Abstract
The biosynthesis of trehalose has been previously shown to serve as an important osmoprotectant and stress protectant in Escherichia coli. Our results indicate that overproduction of trehalose (integrated lacI-Ptac-otsBA) above the level produced by the native regulatory system can be used to increase the growth of E. coli in M9-2% glucose medium at 37°C to 41°C and to increase growth at 37°C in the presence of a variety of osmotic-stress agents (hexose sugars, inorganic salts, and pyruvate). Smaller improvements were noted with xylose and some fermentation products (ethanol and pyruvate). Based on these results, overproduction of trehalose may be a useful trait to include in biocatalysts engineered for commodity chemicals.
Bacteria have a remarkable capacity for adaptation to environmental stress (39). A part of this defense system involves the intracellular accumulation of protective compounds that shield macromolecules and membranes from damage (9, 24). Escherichia coli utilizes a variety of compounds for this purpose, including proline, trehalose, betaine, and dimethylsulfoniopropionate. Although glutamate and proline provide transient relief from osmotic stress, allosteric control of proline biosynthesis in E. coli and the negative charge of glutamate limit their effectiveness. Recent studies have shown that the addition of betaine and dimethylsulfoniopropionate stimulated the growth of and ethanol production by recombinant E. coli (41). Betaine also improved the growth of Bacillus subtilis at elevated temperatures (20). However, neither of these protective compounds can be synthesized de novo by E. coli. In the absence of supplements, trehalose serves as the primary protective osmolyte (22).
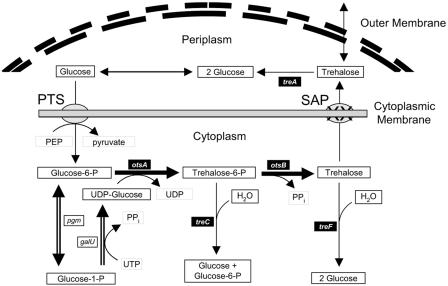
Genes encoding trehalose biosynthesis are widely distributed in nature (14, 35) and have been extensively studied in E. coli and Saccharomyces spp. In E. coli, the trehalose biosynthetic operon (otsBA) is induced by osmotic shock (16), extreme heat (13), extreme cold (23), desiccation (43), and entry into stationary phase (19). Two enzymes are unique to trehalose biosynthesis: trehalose-6-phosphate synthase, encoded by otsA, and trehalose-6-phosphate phosphatase, encoded by otsB (Fig. 1). Mutations in these genes decreased tolerance to osmotic stress and decreased survival during storage. The trehalose biosynthetic operon (otsBA) from E. coli has been used to genetically engineer increased stress tolerance in plants (15) and in mammalian cells (18, 40). This operon has recently been overexpressed in Corynebacterium glutamicum for the production of extracellular trehalose (33).
FIG. 1.
Trehalose metabolism in E. coli. Glucose is transported into the cell and activated by the phosphoenolpyruvate (PEP)-dependent phosphotransferase system (PTS). The biosynthetic pathway for trehalose is indicated by the thicker arrows (double arrows, synthesis of UDP-glucose; filled arrows, condensation and dephosphorylation). Genes deleted in JP10 and JP20 are shown in black boxes. JP20 also contains a functional, regulated ots operon (lacI-Ptac-otsBA) integrated into the ampH gene. Trehalose is degraded by both periplasmic (TreA [4]) and cytoplasmic (TreF [21]) enzymes. Trehalose biosynthesis is also controlled by the synthesis of TreC, which dephosphorylates trehalose-6-phosphate (36). Stretch-activated proteins (SAP) in the plasma membrane facilitate the exit of trehalose under hypotonic conditions (37).
Recent interest in developing microbial biocatalysts for the production of commodity chemicals offers the potential to replace petroleum feedstocks with renewable sugars from starch and lignocellulose (6, 7, 10, 28, 30, 31, 32, 45). Biobased production of these renewable chemicals would be facilitated by improved growth under thermal stress and by increased tolerance to osmotic stress from high concentrations of sugars and salts present in industrial feedstocks. Most prior E. coli studies concerning trehalose have focused primarily on cell survival under osmotic stress from NaCl or glucose or during desiccation. In this paper, we have constructed isogenic derivatives of E. coli K-12 to examine the importance of trehalose biosynthesis for growth during stress (caused by salts, sugars, fermentation products, and environmental extremes) and the effect of trehalose overproduction.
MATERIALS AND METHODS
Bacteria, plasmids, and culture conditions.
Strains and plasmids used in this study are listed in Table 1. Cultures were grown at 37°C in LB alone (2) or on LB solidified with 1.5% agar during strain construction. Ampicillin (50 mg liter−1), kanamycin (50 mg liter−1), and tetracycline (12.5 mg liter−1) were added as appropriate for selection. For studies of osmotic tolerance, cultures were grown in M9 medium (pH 7.0) (2) containing 2% glucose (without antibiotics). Growth in tube cultures was monitored spectrophotometrically at 550 nm using a Spectronic 70 spectrophotometer (Bausch & Lomb, Inc., Rochester, N.Y.). Growth in shaken flasks was monitored at 550 nm by using a Beckman DU 640 spectrophotometer.
TABLE 1.
Sources and characteristics of strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| DH5α | lacZΔM15 recA | Invitrogen |
| W3110 | Wild type | ATCC 27325 |
| TOP10F′ | lacIq (episome) | Invitrogen |
| S17-1λpir | thi pro hsdR hsdM+ recA RP4-2-Tc::Mu-Km::Tn7 λpir | 12 |
| JP10 | W3110 ΔotsBA::FRT ΔtreA::FRT ΔtreC::FRT ΔtreF::FRT | This study |
| JP15 | W3110 ΔotsBA::FRT ΔtreA::FRT-tet-FRT ΔtreC::FRT ΔtreF::FRT | This study |
| JP20 | W3110 ΔotsBA::FRT ΔtreA::FRT ΔtreC::FRT ΔtreF::FRT ΦampH::lacI-Ptac-otsBA-FRT | This study |
| JP21 | W3110 ΔotsBA::FRT ΔtreA::FRT ΔtreC::FRT ΔtreF::FRT ΦalsA::lacI-Ptac-otsBA-FRT | This study |
| JP22 | W3110 ΔotsBA::FRT ΔtreA::FRT ΔtreC::FRT ΔtreF::FRT ΦchiA::lacI-Ptac-otsBA-FRT | This study |
| JP23 | W3110 ΔotsBA::FRT ΔtreA::FRT ΔtreC::FRT ΔtreF::FRT Φ::lacI-Ptac-otsBA-FRT | This study |
| JP24 | W3110 ΔotsBA::FRT ΔtreA::FRT ΔtreC::FRT ΔtreF::FRT Φ::lacI-Ptac-otsBA-FRT | This study |
| Plasmids | ||
| pCR2.1-TOPO | bla kan TOPO TA cloning vector | Invitrogen |
| pFT-A | bla flp low-copy-number vector containing FLP recombinase and temperature-conditional pSC101 replicon | 34 |
| pKD46 | bla γ β exo low-copy-number vector containing red recombinase and temperature-conditional pSC101 replicon | 11 |
| pFLAG-CTC | bla lacI Ptac-controlled expression vector | Sigma |
| pLOI2065 | bla SmaI fragment with FRT-flanked tet gene | 42 |
| pLOI2511 | bla SmaI fragment with FRT-flanked kan gene | 42 |
| mini Tn5 Km2 | bla kan tnp λpir-dependent Tn5 transposase vector | 12 |
| pLOI3469 | bla tnp λpir-dependent Tn5 transposase vector | This study |
| pLOI3601 | pCR2.1-TOPO bla kan otsBA (PCR) | This study |
| pLOI3604 | pLOI3601 bla otsBA (StyI deletion of pLOI3601) | This study |
| pLOI3605 | pLOI3604 bla otsBA-FRT-kan-FRT (SmaI fragment from pLOI2511 ligated into NruI site of pLOI3604) | This study |
| pLOI3607 | bla Ptac-otsBA-FRT-kan-FRT (EcoRI fragment from pLOI3605 ligated into EcoRI site of pFLAG-CTC) | This study |
| pLOI3609 | pLOI3604 bla ΔotsBA::FRT-kan-FRT (1698-bp SspI deletion) | This study |
| pLOI3621 | pCR2.1-TOPO bla kan treA (PCR) | This study |
| pLOI3625 | pLOI3621 bla kan ΔtreA::FRT-tet-FRT (920-bp HpaI deletion) | This study |
| pLOI3631 | pCR2.1-TOPO bla kan treF (PCR) | This study |
| pLOI3635 | pLOI3631 bla kan ΔtreF::FRT-tet-FRT (694-bp AgeI deletion) | This study |
| pLOI3641 | pCR2.1 TOPO bla kan treC (PCR) | This study |
| pLOI3645 | pLOI3641 bla kan ΔtreC::FRT-tet-FRT (579-bp PshAI deletion) | This study |
| pLOI3650 | bla tnp λpir-dependent vector containing transposable Tn5 element (lacI-Ptac-otsBA-FRT-kan-FRT) from pLOI3607 | This study |
Construction of strain JP10.
Standard genetic methods were used during this study (2). Strain JP10 was constructed by sequentially inserting deletions of the genes involved in trehalose biosynthesis and degradation (Fig. 1). Coding regions for treA, treC, and treF were each amplified from W3110 using the respective ORFmer primers (Sigma-Genosys, The Woodlands, TX) and cloned into pCR2.1-TOPO. Coding regions for otsBA genes were amplified by PCR using the following primers: N terminus, 5′AAGGAGGAGAACCGGGTGACA3′, and C terminus, 5′ACGCAGCGTGATGCATGAAG3′. An internal deletion was constructed in the coding region of each trehalose catabolic gene and replaced with a SmaI fragment from pLOI2065 containing a FRT (recognition target for recombinase)-flanked tet gene from pLOI2065 (Table 1). Plasmid pLOI3621 was cut with HpaI to remove the central coding region of treA. Similar excisions were made for treF (pLOI3631) and treC (pLOI3641) using the restriction sites AgeI and PshAI, respectively. Deleted coding regions of otsAB genes (1,698-bp SspI deletion of pLOI3601) were replaced with a FRT-flanked kan gene from pLOI2511 (SmaI fragment). Resulting gene fragments with antibiotic markers were amplified by PCR. Chromosomal integration of amplification products was facilitated by pKD46 containing an arabinose-inducible red recombinase (11). Each deletion was sequentially integrated into W3110. The FRT-flanked antibiotic resistance genes were removed using FLP recombinase (27, 34). The treA::FRT-tet-FRT mutation was inserted last to produce JP15. After deletion of the tet gene in JP15, the final construct was designated JP10 (ΔotsBA::FRT ΔtreA::FRT ΔtreC::FRT ΔtreF::FRT). At each step, the strains were tested for appropriate antibiotic resistance. Mutations were verified by analysis of the PCR products.
Construction of JP20, JP21, JP22, JP23, and JP24.
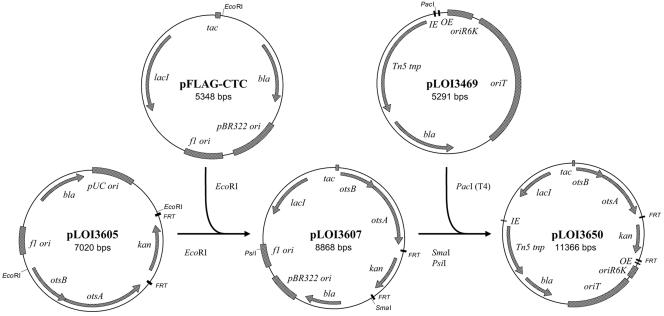
A conjugative transposon vector (pLOI3650) was constructed to integrate a 6.1-kb fragment containing a regulated ots operon (lacI-Ptac-otsBA-FRT-kan-FRT) into JP15. This construct was based on the λpir-dependent transposase vector designed by de Lorenzo et al. (12). The kanamycin gene in the mini Tn5 Km2 plasmid was replaced with a PacI linker by digestion with SfiI and Klenow treatment prior to ligation. The NotI site was eliminated (NotI, Klenow treatment, and self-ligation) to produce pLOI3469 (Fig. 2). The kan gene was also inactivated in pLOI3601 containing otsBA (StyI, Klenow treatment, and self-ligation) to produce pLOI3604. A SmaI fragment from pLOI2511 containing the FRT-flanked kan gene was ligated into the unique NruI site downstream from the coding region of otsBA in pLOI3604 to produce pLOI3605 (Fig. 2). The EcoRI fragment from pLOI3605 containing the promoterless otsBA operon and the kan gene was excised and ligated into the EcoRI site of pFLAG-CTC, immediately downstream from a tac promoter and lacI (pLOI3607). The SmaI-PsiI fragment from pLOI3607 was then ligated into the blunted PacI site of pLOI3469 to produce pLOI3650. The lacI-regulated otsBA cassette from pLOI3650 was integrated into the chromosome of JP15 by conjugation, using S17-1 as the donor. Integration was confirmed with exconjugants selected for kanamycin and tetracycline resistance (ampicillin sensitive) and by analysis of PCR products. Resistance genes used for construction were removed using FLP recombinase, resulting in trehalose-positive strains (JP20, JP21, JP22, JP23, and JP24) that are isogenic with JP10 (JP15 after removal of the tet gene).
FIG. 2.
Diagram summarizing the construction of pLOI3650. This is a conjugative plasmid with a conditional replicon and a Tn5-based transposon for insertion of a regulated ots operon (lacI-Ptac-otsBA-FRT-kan-FRT). The kan gene is bordered by FRT sites that allow removal by FLP recombinase. OE and OI are recognition sites for Tn5 recombinase.
To identify the sites of integration, chromosomal DNA adjacent to Ptac-otsBA-FRT insertions were amplified using arbitrarily primed PCR (44). Sequences of primers used in the first (ARB1 and OUT-OTS) and second (ARB2 and IN-OTS) rounds of amplification were as follows: ARB1, 5′GGCCACGCGTCGACTAGTACNNNNNNNNNNGATAT3′; OUT-OTS, 5′TGGCAGATGCACGGTTACGA3′; ARB2, 5′GGCCACGCGTCGACTAGTAC3′; and IN-OTS, 5′CTATGCGGCATCAGAGCAG3′. Products were recovered from three of the five clones tested. These were gel purified and used as templates for DNA sequencing.
Measurement of intracellular trehalose.
Sufficient culture volume was harvested (10,000 × g, 25°C) to provide 2.0 mg dry cell weight (1 unit of optical density at 550 nm [OD550 nm] equals 0.33 g liter−1 [dry weight] cells). Cells were permeabilized with 50% methanol and extracted for 30 min on ice. The mixture was mixed vigorously and centrifuged at 10,000 × g for 1 min. The supernatant was assayed for trehalose by thin-layer chromatography as described previously (46). After being visualized with N-(1-naphthyl)ethylenediamine reagent, relative amounts of trehalose were determined by densitometry using Quantity One software (Bio-Rad). For estimates of intracellular trehalose concentrations, a volume of 2 ml per gram of dry cells was assumed.
Tolerance assays.
Tolerance was evaluated by measuring growth (defined as final cell mass after 24 h) in M9 minimal medium containing 2% glucose (M9-2% glucose). The MIC was defined as the concentration (interpolated) at which growth was restricted to less than two doublings (fourfold increase in OD) in 24 h. For these tests, cells from a fresh plate were resuspended in M9 medium containing 2% glucose and used as the inoculum (initial level of 0.03 OD550 nm). Cultures were incubated in 13- by 100-mm capped tubes (37°C water bath, 50-rpm reciprocating shaker, 24 h). Results are presented as average values with standard errors from three or more experiments or as averages of three replicates from one or two experiments. In some cases, growth was monitored more frequently using shaken flasks (50 ml in 250-ml flasks, 37°C water bath, 120 rpm). All compounds tested were obtained from either the Sigma Chemical Company or Fisher Scientific.
RESULTS
Native trehalose production by W3110 provided a small benefit for growth during osmotic stress (sugars and salts) and physical stress (elevated temperature and pH) in M9-glucose media.
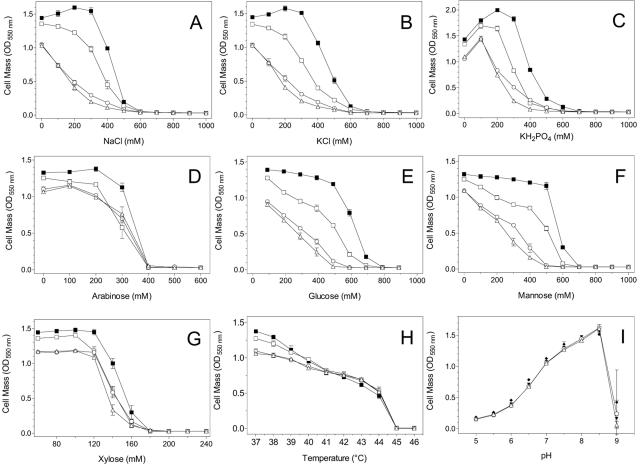
A mutant of W3110 (strain JP10) was constructed in which both biosynthetic genes for trehalose (otsBA), as well as cytoplasmic (treC and treF) and periplasmic (treA) genes concerned with trehalose degradation, were deleted (Fig. 1). Loss of trehalose synthesis in JP10 resulted in a small but measurable reduction in cell growth (cell density after 24 h) during osmotic stress from salts and hexose sugars (Fig. 3; Tables 2 and 3). Differences were most evident at concentrations where the growth of wild-type W3110 was inhibited by half (50% inhibitory concentrations [IC50]). The inability to synthesize trehalose had no effect on growth at elevated temperatures, at high or low pHs, or during arabinose stress.
FIG. 3.
Growth of E. coli W3110 strains during osmotic (inorganic salts and sugars), heat, and pH stresses. Strains were grown for 24 h in M9-2% glucose medium containing NaCl (A), KCl (B), KH2PO4 (C), arabinose (D), glucose (E), mannose (F), or xylose (G). Strain JP20, a trehalose-overproducing derivative (JP10 lacI-Ptac-otsBA), was grown under the same conditions with (▪) and without (□) 0.1 mM IPTG. Strains W3110, JP10, and JP20 were also compared for tolerance to heat (H) and pH (I). Symbols for all panels: ○, W3110 (wild type); ▵, JP10 (trehalose-negative mutant); □, JP20; ▪, JP20 with IPTG.
TABLE 2.
Tolerance of W3110 and derivatives to salts, sugars, and physical stress based on MICs
| Straina | Physical stress
|
MIC (mM)b
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Salt added
|
Sugar added
|
||||||||
| Temp (°C) | pH | KCl | KH2PO4 | NaCl | Arabinose | Glucose | Mannose | Xylose | |
| JP10 | 44.8 | ≤5 | 399 | 376 | 388 | 386 | 452 | 427 | 157 |
| W3110 | 44.8 | ≤5 | 469 | 496 | 463 | 386 | 488 | 469 | 164 |
| JP20 (−) | 44.8 | ≤5 | 572 | 483 | 519 | 391 | 650 | 590 | 159 |
| JP20 (+) | 44.7 | ≤5 | 602 | 608 | 552 | 392 | 729 | 667 | 171 |
Bacteria were grown in M9 medium (93 mM total salts) containing 2% glucose (111 mM). Values for supplements are in addition to the components in basal medium. JP20 was grown with (+) and without (−) an inducer (0.1 mM IPTG).
MIC that restricted growth to less than two doublings in 24 h (OD550 nm, <0.012).
TABLE 3.
Tolerance of W3110 and derivatives to salts, sugars, and physical stress based on IC50s
| Straina | Physical stress
|
IC50 (mM)b
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Salt added
|
Sugar added
|
||||||||
| Temp (°C) | pH | KCl | KH2PO4 | NaCl | Arabinose | Glucose | Mannose | Xylose | |
| JP10 | 44.0 | 6.26 | 166 | 242 | 164 | 333 | 260 | 247 | 134 |
| W3110 | 44.0 | 6.26 | 205 | 291 | 182 | 325 | 314 | 324 | 141 |
| JP20 (−) | 43.9 | 6.21 | 368 | 347 | 385 | 321 | 510 | 508 | 142 |
| JP20 (+) | 43.6 | 6.11 | 496 | 456 | 458 | 354 | 632 | 573 | 153 |
Bacteria were grown in M9 medium (93 mM total salts) containing 2% glucose (111 mM). Values for supplements are in addition to the components in basal medium. JP20 was grown with (+) and without (−) an inducer (0.1 mM IPTG).
IC50 that restricted growth to half that of the wild-type culture grown without stress agents.
Xylose was at least twofold more inhibitory than other sugars. The onset of growth inhibition by xylose was quite abrupt at concentrations above 120 mM, in contrast to other osmolytes that caused a more progressive decrease in growth over a broader range of concentrations (Fig. 3) despite similarity between these sugar structures. Doubling of the glucose concentration from 111 mM to 222 mM did not alter the extent of growth inhibition by 150 mM xylose.
The addition of 100 mM KH2PO4 increased the growth of JP10 and W3110 by 40% after 24 h. No similar increase was observed for NaCl or KCl. The benefit of KH2PO4 appears to result from pH buffering. A similar increase in growth occurred when KH2PO4 was replaced with 100 mM MOPS (morpholinepropanesulfonic acid) (data not shown). Unbuffered cultures that reached final densities of over 1.0 OD550 nm all had a pH of approximately 4.6, consistent with growth being limited by pH under our test conditions.
Construction and characterization of JP10 derivatives containing Ptac-otsBA.
Several plasmids were initially constructed for the controlled expression of the otsBA operon, but all were unstable during growth in M9-2% glucose medium (data not shown). To eliminate this problem, an otsBA-positive strain was constructed from JP15 (JP10 before the elimination of tet) by transposing a single copy of the lacI-regulated otsBA operon into the chromosome. Five exconjugants were selected and confirmed by analysis of PCR products and antibiotic markers. After removal of the tet and kan genes used for selection, the resulting clones (JP20 to JP24) were isogenic with JP10. Arbitrarily primed PCR was successful for three of these clones. Each was integrated at a different chromosomal site: JP20 (ΦampH::lacI-Ptac-otsBA-FRT), JP21(ΦalsA::lacI-Ptac-otsBA-FRT), and JP22 (ΦchiA::lacI-Ptac-otsBA-FRT).
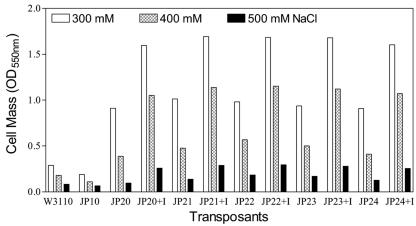
Increased trehalose production in lacI-Ptac-otsBA transposants results in increased NaCl tolerance during growth.
All otsBA transposants exhibited similar increases in cell growth after 24 h in M9-2% glucose medium containing NaCl (300, 400, and 500 mM), fivefold that of the parent (JP10) containing otsAB deletions and threefold that of the wild type, W3110 (Fig. 4). The addition of 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to these integrants resulted in further increases in cell mass (fivefold that of JP10 and eightfold that of the wild type). One strain, JP20, was selected for the measurement of intracellular trehalose. Trehalose levels in JP20 with 0.1 mM IPTG (361 mM trehalose) and without IPTG (28 mM trehalose) were substantially higher than in W3110 (<1.0 mM) and JP10 (<1.0 mM) during growth in M9-2% glucose medium. The low level of trehalose in uninduced JP20 can be attributed to incomplete repression by lacI in a background devoid of periplasmic and cytoplasmic activities that degrade trehalose (TreA, TreC, and TreF).
FIG. 4.
Growth of inducible otsBA+ transposants under osmotic stress. Five strains containing a regulated ots operon (lacI-Ptac-otsBA) were compared during growth for 24 h in M9-2% glucose medium containing 300, 400, and 500 mM NaCl. Strains were grown in both the presence (+I) and the absence of 0.1 mM IPTG as an inducer.
Optimization of otsBA expression for growth under salt and sugar stress (JP20).
The effect of otsBA induction by different levels of IPTG was examined for JP20 during growth under osmotic stress (Fig. 5A through F). For these experiments, concentrations of test agents that are near the respective MICs for JP10 (trehalose negative) were selected. A dose-dependent increase in growth was observed during osmotic stress (inorganic salts and sugars) with an optimum at 0.1 mM IPTG, a fortunate selection for initial tests with NaCl (Fig. 4). At 0.1 mM IPTG, cell growth after 24 h was twofold to threefold that of uninduced JP20 for all osmolytes except mannose (50% increase). Further increases in IPTG were either without consequence or detrimental.
FIG. 5.
Growth optimization of lacI-Ptac-otsBA expression during osmotic stress. JP20 was grown with various concentrations of IPTG and the following stress agents: (A) 400 mM NaCl, (B) 400 mM KCl, (C) 400 mM KH2PO4, (D) 489 mM added glucose (600 mM total glucose), (E) 400 mM mannose, (F) 140 mM xylose, (G) 200 mM acetate, (H) 350 mM pyruvate, and (I) 700 mM ethanol.
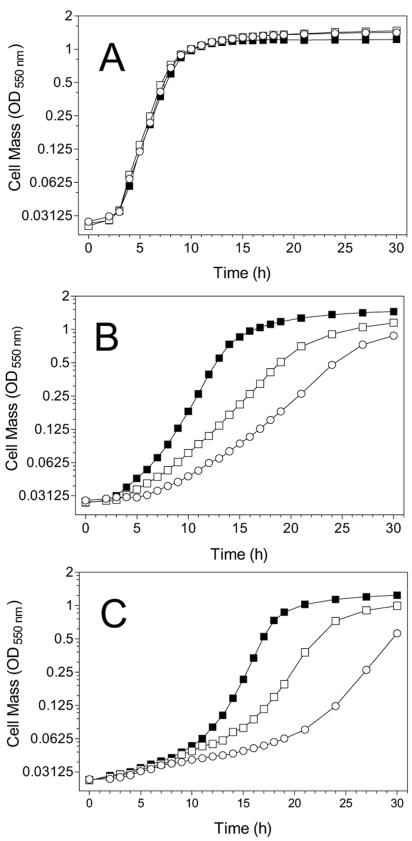
Flask cultures were used to provide more detail concerning the growth of W3110 and JP20 (with and without 0.1 mM IPTG) in M9-2% glucose medium, M9-2% glucose medium containing 400 mM NaCl, and M9-2% glucose medium containing an additional 489 mM of glucose (600 mM total glucose) (Fig. 6). Without added NaCl or glucose, growth rates were similar for W3110, uninduced JP20, and induced JP20, indicating that overproduction of trehalose was not harmful. However, the maximum cell density for W3110 was a lower cell density than that of JP20 (with and without IPTG).
FIG. 6.
Growth of W3110 strains under osmotic stress. W3110 and JP20 were grown in M9-2% glucose medium containing no added chemicals (A), 400 mM NaCl (B), or 600 mM glucose (C). Symbols: ○, W3110; □, JP20; ▪, JP20 with 0.1 mM IPTG.
Increasing the production of trehalose resulted in increased growth under osmotic stress. The production of trehalose by JP20 (induced and uninduced) improved all aspects of growth (shorter lag phase, higher growth rate, and higher final cell density) in comparison to W3110. With IPTG induction, the maximal growth rate for induced JP20 was fivefold that of W3110 and over twice than of uninduced JP20. For these three strains, improvements in growth correlated with the levels of trehalose production measured in the absence of additional osmotic stress.
Increasing the production of trehalose increased growth.
The effects of increasing trehalose production were also examined during growth at elevated temperatures, at high and low pHs, and in response to osmotic stress with sugars and additional inorganic salts (Fig. 3). At 37°C, cell densities after 24 h in M9-2% glucose were consistently highest for induced JP20 (OD550 nm of 1.40 ± 0.05 [mean ± standard deviation]), followed by uninduced JP20 (OD550 nm of 1.31 ± 0.04) and then by JP10 (OD550 nm of 1.06 ± 0.03; equivalent density for W3110). Densities of JP20 (induced and uninduced) were also higher than those of W3110 during growth in shaken flasks (Fig. 6). This beneficial effect of increased trehalose biosynthesis on growth was observed from 37°C to 41°C but did not alter the maximum temperature (41°C) permitting growth. Trehalose biosynthesis did not alter the pH range permitting growth.
Resistance to growth inhibition by inorganic salts and hexose sugars (Fig. 3; Tables 2 and 3) correlated with the levels of trehalose production (measured in the absence of stress agent): induced JP20 > uninduced JP20 > W3110 parent > JP10 (trehalose negative). Strain JP20 without induction (leaky) was approximately 40% more stress tolerant to salts and hexoses than the trehalose-negative mutant, JP10 (Fig. 3; Tables 2 and 3). Induction of otsBA (JP20) with 0.1 mM IPTG further increased growth with these stress agents by approximately 20%.
Trehalose biosynthesis was less beneficial with pentose sugars than with hexose sugars or inorganic salts (Fig. 3; Tables 2 and 3). A small benefit was observed for otsBA induction with arabinose at concentrations which partially inhibited JP10. Xylose was more than twice as inhibitory as the other sugars on a molar basis. Induction of otsBA was beneficial for growth only over a narrow range of xylose concentrations. Together, these results suggest that elevated levels of pentose sugars inhibit E. coli growth by some mechanism other than osmotic stress.
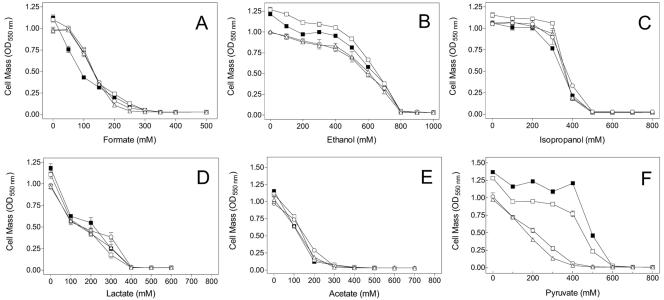
Effects of increased trehalose production on tolerance to fermentation products.
Increased trehalose biosynthesis was beneficial for growth in the presence of pyruvate (Fig. 7). The concentration of pyruvate required to inhibit growth in JP20 was over twice that for W3110 and JP10. Induction of otsBA with 0.1 mM IPTG (JP20) further increased pyruvate tolerance during growth, quite similar to the effects observed with sugars and inorganic salts (Fig. 3; Tables 2 and 3). This IPTG concentration (0.1 mM) was confirmed as being optimal for pyruvate tolerance (data not included). In contrast, increased biosynthesis of trehalose (JP20) was not beneficial for growth in the presence of three weaker organic acids (formate, acetate, and lactate). These three weak acids inhibited growth at less than half the inhibitory concentrations of pyruvate and inorganic salts, consistent with a mechanism of damage other than simple osmotic stress. Different levels of IPTG induction were also examined, but none was found beneficial for growth with formate, acetate, and lactate (data not included).
FIG. 7.
Growth of W3310 strains under stress from fermentation products. Cultures were grown for 24 h in M9-2% glucose medium containing formate (A), ethanol (B), isopropanol (C), lactate (D), acetate (E), or pyruvate (F). Symbols: ○, W3110 (wild type); ▵, JP10 (trehalose-negative mutant); □, JP20 (JP10, lacI-Ptac-otsBA); ▪, JP20 with 0.1 mM IPTG.
Trehalose biosynthesis was beneficial for growth in the presence of ethanol but did not alter the concentration at which growth was completely inhibited (Fig. 7). Previous studies have provided evidence that trehalose also contributes to ethanol tolerance in Saccharomyces cerevisiae (26). The level of trehalose produced by uninduced JP20 appeared optimal for growth with ethanol. IPTG induction (0.1 mM) decreased growth in the presence of ethanol. Results with isopropyl alcohol were qualitatively similar, although differences were smaller. Different levels of IPTG induction (JP20) were also examined with both ethanol and isopropanol, but none were found beneficial (data not included).
DISCUSSION
The unusual effectiveness of trehalose in preserving biological function under diverse stress conditions has been attributed to its unique physical properties. Trehalose is a nonreducing sugar in which the [1—1] glucosyl bond conceals the reactive ends of both glucose monomers (8, 17, 33, 35). The inertness of this disaccharide is presumed to allow the accumulation of high intracellular concentrations without disturbing biochemical processes. In addition, trehalose has an unusually high glass transition temperature, which slows the general reactivity in solutions during desiccation by making macromolecular movement difficult. Under dehydrating conditions, trehalose appears to protect cells by replacing water at the surfaces of macromolecules, holding proteins and membranes in their native conformations until water content is restored. Sola-Penna and Meyer-Fernandes (38) have correlated the stabilizing effect of trehalose on biological processes with its large hydrated volume. When present at concentrations that achieve equivalent viscosities, other sugars provided similar levels of protection.
Our studies indicate that genetically increasing trehalose biosynthesis could potentially improve E. coli-based biocatalysts for the production of commodity chemicals by increasing thermal tolerance. Overproduction of trehalose in E. coli JP20 (M9-2% glucose medium, 24 h) resulted in an increase in final cell densities at temperatures between 37°C and 41°C. Quite analogous improvements in growth were reported for betaine-supplemented B. subtilis for temperatures near the growth maximum (20). In both cases, the protective osmolytes did not increase the upper temperature boundary for growth. Previous studies have also associated trehalose production with the growth of Salmonella enterica at elevated temperatures (5). S. enterica mutants defective in trehalose biosynthesis exhibited a temperature-sensitive phenotype under moderate osmotic stress.
Overproduction of trehalose in E. coli-based biocatalysts may also increase tolerance to high concentrations of salts and sugars present in feedstocks for commercial fermentations and improve tolerance to some fermentation products. Increasing the synthesis of trehalose above that provided by native regulatory systems improved growth in the presence of osmotic-stress agents, such as hexose sugars, inorganic salts, and pyruvate. Additional but smaller benefits to cell growth (24 h) were observed with ethanol and isopropanol. Under these stress conditions, JP20 with a (leaky) lacI-regulated otsBA operon grew to higher densities than the wild-type parent (W3110) or JP10 (defective in trehalose biosynthesis and degradation). For most stress agents, tolerance was further increased by induction of otsBA with 0.1 mM IPTG. These beneficial actions were correlated with differences in intracellular trehalose during growth in M9-2% glucose medium without stress agents.
Osmotic stress is associated with particles in solution, with salts typically providing two- to threefold-higher levels of particles than sugars on a molar basis. Despite this relationship, levels of growth inhibition by hexose sugars and many salts were roughly equivalent on a molar basis. Previous studies have shown that a functional pathway for trehalose biosynthesis contributes to osmotic tolerance with both NaCl and glucose (8, 9, 22). However, the large benefit of increased production from independently regulated otsBA (and IPTG induction) was unexpected. Native otsBA genes cloned from W3110 were functional and used to increase expression from an artificial promoter (Ptac). Although laboratory strains of E. coli, such as W3110, may be less responsive than strains in nature, the otsBA operon in this K-12 line has been reported to retain full trehalose inducibility (3).
Trehalose biosynthesis was less beneficial during growth with pentose sugars (arabinose and xylose) than with hexose sugars (glucose and mannose). On a molar basis, pentoses were more inhibitory than hexoses or inorganic salts (two to four particles), with xylose being the most inhibitory. Inhibition was not substantially reduced by increased biosynthesis of trehalose, an osmoprotectant. The hydrated structure of xylose is similar to that of glucose, and xylose is often used as an analogue for studies of glucose uptake systems in eukaryotes (25). However, competitive inhibition of glucose transport does not appear responsible for the low xylose tolerance of W3110 strains in M9-2% glucose medium. Doubling the glucose concentration in this medium did not alter the sensitivity of W3110 strains to xylose (data not shown).
The inhibition of growth by three weak organic acids (formate, acetate, and lactate) was also examined. Growth was not substantially improved by trehalose biosynthesis in JP20, consistent with other modes of damage, such as a collapse of ΔpH (29) or a disruption of the envelope (1). These three acids have higher pKa values than pyruvate, a stronger acid, and were approximately twice as inhibitory as pyruvate for growth. The extent of growth inhibition by pyruvate was more similar to that of inorganic salts on a molar basis. Inhibition by pyruvate was substantially reduced by overproduction of trehalose.
Analysis of cell extracts revealed that intracellular trehalose levels correlated well with osmotic tolerance to inorganic salts and sugars and with thermal tolerance. Even in the absence of inducer, JP20 was more tolerant to osmotic stress than the wild type. Cell extracts from JP20 were found to contain significant amounts of trehalose without induction (28 mM). Accumulation of trehalose by uninduced cells probably reflects a low level of gene expression combined with the absence of activities known to degrade this compound. Csonka (9) has estimated that E. coli produces sufficient trehalose to equal about 20% of the osmolar concentration of solutes in the growth medium. Our results are in general agreement. Induced levels of trehalose in JP20 (361 mM) are equivalent to 20 to 60% of the osmolar level at which growth was fully inhibited (the MIC) by inorganic salts, pyruvate, and hexose sugars.
Acknowledgments
This research was supported by the Florida Agricultural Experiment Station (grant no. R-09127) and grants from the U.S. Department of Agriculture (01-35504-10669) and the U.S. Department of Energy (FG02-96ER20222).
Footnotes
Florida Agricultural Experiment Station publication no. R-10904.
REFERENCES
- 1.Alakomi, H.-L., E. Skyttä, M. Saarela, T. Mattila-Sandholm, K. Latva-Kala, and I. M. Helander. 2000. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66:2001-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Deidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bachmann, B. J. 1972. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 36:525-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boos, W., U. Ehmann, H. Forkl, W. Klein, M. Rimmele, and P. Postma. 1990. Trehalose transport and metabolism in Escherichia coli. J. Bacteriol. 172:3450-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cánovas, D., S. A. Fletcher, M. Hayashi, and L. N. Csonka. 2001. Role of trehalose in growth at high temperature of Salmonella enterica serovar Typhimurium. J. Bacteriol. 183:3365-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Causey, T. B., K. T. Shanmugam, L. P. Yomano, and L. O. Ingram. 2004. Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc. Natl. Acad. Sci. USA 101:2235-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chotani, G., T. Dodge, A. Hsu, M. Kumar, R. LaDuca, D. Trimbur, W. Weyler, and K. Sanford. 2000. The commercial production of chemicals using pathway engineering. Biochim. Biophys. Acta 1543:434-455. [DOI] [PubMed] [Google Scholar]
- 8.Crowe, L. M. 2002. Lessons from nature: the role of sugars in anhydrobiosis. Comp. Biochem. Physiol. 131:505-513. [DOI] [PubMed] [Google Scholar]
- 9.Csonka, L. N. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53:121-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danner, H., and R. Braun. 1999. Biotechnology for the production of commodity chemicals from biomass. Chem. Soc. Rev. 28:395-405. [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 12:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Virgilio, C., T. Hottiger, J. Dominguez, T. Boller, and A. Wiemken. 1994. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur. J. Biochem. 219:179-186. [DOI] [PubMed] [Google Scholar]
- 14.Elbein, A. D. 1974. The metabolism of α,α-trehalose. Adv. Carbohydr. Chem. Biochem. 30:227-256. [DOI] [PubMed] [Google Scholar]
- 15.Garg, A. K., J. K. Kim, T. G. Owens, A. P. Ranwala, Y. D. Choi, L. V. Kochian, and R. J. Wu. 2002. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 99:15898-15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giæver, H. M., O. B. Styrvold, I. Kaasen, and A. R. Strøm. 1988. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J. Bacteriol. 170:2841-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson, R. P., J. P. Turkenburg, S. J. Charnock, R. Lloyd, and G. J. Davies. 2002. Insights into trehalose synthesis provided by the structure of the retaining glucosyltransferase OtsA. Chem. Biol. 9:1337-1346. [DOI] [PubMed] [Google Scholar]
- 18.Guo, N., I. Puhlev, D. R. Brown, J. Mansbridge, and F. Levine. 2000. Trehalose expression confers desiccation tolerance on human cells. Nat. Biotechnol. 18:168-171. [DOI] [PubMed] [Google Scholar]
- 19.Hengge-Aronis, R., W. Klein, R. Lange, M. Rimmele, and W. Boos. 1991. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J. Bacteriol. 173:7918-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtmann, G., and E. Bremer. 2004. Thermoprotection of Bacillus subtilis by exogenously provided glycine betaine and structurally related compatible solutes: involvement of Opu transporters. J. Bacteriol. 186:1683-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horlacher, R., K. Uhland, W. Klein, M. Ehrmann, and W. Boos. 1996. Characterization of a cytoplasmic trehalase of Escherichia coli. J. Bacteriol. 178:6250-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida, A., N. Otsuka, S. Nagata, K. Adachi, and H. Sano. 1996. The effect of salinity stress on the accumulation of compatible solutes related to the induction of salt-tolerance in Escherichia coli. J. Gen. Appl. Microbiol. 42:331-336. [Google Scholar]
- 23.Kandror, O., A. DeLeon, and A. L. Goldberg. 2002. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 99:9727-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 25.Lee, W. J., M. D. Kim, Y. W. Ryu, L. F. Bisson, and J. H. Seo. 2002. Kinetic studies on glucose and xylose transport in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 60:186-191. [DOI] [PubMed] [Google Scholar]
- 26.Mansure, J. J., A. D. Panek, L. M. Crowe, and J. H. Crowe. 1994. Trehalose inhibits ethanol effects on intact yeast cells and liposomes. Biochim. Biophys. Acta 1191:309-316. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Morales, F., A. C. Borges, A. Martinez, K. T. Shanmugam, and L. O. Ingram. 1999. Chromosomal integration of heterologous DNA in Escherichia coli with precise removal of markers and replicons used during construction. J. Bacteriol. 181:7143-7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKendry, P. 2002. Energy production from biomass (part 1): overview of biomass. Biores. Technol. 83:37-46. [DOI] [PubMed] [Google Scholar]
- 29.Minamino, T., Y. Imae, F. Oosawa, Y. Kobayashi, and K. Oosawa. 2003. Effect of intracellular pH on rotational speed of bacterial flagellar motors. J. Bacteriol. 185:1190-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moniruzzaman, M., and L. O. Ingram. 1998. Ethanol production from dilute acid hydrolysate of rice hulls using genetically engineered Escherichia coli. Biotechnol. Lett. 20:943-947. [Google Scholar]
- 31.Nakamura, C. E., A. A. Gatenby, A. K.-H. Hsu, R. D. La Reau, S. L. Haynie, M. Diaz-Torres, D. E. Trimbur, G. M. Whited, V. Nagarajan, M. S. Payne, S. K. Picataggio, and R. V. Nair. January2000. Method for the production of 1,3-propanediol by recombinant microorganisms. U.S. patent 6,013,494.
- 32.Niu, W., K. M. Draths, and J. W. Frost. 2002. Benzene-free synthesis of adipic acid. Biotechnol. Prog. 18:201-211. [DOI] [PubMed] [Google Scholar]
- 33.Padilla, L., R. Krämer, G. Stephanopoulos, and E. Agosin. 2004. Overproduction of trehalose: heterologous expression of Escherichia coli trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in Corynebacterium glutamicum. Appl. Environ. Microbiol. 70:370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pósfai, G., M. D. Koob, H. A. Kirkpatrick, and F. C. Blattner. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J. Bacteriol. 179:4426-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richards, A. B., S. Krakowka, L. B. Dexter, H. Schmid, A. P. M. Wolterbeek, D. H. Waalkens-Berendsen, A. Shigoyuki, and M. Kurimoto. 2002. Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 40:871-898. [DOI] [PubMed] [Google Scholar]
- 36.Rimmele, M., and W. Boos. 1994. Trehalose-6-phosphate hydrolase of Escherichia coli. J. Bacteriol. 176:5654-5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sleator, R. D., and C. Hill. 2001. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26:49-71. [DOI] [PubMed] [Google Scholar]
- 38.Sola-Penna, M., and J. R. Meyer-Fernandes. 1998. Stabilization against thermal inactivation promoted by sugars on enzyme structure and function: why is trehalose more effective than other sugars? Arch. Biochem. Biophys. 360:10-14. [DOI] [PubMed] [Google Scholar]
- 39.Storz, G., and R. Hengge-Aronis (ed.). 2000. Bacterial stress responses. ASM Press, Washington, D.C.
- 40.Tunnacliffe, A., A. G. de Castro, and M. Manzanera. 2001. Anhydrobiotic engineering of bacterial and mammalian cells: is intracellular trehalose sufficient? Cryobiology 43:124-132. [DOI] [PubMed] [Google Scholar]
- 41.Underwood, S. A., M. L. Buszko, K. T. Shanmugam, and L. O. Ingram. 2004. Lack of protective osmolytes limits final cell density and volumetric productivity of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl. Environ. Microbiol. 70:2734-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Underwood, S. A., S. Zhou, T. B. Causey, L. P. Yomano, K. T. Shanmugam, and L. O. Ingram. 2002. Genetic changes to optimize carbon partitioning between ethanol and biosynthesis in ethanologenic Escherichia coli. Appl. Environ. Microbiol. 68:6263-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Laere, A. 1989. Trehalose, reserve and/or stress metabolite? FEMS Microbiol. Rev. 63:201-210. [Google Scholar]
- 44.Wang, X., J. F. Preston III, and T. Romeo. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186:2724-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, S., T. B. Causey, A. Hasona, K. T. Shanmugam, and L. O. Ingram. 2003. Production of optically pure d-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 69:399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou, S. D., and L. O. Ingram. 2001. Simultaneous saccharification and fermentation of amorphous cellulose to ethanol by recombinant Klebsiella oxytoca SZ21 without supplemental cellulase. Biotechnol. Lett. 23:1455-1462. [Google Scholar]