 23.5 |
Differences in the prevalence values for BTPC among the regions were significant (P < 0.001).
The distribution of BTPC among the geographical regions was not homogeneous. The west and northwest regions exhibited higher prevalence values (38.0% and 30.0%, respectively), whereas the northeast and south regions showed low prevalence values (4.4% and 14.7%, respectively) (Table 2). These regions have different climatic and geographical characteristics, and probably, a combination of these characteristics influence the prevalence of BTPC in soils. Factors that could specifically affect the presence of the clostridia in soils are unknown. In studies carried out with soils of other countries, an association was found between the presence of C. botulinum and a low organic matter content but not with the soil pH (4). However, in another study, the presence of C. botulinum was associated with soils having high organic matter contents (20).
We observed a higher prevalence (P < 0.001) in nonvirgin soils than in virgin soils (Table 3), except in the central region. Coincident with this, in Danish soils (9) C. botulinum was found in 41.0% of cultivated farmland samples, whereas positive samples were not obtained from virgin forest. In addition, Parry (16) reported a higher incidence of C. botulinum in farmed soils than in virgin soils in New York (United States). In the central region we observed a higher prevalence in virgin soils. However, a low number of samples was collected from this type of soil due to the great human activity in this region.
TABLE 3.
Prevalence of BTPC in virgin soils and nonvirgin soilsa
| Region | Nonvirgin soil samplesb
|
Virgin soil samplesc
|
||||
|---|---|---|---|---|---|---|
| No. positive | Total no. | % | No. positive | Total no. | % | |
| Central | 89 | 397 | 22.4 | 31 | 88 | 35.2 |
| Northeast | 9 | 151 | 6.0 | 3 | 119 | 2.5 |
| Northwest | 60 | 174 | 34.5 | 25 | 109 | 22.9 |
| South | 59 | 288 | 20.5 | 13 | 202 | 6.4 |
| West | 147 | 338 | 43.5 | 36 | 143 | 25.2 |
| Total | 364 | 1,348 | 27.0 | 108 | 661 | 16.3 |
Differences in the prevalence values for virgin soils and nonvirgin soils were significant (P < 0.001) in all cases.
Nonvirgin soils were defined as soils that had been changed by people (cultivated, urbanized, and industrialized soils).
Virgin soils were defined as soils that had not been changed by people.
Type A was the type that was most frequently detected (Table 4). Subtype Af and mixtures of types were detected in 3.6% and 3.4% of the positive samples, respectively. For 16.6% of the toxic samples typing was not possible. We did not find reports of subtype Af in soils of any other country, and there was a low incidence of type G in soils of Switzerland (21). It is important to note that this diversity was observed principally in the west region of Argentina. In contrast, only type A was found in the northeast region. We did not find type C, type D, or type E in the samples studied. Types C and D seem to predominate in tropical environments (13, 23), and type E organisms are mainly aquatic, especially in cold waters (9).
TABLE 4.
Prevalence of BTPC in 470 positive soil samplesa
| Region | % of the following types
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | F | G | Af | A+B | A+F | B+F | NSb | |
| Central | 45.0 | 29.2 | 1.7 | 3.3 | 20.8 | ||||
| Northeast | 75.0 | 25.0 | |||||||
| Northwest | 67.1 | 3.5 | 7.1 | 1.2 | 21.1 | ||||
| South | 51.4 | 29.2 | 1.4 | 18.0 | |||||
| West | 61.2 | 7.1 | 4.9 | 1.1 | 9.3 | 5.5 | 0.5 | 10.4 | |
| Total | 56.7 | 15.3 | 3.8 | 0.4 | 3.6 | 3.0 | 0.2 | 0.2 | 16.6 |
Types C, D, and E were not found in the soil samples examined.
NS, nonspecific type (positive samples with low toxin titers that could not be isolated in solid media and were not typed by toxin neutralization tests).
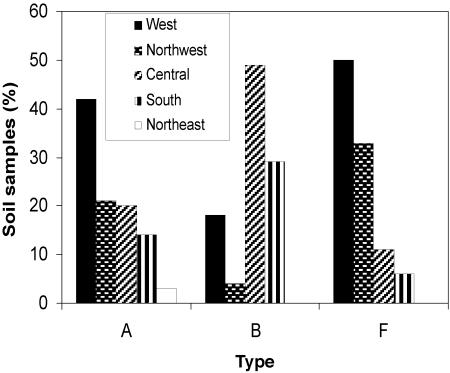
The distribution of types A, B, and F in the five regions was not homogeneous (Fig. 2). Type A was detected in 267 of the 472 positive samples (41.9% from the west region, 20.6% from the northwest region, 20.2% from the central region, 13.8% from the south region, and 3.4% from the northeast region). The distribution of type F was similar. In contrast, 48.6% of the type B samples were from the central region, 29.2% were from the south region, and the rest were from the west and northwest regions. Because the central region is the principal cattle and agricultural area of Argentina, we suggest that the human use of soils could affect the survival of the organisms. Coincident with this, in the Danish environment (9) type B is the predominant type in farmed or forested soils.
FIG. 2.
Distribution of types A, B, and F in soils from five geographical regions of Argentina. Type A was detected in 269 positive samples (41.9% from the west region, 20.6% from the northwest region, 20.2% from the central region, 13.8% from the south region, and 3.4% from the northeast region). Type F was detected in 18 positive samples (50.0% from the west region, 33.3% from the northwest region, 11.1% from the central region, and 5.56% from the south region). Type B was detected in 72 positive samples (48.6% from the central region, 29.2% from the south region, 18.1% from the west region, and 4.1% from the northwest region).
Acknowledgments
This work was supported by grants from Facultad de Ciencias Médicas and Secretaría de Ciencia y Técnica, Universidad Nacional de Cuyo. C.L. had fellowship assistance from CONICET.
REFERENCES
- 1.Aianin, K. M., T. I. Bulatova, and K. I. Matveev. 1968. The distribution of Clostridium botulinum and C. tetani in the soil in Armenia SSR. Gig. Sanit. 33:114-116. [PubMed] [Google Scholar]
- 2.Bulatova, T. I., K. I. Matveev, and I. S. Kazdobina. 1969. Distribution of Clostridium botulinum in the soil of the shores of Lake Balkhash. Gig. Sanit. 34:114-116. [PubMed] [Google Scholar]
- 3.Dezfulian, M., L. M. McCroskey, C. L. Hatheway, and V. R. Dowell. 1981. Selective medium for isolation of Clostridium botulinum from human feces. J. Clin. Microbiol. 13:526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamboa, M. M., E. Rodríguez, and B. Fernandez. 1993. Clostridium botulinum in Costa Rica soils. Rev. Biol. Trop. 41:359-363. [PubMed] [Google Scholar]
- 5.Gao, Q. Y. 1984. Distribution of toxigenic Clostridium botulinum in coastal areas in China. Zhonghua Yufang Yixue Zazhi 18:129-131. [PubMed] [Google Scholar]
- 6.Giménez, D. F., and A. S. Ciccarelli. 1970. Distribución de Clostridium botulinum en Mendoza, Argentina. Bol. Of. Sanit. Panama 69:505-510. [PubMed] [Google Scholar]
- 7.Giménez, F. G., and A. G. Giménez. 1993. Serological subtypes of botulinal neurotoxins, p. 421-431. In B. R. Das Gupta (ed.), Botulinum and tetanus neurotoxins. Plenum Press, New York, N.Y.
- 8.Haq, I., and F. Suhadi. 1981. Epidemiological report: incidence of Clostridium botulinum in coastal and inland areas of West Java. Jpn. J. Med. Sci. Biol. 34:231-235. [DOI] [PubMed] [Google Scholar]
- 9.Huss, H. H. 1980. Distribution of Clostridium botulinum. Appl. Environ. Microbiol. 39:764-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karasawa, T., X. Wang, T. Maegawa, S. Nakamura, B. M. Hang'ombe, and E. Isogai. 2000. Demonstration of botulinum toxins of types B and D in soil samples from Zambia. Ann. Trop. Med. Parasitol. 94:409-411. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi, T., K. Watanabe, and K. Ueno. 1992. Distribution of Clostridium botulinum and Clostridium tetani in Okinawa Prefecture. Kansenshogaku Zasshi 66:1639-1644. [DOI] [PubMed] [Google Scholar]
- 12.Meyer, K. F., and Dubovsky, B. J. 1922. The distribution of the spores of B. botulinus in the United States. IV. J. Infect. Dis. 31:559-594. [Google Scholar]
- 13.Mortojudo, J. W., E. G. Siagian, F. Suhadi, B. Q. Ward, and W. M. S. Ward. 1973. The presence of Clostridium botulinum in Indonesian waters. J. Appl. Bacteriol. 36:437-440. [DOI] [PubMed] [Google Scholar]
- 14.Notermans, S. H., and J. Nagel. 1989. Assays for botulinum and tetanus toxins, p. 319-331. In L. L. Simpson (ed.), Botulinum and tetanus neurotoxins. Academic Press, San Diego, Calif.
- 15.Notermans, S. H., A. H. Havelaar, J. B. Dufrenne, and J. Oosterom. 1985. Incidence of Clostridium botulinum on cattle farms. Tijdschr. Diergeneeskd. 110:175-180. [PubMed] [Google Scholar]
- 16.Parry, E. W. 1946. Prevalence of Clostridium botulinum in soils of central New York State. Food Res. 11:203-209. [DOI] [PubMed] [Google Scholar]
- 17.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty per cent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 18.Smith, G. R., and A. M. Young. 1980. Clostridium botulinum in British soil. J. Hyg. 85:271-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith, G. R., and R. A. Milligan. 1979. Clostridium botulinum in soil on the site of the former Metropolitan (Caledonian) Cattle Market, London. J. Hyg. 83:237-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, L. D. 1978. The occurrence of Clostridium botulinum and Clostridium tetani in the soil of the United States. Health Lab. Sci. 15:74-80. [PubMed] [Google Scholar]
- 21.Sonnabend, W. F., U. P. Sonnabend, and T. Krech. 1987. Isolation of Clostridium botulinum type G from Swiss soil specimens by using sequential steps in an identification scheme. Appl. Environ. Microbiol. 53:1880-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne, M., and L. M. Wentzel. 1950. A new method for the large-scale production of high-titre botulinum formol-toxoid types C and D. J. Inmunol. 65:175-183. [PubMed] [Google Scholar]
- 23.Ward, B. Q., E. S. Garret, and G. B. Reese. 1967. Further indications of Clostridium botulinum in Latin American waters. Appl. Microbiol. 15:1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wobeser, G., S. Marsden, and R. J. MacFarlane. 1987. Occurrence of toxigenic Clostridium botulinum type C in the soil of wetlands in Saskatchewan. J. Wildl. Dis. 23:67-76. [DOI] [PubMed] [Google Scholar]
- 25.Yamakawa, K., S. Kamiya, K. Yoshimura, S. Nakamura, and T. Ezaki. 1990. Clostridium botulinum in the soil of Kenya. Ann. Trop. Med. Parasitol. 84:201-203. [DOI] [PubMed] [Google Scholar]
- 26.Yamakawa, K., S. Kamiya, S. Nishida, K. Yoshimura, H. Yu, D. Y. Lu, and S. Nakamura. 1988. Distribution of Clostridium botulinum in Japan and in Shinkiang district of China. Microbiol. Immunol. 32:579-587. [DOI] [PubMed] [Google Scholar]