Abstract
Biocontrol agents generally do not perform well enough under field conditions to compete with chemical fungicides. We determined whether transgenic strain SJ3-4 of Trichoderma atroviride, which expresses the Aspergillus niger glucose oxidase-encoding gene, goxA, under a homologous chitinase (nag1) promoter had increased capabilities as a fungal biocontrol agent. The transgenic strain differed only slightly from the wild-type in sporulation or the growth rate. goxA expression occurred immediately after contact with the plant pathogen, and the glucose oxidase formed was secreted. SJ3-4 had significantly less N-acetylglucosaminidase and endochitinase activities than its nontransformed parent. Glucose oxidase-containing culture filtrates exhibited threefold-greater inhibition of germination of spores of Botrytis cinerea. The transgenic strain also more quickly overgrew and lysed the plant pathogens Rhizoctonia solani and Pythium ultimum. In planta, SJ3-4 had no detectable improved effect against low inoculum levels of these pathogens. Beans planted in heavily infested soil and treated with conidia of the transgenic Trichoderma strain germinated, but beans treated with wild-type spores did not germinate. SJ3-4 also was more effective in inducing systemic resistance in plants. Beans with SJ3-4 root protection were highly resistant to leaf lesions caused by the foliar pathogen B. cinerea. This work demonstrates that heterologous genes driven by pathogen-inducible promoters can increase the biocontrol and systemic resistance-inducing properties of fungal biocontrol agents, such as Trichoderma spp., and that these microbes can be used as vectors to provide plants with useful molecules (e.g., glucose oxidase) that can increase their resistance to pathogens.
Trichoderma atroviride is a filamentous soil fungus that functions as a biocontrol agent for a wide range of economically important aerial and soilborne plant pathogens (5, 28). The mycoparasitic activity of this organism is attributed to a combination of successful nutrient competition (5), the production of cell wall-degrading enzymes (32), and antibiosis (11, 30). Several strains of the genus Trichoderma are being tested as alternatives to chemical fungicides (12). However, full-scale application of Trichoderma for biological control of plant pathogens has not been widespread. At a molecular genetic level, attempts to increase the biocontrol ability of Trichoderma have been focused on increasing chitinase or proteinase activity either by increasing the number of copies of the appropriate genes or by fusing them with strong promoters (e.g., pcbh1::ech42). These strategies have not always resulted in the expected increase in biocontrol activity (20, 24).
A different strategy for biocontrol is used by Talaromyces flavus, a potential biocontrol agent for the plant pathogens Verticillium dahliae (25), Sclerotinia sclerotiorum (26), and Rhizoctonia solani (3). In vitro experiments performed with culture filtrates of T. flavus suggested that glucose oxidase is responsible for most of the growth inhibition of V. dahliae microsclerotia and hyphae (27, 31). A glucose oxidase-deficient strain of T. flavus also failed to antagonize Verticillium wilt of eggplant in greenhouse experiments (8). Glucose oxidase catalyzes the oxygen-dependent oxidation of d-glucose to d-glucono-1,5-lactone and H2O2. Glucose oxidase, glucose, and gluconate (which is spontaneously formed from d-glucono-1,5-lactone in aqueous solutions) do not inhibit V. dahliae when they are used individually (18), but low concentrations of H2O2 significantly inhibit the growth of Pythium ultimum, Pythium aphanidermatum, R. solani, and V. dahliae. Therefore, the antifungal effect of the glucose oxidase system is due to increased levels of H2O2 (17).
Several species of Trichoderma are more resistant to the products of glucose oxidase activity than the plant pathogens mentioned above (18), although Trichoderma does not have a glucose oxidase ortholog (23). The availability of a versatile expression system for T. atroviride based on application of recently characterized biocontrol-related promoters (23, 36) suggested that it might be possible to improve the disease control effect of this biocontrol agent. We used a transgenic progeny of T. atroviride strain P1, which contains 12 to 14 copies of the Aspergillus niger goxA (glucose oxidase) gene under the nag1 (N-acetyl-β-d-glucosaminidase) promoter, to demonstrate in vivo a novel method for improving the ability of this fungus to directly inhibit phytopathogens and to induce systemic resistance in plants.
MATERIALS AND METHODS
Strains.
T. atroviride strain P1 (“Trichoderma harzianum” ATCC 74058) was used throughout this study and was maintained on potato dextrose agar (PDA) (Merck, Darmstadt, Germany). The glucose oxidase-producing strain used in these experiments (T. atroviride SJ3-4) has in general been described previously and was shown to produce the highest glucose oxidase activity of all strains tested (23). Botrytis cinerea strain 26 was cultivated on malt extract peptone agar (3% malt extract, 1% peptone, 1.5% agar), and R. solani strain 19 and P. ultimum strain 8 were grown on potato dextrose agar. Botrytis, Rhizoctonia, and Pythium strains were obtained from the collection of the Institute of Plant Pathology, Università degli Studi di Napoli “Federico II” (Naples, Italy).
Soil.
We used commercial peat soil (60% light peat and 40% heavy dark peat, partially decomposed) sold by Klasmann-Deilmann (Geeste-Gross Hesepe, Germany); the pH was 4.5.
Cultivation conditions.
T. atroviride strains were grown in liquid synthetic medium (SM) containing (per liter) 2 g of KH2PO4, 1.4 g of (NH4)2SO4, 0.3 g of CaCl2 · 2H2O, 0.3 g of MgSO4 · 7H2O, 0.6 g of urea, 10 mg of FeSO4 · 7H2O, 2.8 g of ZnSO4 · 2H2O, and 3.2 g of CoCl2 · 6H2O (pH 5.4) and supplemented with either glucose or glycerol as a carbon source (15 g/liter, unless indicated otherwise).
Cell wall-degrading enzyme activities.
T. atroviride was precultivated in shake flasks (250 rpm) in potato dextrose broth (PDB) (Merck) for 48 h at 25°C, harvested by filtration through Miracloth (Calbiochem, La Jolla, CA), washed with sterile tap water, and transferred to SM containing either 1.5% (wt/vol) glucose or colloidal chitin. After 3 days culture filtrates were obtained following filtration through a 0.22-μm filter. The culture filtrate was dialyzed against 20 volumes of distilled water for 24 h at 4°C. To obtain more concentrated culture filtrates, the dialysis bags were covered with 1.5 g of polyethylene glycol 8000 (Fluka Biochemika, Buchs, Switzerland) per cm2 of dialysis tube surface and then left for 10 h at 4°C, which resulted in a 20-fold-concentrated culture filtrate. The concentrated culture filtrates were stored at −20°C with 20% (vol/vol) (final concentration) glycerol until use. Enzyme assays were performed as described previously (18) with p-nitrophenyl N-acetyl-β-d-glucosaminidine as the substrate for N-acetyl-β-d-glucosaminidase, p-nitrophenyl β-d-N,N′-diacetylchitobiose as the substrate for 1,4-β-chitobiosidase, and p-nitrophenyl β-d-N′,N"-triacetylchitotriose as the substrate for endochitinase (all substrates were obtained from Sigma-Aldrich, Traufkirchen, Germany).
Determination of glucose oxidase activity.
Culture filtrates were prepared by using essentially the same growth conditions described above. Crude culture supernatants were tested for glucose oxidase activity as described previously (9, 23). For determination of glucose oxidase activity produced during plate confrontation assays with B. cinerea, the plates contained (per liter) 10 g of glucose, 6 g of (NH4)2SO4, 1 g of K2HPO4, 0.5 g of MgSO4 · 7H2O, 0.5 g of KCl, 15 g of agar, and the following trace elements: FeSO4 · 7H2O (10 mg), ZnSO4 · 2H2O (2.8 mg), and CoCl2 · 6H2O (3.2 mg). The plates also contained the pH indicator methyl red (10 mg/liter). Disks (diameter, 3 mm) of Botrytis and Trichoderma were placed on the plates 1.5 cm from each other and incubated in the dark at 25°C for 32 h. We measured the formation of red color due to a pH shift caused by the oxidation of glucose to gluconate.
Biocontrol assays.
In vitro B. cinerea spore germination inhibition was tested in 96-well microtiter plates essentially as previously described (21). Briefly, a suspension of 3 × 103 Botrytis spores and 50 μl of PDB with 5 mM potassium phosphate buffer (pH 6.7) were placed in a well of a microtiter plate, and 10-μl portions of the 20-fold-concentrated culture supernatants of strain P1 and SJ3-4 grown on colloidal chitin were added. Addition of 100 mM H2O2 instead of culture filtrates was used as a control. The number of germinated spores was counted after 8 h of incubation.
For plate confrontation assays, 5-mm disks containing T. atroviride, R. solani, and P. ultimum were placed on PDA 4 cm apart. The plates were incubated at 25°C for 4 days in the absence of light.
To test the germination of bean seeds (Phaseolus vulgaris cv. Borlotto) in pathogen-infested soil, the seeds were coated with a 10% (wt/vol) suspension of Pelgel (Liphatech, Milwaukee, WI) in 20 mM potassium phosphate buffer (pH 6.7) containing 20 mM glucose. One milliliter of a Trichoderma conidial suspension containing 1 × 108 conidia/ml was used for coating 10 g of seeds. As a control, the same suspension without Trichoderma was used.
Pathogen-infested soil was prepared by inoculating 500 ml of PDB with R. solani mycelium from a 4-day-old 8-cm PDA plate. Two grams (wet weight) of the resulting biomass was used to inoculate 1 liter of sterile soil. For P. ultimum, 1 liter of sterile soil was infested with the contents of four 3-day-old 8-cm plates of the pathogen homogenized in a blender for 30 s. After 2 days the infested soil was diluted 1:4 with sterile soil and used for biocontrol assays as described above. The coated seeds were planted 4 cm deep in infested soil, and the germination and growth were monitored for 3 weeks.
Tests of induced resistance.
We performed in vivo tests to determine the ability of T. atroviride SJ3-4 mutants to induce resistance in bean to the foliar pathogen B. cinerea. Bean seeds were coated with a 10% (wt/vol) aqueous suspension of Pelgel containing 1 × 108 spores/ml of wild-type T. atroviride strain P1 or transgenic strain SJ3-4 or were not treated with Trichoderma (control) and then were left in an open petri dish to air dry overnight in a laminar flow hood. Seven seeds were planted in 14-cm vases containing sterile soil (sterilized for 1 h at 122°C) at a depth of 4 cm, incubated at 25°C with light, and kept under humid conditions. In experiments with soil containing R. solani cultures, 3 g of fresh fungal biomass, grown in PDB, was homogenized in distilled water and mixed with 1 kg of sterile soil. In soil with killed R. solani cultures, the liquid cultures of the pathogen were autoclaved before use. When the plants had developed to the stage where four true leaves had emerged, a 15-μl Botrytis spore suspension (1 × 106 or 5 × 106 spores/ml) in germination buffer (20 mM glucose and 20 mM KH2PO4) was inoculated onto the leaf surface. Inoculated plants were incubated at 25°C with light in a humid chamber. After 48 h the leaves were evaluated for disease, and the diameter of each necrotic zone was measured (two perpendicular axes [diameters A and B]). After this, the plants were removed from the humid chamber and evaluated every 24 h for further Botrytis development.
Two inoculations were made per leaf on four leaves per plant for three plants per treatment and two replicates for each experiment. The experiments were repeated at two different times. The lesion size was calculated on the basis of area, as follows: area = π × (measured diameter A/2) × (measured diameter B/2), where the diameters were the treatment means for each experiment. The statistical analyses included an analysis of variance of treatment means with a significance level of P < 0.05. Unpaired t tests were conducted for the different Trichoderma seed coat treatments for each experiment (P < 0.05).
RESULTS
Physiological properties of T. atroviride transformant SJ3-4.
Recombinant strain SJ3-4, which carries 12 to 14 copies of the pnag1::goxA fusion, and wild-type strain P1 grew at similar rates on PDA, on SM containing 1% glucose, and in liquid cultures, but they had reduced growth rates on SM containing 1% N-acetylglucosamine (Table 1). Chitinolytic activity was not detected in filtrates from either Trichoderma strain growing on glucose or glycerol; however, there was clearly detectable chitinolytic enzyme activity in filtrates from both strains following transfer to a medium containing colloidal chitin as the sole carbon source. Under these conditions, SJ3-4 had 55% and 70% of the N-acetyl-β-glucosaminidase and endochitinase activities produced by the wild type (Table 2) but a similar level of chitobiosidase activity. Based on these data, we concluded that SJ3-4 does not suffer from a general reduction in viability but expresses two biocontrol-related chitinase genes at lower levels than the nontransformed parent expresses the genes.
TABLE 1.
Comparison of growth rates of T. atroviride P1 and SJ3-4
| Strain | Colony diam (mm) ona:
|
Biomass (g [dry wt]/ 100 ml PDB)b | ||
|---|---|---|---|---|
| PDA | SM + glucose | SM + NAGAd | ||
| P1 | 50 ± 6c | 32 ± 4 | 22 ± 3 | 0.8 ± 0.1 |
| SJ3-4 | 46 ± 4 | 32 ± 4 | 15 ± 3 | 0.7 ± 0.1 |
Growth on solid media.
Biomass in liquid culture
The values are means ± standard deviations for five separate experiments.
NAGA, N-acetylglucosamine.
TABLE 2.
Enzyme production by T. atroviride strains P1 and SJ3-4 on colloidal chitin
| Strain | Enzyme activities (mU/ml)a
|
|||
|---|---|---|---|---|
| Nagb | Chbc | Echd | GoxAe | |
| P1 | 290 ± 13f | 65 ± 2 | 40 ± 3 | NDg |
| SJ3-4 | 160 ± 13 | 63 ± 3 | 29 ± 2 | 300 ± 19 |
One unit was defined as the conversion of 1 μmol substrate per min. Values were normalized to the biomass production of strain P1.
Nag, N-acetyl-β-d-glucosaminidase.
Chb, 1,4-β-Chitobiosidase.
Ech, endochitinase.
GoxA, glucose oxidase.
The values are means ± standard deviations for five separate experiments.
ND, not determined.
Induction of glucose oxidase expression in SJ3-4.
We previously reported that T. atroviride P1 has no glucose oxidase activity (20). Strain SJ3-4 produced 4 ± 1 and 300 ± 19 mU/ml of glucose oxidase activity on media containing glucose and colloidal chitin, respectively. These results are consistent with previous observations that nag1 expression is induced by chitin (23, 29). The low level of glucose oxidase activity on medium containing glucose probably was due to the high number of integrated copies of the pnag1::goxA fusion (12 to 14 copies [23]) in this strain, as no such expression was measured in four other nag1::goxA transgenic strains that carried fewer copies (2 to 6 copies) (R. L. Mach, K. Payer, and S. Jaksits, unpublished data).
To prove that glucose oxidase expression was induced by direct contact with a potential host, we conducted plate confrontation assays with B. cinerea. A red halo indicative of glucose oxidase activity was observed around Trichoderma strain SJ3-4 after it contacted the host (1 to 2 h). Neither the wild type nor strains transformed with the hygromycin B resistance-conferring vector pHATα (14) exhibited such a fast pH shift. Also, SJ3-4 grown without a pathogen failed to form a red halo. General acidification leading to the occurrence of red halos was observed with all strains during later stages of biocontrol (18 to 24 h after contact). These data strongly suggest that host contact-induced nag1 gene expression and glucose oxidase production are correlated in T. atroviride strain SJ3-4.
Botrytis spore germination assay.
Culture filtrates obtained from SJ3-4 grown on colloidal chitin produced 310 ± 16 mU/ml glucose oxidase activity and showed significantly higher levels of antifungal activity (threefold-higher inhibition of B. cinerea spore germination) than similar wild-type culture filtrates. Wild-type filtrates augmented with H2O2 (8 mM [final concentration] in the assay mixture) resulted in only 27% germinated Botrytis spores, compared to 38% without H2O2. Culture filtrates denatured for 5 min at 95°C had no inhibitory effect. Addition of H2O2 (16 mM [final concentration] in the assay mixture) instead of Trichoderma culture filtrates resulted in germination of 39% of the spores. These values are relative to germination of 100% of the spores in a control assay using sterile water instead of culture filtrate.
Mycoparasitism of T. atroviride strain SJ3-4.
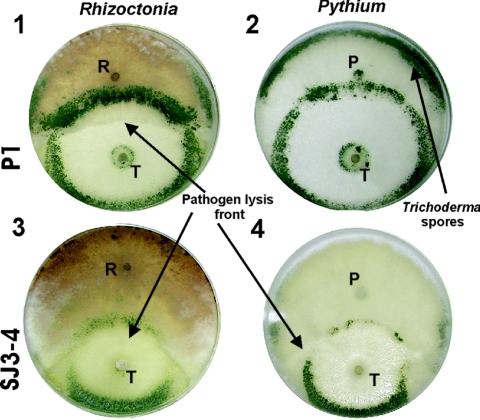
SJ3-4 had increased antagonism (host lysis) against R. solani even though there were no significant differences in the rate of growth on the host. After only 5 days, SJ3-4 began to sporulate on the opposite edge of the plate, while P1 required two more days (Fig. 1). An even stronger difference was observed during confrontation with the oomycete P. ultimum. Wild-type strain P1 had only minor mycoparasitic activity and failed to lyse the entire host even after 1 week, whereas after 7 days SJ3-4 had completely lysed the pathogen and begun to sporulate on the outer regions of the plate (Fig. 1).
FIG. 1.
Performance of T. atroviride strains P1 and SJ3-4 in plate confrontation assays on PDA with Rhizoctonia (R) and Pythium (P) as hosts. The photographs were taken 7 days after inoculation.
In planta biocontrol activity.
Bean seeds coated with conidia of either strain P1 or SJ3-4 were planted in pathogen-infested soil. Germination of the beans was monitored for 2 weeks. In soil tests with small amounts of pathogen (1 g of R. solani biomass or four homogenized plates of P. ultimum per liter of soil), the glucose oxidase-producing strain provided approximately the same level of biocontrol against R. solani and P. ultimum as the wild-type strain (data not shown). Both the number of germinated seeds and the plant height were comparable to the results of Woo et al. (32). Increasing the disease pressure by doubling the inoculum resulted in nearly complete rotting of uncoated seeds and of seeds coated with wild-type conidia. Almost all bean seeds treated with the glucose oxidase-producing strain could germinate in soil infested with large amounts of either of the two pathogens (Table 3 and Fig. 2).
TABLE 3.
In planta biocontrol assays in soil with high pathogen concentrationa
| Mycoparasite | No. of germinated seeds
|
Plant height (cm)
|
||||
|---|---|---|---|---|---|---|
| Unprotected | P1 treated | SJ3-4 treated | Unprotected | P1 treated | SJ3-4 treated | |
| Control without pathogen | 13 ± 3.0 | 13 ± 3.0 | 13 ± 3.3 | 17 ± 2.4 | 21 ± 3.1 | 22 ± 3.8 |
| Rhizoctonia | 0 | 1.3 ± 1.0 | 12 ± 2.3 | 0 | 16 ± 1.8 | 22 ± 3.0 |
| Pythium | 0 | 1.0 ± 0.7 | 12 ± 3.7 | 0 | 15 ± 2.7 | 20 ± 4.3 |
Fourteen beans were used for each of three independently repeated assays.
FIG. 2.
Biocontrol activities of T. atroviride P1 and SJ3-4 in in planta assays under high disease pressure. Beans were coated with Trichoderma strain P1 and SJ3-4 spores and were planted in Rhizoctonia-infested soil. The beans not protected by either Trichoderma strain were designated the disease pressure control (dpc). The nonpathogen control (Control) was prepared by planting seeds in sterile soil. (A) Seeds 1 week after planting; (B) plants emerged from soil 3 weeks after planting.
Induced resistance.
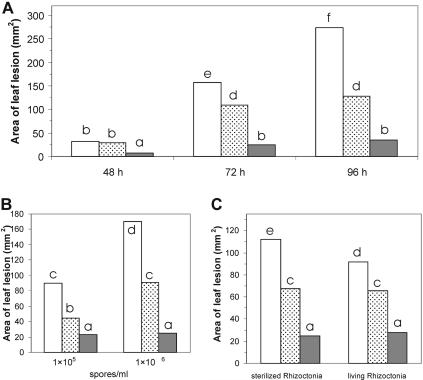
Bean plants subjected to either of the two Trichoderma seed-coating treatments had reduced leaf lesions compared to the untreated control when they were planted in sterile soil and then inoculated with B. cinerea (P < 0.001). Under these conditions, the Trichoderma SJ3-4 mutant performed significantly better than wild-type strain P1 for controlling the development of leaf lesions caused by B. cinerea (P < 0.001) (Fig. 3A). The induced resistance effect was most obvious when plants were observed 72 h after inoculation with the foliar pathogen. After 96 h, treatment comparisons were no longer possible since the disease symptoms on the controls (no seed treatment) were too extensive and the merging lesions could no longer be accurately measured. B. cinerea spores applied at concentrations of 1 × 106 and 5 × 106 spores/ml produced clear disease symptoms on bean leaves derived from nontreated and Trichoderma-treated seeds (Fig. 3B). The resistance effect induced by SJ3-4 was more pronounced at higher spore concentrations (Fig. 3B). However, in the case of high levels of Botrytis inoculum, disease development progressed too rapidly on the untreated plants, and thus the time course experiments were performed with 1 × 106 spores/ml (Fig. 3A).
FIG. 3.
Resistance induced by T. atroviride P1 and SJ3-4 in bean against disease caused by B. cinerea. Antagonists were applied as seed treatments, and B. cinerea was inoculated on the leaves of emerged plants. Disease development was determined at different times after inoculation with B. cinerea (1 × 106 spores/ml) (A), at 24 h after inoculation with different B. cinerea spore concentrations (B), and at 72 h in soil infested with autoclaved or live mycelia of R. solani (C). The mean values for lesion areas are the results for experiments performed with four leaves per plant, three plants, and two replicates. The different bars indicate the strains used for protection of beans, as follows: open bars, no Trichoderma; dotted bars, wild-type strain P1; and grey bars, SJ3-4. Bars with the same letter are not significantly different (P < 0.05).
In the absence of Trichoderma seed coating, the addition of living or sterilized mycelia of R. solani to the soil also reduced the development of B. cinerea lesions on the emerging plants relative to controls in sterile soil (P < 0.001) (Fig. 3A and C). If the seeds were coated with Trichoderma spp. and then placed in the presence of R. solani, the level of plant emergence, survival, and vigor were generally improved compared to controls (Table 3). The combination of Trichoderma and Rhizoctonia induced a higher level of resistance than the level that resulted from the application of either fungus alone (Fig. 3). In all experiments with soil containing R. solani mycelium, SJ3-4 consistently induced a higher level of resistance to B. cinerea than wild-type strain P1 induced (P < 0.001).
DISCUSSION
Glucose oxidase plays a key role in the biocontrol of Verticillium wilt by T. flavus (8). Since T. atroviride does not produce glucose oxidase, we hypothesized that (over)expression of a glucose oxidase in T. atroviride would increase its biocontrol capability. We evaluated a transgenic strain of T. atroviride (SJ3-4) that contains multiple copies of a pnag1::goxA construct. To test the influence of extracellularly secreted glucose oxidase on biocontrol, we used the strain containing the highest copy number from a set of previously constructed and described transformants (23). The nag1 promoter permits glucose oxidase production only after contact with a host fungus. Zeilinger et al. (36) reported that the nag1 gene is active after Trichoderma hyphae contact their host but that it is not expressed constitutively. However, due to the high copy number (12 to 14 copies) of goxA in SJ3-4, this strain expresses a low level of glucose oxidase constitutively (23). Attempts to constitutively express glucose oxidase under the control of the pki promoter (pyruvate kinase) failed, as no viable transformants were recovered (Mach, unpublished data). Therefore, the pnag1::goxA construct appeared to be the most desirable construct for biocontrol applications.
T. atroviride SJ3-4 grew at a rate comparable to that of the wild-type strain on solid media that do not induce the nag1 promoter and in liquid culture, proving that its general physiological characteristics had not been altered by the genomic location or expression of the pnag1::goxA inserts. However, a moderate reduction in growth was observed when the transgenic strain was grown on N-acetyl-β-d-glucosamine-containing media. The N-acetyl-β-d-glucosaminidase activity was reduced to about 55% that of the wild type during growth on colloidal chitin. This reduction may have been due to competition for transcriptional factors by the multiple copies of the nag1 promoter. The significant reduction in endochitinase activity could be explained if both the nag1 gene and the endochitinase genes have the same transcriptional regulatory factors. However, nag1 and ech42, which encodes a 42-kDa endochitinase, are not induced by the same chitooligomers (23), which argues against this hypothesis. Another possibility is that the product(s) of the enzymatic activity of N-acetyl-β-d-glucosaminidase are required for full endochitinase gene expression. Support for this hypothesis comes from our previous findings that a nag1 T. atroviride strain produces only about 1% of the wild-type endochitinase activity (4). Whatever the mode of action, chitobiosidase activity was not affected by nag1::goxA overexpression, suggesting that its gene is part of a different regulatory circuit, which is consistent with the previous results of Brunner et al. (4).
Despite the reduction in the activities of the cell wall lytic enzymes, the pnag1::goxA multicopy strain compensated for this handicap and exceeded the wild-type strain's biocontrol abilities in several standard assays; e.g., SJ3-4 culture filtrates had a threefold-greater inhibitory effect in a Botrytis spore germination assay. In plate confrontation assays the transgenic strain also performed better than the wild type in terms of overgrowth and lysis of Rhizoctonia. Although glucose is known to be a repressor of nag1 induction by N-acetylglucosamine (29), the viable host hyphae induced the nag1 promoter even on media containing glucose. This result is consistent with the results of Zeilinger et al. (36), who used pnag1::gfp reporter strains to demonstrate induction of nag1 after contact with Rhizoctonia on synthetic media containing 1% glucose.
SJ3-4 was clearly superior to the wild type in confrontation assays with Pythium. The wild-type strain only moderately controlled this host, but SJ3-4 overgrew the entire plate and lysed the Pythium cells. The difference between the two strains could be due to the lack of chitin in the Pythium cell wall and the resulting insensitivity of this host to chitinase and to an increased sensitivity of Pythium to H2O2 (18). Apparently, the nag1 promoter may be induced by contact with a host that lacks chitin in its cell wall, which is consistent with the findings of Inglis and Kawchuk (16), who showed that T. harzianum produced various chitinases when it was cultivated on Pythium cell walls. In contrast, this chitinase promoter was not induced by sophorose, a strong inducer of T. reesei cellulases, cellobiose, or PDB, the medium used for confrontation assays (Brunner, unpublished data). This result suggests that further work is needed to understand the induction of nag1 during mycoparasitism.
As the disease threshold in the field caused by soilborne plant pathogens is highly variable (depending on, e.g., the soil, the climate conditions, the crop species and variety, and the infesting phytopathogen [1]), the concentrations of the fungal soilborne pathogens were determined by determining the level at which 50% of the plants exhibited symptoms. This value was defined as low disease pressure. We found no difference between SJ3-4 and the wild-type strain in in planta assays under low disease pressure. At higher pathogen concentrations, however, only seeds coated with SJ3-4 germinated. The difference may be explained by the use of glucose (20 mM) in the coating procedure, which provided a carbon source for spore germination and the initial substrate for the glucose oxidase produced by SJ3-4. Once roots were formed, the glucose necessary for enhanced performance of SJ3-4 might have been available in root exudates (11), as observed for Talaromyces control of Verticillium wilt on eggplant (8, 10). Thus, the impact of glucose oxidase on fungal biocontrol efficiency depends on both the pathogen and the host plant.
Several Trichoderma species can activate systemic induced resistance in plants (7, 13, 34, 35), although this has not previously been documented for the bean-T. atroviride interaction. The addition of sterilized or living R. solani mycelia to the soil also increases resistance to B. cinerea infection of bean leaves. The simultaneous presence of R. solani in soil and T. atroviride on the seed or root surface produced the highest level of systemic resistance. This result may have occurred as a result of a direct additive effect of the two fungi on the plant or because the presence of R. solani stimulated T. atroviride to interact with the plants (e.g., by releasing resistance-inducing molecules). The mechanism of the Trichoderma spp.-plant interaction that activates the systemic plant resistance response has only recently been studied at a molecular level, and our understanding of this response currently is limited to the identification of some of the plant factors accumulated in response to root contact with this fungus (2, 15, 34, 35).
The results of the present study show that the availability of a biocontrol-related promoter sequence combined with a reliable transformation system permits genetic improvement of fungal biocontrol agents through the use of novel transgenes to enhance disease control mechanisms (22). In particular, we increased the ability of a biocontrol strain of T. atroviride to activate systemic resistance responses in the host plants by using a glucose oxidase-encoding gene obtained from another fungus (A. niger) not known as a biocontrol agent.
To our knowledge, our study was the first successful attempt to directly modify the molecular interaction between a plant and a beneficial fungus such that systemic resistance against plant pathogens was increased. The transgene that we used was selected because the encoded enzyme can produce H2O2 in the presence of glucose. Hydrogen peroxide is known to induce systemic acquired resistance in plants by inducing the expression of pathogenesis-related proteins during the oxidative burst following a pathogen attack (6, 11, 19).
Potatoes transformed with the A. niger glucose oxidase-encoding gene produced elevated levels of H2O2 following intercellular secretion of glucose oxidase and were more resistant to Erwinia carotovora and Phytophthora infestans (33). A similar result was obtained by the use of a transgenic Trichoderma strain expressing glucose oxidase in combination with its previously described (34, 35) capability to penetrate the root. The use of SJ3-4 for plant pathogen control has the advantage that H2O2 accumulation occurs only in the presence of the pathogen, due to the host-induced regulation of the nag1 promoter. This eliminates or at least strongly reduces the accumulation of H2O2 in the absence of a pathogen attack. Furthermore, the induction of systemic resistance in plants might occur before the pathogen attacks the plant roots, since the nag1 promoter could trigger glucose oxidase expression as soon as the Trichoderma hyphae contact the pathogen. The plausibility of this hypothesis is further strengthened by the increase in control at high levels of the pathogen. The observed increase in plant protection might be due more to the increased induction of systemic plant resistance than to the direct mycoparasitic abilities of the transgenic strain.
In conclusion, we genetically engineered and characterized a novel biocontrol strain of Trichoderma. We demonstrated that the transgenic use of biocontrol-related promoters associated with an appropriately selected heterologous gene is a powerful method to improve both biocontrol and the ability of soilborne fungal biocontrol agents to systemically induce disease resistance against foliar pathogens.
Acknowledgments
This study was supported by grant AGR/PR(96)FS/A from OECD and by a grant from OEAD (Wissenschaftlich-Technische Zusammenarbeit mit Italien, project 19) to R.L.M. and by grant APART 10764 from the Austrian Academy of Science and by grant P15483 from the Fonds zur Förderung Wissenschaftlicher Forschung to S.Z.
We thank A. Herrera-Estrella for providing pHATα.
REFERENCES
- 1.Agrios, G. N. 1997. Plant pathology, 4th ed. P. 635-646. Academic Press, San Diego CA., USA.
- 2.Ahmed, A. S., C. P. Sanchez, and M. E. Candela. 2000. Evaluation of induction of systemic resistance in pepper plants (Capsicum annuum) to Phytopthora capsici using Trichoderma harzianum and its relation with capsidiol accumulation. Eur. J. Plant Pathol. 106:817-824. [Google Scholar]
- 3.Boosalis, M. G. 1956. Effect of soil temperature and green-manure amendment of unsterilized soil on parasitism of Rhizoctonia solani by Penicillium vermiculatum and Trichoderma sp. Phytopathology 46:473-478. [Google Scholar]
- 4.Brunner, K., C. K. Peterbauer, R. L. Mach, M. Lorito, S. Zeilinger, and C. P. Kubicek. 2003. The Nag1 N-acetylglucosaminidase of Trichoderma atroviride is essential for chitinase induction by chitin and of major relevance to biocontrol. Curr. Genet. 43:289-295. [DOI] [PubMed] [Google Scholar]
- 5.Chet, I. 1987. Trichoderma--application, mode of action, and potential as a biocontrol agent of soilborne plant pathogenic fungi. Wiley & Sons, New York, N.Y.
- 6.Dong, X. 1998. SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Biotechnol. 1:316-323. [DOI] [PubMed] [Google Scholar]
- 7.Elad, Y. 2000. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 19:709-714. [Google Scholar]
- 8.Fravel, D. R., and D. P. Roberts. 1991. In situ evidence for the role of glucose oxidase in the biocontrol of Verticillium wilt by Talaromyces flavus. Biocontrol Sci. Technol. 1:91-99. [Google Scholar]
- 9.Geisen, R. 1995. Expression of the Aspergillus niger glucose oxidase gene in Penicillium nalgiovense. World J. Microbiol. Biotechnol. 11:322-325. [DOI] [PubMed] [Google Scholar]
- 10.Ghisalberti, E. L., and C. Y. Rowland. 1993. Antifungal metabolites from Trichoderma harzianum. J. Nat. Prod. 56:1799-1804. [DOI] [PubMed] [Google Scholar]
- 11.Grayston, S. J., D. Vaugham, and D. Jones. 1996. Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 5:29-56. [Google Scholar]
- 12.Harman, G. E., and C. P. Kubicek. 1998. Trichoderma & Gliocladium, vol. 2. Enzymes, biological control and commercial applications. Taylor & Francis Inc., Bristol, PA.
- 13.Harman, G. E., C. R. Howell, A. Viterbo, I. Chet, and M. Lorito. 2004. Trichoderma species: opportunistic, avirulent plant symbionts. Nat. Microbiol. Rev. 2:43-56. [DOI] [PubMed] [Google Scholar]
- 14.Herrera-Estrella, A., G. H. Goldman, and M. Van Montagu. 1990. High-efficiency transformation system for the biocontrol agents, Trichoderma spp. Mol. Microbiol. 4:839-843. [DOI] [PubMed] [Google Scholar]
- 15.Howell, C. R., L. E. Hanson, R. D. Stipanovic, and L. S. Puckhaber. 2000. Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathology 90:248-252. [DOI] [PubMed] [Google Scholar]
- 16.Inglis, G. D., and L. M. Kawchuk. 2002. Comparative degradation of oomycete, ascomycete, and basidiomycete cell walls by mycoparasitic biocontrol fungi. Can. J. Microbiol. 48:60-70. [DOI] [PubMed] [Google Scholar]
- 17.Kim, K. K., D. R. Fravel, and G. C. Papavizas. 1988. Identification of a metabolite produced by Talaromyces flavus as glucose oxidase and its role in the biocontrol of Verticillium dahliae. Phytopathology 78:488-492. [Google Scholar]
- 18.Kim, K. K., D. R. Fravel, and G. C. Papavizas. 1993. Glucose oxidase as the antifungal principle of talaron from Talaromyces flavus. Can. J. Microbiol. 36:760-764. [DOI] [PubMed] [Google Scholar]
- 19.Klessing, D. F., and J. Malamy. 1994. The salicylic acid signals in plants. Plant Mol. Biol. 26:1439-1458. [DOI] [PubMed] [Google Scholar]
- 20.Limon, M. C., J. A. Pintor-Toro, and T. Benitez. 1999. Increased antifungal activity of Trichoderma harzianum transformants that overexpress a 33-kDa chitinase. Phytopathology 89:254-261. [DOI] [PubMed] [Google Scholar]
- 21.Lorito, M., C. Peterbauer, C. K. Hayes, and G. E. Harman. 1994. Synergistic interaction between fungal cell wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiology 140:623-629. [DOI] [PubMed] [Google Scholar]
- 22.Lorito, M., F. Scala, A. Zoina, and S. L. Woo. 2001. Enhancing biocontrol of fungal pests by exploiting the Trichoderma genome, p. 248-259. In J. Gressel and M. Vurro (ed.), Enhancing biocontrol agents and handling risks. IOS Press, Amsterdam, The Netherlands.
- 23.Mach, R. L., C. K. Peterbauer, K. Payer, S. Jaksits, S. L. Woo, S. Zeilinger, C. M. Kullnig, M. Lorito, and C. P. Kubicek. 1999. Expression of two major chitinase genes of Trichoderma atroviride (T. harzianum P1) is triggered by different regulatory signals. Appl. Environ. Microbiol. 65:1858-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margolles-Clark, E., C. K. Hayes, G. E. Harman, and M. Penttila. 1996. Improved production of Trichoderma harzianum endochitinase by expression in Trichoderma reesei. Appl. Environ. Microbiol. 62:2145-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marois, J. J., M. T. Dunn, and G. C. Papavizas. 1982. Biological control of Verticillium wilt of eggplant in the field. Plant Dis. 66:1166-1168. [Google Scholar]
- 26.McLaren, D. L., and H. C. Huang. 1986. Hyperparasitism of Sclerotinia sclerotiorum by Talaromyces flavus. J. Plant Pathol. 8:43-48. [Google Scholar]
- 27.Murray, F. R., D. J. Llewellyn, W. J. Peacock, and E. S. Dennis. 1997. Isolation of the glucose oxidase gene from Talaromyces flavus and characterization of its role in the biocontrol of Verticillium dahliae. Curr. Genet. 32:367-375. [DOI] [PubMed] [Google Scholar]
- 28.Papavizas, G. C. 1985. Trichoderma and Gliocladium: biology, ecology and potential for biocontrol. Annu. Rev. Phytopathol. 23:23-54. [Google Scholar]
- 29.Peterbauer, C. K., M. Lorito, C. K. Hayes, G. E. Harman, and C. P. Kubicek. 1996. Molecular cloning and expression of the nag1 gene (N-acetyl-β-d-glucosaminidase-encoding gene) from Trichoderma harzianum P1. Curr. Genet. 30:325-331. [DOI] [PubMed] [Google Scholar]
- 30.Schirmböck, M., M. Lorito, Y. L. Wang, C. K. Hayes, I. Arisan-Atac, F. Scala, G. E. Harman, and C. P. Kubicek. 1994. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics: molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 60:4364-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stosz, S. K., D. R. Fravel, and D. P. Roberts. 1996. In vitro analysis of the role of glucose oxidase from Talaromyces flavus in biocontrol of the plant pathogen Verticillium dahliae. Appl. Environ. Microbiol. 62:3183-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo, S. L., B. Donzelli, F. Scala,R. L. Mach, G. E. Harman, C. P. Kubicek, G. Del Sorbo, and M. Lorito. 1999. Disruption of the ech42 (endochitinase-encoding) gene affects biocontrol activity in Trichoderma harzianum P1. Mol. Plant-Microbe Interact. 12:419-429. [Google Scholar]
- 33.Wu, G., B. J. Shortt, E. B. Lawrence, E. B. Levine, K. C. Fitzsimmons, and D. M. Shah. 1995. Disease resistance conferred by expression of a gene encoding H2O2-generating glucose oxidase in transgenic potato plants. Plant Cell 7:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yedidia, I., N. Benhamou, and I. Chet. 1999. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 65:1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yedidia, I., N. Benhamou, Y. Kapulnik, and I. Chet. 2000. Induction and accumulation of PR protein activity during early stages of root colonization by the mycoparasite Trichoderma harzianum strain T-203. Plant Physiol. Biochem. 38:863-873. [Google Scholar]
- 36.Zeilinger, S., C. Galhaup, K. Payer, S. L. Woo, R. L. Mach, C. Fekete, M. Lorito, and C. P. Kubicek. 1999. Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fungal Genet. Biol. 26:131-140. [DOI] [PubMed] [Google Scholar]