Abstract
Approximately 550 to 600 yersiniosis patients are reported annually in Sweden. Although pigs are thought to be the main reservoir of food-borne pathogenic Yersinia enterocolitica, the role of pork meat as a vehicle for transmission to humans is still unclear. Pork meat collected from refrigerators and local shops frequented by yersiniosis patients (n = 48) were examined for the presence of pathogenic Yersinia spp. A combined culture and PCR method was used for detection, and a multiplex PCR was developed and evaluated as a tool for efficient identification of pathogenic food and patient isolates. The results obtained with the multiplex PCR were compared to phenotypic test results and confirmed by pulsed-field gel electrophoresis (PFGE). In all, 118 pork products (91 raw and 27 ready-to-eat) were collected. Pathogenic Yersinia spp. were detected by PCR in 10% (9 of 91) of the raw pork samples (loin of pork, fillet of pork, pork chop, ham, and minced meat) but in none of the ready-to-eat products. Isolates of Y. enterocolitica bioserotype 4/O:3 were recovered from six of the PCR-positive raw pork samples; all harbored the virulence plasmid. All isolates were recovered from food collected in shops and, thus, none were from the patients' home. When subjected to PFGE, the six isolates displayed four different NotI profiles. The same four NotI profiles were also present among isolates recovered from the yersiniosis patients. The application of a multiplex PCR was shown to be an efficient tool for identification of pathogenic Y. enterocolitica isolates in naturally contaminated raw pork.
Yersinia enterocolitica comprises a wide spectrum of phenotypic variants, of which only a few are known to cause disease in humans. The bioserotypes most frequently associated with human yersiniosis are 4/O:3, 2/O:9, 1B/O:8, and 2/O:5,27, with bioserotype 4/O:3 dominating both globally and in Sweden (5; see also http://www.smittskyddsinstitutet.se). Y. enterocolitica has been isolated from a variety of wild or domestic animals, but the only animal from which Y. enterocolitica of bioserotype 4/O:3 is frequently isolated is the pig. Therefore, pork meat is thought to be an important source of the infection. However, the prevalence of pathogenic Y. enterocolitica in pork meat, except for tongues and offal, seems to be very low (24, 29).
That most Yersinia infections occur sporadically and the lack of a sensitive and specific method for detecting Yersinia in food have hampered the identification of infection sources (16, 29). For example, with the commonly used medium for isolation of Y. enterocolitica, cefsulodin-irgasan-novobiocin (CIN) agar, it is difficult to distinguish pathogenic from nonpathogenic colonies. Also, colonies of other species can resemble pathogenic Y. enterocolitica on this medium (11). An additional problem is that the serotype O:3 antigen not only exists in pathogenic strains of Y. enterocolitica but also in biovar 1A and in nonpathogenic Yersinia spp. such as Y. frederiksenii and Y. kristensenii (1, 10). In recent years, multiplex PCR assays have been developed as an efficient tool for identifying pathogenic Y. enterocolitica. If four pairs of primers are included in a multiplex PCR targeting the genes yst, rfbC, ail, and virF, it is possible to precisely distinguish pathogenic from nonpathogenic Yersinia isolates (35). In the same reaction it is also possible to differentiate pathogenic Y. enterocolitica serotype O:3 from other pathogenic Y. enterocolitica serotypes and from Y. pseudotuberculosis. All four genes are associated with disease in humans. The ail gene (named for attachment and invasion locus) (26); the yst gene, which encodes a heat-stable enterotoxin (21); and the rfbC gene can be used to identify pathogenic Y. enterocolitica O:3 strains (35). Finally, the virF gene (called lcrF in Y. pseudotuberculosis), located on the virulence plasmid, enables determination of the presence or absence of the plasmid (33). The presence of the virulence plasmid is a prerequisite for full virulence of Yersinia spp. (6).
Until recently it has been considered enough to show that Y. enterocolitica isolates recovered from food and patients shared the same bioserotype to prove similarity between the isolates. Now, however, molecular typing is also necessary. In a comparison of three molecular methods—pulsed-field gel electrophoresis (PFGE), ribotyping, and restriction enzyme analysis of the virulence plasmid (REAP)—PFGE was shown to be the most suitable technique for subtyping (23). However, one problem is that the NotI enzyme, which Fredriksson-Ahomaa et al. (13) selected as the primary enzyme to give the best resolution from a set of 35 tested enzymes, produces a large number (>40) of restriction fragments (8). This makes the profiles difficult to score and analyze. In order to reduce the number of fragments the reading range has been limited to fragments larger than 75 to 100 kb or restricted to comprise the 12 to 15 largest bands only (2, 31).
The objectives of the present study were (i) to develop a multiplex PCR targeting four virulence genes as an effective tool for identifying pathogenic Yersinia isolates, (ii) to evaluate the assay on a panel of culture collection strains and on isolates from yersiniosis patients and pork meat and, finally, (iii) to reveal the PFGE patterns within the patient and food isolates by using NotI digestion.
MATERIALS AND METHODS
Culture collection strains.
A total of 145 Yersinia strains, including 15 reference strains (Tables 1 and 2) were used to test the specificity of the multiplex PCR developed in the present study. The strains were from human (n = 85), pig (n = 14), food (n = 14), water (n = 1), and unknown (n = 31) sources. The pathogenic strains were chosen to represent the most common bioserotypes associated with human or animal disease, and the nonpathogenic strains were chosen to represent the most commonly encountered isolates recovered from food, human, and environmental sources (24). All strains were recognized as pathogenic or nonpathogenic by virtue of their origin and/or biochemical classification. Briefly, pure colonies were grown on nutrient agar (Oxoid CM3) overnight at 30°C. Per sample, five to six colonies were transferred to a tube containing 110 μl of Millipore water including 10 μl of 0.8 M NaOH. The tubes were incubated for 10 min at 70 to 75°C, and subsequently 24 μl of equal volumes of 0.8 M HCl and 1.0 M Tris (pH 8.3) were added. The samples were mixed and centrifuged. DNA concentrations were measured (GeneQuant; Amersham Pharmacia Biotech, Uppsala, Sweden), and 10 to 50 ng of DNA/μl per sample was accepted. In case of a negative multiplex PCR result obtained for any of the four separate targets, the analysis was repeated. In the reanalysis only the primer pair(s) that did not generate amplicon(s) was used, and the original PCR protocol for each primer pair was applied (21, 33, 35). The presence or absence of the virulence plasmid for all of the 145 strains was biochemically confirmed by growth on CR-BHO agarose plates (see below).
TABLE 1.
PCR detection of four virulence genes in Y. enterocolitica, Y. pseudotuberculosis, and other Yersinia spp. selected from our culture collection
| Species and bioserotype (no. of strains)a | No. of strains with chromosomal gene:
|
No. of strains with plasmid-borne gene virF | ||
|---|---|---|---|---|
| yst | rfbC | ail | ||
| Y. enterocolitica (n = 98) | ||||
| Pathogenic | ||||
| 4/O:3 (85) | 85 | 85 | 85 | 79 |
| 2/O:9 (1) | 1 | 0 | 1 | 0 |
| 1B/O:8 (6) | 6 | 0 | 6 | 0 |
| NT/O:5,27 (1) | 1 | 0 | 1 | 0 |
| 1B/O:18 (1) | 1 | 0 | 1 | 0 |
| 1B/O:20 (1) | 1 | 0 | 1 | 0 |
| 1B/O:21 (1) | 1 | 0 | 1 | 0 |
| 3/O:1,2,3 (1) | 1 | 1 | 1 | 0 |
| 5/O:2,3 (1) | 1 | 1 | 1 | 0 |
| Y. enterocolitica (n = 18) | ||||
| Nonpathogenic | ||||
| 1A, diverse (11) | 0 | 0 | 0 | 0 |
| Diverse (7) | 0 | 0 | 0 | 0 |
| Nonpathogenic Yersinia spp. (n = 15) | ||||
| Y. frederiksenii (9) | 0 | 0 | 0 | 0 |
| Y. kristensenii (3) | 0 | 0 | 0 | 0 |
| Y. intermedia (3) | 0 | 0 | 0 | 0 |
| Y. pseudotuberculosis (14) | 0 | 0 | 14 | 8 |
Total of 145 strains.
TABLE 2.
Bioserotypes of Y. enterocolitica and Y. pseudotuberculosis reference strains used in this study
| Species | Bioserotype | Origina |
|---|---|---|
| Y. enterocolitica | 4/O:3 | CCUG 34604 |
| 4/O:3 | CCUG 4586 | |
| 4/O:3 | CCUG 21476 | |
| 4/O:3 | CCUG 8233 | |
| 3/O:9 | CCUG 8239 | |
| 2/O:8 | CCUG 8238 | |
| 1B/O:18 | IP-846 | |
| 1B/O:20 | IP-845 | |
| 1B/O:21 | IP-1110 | |
| 3/O:1,2,3 | IP-64 | |
| 5/O:2,3 | IP-178 | |
| Y. enterocolitica | 1A/O:8 | IP-1105 |
| 1A/O:5 | IP-124 | |
| Y. intermedia | Not specified | ATCC 29909 |
| Y. pseudotuberculosis | Not specified | CCUG 36765 |
CCUG, Culture Collection, University of Göteborg, Göteborg, Sweden; IP, Pasteur Institute, University of Louvain, Louvain, Belgium; ATCC, American Type Culture Collection, Manassas, Va.
Patient isolates.
Isolates from yersiniosis patients were bioserotyped at the following clinical hospital laboratories in Sweden: Kristianstad and Malmö (Malmö county); Karolinska, Huddinge and St Göran (Stockholm county); Uppsala (Uppsala county); and Sundsvall and Härnösand (Västernorrland county). Isolates were then sent to the National Food Administration, Uppsala, Sweden, where the species and bioserotype determination was confirmed by phenotypic and genotypic tests. The latter included tests by multiplex PCR and PFGE analysis (see below).
Patient data.
Yersiniosis patients from four counties in Sweden (Skåne, Stockholm, Uppsala, and Västernorrland) were enrolled in this project. The four counties represented three different geographical regions in Sweden: south (Skåne); central, i.e., the region of Stockholm (Stockholm and Uppsala); and middle-north (Sundsvall/Härnösand). The project was conducted during January through June in 1999. Medical doctors were engaged to help in early recruitment of the patients. Only domestic cases were accepted. The patients were contacted by telephone immediately after the diagnosis and asked about their consumption of pork meat within 2 weeks before the onset of illness. Each patient received in addition a questionnaire concerning age, sex, symptoms, pets, water supply, handling of home-slaughtered meat, etc., and details about food eaten 2 weeks before the first sign of infection, such as milk, cream, hard and soft cheese, graved meat, fillet of pork, loin of pork, ham, pork chop, minced meat, sausages, paté, brawn, black pudding, beef, wild meat, fish, seafood, and beans. One control was matched to each patient. The criteria used for acceptance of a control was: born the same year and month as the patient, of the same sex, living in the same municipality, and with no sign of gastrointestinal disease within 1 month before the infection presented in the corresponding patient. Cases and controls were compared statistically. Thus, a case-control study was performed.
Pork meat samples.
Local public food inspectors conducted the sampling. Pork meat (both raw and ready-to-eat), the same kind as the patients had eaten before the infection, was collected from the patients' refrigerator or freezer and/or at their local stores. Sampling in the homes was chosen in the first place. The specimens were packed in individual sterile plastic containers and shipped on ice to the National Food Administration. The samples arrived within 24 h after sampling, and analysis commenced on the day of arrival.
Pork meat isolates.
The detection was performed by using a combined culture and PCR method (33). The PCR targeted the chromosomally located ail gene that is present in all pathogenic Y. enterocolitica strains. In brief, 10 g of sample was homogenized in 90 ml of tryptone soy broth (CM 0129) and enriched at 25°C for 18 to 20 h. A 30-s Percoll buoyant density centrifugation (16,000 × g) was then performed. After the centrifugation, one portion (10 μl) was taken for PCR analysis, and another portion (25 μl) was spread on CIN agar (agar base Oxoid CM653 and SR 109). The plates were incubated overnight at 30°C. If the enrichment was indicated as positive by the ail-PCR, up to five dark red “bull's-eye” colonies grown on the CIN agar were analyzed by multiplex PCR (see below). PCR-negative colonies were tested for presence of urease. Urease-positive isolates were further analyzed by biochemical methods (see below).
Multiplex PCR.
Four pairs of primers were combined in a multiplex PCR (Table 3). The optimal annealing temperature of the PCR was determined by a gradient PCR between 50 and 65°C with 1.1°C increments (Eppendorf Mastercycle gradient). A step-by-step empirical approach was used to balance the individual concentrations of primers and of the concentrations of MgCl2 and deoxynucleoside triphosphate. The final PCR protocol was as follows: 1× PCR buffer II (without MgCl2), 1.5 mM MgCl2, 1 U of AmpliTaq polymerase (Applied Biosystems, Foster City, CA), and 100 μM concentrations of each deoxynucleoside triphosphate (Amersham Biosciences); primer set Pr2a and Pr2c (yst gene), 2×5 pmol; primer set 9A and 10A (ail gene), 2×10 pmol; primer set rfbCa and rfbCb (rfbC gene), 2×20 pmol; and finally, primer set 11A and 12A (virF gene), 2×15 pmol. Millipore water was added to a final volume of 40 μl. We used 10 μl of each template DNA. The amplification conditions were as follows: an initial denaturation of 94°C for 3 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 1 min, and extension at 72°C for 1 min, with a final extension at 72°C for 5 min. The PCR products were separated in a 2% agarose gel and stained with ethidium bromide.
TABLE 3.
Primers used in the multiplex PCR for identification and virulence determination of food-borne pathogenic Yersinia spp.
| Primera | Sequence (5′-3′) | Product length (bp) | Reference |
|---|---|---|---|
| 9A, ail | GTT TAT CAA TTG CGT CTG TTA ATG TGT ACG | 454 | 33 |
| 10A | CTA TCG AGT TTG GAG TAT TCA TAT GAA GCG | ||
| 11A, virF | AAG GTT GTT GAG CAT TCA CAA GAT GG | 700 | 33 |
| 12A | TTT GAG TGA AAT AAG ACT GAC TCG AGAACC | ||
| rfbCa, rfbC | CGC ATC TGG GAC ACT AAT TCG | 405 | 35 |
| rfbCb | CCA CGA ATT CCA TCA AAA CCA CC | ||
| Pr2a, yst | AAT GCT GTC TTC ATT TGG AGC | 145 | 21 |
| Pr2c | ATC CCA ATC ACT ACT GAC TTC |
ail, for attachment invasion locus; virF, for virulence regulatory functions located on the virulence plasmid; rfbC, of unknown function, located within the rfb cluster responsible for the biosynthesis of the O side chain of Y. enterocolitica O:3; yst, for Yersinia heat-stable toxin.
Genotypic and phenotypic analysis of food and patient isolates.
Multiplex PCR-positive or urease-positive isolates were characterized with API 20E (bioMérieux, Marcy l'Etoile, France). Also, a number of isolates resembling pathogenic Yersinia strains on CIN agar yielding PCR- and urease-negative reactions were analyzed phenotypically with API 20E. The API strips were incubated at 30°C for 18 to 20 h. Isolates identified as Y. enterocolitica by API 20A were further biotyped by methods based on the revised biotyping scheme proposed by Wauters et al. (34) given in Tables 4 and 5 and serotyped with a commercial slide agglutination kit for the O:3 and O:9 antigens (Reagensia AB). The presence or absence of the virulence plasmid for the pathogenic Y. enterocolitica isolates was determined with CR-BHO agarose plates (Congo red-brain heart infusion [BHI]), i.e., agarose plates testing the calcium absorption and Congo red uptake (4).
TABLE 4.
Phenotypic and genotypic characterization of 48 Y. enterocolitica strains isolated from yersiniosis patients
| Patient no. | Phenotypic characterization
|
Genotypic characterization
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Biotyping testsd
|
Biosero- groupa | CR-BHOb | PFGE (NotI)c | Multiplex PCR (yst rfbC ail virF)d | |||||
| Sal/Escul/Pyz | Xyl | Treh | VP | Lip | |||||
| 1, 16, 18, 22, 25, 42, 49, 52, 53, 54, 55, 58 | − | − | + | + | − | 4/O:3 | +++ | NA | + + + + |
| 39, 44, 46 | − | − | + | − | − | 4/O:3 | +++ | NA | + + + + |
| 32 | − | − | + | + | − | 4/O:3 | ++ | NA | + + + + |
| 13, 24, 40, 57 | − | − | + | + | − | 4/O:3 | + | NA | + + + + |
| 23, 28, 37, 48 | − | − | + | + | − | 4/O:3 | +++ | NB | + + + + |
| 4, 47 | − | − | + | + | − | 4/O:3 | + | NB | + + + + |
| 3 | − | − | + | + | − | 4/O:3 | ++ | NB | + + + + |
| 26 | − | − | + | − | − | 4/O:3 | +++ | NB | + + + + |
| 8, 56 | − | − | + | + | − | 4/O:3 | +++ | NB | + + + + |
| 7 | − | − | + | + | − | 4/O:3 | + | NE | + + + + |
| 11 | − | − | + | + | − | 4/O:3 | − | NE | + + + − |
| 14 | − | − | + | + | − | 4/O:3 | +++ | NI | + + + + |
| 5 | − | − | + | + | − | 4/O:3 | − | NI | + + + + |
| 21 | − | − | + | + | − | 4/O:3 | +++ | NG | + + + + |
| 29 | − | − | + | − | − | 4/O:3 | − | NH | + + + + |
| 17 | − | − | + | − | − | 4/O:3 | + | NC | + + + + |
| 27 | − | − | + | + | − | 4/O:3 | +++ | NF | + + + + |
| 9 | − | − | + | − | − | 4/O:3 | +++ | ND | + + + + |
| 2 | − | − | + | + | − | 4/O:3 | ++ | ND | + + + + |
| 2(200) | − | − | + | − | − | 4/O:3 | ++ | ND | + + + + |
| 19, 45 | − | − | + | + | − | 4/O:3 | + | ND | + + + + |
| 10 | − | − | + | + | − | 4/O:3 | ++ | NJ | + + + + |
| 34, 36, 50, 51 | + | + | + | + | + | IA | − | Nx | − − − − |
Biotyped according to the reduced biotyping schema proposed by the Wauters schema and serotyped using monovalent antiserum agglutination tests.
CR-BHO results: +/−, presence/absence of pinpoint colonies (numbers of visible pinpoint colonies are indicated as +, ≤10; ++, 10 to 150; or +++, >150).
Nx, NotI profiles different from those found in the Y. enterocolitica 4/O:3 strains.
+/−, positive and negative reactions, respectively. Sal, salicin; Escul, esculin; Pyz, pyrazinamidase; Xyl, xylose; Treh, trehalose; VP, Voges-Proskauer; Lip, lipase.
TABLE 5.
Phenotypic and genotypic characterization of seven pathogenic and four nonpathogenic Y. enterocolitica strains isolated from food and/or feed
| Patient no.a | Strain no./food type | Phenotypic characterization
|
Genotypic characterization
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biotyping testse
|
Bioserogroupb | CR-BHOc | PFGE (NotI)d | Multiplex PCR (yst rfbC ail virF)e | ||||||||
| Sal | Escul | Pyz | Xyl | Treh | VP | Lip | ||||||
| 7 | 50A/minced pork | − | − | − | − | + | + | − | 4/O:3 | +++ | NA | + + + + |
| 10 | 75A/minced pork | − | − | − | − | + | + | − | 4/O:3 | +++ | NA | + + + + |
| 19 | 3a/ham | − | − | (+) | − | + | + | − | 4/O:3 | ++ | NJ | + + + + |
| 5a/loin of pork | − | − | − | − | + | + | − | 4/O:3 | +++ | ND | + + + + | |
| 22 | 47/minced pork | − | − | − | − | + | + | − | 4/O:3 | +++ | NA | + + + + |
| 32 | 237A1/fillet of pork | − | − | − | − | + | + | − | 4/O:3 | +++ | NC | + + + + |
| 49 | 303/dog feed | − | − | − | − | + | + | − | 4/O:3 | +++ | NI | + + + + |
| 11 | 43 1/pork chop | + | + | + | + | + | + | + | 1A | − | Nx | − − − − |
| 32 | 235A4/loin of pork | + | + | + | + | + | + | + | 1A | − | Nx | − − − − |
| 54 | 365A1/minced pork | + | + | + | + | + | + | + | 1A | − | Nx | − − − − |
| 54 | 366 2/ham | + | + | + | + | + | + | + | 1A | − | Nx | − − − − |
Refers to the patient numbers listed in Table 4.
Biotyped according to the reduced biotyping scheme proposed by Wauters et al. (34) and serotyped by using monovalent antisera agglutination tests.
CR-BHO results: +/−, presence/absence of pinpoint colonies (numbers of visible pinpoint colonies are indicated as +, ≤10; ++, 10 to 150; or +++, >150).
Nx, NotI profiles different from those found in the Y. enterocolitica 4/O:3 strains.
+/−, positive and negative reactions, respectively; (+), weak positive reaction. See footnote d of Table 4 for abbreviations.
PFGE.
One or two pure colonies of each of the examined strains were transferred to 10 ml of BHI broth (Difco) and grown overnight at 28°C under shaking. The bacterial suspension was centrifuged at 1,500 rpm for 10 min, the supernatant was discarded, and the pellet was resuspended in 10 ml of PETT IV solution (10 mM Tris-HCl [pH 8.0], 1 M NaCl, 10 mM EDTA). The bacterial suspension was recentrifuged at 1,500 rpm for 10 min, the supernatant was discarded, and the pellet was resuspended in 1.00 ml of EC buffer (i.e., lysis buffer containing 1 M NaCl, 10 mM Tris [pH 8.0], 200 mM EDTA, 0.5% sarcosyl, and 0.2% sodium deoxycholate). Agarose 1% (agarose prep, 80-113007; Amersham Biosciences) was prepared in Millipore water. A volume of 300 μl of the bacterial suspension was heated to 40°C. We then added 18 μl of 50 mg of lysozyme/ml (Roche Diagnostics GmbH, Mannheim, Germany) and 700 μl of heated (40°C) 1% agarose solution. The mixture was immediately dispensed into wells and chilled for 10 min at 8 to 10°C. The plugs were placed in 2.5 ml of EC buffer, and 71 μl of proteinase K (0.5 mg/ml; Roche) was added before incubation at 56°C for ca. 20 h. The plugs were rinsed once with 1× TE buffer (10 mM Tris, 1 mM EDTA). We added 5 μl of 1× TE buffer and 50 μl of 29 mM Pefablock (Roche) before incubation at 37°C for 2 h. The plugs were washed with 5 ml of 1× TE buffer twice for 30 min each time at 37°C and then stored in 1 ml of 1× TE buffer at 4°C. PFGE (CHEF-DR III; Bio-Rad Laboratories, Inc.) was carried out in a 1% agarose gel (Agarose NA, 17-0554-02; Amersham Biosciences) in 0.5× TBE buffer with a switching time of 7 to 23 s for 45 h at 14°C (vertical position). DNA was cleaved with 5 U of NotI enzyme (New England BioLabs) per μg of DNA. The DNA was digested for 4 h at 37°C. Lambda DNA-PFGE marker (catalog no. 03405; New England BioLabs) was used. The gels were stained in ethidium bromide solution.
Two NotI profiles were considered different if a one-band difference could be observed between fragments exceeding 75 kb.
Statistical analysis.
The matched odds ratio was calculated according to McNemar's test.
RESULTS
Multiplex PCR analysis on strains selected from our culture collection.
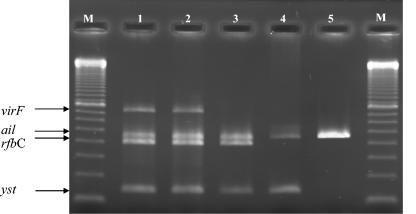
The optimal annealing temperature for the four primer pairs included in the multiplex PCR was determined to be 60.0°C. The results from the selectivity test of the multiplex PCR on 145 Yersinia strains are shown in Table 1. The strains were divided into four groups by means of the PCR results: (i) pathogenic Y. enterocolitica serotype O:3 strains with the genotype yst+ rfbC+ ail+ virF+/− (virF+/− for presence of the virulence plasmid in some strains and absence in others); (ii) pathogenic Y. enterocolitica serotypes other than serotype O:3 with the genotype yst+ ail+ virF+/− (all lacking rfbC); (iii) Y. pseudotuberculosis strains with the genotype ail+ lcrF+/− (all lacking yst and rfbC); and finally (iv) the nonpathogenic Yersinia strains, all lacking the four genes yst, rfbC, ail, and virF. The virF/lcrF gene was detected in 93% (79 of 85) of the Y. enterocolitica 4/O:3 strains and in 57% (8 of 14) of the Y. pseudotuberculosis strains. The virulence plasmid was not detected in any of the nonpathogenic Yersinia strains (Table 1). The different multiplex PCR profiles found are illustrated in Fig. 1. Strains with or without the virulence plasmid are shown in lanes 2 or 3, respectively. The 145 strains were also tested (with CR-BHO agarose) for presence of the virulence plasmid. Isolates that harbored the virulence plasmid (virF+/lcrF+) similar to the multiplex PCR also produced pinpoint colonies when grown on CR-BHO agarose. None of the virF− isolates produced visible pinpoint colonies.
FIG. 1.
Multiplex PCR targeting genes encoding four virulence-associated properties: yst (145 (bp), rfbC (405 bp), ail (454 bp), and virF (700 bp). Lanes: 1, 2, and 3, Y. enterocolitica 4/O:3; 4, Y. enterocolitica O:8; 5, Y. pseudotuberculosis; M, 100-bp ladder as the DNA size control (no. 27-4007-01; Amersham Biosciences).
Patient data.
Between 2 January and 30 June 1999, 212 cases of yersiniosis occurred in Sweden (http://www.smittskyddsinstitutet.se). A total of 101 of these originated from the four counties included in the present study. However, 53 (52.5%) were not enrolled in the project either because of patient refusal, because patients were infected abroad, or because we were unable to contact the patient. Thus, 48 patients (26 males and 22 females) participated in the case-control study. They lived in the counties of Skåne (n = 24), Stockholm (n = 17), Uppsala (n = 5), and Västernorrland (n = 2). Approximately 38% of the cases were among children younger than 5 years. The ages ranged from 9 month to 77 years, with a median age of 12 years. Age and sex were distributed fairly evenly. None of the patients were vegetarian. Patients were as likely as controls to have used private well water in their households. Patients younger than 16 years displayed a significant difference compared to the corresponding control group for having pets, i.e., dogs or cats (matched odds ratio = 8; 95% confidence interval of 1 to 355; P = 0.045). Cases were no more likely than controls to have eaten any of the requested food items.
Pathogenic Yersinia spp. in pork meat.
In all, 118 pork meat samples were collected and analyzed. In addition, one sample of dog feed was included (because one of the patients, a 10-month-old boy, was suspected by his parents to have eaten dog feed). Food and the dog feed are treated separately in the present study. The typical food collected from a patient's home was frozen pork meat from the batch that the patient used before onset of illness, and the typical food collected from a shop was unfrozen raw pork meat of the same kind as the patient had eaten before onset of illness. Approximately 10% (9 of 91) of the raw pork products tested ail-PCR positive (two loin of pork [2 of 7], two fillet of pork [2 of 7], one pork chop [1 of 20], one ham [1 of 10], and three minced meat [3 of 24]): three of the PCR positives were collected from homes (3 of 30), and six were from local shops (6 of 61). Isolates of Y. enterocolitica 4/O:3 were recovered from six ail-PCR positive samples, and all were collected in shops, including one sample of loin of pork, one sample of fillet of pork, one sample of ham, and three samples of minced pork. Thus, none were isolated from food collected in homes. However, one strain of Y. enterocolitica 4/O:3 was found in dog-feed meat sampled in one patient's home. Samples of raw pork with both PCR- and culture-negative results (where n is the number of samples tested) included sliced ham (n = 8), flesh (n = 3), ribs (n = 5), liver (n = 1), leg of pork (n = 2), shoulder (n = 1), pig's neck (n = 2), hand/knuckle of pork (n = 1), schnitzel (n = 1), and samples of ready-to-eat with negative results included meat balls (n = 3), Falun sausage (n = 2), smoked loin of pork (n = 3), Wiener sausage (n = 5), liver paste (n = 2), pork sausage (n = 4), Hot dogs (n = 2), bacon (n = 2), cold-smoked sausage, Mittwurst (n = 3), and small sausage (n = 1).
Phenotypic and genotypic characterization of patient and food isolates.
Of the 48 human Y. enterocolitica isolates examined, 44 belonged to Y. enterocolitica bioserogroup 4/O:3. The remaining four human isolates were identified as biogroup 1A (see Table 4). Also, four biotype 1A strains were isolated from four food samples: two collected from refrigerators and two from local stores (see Table 5). A total of 41 of the human 4/O:3 isolates and the 6 4/O:3 food isolates produced pinpoint colonies when grown on CR-BHO agarose, indicating the presence of the virulence plasmid. Of the 44 human 4/O:3 strains, 8 were Voges-Proskauer (VP) negative (Table 4). Food strain 3a produced a weak pyrazinamidase reaction and, therefore, was interpreted as positive. Twelve urease-positive but PCR-negative food isolates were biochemically identified with API 20E as Y. fredriksenii-intermedia (eight strains), Y. kristensenii (one strain), and Yersinia spp. (three strains). Seven urease- and multiplex PCR-negative food isolates were found to be Pantoea spp. (five strains), Enterobacter spp. (one strain), and Klebsiella spp. (one strain). When subjected to the multiplex PCR, 43 of the 44 human 4/O3 isolates, and all of the six food isolates displayed the predominant genotype for fully virulent Y. enterocolitica O:3 strains: yst+ rfbC+ ail+ virF+. PCR analysis of patient strain number 11 did not result in a detectable product for virF. Of the 48 Y. enterocolitica isolates from patients, 4 were identified as biogroup 1A, and 4 biotype 1A strains were also isolated from some of the pork meat samples. All 1A strains were negative in the multiplex PCR targeting the virulence genes yst, rfbC, ail, and virF.
PFGE analysis of Y. enterocolitica 4/O:3 isolates recovered from patients and foods.
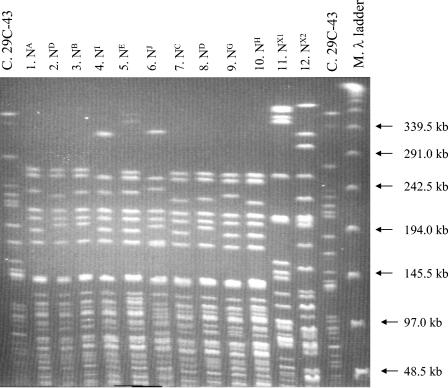
All patient (n = 48) and food (n = 10) Y. enterocolitica strains were genotypically characterized by PFGE. Ten NotI profiles were revealed within the 44 Y. enterocolitica 4/O:3 strains isolated from yersiniosis patients (Fig. 2, lanes 1 to 10). In Table 4 the profiles are designated NA to NJ and are assigned to the patients. A single NotI profile, NA, predominated and was found in 46% (20 of 44) of the human isolates. The second most common NotI profile, NB, covered 23% (10 of 44) of the human isolates. The remaining eight NotI profiles, NC-J, were represented by only a few strains each. Four NotI profiles were found in the six Y. enterocolitica 4/O:3 strains isolated from the food samples (Table 5). They were designated NA, NC, ND, and NJ. Three of the six pork strains, all isolated from minced pork, revealed the same NotI profile, NA. Strain 303 isolated from raw dog feed of pork sampled in the home of patient number 49 displayed the NotI profile NI, whereas the patient strain belonged to NotI profile NA. One pair of isolates from two brothers displayed similar profiles, i.e., ND, patient strain numbers 2 and 2(200), respectively (Table 4). Two strains isolated from a couple living together, patient strain numbers 46 and 45, displayed different NotI profiles, NA and ND, respectively.
FIG. 2.
PFGE patterns of NotI-restricted Y. enterocolitica strains from yersiniosis patients in Sweden during January to June 1999. Lanes: C, control strain; 1 to 10, Y. enterocolitica 1A strains (patients 1, 2, 3, 5, 7, 10, 17, 19, 21, and 29, respectively); 11 and 12, Y. enterocolitica 1A strains (patients 34 and 36, respectively); M, molecular size marker (lambda ladder).
DISCUSSION
Weynants et al. (35) developed a multiplex PCR that both identified and differentiated three groups of food-borne pathogenic Yersinia isolates: (i) pathogenic Y. enterocolitica O:3, (ii) pathogenic Y. enterocolitica serogroups other than O:3, and (iii) Y. pseudotuberculosis. This multiplex PCR, in addition, allowed the detection of Y. pestis. As is shown in Fig. 1, our multiplex PCR identified and distinguished the same three groups as mentioned above. However, in contrast to Weynants et al. (35), we based the detection on the chromosomal yst gene (named for yersinia heat-stable toxin) as the target instead of the inv gene and amplified other sequences of the ail and the virF genes. These differences allowed a common detection of all pathogenic yersiniae in the same PCR (by amplification of a certain sequence of the ail gene shared by these isolates). Furthermore, these changes led to amplification of two chromosomal sequences (yst and ail) as a minimum instead of one to achieve a more reliable basis for the detection. In addition to the evaluation of the multiplex PCR assay performed on a number of strains from our culture collection (Tables 1 and 2) and in contrast to the approach of Weynants et al. (35), we tested the multiplex PCR on isolates (uncharacterized) obtained in the present study from yersiniosis patients and pork meat (Tables 4 and 5).
Several investigators have described multiplex PCR assays for Y. enterocolitica, some for detection (applied to enrichment media) (3, 25) and others for identification (applied to pure cultures) (20, 22). When the former strategy is used, however, problems with PCR inhibitors are often encountered, and an additional sample preparation step prior to the PCR is required (30). Moreover, most of the multiplex PCR assays referred to above typically include two sets of primers, sometimes with one of the primer sets used to amplify an internal control to verify the integrity of the PCR, while the second is targeted to the DNA sequence of interest (25). In our multiplex PCR, four pairs of primers simultaneously amplified four DNA fragments in one PCR detecting four virulence factors. This more complex PCR system required careful optimization in order to minimize problems such as competing primer pairs, the increased risk of primer-dimer formation, and amplicons with different efficiencies. For this reason, to maintain a well-balanced set of amplicons throughout the reaction and avoid interference from PCR-inhibiting components such as food and media components (30), this multiplex PCR is intended for use only on colonies to replace the final step of the conventional culture, i.e., the biochemical and serological tests.
Although pork meat contaminated with Y. enterocolitica is thought to be an important source of the human infection, the occurrence of Y. enterocolitica in pork meat collected at the retail level is reported to be low (34). Therefore, in the present study we examined food collected from homes and/or local shops frequented by yersiniosis patients. We limited our study to raw pork meat and products containing pork of the type the patients had eaten within 2 weeks prior to the first sign of infection. Medical doctors were called upon to help in early recruitment of the patients so that the local public food inspectors could sample food either the same day or the day after the patient's diagnosis. Despite these efforts, foods were not collected until on average 2 weeks (average time, 13 days; range, 5 to 40 days) after the initial onset of illness and, if we assume an incubation time of 1 to 11 days, foods were not collected until ca. 6 to 50 days after the patient's exposure. Therefore, the source of the yersiniosis infection for the majority of the patients in the present study was most probably a food no longer present in the refrigerator or a food eaten outside the home, and thus the findings in the present study may just as well reflect any consumer being exposed to contaminated food. Therefore, no attempts were made to match any of the isolates in order to identify possible outbreaks. Accordingly, the PFGE analysis of the obtained human and food isolates was limited to cleavage by the NotI enzyme, which is suitable for confirmation of the strain typing results, and to reveal the diversity of NotI profiles present within the isolates.
In addition, during our first contact with patients by phone we asked about their consumption of pork meat; this means was necessary to allow a rapid and accurate sampling. On account of this, the information given by the patients in the subsequent questionnaire (the case-control study) may have been biased, especially with regard to the information about food consumption. Thus, to point out pork (or any other food) as a risk factor by means of the case-control study results was not possible.
We used a combined culture and PCR method for the detection in food (33) and isolated strains from various samples of raw pork meat. The human strains, in contrast, were sent to us from clinical laboratories and were already identified to the species level. Despite of this, all isolates were typed by both phenotypic and genotypic tests, the latter including multiplex PCR and PFGE analysis. The multiplex PCR rapidly and effectively identified the isolates and discriminated the pathogenic from the nonpathogenic serotypes, whereas the phenotypic tests were laborious and varied in results (Table 5). For example, we found that the human isolates gave variable VP results despite a positive VP reaction being stated for all isolates belonging to biotype 4 (34). Also, two patient strains indicated as plasmid bearing by the multiplex PCR gave no visible pinpoint colonies when grown on CR-BHO agarose; plasmid-bearing cells appear as red pinpoint colonies on this medium when incubated overnight at 37°C. It is, in addition, worth noting that phenotypic tests may have limited predictive values for the pathogenicity of Yersinia isolates (12). Furthermore, they cannot be used until a strain has been identified to the species level. A disadvantage of a gene-based assay, on the other hand, is that isolates can be identified only as potentially pathogenic because some genes may be unexpressed and thus silent. The inv gene for example, is carried by both pathogenic and nonpathogenic strains of Y. enterocolitica but confers an invasive phenotype on the pathogens only; the nonpathogenic strains contain nonfunctional inv homologous sequences (29).
In the present study, the 51 Y. enterocolitica 4/O:3 isolates examined (6 raw pork, 1 dog feed, and 44 human) generated 10 different NotI profiles: two NotI profiles predominated and comprised >50% of the isolates, whereas the rest were about evenly distributed between the remaining profiles. Thus, to reach higher resolution, which is especially important for the two major groups, it is necessary to involve cleavage by a series of restriction nucleases (13). Only minor variations were observable between all of the profiles. These findings are consistent with previous studies (14, 27). Furthermore, and in accordance with results reported by Najdenski et al. (27), we found that the PFGE results based on the NotI profiles unequivocally differentiated pathogenic from nonpathogenic strains and could simultaneously distinguish strains belonging to bioserotype 4/O:3 from other bioserotypes associated with pathogenicity. Thus, besides being a molecular technique for epidemiological tracing of pathogenic Y. enterocolitica isolates (13), PFGE is a valuable tool to confirm strain typing results.
Four patient isolates and four food isolates were identified as biotype 1A. This biotype is frequently isolated from animals, foods, waters, and environmental sources and is generally regarded as nonpathogenic. Occasionally, 1A strains have been isolated from humans with a clinical diagnosis of yersiniosis (9, 18). It has therefore been suggested that some of the biotype 1A strains, although lacking the classical virulence markers of Y. enterocolitica, may be able to cause gastroenteritis in humans (9). However, it was not within the scope of the present study to perform work on the 1A strains other than to demonstrate the ease with which they could be distinguished from the classical Y. enterocolitica pathogens by the multiplex PCR.
Boyapalle et al. (7) analyzed 350 samples of minced pork collected from four plants in the United States and detected pathogenic Y. enterocolitica in 10% of the samples by use of a 40-cycle single PCR targeting the chromosomal ail gene. In another study, Fredriksson-Ahomaa et al. (15) detected pathogenic Yersinia strains in 25% of 255 minced-meat samples purchased at 40 retail outlets in Helsinki by using a nested PCR. In our study, we used for the detection a 34-cycle conventional single PCR targeting the chromosomally located ail gene and found 10% PCR-positive results when we tested 91 raw pork samples. Although these findings tallied well with the observations by Boyapalle et al. (6), we did not match the occurrences found by Fredriksson-Ahomaa et al. (13). The reason for this could be that we limited our analysis to a single PCR format. A study of Rijpens et al. (28) showed that the addition of an inner primer pair, i.e., a nested PCR, when applied to the obtained negative single PCR products, could provide up to 100-fold greater sensitivity than a single PCR. Thus, if the pathogen was present in the examined foods at levels not detectable by the single PCR or if PCR inhibitors were present and caused a lower sensitivity, the addition of a nested PCR might have increased our findings.
Interestingly, one of the patients, a 10-month-old boy, was observed by his parents when crawling around on the floor to be interested in the dog's feed bowl. Transmission of Y. enterocolitica 4/O:3 to pets via contaminated pork was studied by Fredriksson-Ahomaa et al. (17) with the conclusion that raw pork should not be given to pets. Furthermore, in the present study the patients were significantly more likely than controls to have pets. The dog mentioned above was fed with raw pig offal, and Y. enterocolitica 4/O:3 was isolated both from the boy and from the dog food. However, the NotI profiles from the two isolates were different. Finding more than one clonal type may result from genomic rearrangements affecting restriction sites. The NotI profiles displayed a two-band difference, which, according to Tenover et al. (32), means that the isolates are closely related and probably part of the same outbreak. Unfortunately, only a single isolate was analyzed from each patient. Multiple isolates maybe could have confirmed the suggested relatedness. When examining strains isolated from a pair of twins, Gray et al. (19) found that more than one strain of Y. enterocolitica 4/O:3 had colonized in one of the twins. Therefore, we suggest that, if possible, up to three isolates of the pathogen per positive sample should be used for this kind of investigation.
In conclusion, the multiplex PCR developed in the present study was shown to be an efficient tool for identification of pathogenic Y. enterocolitica isolates. It both identified the virulence genes—ail, yst, rfbC, and virF—and simultaneously differentiated three groups of food-borne pathogenic Yersinia isolates: (i) pathogenic Y. enterocolitica O:3, (ii) pathogenic Y. enterocolitica serogroups other than O:3, and (iii) Y. pseudotuberculosis. Further, as is apparent from this and other studies, it is very difficult to isolate colonies of pathogenic Y. enterocolitica from food; the pathogen was not isolated from all samples indicated as positive by PCR. Strain isolation and further characterization is a prerequisite in order to obtain epidemiological information. Therefore, work to improve the culture methodology to facilitate the isolation is of prime importance. Furthermore, in the present study we used a combined culture and PCR method, which included buoyant density centrifugation as sample preparation, for the detection in food. Thus, the same sample preparation method was applied for a number of different food types and may not be optimal for all. Therefore, the effect of different sample preparation methods for different food types needs to be investigated.
Acknowledgments
We thank the County Medical Officers of Infectious Disease Control of Skåane County, Stockholm County, Uppsala County, and Västernorrland County, as well as all of the local public food inspectors, for providing the clinical strains and food samples, respectively.
This study was partly supported by the Elsa and Ivar Sandberg Foundation of Sweden.
REFERENCES
- 1.Aleksic, S. 1995. Occurrence of Y. enterocolitica antigens O:3, O:9 and O:8 in different Yersinia species, their corresponding H antigens and origin. Contrib. Microbiol. Immunol. 13:89-92. [PubMed] [Google Scholar]
- 2.Asplund, K., T. Johansson, and A. Siitonen. 1998. Evaluation of pulsed-field gel electrophoresis of genomic restriction fragments in the discrimination of Yersinia enterocolitica O:3. Epidemiol. Infect. 121:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaduri, S., and B. Cottrell. 1998. A simplified sample preparation method from various foods for PCR detection of pathogenic Yersinia enterocolitica: a possible model for other food pathogens. Mol. Cell. Probes 12:79-83. [DOI] [PubMed] [Google Scholar]
- 4.Bhaduri, S., C. Turner-Jones, M. M. Taylor, and R. V. Lachica. 1990. Simple assay of calcium dependency for virulent plasmid-bearing clones of Yersinia enterocolitica. J. Clin. Microbiol. 28:798-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottone, E. J. 1999. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1:323-333. [DOI] [PubMed] [Google Scholar]
- 6.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyapalle, S., I. V. Wesley, H. S. Hurd, and P. Gopal Reddy. 2001. Comparison of culture, multiplex, and 5′ nuclease polymerase chain reaction assays for the rapid detection of Yersinia enterocolitica in swine and pork products. J. Food Prot. 64:1352-1361. [DOI] [PubMed] [Google Scholar]
- 8.Buchrieser, C., S. D. Weagant, and C. W. Kaspar. 1994. Molecular characterization of Yersinia enterocolitica by pulsed-field gel electrophoresis and hybridization of DNA fragments to ail and pYV probes. Appl. Environ. Microbiol. 60:4371-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnens, A. P., A. Frey, and J. Nicolet. 1996. Association between clinical presentation, biogroups and virulence attributes of Yersinia enterocolitica strains in human diarrhoeal disease. Epidemiol. Infect. 116:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiesa, C., L. Pacifico, and G. Ravagnan. 1993. Identification of pathogenic serotypes of Yersinia enterocolitica. J. Clin. Microbiol. 31:2248-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devenish, J. A., and D. A. Schiemann. 1981. An abbreviated scheme for identification of Yersinia enterocolitica isolated from food enrichments on CIN (cefsulodin-irgasan-novobiocin) agar. Can. J. Microbiol. 27:937-941. [DOI] [PubMed] [Google Scholar]
- 12.Farmer, J. J., III, G. P. Carter, V. L. Miller, S. Falkow, and I. K. Wachsmuth. 1992. Pyrazinamidase, CR-MOX agar, salicin fermentation-esculin hydrolysis, and d-xylose fermentation for identifying pathogenic serotypes of Yersinia enterocolitica. J. Clin. Microbiol. 30:2589-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredriksson-Ahomaa, M., T. Autio, and H. Korkeala. 1999. Efficient subtyping of Yersinia enterocolitica bioserotype 4/O:3 with pulsed-field gel electrophoresis. Lett. Appl. Microbiol. 29:308-312. [DOI] [PubMed] [Google Scholar]
- 14.Fredriksson-Ahomaa, M., S. Hallanvuo, T. Korte, A. Siitonen, and H. Korkeala. 2001. Correspondence of genotypes of sporadic Yersinia enterocolitica bioserotype 4/O:3 strains from human and porcine sources. Epidemiol. Infect. 127:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredriksson-Ahomaa, M., S. Hielm, and H. Korkeala. 1999. High prevalence of yadA-positive Yersinia enterocolitica in pig tongues and minced meat at the retail level in Finland. J. Food Prot. 62:123-127. [DOI] [PubMed] [Google Scholar]
- 16.Fredriksson-Ahomaa, M., and H. Korkeala. 2003. Low occurrence of pathogenic Yersinia enterocolitica in clinical, food, and environmental samples: a methodological problem. Clin. Microbiol. Rev. 16:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredriksson-Ahomaa, M., T. Korte, and H. Korkeala. 2001. Transmission of Yersinia enterocolitica 4/O:3 to pets via contaminated pork. Lett. Appl. Microbiol. 32:375-378. [DOI] [PubMed] [Google Scholar]
- 18.Grant, T., V. Bennett-Wood, and R. M. Robins-Browne. 1998. Identification of virulence-associated characteristics in clinical isolates of Yersinia enterocolitica lacking classical virulence markers. Infect. Immun. 66:1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray, J. T., M. WaKabongo, F. E. Campos, A. A. Diallo, C. Tyndal, and C. A. Tucker. 2001. Recognition of Yersinia enterocolitica multiple strain infection in twin infants using PCR-based DNA fingerprinting. J. Appl. Microbiol. 90:358-364. [DOI] [PubMed] [Google Scholar]
- 20.Harnett, N., Y. P. Lin, and C. Krishnan. 1996. Detection of pathogenic Yersinia enterocolitica using the multiplex polymerase chain reaction. Epidemiol. Infect. 117:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim, A., W. Liesack, M. W. Griffiths, and R. M. Robins Browne. 1997. Development of a highly specific assay for rapid identification of pathogenic strains of Yersinia enterocolitica based on PCR amplification of the Yersinia heat-stable enterotoxin gene (yst). J. Clin. Microbiol. 35:1636-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim, A., W. Liesack, S. Pike, and E. Stackebrandt. 1992. The polymerase chain reaction: an epidemiological tool to differentiate between two clusters of pathogenic Yersinia enterocolitica strains. FEMS Microbiol. Lett. 97:63-66. [DOI] [PubMed] [Google Scholar]
- 23.Iteman, I., A. Guiyoule, and E. Carniel. 1996. Comparison of three molecular methods for typing and subtyping pathogenic Yersinia enterocolitica strains. J. Med. Microbiol. 45:48-56. [DOI] [PubMed] [Google Scholar]
- 24.Kapperud, G. 1991. Yersinia enterocolitica in food hygiene. Int. J. Food Microbiol. 12:53-65. [DOI] [PubMed] [Google Scholar]
- 25.Lantz, P. G., R. Knutsson, Y. Blixt, W. A. Al Soud, E. Borch, and P. Radstrom. 1998. Detection of pathogenic Yersinia enterocolitica in enrichment media and pork by a multiplex PCR: a study of sample preparation and PCR-inhibitory components. Int. J. Food Microbiol. 45:93-105. [DOI] [PubMed] [Google Scholar]
- 26.Miller, V. L., J. J. Farmer III, W. E. Hill, and S. Falkow. 1989. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect. Immun. 57:121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najdenski, H., I. Iteman, and E. Carniel. 1994. Efficient subtyping of pathogenic Yersinia enterocolitica strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 32:2913-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rijpens, N. P., G. Jannes, M. Van Asbroeck, R. Rossau, and L. M. Herman. 1996. Direct detection of Brucella spp. in raw milk by PCR and reverse hybridization with 16S-23S rRNA spacer probes. Appl. Environ. Microbiol. 62:1683-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins Browne, R. M. 1997. Yersinia enterocolitica, p. 192-215. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers. ASM Press, Washington, D.C.
- 30.Rossen, L., P. Norskov, K. Holmstrom, and O. F. Rasmussen. 1992. Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int. J. Food Microbiol. 17:37-45. [DOI] [PubMed] [Google Scholar]
- 31.Saken, E., A. Roggenkamp, S. Aleksic, and J. Heesemann. 1994. Characterisation of pathogenic Yersinia enterocolitica serogroups by pulsed-field gel electrophoresis of genomic NotI restriction fragments. J. Med. Microbiol. 41:329-338. [DOI] [PubMed] [Google Scholar]
- 32.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thisted Lambertz, S., R. Lindqvist, A. Ballagi Pordany, and M. L. Danielsson Tham. 2000. A combined culture and PCR method for detection of pathogenic Yersinia enterocolitica in food. Int. J. Food Microbiol. 57:63-73. [Google Scholar]
- 34.Wauters, G., K. Kandolo, and M. Janssens. 1987. Revised biogrouping scheme of Yersinia enterocolitica. Contrib. Microbiol. Immunol. 9:14-21. [PubMed] [Google Scholar]
- 35.Weynants, V., V. Jadot, P. A. Denoel, A. Tibor, and J. J. Letesson. 1996. Detection of Yersinia enterocolitica serogroup O:3 by a PCR method. J. Clin. Microbiol. 34:1224-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]