Abstract
Listeria monocytogenes is a foodborne pathogen able to cause serious disease in humans and animals. Not all isolates are equally pathogenic, however, and several isolates have been characterized as naturally virulence attenuated. We sought to identify the genetic basis of natural virulence attenuation using cell culture assays and molecular techniques. By comparing the phenotypes of naturally virulence-attenuated isolates to those of defined virulence gene mutants in plaque, cytotoxicity, and hemolysis assays and by characterizing selected virulence genes and their expression using DNA sequencing and TaqMan reverse transcriptase PCR, we classified virulence-attenuated isolates into four categories. Both group A and group B isolates were noncytotoxic and nonhemolytic; however, group A isolates underexpressed listeriolysin O (LLO, encoded by hlyA), while group B isolates produced LLO proteins that were inactive. The single isolate in group C was fully cytotoxic, had higher than wild-type hemolytic activity, and was, therefore, likely virulence attenuated due to overexpression of LLO. Group D isolates were characterized by normal cytotoxicity, hemolytic activity, and hlyA expression but had reduced intracellular growth. The genetic mechanisms causing virulence-attenuated phenotypes among the group D isolates could not be determined definitively but may involve defects in the expression of actA or the function of the ActA protein. Our results show (i) that the combination of cell culture assays and molecular techniques used in this study allows for identification and characterization of naturally virulence-attenuated isolates and (ii) that multiple distinct genetic mechanisms are responsible for natural virulence attenuation in L. monocytogenes.
The bacterial pathogen Listeria monocytogenes is a significant public health concern and a leading cause of hospitalization and death due to foodborne illness. There are approximately 2,500 cases of invasive listeriosis, resulting in approximately 500 deaths, each year in the United States (26). Not all L. monocytogenes strains are equally capable of causing disease in humans, however (13, 25, 36). Of the 13 serovars of L. monocytogenes, for example, only three serovars, 1/2a, 1/2b, and 4b, cause more than 90% of human cases (16). Several groups have identified food and environmental L. monocytogenes isolates that are naturally avirulent or virulence attenuated as indicated by their decreased ability to colonize and multiply in the spleens of infected mice (6, 10, 35). Furthermore, work by Roche et al. demonstrated that these virulence-attenuated and avirulent phenotypes are stable (35). Previous studies that describe genetic mechanisms of natural virulence attenuation characterized only a single isolate or a few isolates; for example, Leimeister-Wachter et al. describe a natural 300-bp deletion in prfA in strain NCTC 7973, which results in a nonhemolytic phenotype (22). Chakraborty et al. identified three naturally virulence-attenuated food isolates and suggested that one strain (L99) was virulence attenuated due to its slightly truncated form of actA (10). A more comprehensive understanding of the mechanisms of natural virulence attenuation in L. monocytogenes is thus needed to develop better cell culture or PCR-based assays to detect virulence-attenuated L. monocytogenes and to better understand the development and evolution of virulence attenuation in L. monocytogenes.
Virulence attenuation in L. monocytogenes has been detected and characterized using in vitro, cell culture, and animal models. In vitro assays can be designed to detect phenotypic or genotypic markers of virulence attenuation. For example, defined mutants that were avirulent in mice were also phenotypically unable to lyse red blood cells in a semiquantitative hemolysis assay (33). Likewise, molecular techniques, such as mixed-genome microarrays, can detect genetic markers specific to clonal groups of L. monocytogenes that have various associations to human listeriosis (8). Examples of cell culture models that have been used to characterize L. monocytogenes virulence include the plaque-forming assay, as well as cytotoxicity-based assays that depend on measuring the release of intracellular enzymes or microscopically monitoring indicators of cell damage (4, 32, 42). The plaque-forming assay has been used extensively to characterize L. monocytogenes cytopathogenicity in several host cell types, including human enterocyte-like Caco-2 cells (15, 42), mouse fibroblast L2 cells, and human epithelial Henle cells (40). Cell culture assays measuring cytotoxicity include assays to assess penetration into and multiplication within intestinal epithelial cells (42), alkaline phosphatase release from infected hybrid B lymphocytes (4), and lactate dehydrogenase release from infected mouse macrophage-like cells (33). While in vitro assays that characterize virulence are often relatively fast and simple to perform, they may not necessarily be relevant to mammalian, particularly human, virulence, and animal models may provide better means of assessing the virulence of a given pathogen. Some of the in vivo models frequently used to determine L. monocytogenes virulence include determination of 50% lethal dose in mice and recovery of L. monocytogenes from the livers and spleens of immunocompetent or immunocompromised mice following intravenous or oral inoculation (1, 2). Oftentimes, however, the use of laboratory animals is impractical for a particular study, or the best available animal models have significant limitations, as was recently demonstrated by Lecuit et al. in their study identifying the single amino acid substitution in mouse E-cadherin that renders it unable to bind to L. monocytogenes surface protein internalin A (21).
In light of the current limitations of individual in vitro assays and animal models to characterize and identify virulence-attenuated L. monocytogenes, we sought (i) to develop and apply a combination of cell culture and other in vitro assays to detect and characterize naturally virulence-attenuated L. monocytogenes isolates and (ii) to define the genetic mechanisms underlying natural virulence attenuation by comparing naturally virulence-attenuated isolates to defined virulence gene mutants.
MATERIALS AND METHODS
Bacterial strains.
Fully virulent control strain 10403S, 11 virulence-attenuated natural isolates, and 10 in-frame virulence gene deletion mutants were used in this study (Table 1); in addition, strain EGD (Table 1) was included as a control in the cytotoxicity assay. Eight of the 11 natural isolates were defined as virulence attenuated due to their previously reported zero-plaque-forming phenotypes (18, 31), while three natural isolates, X1-002, X1-004, and X1-005, had been shown to be virulence attenuated in a mouse model of infection (10). Among the natural isolates, nine came from food products, one from a food-processing environment, and one from a human clinical case. All natural isolates had previously been confirmed as L. monocytogenes by EcoRI ribotyping and by PCR-restriction fragment length polymorphism analysis of hlyA using L. monocytogenes-specific primers for this gene; ribotyping and PCR-restriction fragment length polymorphism data had also previously been used to assign isolates into genetic lineages as described previously (18, 31, 44). Stock cultures were stored at −80°C in 15% glycerol, and unless otherwise noted, maintained on brain heart infusion (BHI) agar (Difco, Sparks, MD).
TABLE 1.
Listeria monocytogenes strains used
| Strain | Previous name | Genotype | Ribotype | Lineage | Source | Reference |
|---|---|---|---|---|---|---|
| Control | ||||||
| M2-013 | EGD | 1039C | II | M. Kuhn | ||
| X1-001 | 10403S | 1030A | II | D. Portnoy | ||
| Defined isogenic mutants | ||||||
| R3-001 | 10403S ΔactA | 1030A | II | M. Wiedmann | ||
| R3-002 | DP-L1465 | 10403S Δmpl | 1030A | II | D. Portnoy | 37 |
| R3-003 | DP-L2161 | 10403S ΔhlyA | 1030A | II | D. Portnoy | 20 |
| R3-004 | DP-L1552 | 10403S ΔplcA | 1030A | II | D. Portnoy | 9 |
| R3-005 | DP-L1935 | 10403S ΔplcB | 1030A | II | D. Portnoy | 38 |
| R3-006 | DP-L1936 | 10403S ΔplcAB | 1030A | II | D. Portnoy | 38 |
| R3-007 | DP-L1075 | 10403S prfA Tn917 insertion | 1030A | II | D. Portnoy | 14 |
| R3-008 | WL-112 | EGD ΔinlAB | 1039C | II | M. Kuhn | 19 |
| R3-009 | WL-111 | EGD ΔinlB | 1039C | II | M. Kuhn | 19 |
| R3-010 | A76 | EGD ΔinlA | 1039C | II | M. Kuhn | 19 |
| Natural virulence-attenuated isolates | ||||||
| N1-047 | 1053B | II | Salmon | 31 | ||
| N1-302 | 1053B | II | Sable | 31 | ||
| N1-304 | 1053B | II | Fish plant floor | 31 | ||
| R2-006 | 1042B | I | Deli salad | 18 | ||
| R2-303 | 1053A | II | Human sporadic | 18 | ||
| R2-319 | 1051D | I | Lunch meat | 18 | ||
| R2-394 | 1043A | I | Deli salad | 18 | ||
| X1-002 | L99 | 1059B | III | Food | 10 | |
| X1-004 | L83 | Unique | III | Food | 10 | |
| X1-005 | L19 | 1062D | II | Food | 10 | |
| W1-038 | 16618 | Unknown | Salmon | 31 |
Growth curve.
To assess the in vitro growth of L. monocytogenes isolates, we used a fusion spectrophotometer (PerkinElmer Life and Analytical Sciences, Boston, MA) to measure the optical density (at 600 nm) every 30 min for 10 h of L. monocytogenes cultures growing at 37°C. Each control and each natural isolate was tested in four different media: two relatively rich media, BHI broth and Luria-Bertani (LB) broth; a cell culture medium, Dulbecco's modified Eagle medium (DMEM); and a minimal defined medium previously described by Premaratne et al. (34). Using a round-bottomed 96-well plate, 150 μl of each test media was inoculated in triplicate with 15 μl of a 1:10 dilution (containing 3 × 106 CFU) of a L. monocytogenes culture that had been grown for 12 h in BHI broth. Bacterial cells were washed once in sterile phosphate-buffered saline (PBS) and diluted in PBS before inoculation.
Plaque assay.
A previously described plaque assay (40) was used to initially characterize all natural isolates and defined mutants. Briefly, mouse fibroblast L2 cells were grown to confluence in 2 ml of DMEM containing 10% fetal bovine serum in six-well cell culture plates. Bacteria were grown overnight at 30°C in BHI broth, pelleted, and resuspended in PBS. For each bacterial strain tested, one well was infected with 1 × 105 CFU and one well was infected with 3 × 104 CFU. After a 1-hour incubation at 37°C, the cell monolayers were washed three times with PBS and overlaid with 3 ml of DMEM containing 10 μg/ml gentamicin and 1.4% Bacto agar (Difco, Sparks, MD). After 3 days of incubation at 37°C, a second 2-ml overlay of DMEM containing 6% neutral red solution and 1.4% Bacto agar was added. After a final day of incubation, plaques were enumerated and their size measured by projecting the plaque images against a wall using an overhead projector. For each test and control strain, the diameters of 25 plaques were measured with a ruler and averaged. Plaque size and number were normalized by expressing them as a percentage of the wild-type internal control strain, 10403S, included in each assay.
Cytotoxicity assay.
To further characterize the virulence of our collection of natural isolates and defined virulence gene mutants, we utilized a cytotoxicity assay (Cytotox 96 Non-Radioactive cytotoxicity assay; Promega, Madison, WI) to colorimetrically measure host cell lactate dehydrogenase (LDH) release as an indicator of bacterium-induced host cell membrane damage. Mouse macrophage-like J774 cells were grown to confluence in 24-well cell culture plates and infected in triplicate at a multiplicity of infection (MOI) of 100 bacterial cells per J774 cell. After 6 h of incubation at 37°C, the cell supernatant was assayed for LDH according to the kit instructions. Percent cytotoxicity was then calculated as described by Decatur and Portnoy (12): percent cytotoxicity = 100 × [(experimental LDH release − spontaneous LDH release)/(maximum LDH release − spontaneous LDH release)]. Cytotoxicity results were then normalized by expressing them as a percentage of the cytotoxicity of the wild-type internal control strain, 10403S.
Intracellular growth assay.
To assess the abilities of the natural isolates to infect and multiply within host cells, we used an intracellular growth assay originally described by Portnoy et al. (33). Briefly, mouse macrophage-like J774 cells were grown to confluence in 2 ml of DMEM in six-well plates containing three 12-mm round glass coverslips. Cell monolayers were infected in duplicate with 2 × 105 CFU, washed three times with PBS after 30 min, incubated with 2 ml of DMEM for another 30 min, and finally treated with gentamicin at a final concentration of 50 μg/ml. At 3, 6, and 12 h postinfection, two coverslips, one from each of the duplicate wells, were removed and vortexed together in 5 ml deionized water containing 0.1% Triton X. Intracellular bacteria were enumerated by plating on BHI agar and expressed relative to the number of bacteria in the inoculum. Relative intracellular growth was normalized by expressing it as a percentage of the relative intracellular growth of the wild-type internal control strain 10403S.
Semiquantitative hemolysis assay.
We used a semiquantitative hemolysis assay originally described by Portnoy et al. (33) as an in vitro screen for hemolysis activity. Briefly, tubes of LB broth containing 2.5 mM CaCl2, 20 mM MgCl2, and 20 mM MgSO4 (termed LB medium plus salts) were inoculated 1:10 with overnight cultures of the different L. monocytogenes isolates and strains and incubated at 37°C with shaking for 5 h. A 1% sheep erythrocyte solution was prepared by centrifuging 10 ml of sheep red blood cells for 30 min at 1,000 × g, washing the pellet once in 10-ml sterile PBS, and resuspending the pellet in 50 ml of PBS. After confirming that the optical densities of the bacterial cultures did not vary significantly from one another, we pelleted 1 ml of each culture, resuspended the pellets in 0.5 ml of sterile PBS containing 6 mM cysteine at pH 5.8, and made seven 1:2 dilutions in the PBS-cysteine solution. The bacterial dilutions were then incubated at 37°C for 30 min without shaking. Finally, 0.5 ml of the 1% sheep erythrocyte solution was added to each dilution, followed by incubation for 1 h at 37°C without shaking. The tubes were then examined visually and scored as having achieved zero, partial, or complete hemolysis by comparison to a fully hemolyzed positive control containing 0.05% Triton X. We calculated the number of hemolytic units for each strain by taking the inverse of the last dilution to show complete hemolysis and then expressed the hemolytic units as a percentage of the hemolysis of the control strain, 10403S.
Sequencing of the hlyA and prfA promoters and open reading frames.
All PCR amplifications were performed using Taq polymerase, MgCl2 at a final concentration of 1.5 mM, 1× PCR buffer, deoxynucleoside triphosphates at a final concentration of 50 μM (each), and 12.5 μM of each primer (Table 2). Negative-control reactions were included in each PCR. DNA sequencing was performed in both the forward and reverse directions to generate completely overlapping sequences at Cornell University's Bioresource Center using an ABI 3700 sequencer (PerkinElmer). Completed DNA sequences were proofread, and any discrepancies in base calling were resolved by inspecting the respective DNA sequencing electropherograms. The plcA-hlyA intergenic region, containing the hlyA promoter, was amplified and sequenced using primers plcAhly-F and plcAhly-R (Table 2) with the following thermocycling conditions: 94°C for 5 min; 35 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 1 min; followed by a final extension at 72°C for 7 min. The coding region of hlyA was amplified using primers hly-F and hly-R (Table 2) and the following thermocycling conditions: 94°C for 3 min; 40 cycles of 94°C for 1 min, 54°C for 1 min, and 72°C for 2 min; followed by a final extension at 72° for 7 min. The hlyA coding region was sequenced using primers hly-F, hly-R, internal-hlyF, internal-hlyR, hly-int2F, and hly-int2R (Table 2). The prfA promoter region and open reading frame were amplified and sequenced using primers prfA-F(CB) and prfA-R (Table 2). Thermocycling conditions were 94°C for 3 min; 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; followed by a final extension at 72° for 5 min. All DNA sequences are available online through Pathogen Tracker (www.pathogentracker.net).
TABLE 2.
Primers and probes used
| Primer or probe | Purpose | Sequence (5′ → 3′) |
|---|---|---|
| plcA-hly-F | hlyA promoter PCR or sequencing | CCGCCTAATGGGAAAGTAAA |
| plcA-hly-R | hlyA promoter PCR or sequencing | AGGCGGAGATGCTGGTG |
| ORFa PCR hly-F | hlyA ORFa PCR or sequencing | TGCGTTTCATCTTTAGAAGC |
| hly-R | hlyA ORF PCR or sequencing | AAGCCTGTTTCTACATTCTTCA |
| Internal-hly-F | hlyA ORF sequencing | TTTGCCAGGAATGACTAATC |
| Internal-hly-R | hlyA ORF sequencing | TTTGAAGGAAGAATTTTTGATGA |
| hly-int2-F | hlyA ORF sequencing | GCTGCTTTTGACGCTGCCG |
| hly-int2-R | hlyA ORF sequencing | CTGATTGCGCCGAAGTTTACA |
| prfA-F(CB) | prfA PCR or sequencing | CTCCGAGCAACCTCGGAACC |
| prfA-R | prfA PCR or sequencing | TTCTCATTGAGGAATACTG |
| hly-TqMnFa | hlyA TaqMan primer | ATGGCACCACCAGCATCTC |
| hly-TqMnR | hlyA TaqMan primer | ATCCGCGTGTTTCTTTTCGAT |
| hly-TqMn probeb | hlyA TaqMan probe | CCTGCAAGTCCTAAGAC |
| prfAp2 TqMn F | prfA TaqMan primer | GATGGTATCACAAAGCTCACGAGTA |
| prfAp2-TqMn-R | prfA TaqMan primer | AATAAAGCCAGACATTATAACGAAAGC |
| prfA TqMn probeb | prfA TaqMan probe | TGTAAATTCATGATGGTCCCGTTCTCGCT |
| rpoB-TqMnF1 | rpoB TaqMan primer | TGTAAAATATGGACGGCATCGT |
| rpoB-TqMnR1 | rpoB TaqMan primer | GCTGTTTGAATCTCAATTAAGTTTGG |
| rpoB TqMn probeb | rpoB TaqMan probe | CTGATTCGCGCAAAACTTCTACGCG |
ORF, open reading frame.
TaqMan (TaqMan) probes were labeled with the reporter dye 6-carboxyfluorescein at the 5′ end and with the QSY7 dark-quencher dye at the 3′ end (39).
Purification and quantification of PCR products.
For DNA sequencing, PCR products were purified using the Qiaquick PCR purification kit (QIAGEN, Valencia, CA). DNA concentrations of purified PCR products were estimated by comparison of amplicon band intensity with a DNA marker of known size and concentration (pGEM marker; Promega, Madison, WI) using the LabImage software (Kapelan, Germany).
Total RNA isolation and TaqMan quantitative RT-PCR.
RNA was extracted from L. monocytogenes grown for 5 h in LB medium plus salts using the RNeasy Protect Bacteria Midi kit (QIAGEN). For cell lysis, we used the enzymatic and mechanical disruption protocol provided by the manufacturer, with the exception that, instead of bead beating, we used sonication at 21 W for 20 s three times, with cells iced for 30 s between sonication bursts. To quantify the amounts of hlyA and prfA transcripts produced by the natural isolates, we used TaqMan reverse transcriptase PCR (RT-PCR). Oligonucleotide primers and probes (Table 2) were designed with Primer Express 1.0 software (ABI Prism; PerkinElmer-Applied Biosystems), and probes were purchased from Megabases, Inc. The probes were labeled with the reporter dye 6-carboxyfluorescein at the 5′ end and with the QSY7 dark-quencher dye at the 3′ end (39). Quantitative reverse transcriptase PCR (RT-PCR) was performed using the TaqMan One-Step RT-PCR Master Mix Reagents kit (PerkinElmer) essentially as previously described (39). The RT-PCR mixture (25 μl) contained 6.25 U of Multiscribe RT, 10.0 U of RNase inhibitor, 500 nM of each gene-specific primer, 100 nM of each probe, and 25 ng of total RNA template. Amplification and detection of specific products were performed with the ABI Prism 7700 sequence detection system (PerkinElmer) with the following cycling parameters: 48°C for 30 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min. During each run, a standard dilution of 10403S genomic DNA of known quantity, isolated by phenol chloroform extraction, was included to permit transcript quantification. The amount of contaminating chromosomal DNA in each sample was determined with control reaction mixtures that did not contain RT. The quantity of hlyA or prfA cDNA in each experimental sample was normalized to the quantity of rpoB cDNA in each sample.
Cell supernatant protein isolation and immunoblot analysis.
To test whether the natural isolates that expressed hlyA were able to secrete listeriolysin O (LLO), we performed immunoblotting on bacterial supernatant fractions. Bacteria were grown overnight in BHI broth, then diluted 1:10 in LB medium plus salts, and incubated at 37°C with shaking for 5 h. Total protein was precipitated from the bacterial supernatant using 5% trichloroacetic acid and quantified in a microtiter plate protein microassay (tech note 1069; Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. A standard volume (15 μl) of protein was run on a NuPage Novex Bis-Tris gel (Invitrogen, Carlsbad, CA) at 200 V for 35 min in morpholineethanesulfonic acid (MES) buffer using the XCell SureLock Mini-Cell (Invitrogen) and blotted to a nitrocellulose membrane using the XCell II Blot module (Invitrogen) according to the kit instructions. Finally, the membrane was probed with monoclonal anti-LLO primary antibody B3-19 (30) and detected using alkaline phosphatase-conjugated anti-mouse secondary antibodies provided with the WesternBreeze chromogenic Western blot immunodetection kit (Invitrogen) according to the kit instructions.
RESULTS
Growth curve.
To confirm that the previously identified small plaque-forming phenotype of several of the natural isolates was not the result of general growth defects, we tested each natural isolate's ability to grow in four different media, BHI, LB, DMEM, and a defined minimal medium. Within each medium, all isolates showed lag times, growth rates, and final optical densities similar to those of the wild-type control strain, 10403S, indicating normal extracellular growth.
Plaque assay.
We tested the wild-type strain 10403S, the natural L. monocytogenes isolates, and defined L. monocytogenes isogenic mutants in at least three independent plaque assays to determine two measures of virulence: (i) invasion efficiency, represented by the number of plaques, and (ii) rate of cell-to-cell spread, represented by plaque size (Fig. 1 and Table 3). As expected on the basis of other reports (14, 23, 40), the ΔhlyA and ΔprfA mutants failed to form any plaques, while the ΔactA, Δmpl, ΔplcB, and ΔplcAB mutants had median plaque sizes that were smaller (19%, 77%, 66%, and 46%, respectively) than the internal control strain, 10403S. The defined ΔplcA, ΔinlA, ΔinlB, and ΔinlAB mutants formed plaques similar in size to those formed by the internal control strain 10403S (89%, 112%, 92%, and 82%, respectively). Natural isolates N1-047, N1-302, N1-304, R2-006, R2-303, R2-319, R2-394, W1-038, and X1-004 were chosen for inclusion in this study due to their previously recognized inability to form plaques (10, 18, 31), which was confirmed in this study, while isolates X1-002 and X1-005, previously identified as avirulent in mice (10), formed plaques that were 40% and 72%, respectively, the size of those formed by the internal control strain 10403S. While plaque size serves as an indirect indicator of the bacterial rate of cell-to-cell spread, the ratio of CFU to PFU serves as a measure of bacterial invasiveness, with higher CFU/PFU values indicating less efficient invasion. In this study, the normalized CFU/PFU results for each strain were highly variable, but most defined virulence gene mutants and natural isolates had median percent CFU/PFU ratios greater than 150, with the exception of natural isolate X1-002 and the defined ΔplcA mutant, which had median CFU/PFU ratios of 24% and 83%, respectively.
FIG. 1.
Plaque assay. For each strain in each assay, the diameters of 25 plaques were measured, averaged, and normalized by expressing them as a percentage of the average size of the internal control strain, 10403S. Shown are the median normalized plaque sizes from at least three independent experiments. Error bars represent the minimum and maximum normalized size values; no error bars are shown for internal control strain 10403S, since the plaque size value for this strain is set at 100% for each replicate experiment.
TABLE 3.
Results from all virulence characterization assays for natural isolates
| Group | Strain | Relative value for virulence parametersa
|
Cause of virulence-attenuated phenotype | ||||
|---|---|---|---|---|---|---|---|
| Plaque sizeb | Cytotoxicity in J774 cells | Hemolytic activity | hlyA transcript levelsc | Intracellular bacterial no. in J774 cells at 12 h p.i.d | |||
| A | R2-319 | 0 | 7 | 0 | 20 | 4 | |
| N1-047 | 0 | 13 | 0 | 2 | 1 | ||
| N1-302 | 0 | 14 | 0 | NTe | NT | hlyA underexpression | |
| N1-304 | 0 | 21 | 0 | NT | NT | ||
| X1-004 | 0 | 2 | 0 | 1 | 0 | ||
| B | R2-394 | 0 | 3 | 0 | 750 | 3 | Putative inactive LLOf |
| W1-038 | 0 | 1 | 0 | 500 | NT | inactive LLOg | |
| C | X1-005 | 70 | 98 | 200 | 7,000 | 62 | hlyA overexpression |
| D | R2-006 | 0 | 93 | 200 | 650 | 8 | |
| R2-303 | 0 | 91 | 100 | 90 | 7 | Other defect | |
| X1-002 | 39 | 88 | 100 | 130 | 40 | ||
Relative values for various virulence parameters (expressed as percentages of values obtained for wild-type control strain 10403S. Since all values are expressed relative to the value for 10403S, the value for wild-type strain 10403S would be 100% for each category.
Plaque size value of 0 indicates that no plaques were formed in this assay.
hlyA transcript levels were determined by TaqMan RT-PCR.
p.i., postinfection.
NT, not tested.
The inactivity of LLO from R2-394 may be caused by a unique Q253K substitution.
The inactivity of LLO from W1-038 is caused by a frameshift mutation resulting from a single-base-pair deletion after residue 378.
Cytotoxicity assay.
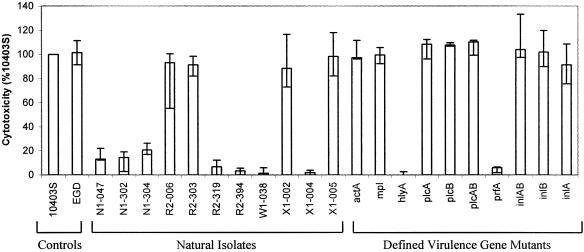
We used a cytotoxicity assay based on lactate dehydrogenase release to characterize further the virulence properties of control strains 10403S and EGD, the natural isolates, and the defined virulence gene mutants (Fig. 2 and Table 3). Among the defined mutants, the ΔhlyA and ΔprfA mutants had significantly (P < 0.01 by the Wilcoxon rank sum test) reduced cytotoxicity (0% and 6%) in murine macrophage-like J774 cells compared to those of the other mutants and the 10403S wild-type control, while the ΔactA, Δmpl, ΔplcA, ΔplcB, ΔplcAB, ΔinlA, ΔinlB, and ΔinlAB mutants had cytotoxicities that did not differ significantly from each other or the EGD and 10403S controls (P > 0.05 by Kruskal-Wallis one-way nonparametric analysis of variance [ANOVA]). On the basis of the cytotoxicity data, the natural isolates fell into two easily distinguishable categories: those that had relative cytotoxicities significantly lower than the cytotoxicities of the EGD and 10403S controls (P < 0.01 by the Wilcoxon rank sum test) and those that had relative cytotoxicities similar to the cytotoxicities of the EGD and 10403S controls. In the first category, isolates R2-319, R2-394, W1-038, and X1-004 all had median cytotoxicities below 10% (7%, 3%, 1%, and 2%, respectively), while isolates N1-047, N1-302, and N1-304 all had median cytotoxicities between 10% and 25% (13%, 14%, and 21%, respectively). In the second category, natural isolates R2-006, R2-303, X1-002, and X1-005, which were virulence attenuated in the plaque assay, had median cytotoxicities that did not differ significantly (P > 0.05 by Kruskal-Wallis one-way nonparametric ANOVA) from each other or the 10403S and EGD controls (93%, 91%, 88%, and 98%, respectively).
FIG. 2.
Cytotoxicity assay. LDH released from host J774 cells upon infection with L. monocytogenes was measured as an indicator of cytotoxicity and normalized to the amount of LDH released from cells infected with wild-type strain 10403S; median normalized cytotoxicity values from at least three independent experiments are shown. Error bars represent the minimum and maximum normalized cytotoxicity values; no error bars are shown for internal control strain 10403S, since the cytotoxicity value for this strain is set at 100% for each replicate experiment.
Intracellular growth assay.
To further probe the cause of virulence-attenuated phenotypes, we examined the intracellular growth, as determined by mean doubling times and total growth at 12 h postinfection, in J774 cells for a representative subset of our natural isolates (Table 3 and Fig. 3). Of the natural isolates tested, only R2-006, R2-303, X1-002, and X1-005 showed growth between 3 and 6 h, as indicated by positive median doubling times (102 min, 75 min, 74 min, and 77 min, respectively). From 6 to 12 h postinfection, all strains, except R2-394 and X1-004 showed growth as indicated by positive median doubling times. The absolute levels of intracellular bacteria at 12 h postinfection were significantly (P < 0.01 by Kruskal-Wallis one-way nonparametric ANOVA) lower for most naturally virulence-attenuated isolates (Table 3 and Fig. 3). Only two isolates (X1-002 and X1-005) had grown to levels of >10% (40% and 62%, respectively) of that observed for the wild-type control strain 10403S; these levels were not statistically different from the level of intracellular bacteria observed for 10403S at 12 h postinfection.
FIG. 3.
Intracellular (I.C.) growth assay. Mouse macrophage-like J774 cells growing on glass coverslips were infected with L. monocytogenes. At 3, 6, and 12 h after infection, coverslips were removed and the bacteria contained within the host cells enumerated. Data points represent the median of five independent experiments. The y axis indicates the number of bacterial cells recovered at each time point expressed relative to the inoculum number. The numbers on the right-hand side indicate the intracellular bacterial numbers at 12 h postinfection expressed as a percentage of the intracellular bacterial numbers of the wild-type strain 10403S.
Semiquantitative hemolysis assay.
To assess the hemolytic capabilities of our defined virulence gene mutants and natural isolates, we performed a semiquantitative hemolysis assay. Among the defined virulence gene mutants, the ΔhlyA and ΔprfA mutants failed to hemolyze sheep red blood cells, while mutants ΔplcA, ΔplcB and ΔplcAB hemolyzed to the same extent as the 10403S wild-type strain did. In agreement with the results from the plaque and cytotoxicity assays, the hemolytic activity of the natural isolates fell into two categories (Table 3), with the majority of isolates severely impaired in their ability to hemolyze red blood cells; isolates N1-047, N1-302, N1-304, R2-319, R2-394, X1-004, and W1-038 exhibited no hemolysis. Natural isolates R2-006, R2-303, X1-002, and X1-005 had hemolytic activities greater than or equal to the control (200%, 100%, 100%, and 200%, respectively).
Sequencing of the hlyA promoter and open reading frame.
Because natural isolates N1-047, N1-302, N1-304, R2-319, R2-394, X1-004, and W1-038 had the same phenotypes in the plaque, cytotoxicity, and semiquantitative hemolysis assays as the ΔhlyA and ΔprfA defined mutants, we decided to use DNA sequencing to probe whether the virulence attenuation in these natural isolates could be explained by loss-of-function mutations in hlyA or prfA. For comparison, hlyA and prfA of isolates R2-006, R2-303, and X1-005 were also sequenced because of these isolates' fully cytotoxic, fully hemolytic, yet zero- or small-plaque-forming phenotypes. When we compared the hlyA promoter region sequences of our natural isolates to the sequences of 15 virulent isolates that had been previously sequenced in our lab (7), the only deviations from the consensus sequence occurred in the −35 region of hlyA promoter P1 (as mapped by Mengaud et al. [27]); some isolates had a P1 −35 sequence of AGGATA, while others had the sequence GGGACA. The two different −35 sequences were distributed randomly among the natural virulence-attenuated and virulent isolates, however, indicating that they reflect evolutionary relationships, rather than virulence phenotype. There were no polymorphisms found among the natural virulence-attenuated isolates in the PrfA box, P1 −10 sequence, or P2 −10 sequence.
When the hlyA open reading frame sequences for all natural virulence-attenuated isolates were compared to the five hlyA open reading frame sequences found in GenBank (accession numbers F5782, F4233, F2365, F6789, and ATCC 9525), only two unique polymorphisms were found among the natural isolates. Isolate R2-394 has a lysine residue at position 253, whereas all other natural isolates, GenBank isolates, and control strain 10403S had a glutamine. Isolate W1-038 had a single-base-pair deletion, resulting in a frameshift mutation after residue 378. The high levels of homology of the hlyA sequences for the naturally virulence-attenuated isolates characterized here with hlyA sequences for other L. monocytogenes also further confirm that these isolates are truly members of the species L. monocytogenes.
Sequencing of the prfA promoter and open reading frame.
We sequenced the prfA promoter regions and prfA open reading frame for a subset of natural isolates (N1-047, N1-304, R2-006, R2-319, X1-004, and X1-005) spanning different genetic lineages, ribotypes, and cytotoxicity assay and hemolysis assay phenotypes. No polymorphisms were found in the prfA promoter regions of the natural isolates compared to prfA promoter sequences found in GenBank (accession numbers X61210, AJ002742, and M55160), and no unique, lineage-independent nonsynonymous substitutions were found in the prfA open reading frames of the natural isolates compared to 28 partial prfA sequences (45). Upon completion of hlyA and prfA sequencing, we concluded that isolates N1-047, N1-302, and N1-304 are clones, as evidenced by their identical hlyA and prfA sequences, their similar cell culture and hemolysis assay phenotypes, their identical ribotypes, and their similar source of isolation; therefore, only one of the three, isolate N1-047, was used for the remainder of the study.
TaqMan RT-PCR.
We utilized TaqMan quantitative RT-PCR to determine the amounts of hlyA and prfA transcripts produced by selected isolates (Table 3). Among the five noncytotoxic and nonhemolytic natural isolates that were assayed for hlyA transcription, three isolates, N1-047, R2-319, and X1-004, had average levels (n = 2) of hlyA transcript that were ≤20% of that found in the wild-type control strain 10403S (2%, 20%, and 1%, respectively), while two isolates, R2-394 and W1-038, had average hlyA transcript levels that were 750% and 500%, respectively, the level of wild type. The two natural isolates that were fully cytotoxic in the LDH release cytotoxicity assay but had greater than wild-type hemolytic activity (R2-006 and X1-005) also expressed higher than wild-type levels of hlyA transcript, 650% and 7000%, respectively. Finally, the two isolates that were fully cytotoxic and had wild-type hemolytic activity, R2-303 and X1-002, also produced wild-type levels of hlyA transcript (94% and 130%, respectively). TaqMan RT-PCR analysis of prfA transcript levels showed that both N1-047 and X1-005 expressed prfA at greater than wild-type levels, 560% and 3,900%, respectively (average of two experiments), even though they showed under- and overexpression of hlyA, respectively. Preliminary results also indicated that isolates R2-006 (an hlyA overexpresser), R2-319 (an hlyA underexpresser), and X1-004 (an hlyA underexpresser) have prfA expression levels that are similar or higher than those of the wild type; TaqMan data for isolates R2-006, R2-319, and X1-004 are preliminary because the prfA TaqMan primers and probes were designed using a lineage II strain sequence and, thus, did not perform optimally with these isolates, which belong to lineages I and III. Our prfA TaqMan RT-PCR data, combined with our hlyA and prfA sequencing results, suggest defects in PrfA activation rather than defects in prfA transcription or PrfA protein function. Due to the complex and multifactorial nature of PrfA activation and regulation, we did not pursue defining these defects.
Immunoblot analysis.
We performed immunoblotting to confirm that the levels of secreted LLO protein were proportional to the levels of hlyA transcript determined by TaqMan RT-PCR (Fig. 4). Visual inspection of the blot confirmed that those isolates that expressed low levels of hlyA (N1-047, R2-319, and X1-004) also failed to secrete detectable levels of LLO, while those isolates that expressed the highest levels of hlyA (R2-006 and X1-005) also secreted high levels of LLO. Those isolates that expressed wild-type levels of hlyA had bands that were similar in size and intensity to those of the 10403S wild-type control, with the exception of R2-394, which produced a band fainter than the 10403S band in this blot but similar in intensity to the 10403S band in other blots. Finally, we did not detect a band for isolate W1-038, indicating that isolate W1-038 either fails to secrete detectable levels of LLO or produces an incomplete protein (due to the stop codon after residue 378) that is degraded intracellularly or that cannot be detected by our monoclonal antibody.
FIG. 4.
Immunoblot of LLO. Total protein from the supernatants of L. monocytogenes cultures grown for 5 h in LB medium plus salts was precipitated and run on a 10% Bis-Tris polyacrylamide gel. Protein was blotted onto a nitrocellulose membrane and probed with anti-LLO monoclonal antibody.
Classification of naturally attenuated L. monocytogenes isolates into distinct attenuation groups.
On the basis of the results of the cell culture, hemolysis, and expression assays as well as the hlyA and prfA sequencing data, we classified the naturally virulence-attenuated isolates into four groups (Table 3). Group A isolates formed no plaques, were noncytotoxic and nonhemolytic, and underexpressed hlyA. Group B isolates also formed no plaques, were noncytotoxic and nonhemolytic, but showed hlyA transcript levels comparable to (or slightly higher than) those observed for the wild-type strain 10403S. Group C included a single isolate, which showed reduced plaque size, was fully cytotoxic, and had higher than wild-type hemolytic activity and hlyA transcript levels. Group D included isolates that were characterized by normal cytotoxicity, hemolytic activity, and hlyA expression but reduced intracellular growth. Isolate R2-006 was placed in group D instead of in group C, because even though it showed a higher expression of hlyA and greater hemolytic activity than the control strain 10403S, it did not grow nearly as well as 10403S in the intracellular growth assay.
DISCUSSION
Driven by the hypothesis that virulence attenuation in natural food and environmental isolates might be at least partially due to defects in one or more of the widely recognized L. monocytogenes virulence genes, we developed and applied a combination of cell culture, gene expression, and other in vitro assays to identify and characterize naturally virulence-attenuated isolates and to define target gene(s) within those isolates that might be responsible for their virulence-attenuated phenotypes. Specifically, our approach was to compare the phenotypes of a series of defined virulence gene mutants to the phenotypes of 11 naturally virulence-attenuated L. monocytogenes isolates in several in vitro assays to identify candidate genes and then to further characterize the genes most likely responsible for virulence attenuation using molecular methods, such as DNA sequencing and quantitative RT-PCR. Our data show that the combination of cell culture assays and molecular techniques used in this study allows for identification and characterization of naturally virulence-attenuated isolates and that multiple distinct genetic mechanisms are responsible for natural virulence attenuation in L. monocytogenes.
Combination of tissue culture assays allows for identification and characterization of naturally virulence-attenuated L. monocytogenes.
A plaque assay that has been used extensively to characterize L. monocytogenes virulence (14, 18, 24, 31) was used to initially characterize the 11 naturally virulence-attenuated isolates included in this study and to phenotypically compare them to defined virulence gene mutants. The plaque sizes we observed for the defined virulence gene mutants were all very similar to the sizes observed in other labs for the same virulence gene mutants (14, 23, 40), which indicates that the plaque assay is a highly reproducible means of characterizing L. monocytogenes virulence. We also showed that, relative to other in vitro assays for virulence characterization, the plaque assay is more sensitive. For example, the plaque assay, but not the LDH release cytotoxicity assay or the semiquantitative hemolysis assay, was able to discriminate between the wild type and the Δmpl, ΔplcB, and ΔplcAB defined mutants. Despite its advantages of sensitivity and reproducibility, the plaque assay has several major drawbacks; it takes up to 5 days to perform, it works with only a limited number of host cell types (i.e., L2 or Henle), and it often fails to yield reproducible invasiveness data, as indicated by highly variable CFU/PFU ratios for the same strain. The plaque assay, therefore, is not ideal for high-throughput virulence characterization applications and may not adequately extend to the identification of isolates with reduced pathogenic potential in clinically relevant human cells.
Due to the disadvantages of the plaque assay, we were interested in developing and applying an assay that would more rapidly characterize the virulence of L. monocytogenes isolates and that could be used with a wider variety of host cell types, including human cell lines. A cytotoxicity assay based on the spectrophotometric measurement of LDH release from infected host cells has been used successfully to quantify L. monocytogenes cytopathogenicity (12). In our study, the LDH release cytotoxicity assay was less discriminatory among the defined virulence gene mutants than the plaque assay was. While mutants, such as ΔhlyA and ΔprfA mutants, were still identifiable by their extremely low cytotoxicities, the remaining defined virulence gene mutants, including the ΔactA mutant, did not show reduced cytotoxicities. While the cytotoxicity assay can be completed in 1 day or less (5), its current insensitivity with regard to the identification of strains with a moderately virulence-attenuated phenotype (such as an Δmpl mutant) indicates that it needs further development to be a high-throughput quantitative virulence characterization assay and to be a useful screen of virulence attenuation. Preliminary work in our lab to optimize the LDH release cytotoxicity assay shows promise. For example, an ΔactA mutant can be identified after 24 h by reducing the MOI from 100:1 to 10:1 and adding 50 μg/ml gentamicin 1 hour postinfection, while an ΔinlAB mutant can be detected after 6 h, using a MOI of 100:1 and adding 50 μg/ml gentamicin 1 hour postinfection. Despite its limitations, the LDH release cytotoxicity assay allowed us to define two distinct phenotypic categories among the natural isolates tested; while one set of isolates was fully cytotoxic (at or above 100% cytotoxicity), another set of isolates showed considerably reduced cytotoxicity values (below 25%).
Natural virulence attenuation in L. monocytogenes is caused by multiple genetic mechanisms.
On the basis of tissue culture assay results, we were able to classify the 11 naturally virulence-attenuated L. monocytogenes isolates into distinct phenotypic categories. For example, 8 of 11 of the natural isolates had the same phenotypes as the defined ΔhlyA and the ΔprfA mutants in both the plaque assay and LDH release cytotoxicity assay, allowing us to hypothesize that defects in either hlyA or prfA expression or in the function of the respective proteins were the cause of the virulence-attenuated phenotypes in these strains. Use of a combination of additional in vitro assays and molecular tools allowed us to test this hypothesis and to classify virulence-attenuated strains into four groups, which are characterized by distinct virulence-attenuating mechanisms.
A common cause of virulence attenuation among the natural isolates could be traced to the expression of hlyA. As determined by TaqMan quantitative RT-PCR, isolates in group A underexpressed hlyA compared to the wild-type control, while the single group C isolate, X1-005, significantly overexpressed hlyA. The phenotypes of the group A and C isolates in the LDH release cytotoxicity assay, semiquantitative hemolysis assay, and LLO immunoblot analysis were consistent with the observed hlyA expression patterns. Specifically, group A isolates were noncytotoxic and nonhemolytic and failed to form plaques because they do not produce sufficient quantities of LLO, which, as has been shown previously, is required for escape from the vacuole upon invasion of a host cell and is essential for virulence (3, 41). The group C isolate, on the other hand, had wild-type cytotoxicity in J774 cells, as well as higher than wild-type hemolytic activity, indicating that it overexpresses the pore-forming, membrane-damaging protein LLO. Several studies have indicated that increased LLO in the host cell cytoplasm, caused either by mutations in the PEST-like sequence that result in a failure of LLO to be targeted for degradation by the proteosome (12) or by mutations that alter LLO's pH optimum and make the protein more active in the cytoplasm (17), results in virulence attenuation due to damage to the host cell membrane. The host cell membrane damage caused by hlyA overexpression likely exposes the bacterium to the extracellular milieu, which results in smaller plaque formation in the plaque assay and a virulence-attenuated phenotype in an animal infection. Unfortunately, it remains unclear why hlyA is being under- or overexpressed in the group A and C isolates, respectively. Sequencing of the hlyA promoter regions of these strains revealed no polymorphisms or other features that would easily suggest altered gene expression. The group C isolate X1-005 may be overexpressing hlyA because it overexpresses prfA, which encodes the positive regulatory factor PrfA. However, when the group A and C isolates' prfA sequences were compared to an alignment of the prfA sequences available in GenBank, no polymorphisms were identified in the prfA promoter region to suggest altered gene expression, and no nonsynonymous substitutions were found in the prfA coding domain region to suggest altered PrfA activity.
The isolates in group B were similar to group A isolates in that they were noncytotoxic and nonhemolytic. Unlike group A isolates, however, group B isolates had hlyA expression levels that were relatively similar to those of the wild-type control strain. Sequencing of the hlyA open reading frames in the group B isolates revealed a single-base-pair deletion, which results in a frameshift mutation and likely a listeriolysin O protein that is truncated and nonfunctional in isolate W1-038, and a unique glutamine-to-lysine substitution at residue 253 in isolate R2-394. Complementation or site-directed mutagenesis experiments, however, will be necessary to prove that this substitution indeed leads to a nonfunctional listeriolysin O. However, other groups have reported that single-residue changes in LLO can reduce in vitro hemolytic activity as well as reduce virulence in vivo. Specifically, Michel et al. observed decreases in the hemolytic activity of culture supernatants from strains with C484A, C484S, W491A, and W492A substitutions in LLO (28), while Glomski et al. reported a single amino acid substitution, L461T, that results in a LLO molecule more active at neutral pH and, thus, more facile at permeabilizing infected cells (17). To our knowledge, however, no group has ever reported in vitro or in vivo virulence attenuation resulting from the substitutions at residue 253.
A final category of virulence-attenuated strains identified in our study includes those isolates that exhibited wild-type cytotoxicity in the J774 LDH release cytotoxicity assay, normal hemolytic activity, and wild-type expression of hlyA (group D), and thus, appear virulence attenuated due to mechanisms unrelated to LLO expression or activity. Interestingly, the one human isolate that was characterized as naturally virulence attenuated was classified into group D. While it is conceivable that even virulence-attenuated L. monocytogenes could cause human disease in severely immunocompromised patients, unfortunately, no information on the clinical history of this case was available. Another possible explanation for the isolation of a virulence-attenuated L. monocytogenes from a human with symptoms consistent with listeriosis could include coinfection with a virulent isolate or loss of virulence during laboratory passage. Alternatively, the reduced plaquing efficiency and the low intracellular bacterial number observed in L cells and J774 cells, both of which are murine cell lines, could also indicate reduced invasion and/or growth capabilities specifically in murine cells. While we were not able to definitively define the mechanism(s) of virulence attenuation in the group D isolates, our phenotypic data suggest several likely mechanisms. First, the group D phenotypes could be caused by general growth defects that impair intracellular growth, but not extracellular growth (such as a mutation in the glucose-6-phosphate translocase homolog, hpt [11], or the transposon mutant DP-l793 described by Sun et al. [40]); second, there could be defects in other virulence genes, not included among our set of defined virulence gene mutants; third, the virulence-attenuated phenotypes could be due to partial defects (reduced expression or activity) in one or more of the well-known virulence genes, which the assays and experimental protocols used in our study did not identify. One likely genetic mechanism that could explain the reduced intracellular growth and small- to no-plaque phenotype of the group D isolates is suboptimal functioning of the actin-polymerizing protein, ActA, which plays a key role in cell-to-cell spread. Indeed, unpublished data from our lab (A. Roberts and M. Wiedmann) indicate that replacing the X1-002 actA allele with the allele from strain 10403S restores wild-type-sized plaques.
While a variety of previous reports have indicated that L. monocytogenes can be separated into three genetic lineages which may differ in their ability to cause human and animal disease (43), classification into the four distinct virulence attenuation groups was entirely independent of lineage and subtype classification. For example, attenuation groups A and D both included isolates representing all three previously described lineages (I, II, and III), indicating that virulence attenuation likely resulted from distinct evolutionary events. While lineage II isolates have previously been hypothesized to have reduced virulence compared to lineage I isolates, as supported by epidemiological evidence and lower than average plaque size in L cells (18, 31, 43), our results show that high levels of virulence attenuation occur independent of lineage. L. monocytogenes thus may be divided into at least three distinct virulence categories, including (i) highly virulence-attenuated strains, which occur across different phylogenetic lineages, (ii) strains with reduced human virulence, which may be predominant among lineage II, and (iii) highly virulent strains with a predisposition of causing human listeriosis cases and outbreaks, which may be predominant among lineage I (i.e., serotypes 1/2b and 4b) strains (18, 29, 31, 43, 44). Highly virulence-attenuated strains appear to represent approximately 3 to 5% of isolates from foods and food-processing environments, as Gray et al. (18) identified three highly virulence-attenuated isolate among 91 food isolates and as Norton et al. (31) identified two independent highly virulence-attenuated isolates among 47 isolates. Rapid methods to identify these virulence-attenuated isolates are thus of considerable importance for the food industry and regulatory agencies.
Conclusions.
While the majority of naturally virulence-attenuated L. monocytogenes isolates characterized in this study showed defects in expression or activity of listeriolysin O, our data also showed that a variety of different specific mechanisms and independent genetic events are responsible for natural virulence attenuation in L. monocytogenes. It is therefore unlikely that a single molecular, cell culture, or animal assay would be able to detect all virulence-attenuated isolates. Rather, it is a combination of cell culture and molecular screening assays that offers the best chance of most accurately and reliably identifying naturally virulence-attenuated L. monocytogenes isolates. The observation that most highly virulence-attenuated isolates show defects in expression or function of LLO may indicate that, in some environments, there is no selective pressure to maintain LLO function, while the fact that no isolates showed phenotypic characteristics typical for complete null mutations in actA may indicate selection for maintenance of functional ActA even in environments where there may be no selection for maintenance of functional LLO. Further studies on the role of ActA in different host species may thus help to develop a better understanding of the role of L. monocytogenes virulence genes in nonmammalian hosts.
Acknowledgments
We thank our colleagues for their assistance: K. Nightingale for statistical analysis; K. Windham, T. Cargioli, and C. Bolger for DNA sequencing; P. Cossart for the gift of antibody B3-19; and M. Gray for reviewing the manuscript. Strains DP-L1465, DP-L2161, DP-L1552, DP-L1935, DP-L1936, and DP-L1075 were gifts from D. Portnoy, while strains EGD, WL-111, WL-112, and A76 were gifts from M. Kuhn.
This work was supported by the National Institutes of Health award R01GM63259 (to M.W.).
REFERENCES
- 1.Barbour, A. H., A. Rampling, and C. E. Hormaeche. 1996. Comparison of the infectivity of isolates of Listeria monocytogenes following intragastric and intravenous inoculation in mice. Microb. Pathog. 20:247-253. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, A. H., A. Rampling, and C. E. Hormaeche. 2001. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infect. Immun. 69:4657-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, R. A., H. G. Bouwer, D. A. Portnoy, and D. J. Hinrichs. 1992. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 60:1625-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhunia, A., and D. Westbrook. 1998. Alkaline phosphatase release assay to determine cytotoxicity for Listeria species. Lett. Appl. Microbiol. 26:305-310. [DOI] [PubMed] [Google Scholar]
- 5.Bhunia, A. K., P. J. Steele, D. G. Westbrook, L. A. Bly, T. P. Maloney, and M. G. Johnson. 1994. A six-hour in vitro virulence assay for Listeria monocytogenes using myeloma and hybridoma cells from murine and human sources. Microb. Pathog. 16:99-110. [DOI] [PubMed] [Google Scholar]
- 6.Brosch, R., B. Catimel, G. Milon, C. Buchrieser, E. Vindel, and J. Rocourt. 1993. Virulence heterogeneity of Listeria monocytogenes strains from various sources (food, human, animal) in immunocompetent mice and its association with typing characteristics. J. Food. Prot. 56:296-301, 312. [DOI] [PubMed] [Google Scholar]
- 7.Cai, S., D. Y. Kabuki, A. Y. Kuaye, T. G. Cargioli, M. S. Chung, R. Nielsen, and M. Wiedmann. 2002. Rational design of DNA sequence-based strategies for subtyping Listeria monocytogenes. J. Clin. Microbiol. 40:3319-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Call, D. R., M. K. Borucki, and T. E. Besser. 2003. Mixed-genome microarrays reveal multiple serotype and lineage-specific differences among strains of Listeria monocytogenes. J. Clin. Microbiol. 41:632-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty, T., F. Ebel, J. Wehland, J. Dufrenne, and S. Notermans. 1994. Naturally occurring virulence-attenuated isolates of Listeria monocytogenes capable of inducing long term protection against infection by virulent strains of homologous and heterologous serotypes. FEMS Immunol. Med. Microbiol. 10:1-9. [DOI] [PubMed] [Google Scholar]
- 11.Chico-Calero, I., M. Suarez, B. Gonzalez-Zorn, M. Scortti, J. Slaghuis, W. Goebel, and J. A. Vazquez-Boland. 2002. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc. Natl. Acad. Sci. USA 99:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decatur, A. L., and D. A. Portnoy. 2000. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science 290:992-995. [DOI] [PubMed] [Google Scholar]
- 13.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freitag, N. E., L. Rong, and D. A. Portnoy. 1993. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 61:2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaillard, J. L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1989. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 261:1313-1320. [PubMed] [Google Scholar]
- 17.Glomski, I. J., M. M. Gedde, A. W. Tsang, J. A. Swanson, and D. A. Portnoy. 2002. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 156:1029-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray, M. J., R. N. Zadoks, M. M. Roma, E. D. Fortes, B. Dogan, S. Cai, Y. Chen, V. N. Scott, D. E. Gombas, K. J. Boor, and M. Wiedmann. 2004. Food and human isolates of Listeria monocytogenes form distinct but overlapping populations. Appl. Environ. Microbiol. 70:5833-5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greiffenberg, L., Z. Sokolovic, H. J. Schnittler, A. Spory, R. Bockmann, W. Goebel, and M. Kuhn. 1997. Listeria monocytogenes-infected human umbilical vein endothelial cells: internalin-independent invasion, intracellular growth, movement, and host cell responses. FEMS Microbiol. Lett. 157:163-170. [DOI] [PubMed] [Google Scholar]
- 20.Jones, S., and D. A. Portnoy. 1994. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62:5608-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leimeister-Wachter, M., W. Goebel, and T. Chakraborty. 1989. Mutations affecting hemolysin production in Listeria monocytogenes located outside the listeriolysin gene. FEMS Microbiol. Lett. 53:23-29. [DOI] [PubMed] [Google Scholar]
- 23.Leimeister-Wachter, M., C. Haffner, E. Domann, W. Goebel, and T. Chakraborty. 1990. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 87:8336-8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquis, H., H. Goldfine, and D. A. Portnoy. 1997. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J. Cell Biol. 137:1381-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLauchlin, J. 1990. Distribution of serovars of Listeria monocytogenes isolated from different categories of patients with listeriosis. Eur. J. Clin. Microbiol. Infect. Dis. 9:210-213. [DOI] [PubMed] [Google Scholar]
- 26.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, et al. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mengaud, J., M. F. Vicente, and P. Cossart. 1989. Transcriptional mapping and nucleotide sequence of the Listeria monocytogenes hlyA region reveal structural features that may be involved in regulation. Infect. Immun. 57:3695-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel, E., K. A. Reich, R. Favier, P. Berche, and P. Cossart. 1990. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol. Microbiol. 4:2167-2178. [DOI] [PubMed] [Google Scholar]
- 29.Nadon, C. A., D. L. Woodward, C. Young, F. G. Rodgers, and M. Wiedmann. 2001. Correlations between molecular subtyping and serotyping of Listeria monocytogenes. J. Clin. Microbiol. 39:2704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nato, F., K. Reich, S. Lhopital, S. Rouyre, C. Geoffroy, J. C. Mazie, and P. Cossart. 1991. Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect. Immun. 59:4641-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norton, D. M., J. M. Scarlett, K. Horton, D. Sue, J. Thimothe, K. J. Boor, and M. Wiedmann. 2001. Characterization and pathogenic potential of Listeria monocytogenes isolates from the smoked fish industry. Appl. Environ. Microbiol. 67:646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pine, L., S. Kathariou, F. Quinn, V. George, J. Wenger, and R. E. Weaver. 1991. Cytopathogenic effects in enterocytelike Caco-2 cells differentiate virulent from avirulent Listeria strains. J. Clin. Microbiol. 29:990-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portnoy, D. A., P. Jacks, and D. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premaratne, R. J., W. J. Lin, and E. A. Johnson. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roche, S. M., P. Gracieux, I. Albert, M. Gouali, C. Jacquet, P. M. Martin, and P. Velge. 2003. Experimental validation of low virulence in field strains of Listeria monocytogenes. Infect. Immun. 71:3429-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz, B., D. Hexter, C. V. Broome, A. Hightower, R. Hirschhorn, J. Porter, P. Hayes, W. Bibb, B. Lorber, and D. Faris. 1989. Investigation of an outbreak of listeriosis: new hypotheses for the etiology of epidemic Listeria monocytogenes infections. J. Infect. Dis. 159:680-685. [DOI] [PubMed] [Google Scholar]
- 37.Shetron-Rama, L. M., H. Marquis, H. G. Bouwer, and N. E. Freitag. 2002. Intracellular induction of Listeria monocytogenes actA expression. Infect. Immun. 70:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, G. A., H. Marquis, S. Jones, N. C. Johnston, D. A. Portnoy, and H. Goldfine. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63:4231-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sue, D., D. Fink, M. Wiedmann, and K. J. Boor. 2004. σB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology 150:3843-3855. [DOI] [PubMed] [Google Scholar]
- 40.Sun, A., A. Camilli, and D. A. Portnoy. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Langendonck, N., E. Bottreau, S. Bailly, M. Tabouret, J. Marly, P. Pardon, and P. Velge. 1998. Tissue culture assays using Caco-2 cell line differentiates virulent from non-virulent Listeria monocytogenes strains. J. Appl. Microbiol. 85:337-346. [DOI] [PubMed] [Google Scholar]
- 43.Wiedmann, M. 2002. Molecular subtyping methods for Listeria monocytogenes. J. AOAC Int. 85:524-531. [PubMed] [Google Scholar]
- 44.Wiedmann, M., J. Bruce, C. Keating, A. Johnson, P. McDonough, and C. Batt. 1997. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 65:2707-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, W., B. M. Jayarao, and S. J. Knabel. 2004. Multi-virulence-locus sequence typing of Listeria monocytogenes. Appl. Environ. Microbiol. 70:913-920. [DOI] [PMC free article] [PubMed] [Google Scholar]