Abstract
Clostridium cellulovorans, an anaerobic bacterium, degrades native substrates efficiently by producing an extracellular enzyme complex called the cellulosome. All cellulosomal enzyme subunits contain dockerin domains that can bind to hydrophobic domains termed cohesins which are repeated nine times in CbpA, the nonenzymatic scaffolding protein of C. cellulovorans cellulosomes. In this study, the synergistic interactions of cellulases (endoglucanase E, EngE; endoglucanase L, EngL) and hemicellulases (arabinofuranosidase A, ArfA; xylanase A, XynA) were determined on the degradation of corn fiber, a natural substrate containing mainly xylan, arabinan, and cellulose. The degradation by XynA and ArfA of cellulose/arabinoxylan was greater than that of corn fiber and resulted in 2.6-fold and 1.4-fold increases in synergy, respectively. Synergistic effects were observed in increments in both simultaneous and sequential reactions with ArfA and XynA. These synergistic enzymes appear to represent potential rate-limiting enzymes for efficient hemicellulose degradation. When mini-cellulosomes were constructed from the cellulosomal enzymes (XynA and EngL) and mini-CbpA with cohesins 1 and 2 (mini-CbpA1&2) and mini-CbpA with cohesins 5 and 6 (mini-CbpA5&6), higher activity was observed than that for the corresponding enzymes alone. Based on the degradation of different types of celluloses and hemicelluloses, the interaction between cellulosomal enzymes (XynA and EngL) and mini-CbpA displayed a diversity that suggests that dockerin-cohesin interaction from C. cellulovorans may be more selective than random.
Plants are the master glycan producers of the planet, and most of this material is contained in their cell wall or extracellular matrix. The plant cell wall is a highly organized network of cellulose and cross-linked glycans embedded in a gel matrix of pectic substances and reinforced with structural proteins and aromatic substances (8). Cellulose and hemicelluloses are the major components of plant cell walls. Hemicelluloses, the second most common polysaccharides in nature, represent about 20 to 35% of lignocellulosic biomass. Xylans are the most abundant hemicelluloses. Xylans of many plant materials are heteropolysaccharides with homopolymeric backbone chains of 1,4-linked β-d-xylopyranose units. Besides xylose, xylans may contain arabinose, glucuronic acid, or its 4-O-methyl ether and acetic, ferulic, and p-coumeric acids. The frequencies and composition of branches depend on the sources of xylan (1). Xylans can thus be categorized as linear homoxylan, arabinoxylan, glucuronoxylan, and glucuronoarabinoxylan. Arabinoxylans have been speculated to have a role in cross-linking of cellulose microfibrils and may thereby regulate cell expansion and strengthen the cell wall (7). These substituent groups in xylan act as a limiting factor in achieving the efficient hydrolysis of the substrate. Complete hydrolysis of hemicellulose requires the interaction of a number of hemicellulosic enzymes that have the ability to cleave main chains and side chains.
In nature, the degradation of hemicellulose is carried out mainly by fungi and bacteria such as clostridia species (3, 9-11, 31, 43). Many sequence-based families of glycosyl hydrolases from Clostridium cellulovorans are known, and their genes have been cloned, expressed, and characterized (18, 21, 22, 26, 40- 42). Our understanding of the activity and binding of these enzymes on different defined substrates has been improved, and many studies have elucidated the mechanism of hydrolysis by individual enzymes (10, 21, 27, 28). However, many questions remain to be answered about the degradation of complex biomass substrates, such as hemicelluloses. Arabinoxylan and cellulose, which are the most abundant components of plant cell walls, are resistant to saccharification (20). However, the enzymatic saccharification of native, crystalline cellulose is potentially of great economic importance with a view to biotechnological applications, such as treatment of cellulosic wastes and conversion of cellulosic substrates into solvents and fuels. Nevertheless, the enzymatic saccharification is not yet cost-effective. This process is difficult because it involves a set of cooperative enzymes which have to hydrolyze a substrate with a heterogeneous structure. Only by synchronous cooperation of enzymes with different modes of activity in a synergistic arrangement can this recalcitrant substrate be effectively and completely decomposed (5).
To solve these problems, cellulolytic anaerobic bacteria have developed a defined arrangement of enzymes along a noncatalytic scaffolding protein. The cellulosome, this large multienzyme complex, contains various cellulase components, which differ in their modes of action (11, 25, 34). In C. cellulovorans, the scaffolding protein called CbpA contains nine reiterated domains (10, 35, 36), which act as receptors recognized by the dockerin domains of the catalytic subunits. The bacterial cellulase systems investigated so far include a variety of nonprocessive endo-β-1,4-glucanases producing new ends at random within a polysaccharide chain and processive cellulases (exo-β-1,4-glucanases) which remain attached to one end of the substrate and split off cellobiose (cellobiohydrolases) or multimers of cellobiose (processive endocellulases). β-1,4-Glucanases with different modes of action work synergistically to effectively degrade crystalline substrate.
The determination of synergistic effects on cellulose and hemicelluloses between cellulases and hemicellulases should help us to understand the cellulolytic mechanism and to know how to improve the efficiency of cellulosomes which will be used for biomass conversion.
The synergistic effects between cellulases on cellulose degradation have been extensively studied (4, 19, 27-29, 33, 38, 44-46). To degrade crystalline cellulose to glucose, at least three enzymes have to cooperate: endoglucanase, exoglucanase, and β-glucosidase. Recent work with C. cellulovorans enzyme degradation related to synergism has been reported (21, 27, 28). Endoglucanases and exoglucanases acted synergistically (27), and the combination of cellulases and xylanases or noncellulosomal and cellulosomal enzymes also contributed to synergistic degradation of corn fiber (21, 28). However, there are few studies concerning synergistic effects between hemicellulosic enzyme and cellulases on “natural substrate.”
Corn stover is a potential substrate for biomass conversion to obtain fermentable sugars. Cleavage of xylan cross-linkage is considered to be one of the key reactions to degrade corn fiber. In this study, we determined the synergistic effects between cellulases (endoglucanase L [EngL] and endoglucanase E [EngE]) and hemicellulases (xylanase A [XynA] and α-l-arabinofuranosidase A [ArfA]) on the degradation of corn cell walls, a more natural substrate than purified cellulose and hemicelluloses. We found that XynA and ArfA represented potential rate-limiting enzymes for efficient degradation of hemicelluloses. In addition to synergism, the composition of cellulosomal enzymes is likely to be an important factor in the enzymatic hydrolysis of substrates. CbpA appears to contribute to specific hemicellulose degradation and to the synergistic reactions of hemicellulases.
MATERIALS AND METHODS
Bacterial strains and media.
Cultures of C. cellulovorans ATCC 35296 were grown anaerobically at 37°C in round-bottom flasks (35, 37). Escherichia coli BL21(DE3) (Novagen) was used as an expression system for mini-CbpA1&2, mini-CbpA5&6, endoglucanase E (EngE), xylanase (XynA), and α-l-arabinofuranosidase (ArfA) production with pET-miniCbpA1&2, pET-miniCbpA5&6, pENGE, pEXynA29, and pET29-ArfA, respectively (21-23). E. coli TOP10 (Invitrogen) was used as an expression host for endoglucanase L (EngL) production with pBAD/Thio-EngL. Recombinant strains were cultivated in Luria-Bertani medium (32) supplemented with ampicillin (50 or 100 μg/ml) or kanamycin (50 μg/ml).
Plasmid construction for EngL, EngE, ArfA, and XynA.
The xynA gene, the arfA gene, and the engE gene were amplified by PCR and inserted into pET29, pET29b, and pET22b, respectively, as described previously (21-23). EngL was expressed by using the pBAD/Thio vector (Invitrogen). For the construction of EngL production vector, the engL gene was amplified by PCR with genomic DNA from C. cellulovorans as a template with primers engL Forward (GCACCTAAATTTGACTATTCTGATGC) and engL Reverse (ACCAAGAAGTAACTTTTTAAGAAGTGC). The fragment obtained was cloned directly into the pBAD/Thio vector by TA cloning to generate pBAD/Thio-EngL.
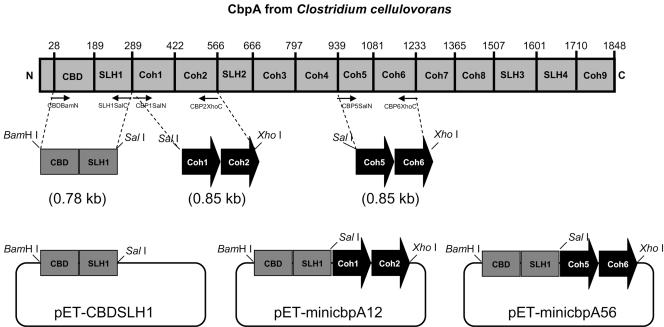
Plasmid construction for mini-CbpA1&2 and mini-CbpA5&6.
Mini-CbpA1&2 and mini-CbpA5&6 were designed to consist of a cellulose-binding domain (CBD), a hydrophilic cellulose-binding domain, and two cohesins of scaffolding protein CbpA (Fig. 1). Mini-CbpA's were expressed with the pET22b vector (Novagen) in E. coli. These genes were designed to fuse the His tag at their C-terminal ends from pET22b. The CBD and SLH1 domain was amplified by PCR using genomic DNA from C. cellulovorans as a template with the pair of primers CBDBamN (5′-GCGCGCGGATCCGGCGACATCATC-3′) and SLH1SalC(5′-GCTGTTACAGCGTCGACTGGTGTGTCAAC-3′) (Fig. 1). The amplified 0.78-kb fragment had a BamHI site at the 5′ end and a SalI site at the 3′ end. This fragment was digested with BamHI and SalI and inserted into pET22b digested with the same pair of the restriction enzymes to generate pET22b-CBDSLH1. Cohesin 1 and 2 domains were amplified with primers CBP1SalN (5′-GTAATTACAGTAGTTGACACACCAGTCGACGCTG-3′) and CBP2XhoC(5′-GCGCGCGCCTCGAGTATAGGATCTCC-3′), respectively, and cohesin 5 and 6 domains were amplified with CBP5SalN (5′-CTAAAACAGTAGTCGACAGCGTTACAATAG-3′) and CBP6XhoC (5′-GGTGGTGGTGGTGCTCGAGACCTGCTACTG-3′), respectively. The amplified 0.85-kb PCR fragments were digested with SalI and XhoI and inserted into pET22b-CBDSLH1 digested with the same pair of the restriction enzymes to generate pET-miniCbpA1&2 and pET-miniCbpA5&6.
FIG. 1.
Construction of mini-CbpA's. Numbers are amino acid residues counted from the translation start of CbpA. The amplified mini-CbpA fragments are also indicated.
Expression of recombinant proteins.
For production of recombinant mini-CbpA's, ArfA, XynA, and EngE, E. coli BL21(DE3) cells harboring pET-miniCbpA1&2, pET-miniCbpA5&6, pET29-ArfA, pEXynA29, and pENGE were grown, and recombinant proteins were induced by adding IPTG (isopropyl-β-d-thiogalactoside) as an inducer. E. coli cells were grown in 1 liter of medium at 37°C to a density of 0.9 at 600 nm, and IPTG was added to a final concentration of 0.4 mM for mini-CbpA expression and 1 mM for the rest. Then, the culture was grown at 30°C for an additional 6 h. For the production of the recombinant EngL, E. coli TOP10 harboring pBAD/Thio-EngL was grown, and recombinant proteins were induced by adding l-arabinose as an inducer. The E. coli cells were grown in 1 liter of medium at 37°C to a density of 0.9 at 600 nm. After the culture broth was cooled on ice for 30 min, l-arabinose was added to a final concentration of 0.6%. Then, the culture was grown at 18°C for 18 h.
Purification of recombinant proteins.
The recombinant proteins mini-CbpA, ArfA, XynA, EngL, and EngE were purified in the same manner as follows. After the E. coli cells grown as described above were collected by centrifugation, the cells were resuspended in 50 ml of the lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 1 mg of lysozyme/ml, pH 8.0). The solution was incubated on ice for 30 min and then sonicated by using a sonicator equipped with a microtip. After centrifugation, the extracted solution was applied to 4 ml of nickel-nitrilotriacetic acid agarose resin (QIAGEN), and the proteins bound to the resin were purified and pooled. The pooled solution was desalted and concentrated into 1 ml of Tris-HCl buffer (pH 8.0) by use of the Ultrafree 10-kDa membrane (Millipore).
Protein concentration.
Protein was measured by using the method of Bradford (6) with a protein assay kit from Bio-Rad with bovine serum albumin as a standard. The molar amount of each recombinant protein was calculated by use of the calculated molecular weight of each protein.
Assembly of recombinant cellulosomes.
The purified mini-CbpA and the recombinant cellulosomal subunits were mixed in the ratio 1:1 (5 nmol of each protein) in 100 μl of binding buffer (25 mM sodium acetic buffer [pH 6.0], 15 mM CaCl2) and kept for 2 h at 4°C. The assembly of mini-CbpA's and cellulosomal subunits was confirmed by native polyacrylamide gel electrophoresis (PAGE) analysis as described by Laemmli but without sodium dodecyl sulfate (SDS) (24).
Stability measurements.
As a control, a time course of the effect of temperature (37°C) on the stability of the complexes or enzymes alone was assessed by enzyme activity measurements. Each aliquot was collected at the appropriate time (0, 5, and 20 h) and the residual activity measured on cellulose/arabinoxylan (CAX) substrate as described below.
Interaction Western blotting.
The binding of cellulosomal enzymes to mini-CbpA's was tested by interaction analysis. Purified EngL and XynA were first subjected to SDS-PAGE, transferred to a polyvinylidene difluoride membrane, washed with blocking buffer (3% gelatin in Tris-buffered saline [TBS]), washed with washing buffer (0.05% Tween 20 in TBS), and then treated with washing buffer containing 0.1 mg of mini-CbpA1&2 or mini-CbpA5&6 for 2 h at room temperature. The membranes were then washed with TBS and treated with washing buffer containing 1% gelatin and anti-CbpA antibody. Signals were detected by using a secondary antibody (goat anti-rabbit immunoglobulin G) conjugated with alkaline phosphatase.
Substrates.
The powder of the water-insoluble fraction of corn stem fiber was supplied by Meiji Seika Kaisha, Ltd. The CAX, corn fiber gum (CFG), and corn fiber were kindly provided by David B. Johnston of the U.S. Department of Agriculture (12). The carboxymethyl cellulose (CMC, medium viscosity) and Avicel were from Sigma.
Enzyme assays.
Enzyme activity was assayed in the presence of a 0.5% (wt/vol) concentration of each polysaccharide at 37°C in 50 mM acetate buffer (pH 6.0). Enzyme concentration was related to the type of substrate. For the soluble substrates (CFG and CMC), samples (500 μl) were collected at appropriate times and immediately mixed with 500 μl chilled 0.38 M sodium carbonate containing 1.8 mM cupric sulfate and 0.2 M glycine. For the insoluble substrates (Avicel, CAX, acid swollen [AS] cellulose, corn stem fiber, and corn fiber), the experiments were done by shaking slowly the mixture reaction; 1-ml aliquots were collected and centrifuged, and the reducing sugars content was examined as in the case of soluble substrate mentioned above. The reducing power was determined by reductometry with the Dygert et al. method (14). All experiments were repeated three times.
RESULTS
Purification of EngL, XynA, and ArfA.
Arabinoxylans are known to be the major hemicelluloses in corn fiber (12). Therefore, we chose EngL, a family 9 endoglucanase, XynA, a family 11 endo-β-xylanase, and ArfA, an l-α-arabinofuranosidase family 51 glycosyl hydrolase, with respect to their individual activities on hemicelluloses. The recombinant enzymes were expressed by E. coli. XynA and ArfA, which could be expressed as soluble protein by the pET29 vector at 30°C, were purified as described previously (21, 22).
EngL was expressed as an inclusion body. We optimized the induction of the expression of EngL from pBAD in order to produce a soluble enzyme. The optimized conditions are described in Materials and Methods. In short, the culture was incubated at 37°C to a density of 0.9 at 600 nm. Induction was performed by adding 0.6% (wt/vol) l-arabinose and incubating the culture for 18 h at 18°C. EngL was purified from the crude extract supernatant by fast protein liquid chromatography purification on a nickel-nitrilotriacetic acid column. SDS-PAGE analysis showed one protein band.
Time course of CAX degradation.
To determine the relationship between reaction period and synergy degree, CAX was degraded by a mixture of equimolar amounts of endo-β-xylanase (XynA) and α-arabinofuranosidase (ArfA) for 5, 15, and 20 h at 37°C. The control was done in the related set of experiments where the activity of each enzyme was assayed on CAX degradation at the beginning and after 20 h of incubation at 37°C. The enzyme activity was not affected during 20 h of incubation; a variation within only ±3% of the original activity was found. Synergy was demonstrated when the enzymes were applied together, since they converted more substrate than the sum of conversions achieved by each of the enzymes. As shown in Fig. 2, the ratio between the synergistic activity and the sum of the individual activities (the degree of synergism) increased from 1.1-fold (5 h) to 2.6-fold (20 h), according to the length of the reaction period. These results suggested that synergistic effects between XynA and ArfA were more effective progressively during substrate degradation.
FIG. 2.
Time course of CAX degradation by mixture of XynA and ArfA. CAX (0.5%) was degraded by a 0.5 μM concentration of each enzyme at 37°C for 5, 15, and 20 h. a, degree of synergy.
Synergistic effect between hemicellulases on different substrates.
The synergistic effects between ArfA and XynA were determined by using different hemicelluloses, such as CAX containing 68% arabinoxylan (13), corn fiber containing 40% arabinoxylan (13), and corn stem fiber containing 20% arabinoxylan (7). Based on preliminary experimental results about the time course of CAX degradation, ArfA and XynA were simultaneously incubated with different hemicelluloses for 20 h at 37°C. The results showed that the amount of reducing sugar released was significantly increased when both XynA and ArfA were present compared with that released with ArfA or XynA alone (Table 1). The highest synergy degree was observed with CAX (2.6-fold) and then corn fiber (2.3-fold), and the lowest synergy was observed with corn stem fiber (1.4-fold). Based on the respective content of arabinoxylan in each substrate (68%, 40%, and 20% for CAX, corn fiber, and corn stem fiber, respectively), the increase of synergism degree could be related to the content of arabinoxylan, or this variation could be due to differences in the accessibility of the substrate in the corn fiber. The results suggest that the combination of ArfA and XynA might play important roles in effective degradation of arabinoxylans and therefore in the hemicellulosic complex digestion of plant cell walls as well.
TABLE 1.
Reducing sugars released from hemicelluloses by XynA and ArfA
| Substrate | Activitya
|
|||
|---|---|---|---|---|
| ArfA (μM) | XynA (μM) | ArfA + XynA (μM) | Synergy | |
| CAX (cellulose/arabinoxylan)b | 12 (0.7) | 16 (0.5) | 73 (1.1) | 2.6 |
| Corn fiber (coarse and fine fiber)b | 3 (0.1) | 5 (0.8) | 19 (1.2) | 2.3 |
| Corn stem fiberc | 14 (0.9) | 50 (4.3) | 91 (2.1) | 1.4 |
Synergy is the ratio of ArfA + XynA activity to the sum of individual ArfA and XynA activities. Hemicelluloses were degraded by 0.5 μM concentrations of each enzyme for 20 h at 37° C. The value of activity is the average of three determinations. The number in parentheses indicates standard deviation.
CAX and corn fiber were prepared as described previously (12).
Corn stem fiber was prepared as described previously (28).
Degradation of hemicellulose by sequential reactions.
Several series of experiments were performed to understand the sequential action of hemicellulolytic enzymes. CAX was treated with either XynA or ArfA for 5 or 15 h in the first reaction. Later, the second enzyme was added immediately after the reaction period of the first reaction for an additional 15 or 5 h, which brought the total reaction time to 20 h. The amount of liberated reducing sugars was then determined (Table 2). The results showed a strong synergy whether the first enzyme was XynA or ArfA. These results also showed that when XynA is the first enzyme, the degree of synergy is higher (2.8-fold) than when the first enzyme is ArfA (2.3-fold) (Table 2). Sequential reactions confirmed the results of the simultaneous reaction, which showed that ArfA acted synergistically with XynA for arabinoxylan degradation.
TABLE 2.
Sequential reactions against CAX by ArfA and XynAa
| Added enzyme(s)
|
Liberated reducing sugar (μM)b | Synergyc | |
|---|---|---|---|
| In first reaction (time of reaction) | In second reaction (time of reaction) | ||
| ArfA (15 h) | XynA (5 h) | 52 (1.5) | 2.3 |
| XynA (15 h) | ArfA (5 h) | 55 (2.7) | 2.8 |
| Control reactions | |||
| XynA + ArfA (20 h) | 68 (1.0) | 2.4 | |
| ArfA (5 h) | 4 (1.0) | ||
| ArfA (20 h) | 12 (0.7) | ||
| XynA (5 h) | 11 (0.9) | ||
| XynA (20 h) | 16 (0.5) | ||
CAX (0.5%) was degraded by 0.5 μM concentrations of each enzyme at 37°C. The second enzyme was added immediately after the end of the incubation period of the first reaction.
The values are the averages of three determinations. The number in parentheses indicates standard deviation.
Synergy is as described in Table 1.
Degradation of CAX by hemicellulases and cellulases.
In order to determine whether hemicellulases (XynA and ArfA) help cellulases (or vice versa) to degrade cellulose, the degradation of CAX was monitored with and without cellulases (EngE and EngL) in the first incubation reaction. The results of sequential action of cellulases, xylanase, and arabinofuranosidase are shown in Table 3. When, in step 1, CAX was incubated with the enzyme mixture (ArfA plus XynA) for 15 h and then treated further, in step 2, with EngL or EngE for an additional 5 h, synergistic effects were found (1.9-fold or 1.7-fold after further treatment with EngE or EngL, respectively). However, only a minor effect was observed in the case when EngE or EngL was added in the first step (1.1-fold when either EngL or EngE was used). The synergism in the latter case is probably due to the cooperation of the mixture of enzymes (ArfA plus XynA) added in step 2. This study demonstrates that the efficiency of cellulose degradation from CAX depends first of all on the degradation of the arabinoxylan backbone by both endoxylanase and arabinofuranosidase.
TABLE 3.
Sequential reactions against CAX by ArfA, XynA, EngE, and EngLa
| Added enzyme(s)
|
Liberated reducing sugar (μM)b | Synergyc | |
|---|---|---|---|
| In first reaction (time of reaction) | In second reaction (time of reaction) | ||
| ArfA + XynA (15 h) | EngE (5 h) | 168 (3.1) | 1.9 |
| EngE (15 h) | ArfA + XynA (5 h) | 114 (5.9) | 1.1 |
| ArfA + XynA (15 h) | EngL (5 h) | 105 (3.0) | 1.7 |
| EngL (15 h) | ArfA + XynA (5 h) | 112 (5.8) | 1.1 |
| Control reactions | |||
| XynA + ArfA + EngE (20 h) | 143 (1.9) | 1.3 | |
| XynA + ArfA + EngL (20 h) | 135 (2.0) | 1.3 | |
| ArfA (5 h) | 14 (0.1) | ||
| ArfA (20 h) | 17 (0.5) | ||
CAX (0.5%) was degraded by 0.8 μM concentrations of EngL, ArfA, and XynA and a 0.05 μM concentration of EngE at 37°C. The second enzyme was added immediately after the end of the incubation period of the first reaction.
The values are the average of three determinations. The number in parentheses indicates standard deviation.
Synergy is as described in Table 1.
Contribution of mini-CbpA to cellulase and hemicellulase activity.
Previous works have shown that individual cellulosomal enzymes from C. cellulovorans retain activity against cellulose, but significant hydrolysis of native cellulose is affected by cellulosomal enzymes associated with CbpA, implying a pivotal role for CbpA (27, 28). In this study, the impact of mini-CbpA's on hemicellulases and cellulase activity was also determined. The mini-cellulosomes were assembled by mixing the cellulosomal enzymes and the mini-CbpA containing the following components: one cellulose-binding domain, one hydrophilic domain, and two cohesin domains of CbpA (cohesins 1 and 2 and cohesins 5 and 6). The hydrolytic activity of recombinant mini-cellulosomes was assayed on various celluloses and hemicelluloses. Initially, the mini-cellulosomes were subjected to nondenaturing PAGE (Fig. 3) and Western blotting interaction in order to verify complex formation. Figure 4A shows the purified proteins. The binding of cellulosomal enzymes EngL and XynA to mini-CbpA1&2 and mini-CbpA5&6 was demonstrated by the interaction Western blotting technique developed in our laboratory (39). These results indicated that mini-CbpA's could bind to both EngL and XynA. EngL showed stronger binding ability to mini-CbpA1&2 and mini-CbpA5&6 than XynA (Fig. 4B).
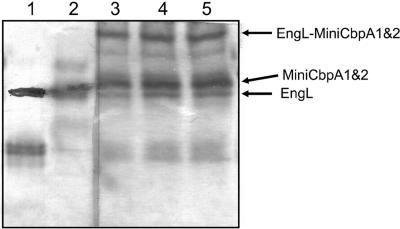
FIG. 3.
Nondenaturing PAGE of the purified EngL and the purified mini-CbpA1&2 complex. The complex EngL-mini-CbpA1&2 (molar ratio, 2:1) was incubated at 37°C. Lanes 3, 4, and 5 represent aliquots collected at 0, 10, and 20 h, respectively, and loaded in the 6% nondenaturing gel. Lane 1, EngL; lane 2, mini-CbpA1&2.
FIG. 4.
Purification of EngL, XynA, and mini-CbpA's and assembly of EngL and XynA cellulosomes. (A) SDS-PAGE analysis of purified XynA, EngL, and mini-CbpA's. Lane M, molecular marker; lane 1, EngL; lane 2, XynA; lane 3, mini-CbpA1&2; and lane 4, mini-CbpA5&6. (B) Interaction Western blot analysis. See Materials and Methods for details. Lane 1, mixture of EngL and mini-CbpA1&2; lane 2, mixture of EngL and mini-CbpA5&6; lane 3, mixture of XynA and mini-CbpA1&2; lane 4, mixture of XynA and mini-CbpA5&6.
Activity of mini-cellulosomes toward celluloses.
Prior to all experiments, the stability measurements of the complexes (enzyme-mini-CbpA) had been measured by means of their hydrolytic activity. The time course of the effect of the temperature on CAX degradation by each complex was not affected even after 20 h at 37°C. Variation in the range of ±3 to 7% of the original activity was found. To investigate whether CbpA enhances the cellulase activity of catalytic subunits of the cellulosome, we compared the activity of free EngL against different forms of celluloses, with an equimolar mixture of EngL with mini-CbpA's (mini-CbpA1&2 and mini-CbpA5&6) (Table 4). Similar hydrolytic activities were obtained for the complex of EngL with either mini-CbpA1&2 or mini-CbpA5&6, which showed a significant increase in cellulolytic activity on AS cellulose and Avicel. On the other hand, the complex of EngL with both types of scaffolding had no impact on CMC degradation. The results showed that whatever the type of cellulose, the complex EngL-mini-CbpA1&2 exhibited lower activity than observed for EngL-mini-CbpA5&6. These results suggested that the properties of mini-CbpA5&6 differed from those of mini-CbpA1&2 and that the mini-CbpA's enhanced the cellulase activity on celluloses with greater crystalline structure.
TABLE 4.
Influence of the type of cellulose on the activity of EngL in the free state or complexed onto mini-scaffolding protein
| Substrate | Free EngL (μM) | EngL-mini-CbpA1&2
|
EngL-mini-CbpA5&6
|
||
|---|---|---|---|---|---|
| Amount (μM)a | SFb | Amount (μM)a | SFb | ||
| CMC | 115 (5.0) | 114 (7.3) | 1.0 | 110 (2.1) | 1.0 |
| AS-cellulose | 168 (6.1) | 182 (4.9) | 1.1 | 207 (10.5) | 1.2 |
| Avicel | 76 (2.9) | 104 (6.1) | 1.4 | 141 (5.2) | 1.9 |
The amount of released reducing sugars from each substrate (0.5%) at 37°C is given. Each enzyme and mini-scaffolding protein were in equimolar amounts. CMC, AS cellulose, and Avicel were degraded by 6 nM for 20 min, 0.1 μM for 20 h, and 0.25 μM for 20 h, respectively. The number in parentheses indicates standard deviation.
SF, ratio of reducing sugar released by mini-cellulosome to reducing sugars released by the corresponding free enzyme.
Activity of mini-cellulosomes toward hemicelluloses.
The impact of the complex of EngL and XynA with mini-CbpA1&2 and mini-CbpA5&6 was also investigated in terms of activity toward the highly arabinoxylan-rich CFG (85% arabinoxylan) and the corn stem containing the least arabinoxylan (20% arabinoxylan). The results are summarized in Table 5. Whatever the substrate, the complex of EngL with both types of mini-CbpA induced increases in activity, and the difference between EngL-mini-CbpA1&2 and EngL-mini-CbpA5&6 activity was less marked except in the case of the CAX degradation, where the difference in hydrolytic activity was particularly significant (2.2-fold versus 3.9-fold for EngL-mini-CbpA1&2 and EngL-mini-CbpA5&6, respectively). In contrast to EngL, the difference in the hemicellulosic activity of the complex of XynA with mini-CbpA1&2 and mini-CbpA5&6 was more marked. Indeed, the XynA-mini-CbpA1&2 displayed much higher activity than the free enzyme (2.3-fold and 2-fold on the CFG and corn stem, respectively). XynA-mini-CbpA5&6 had activity levels almost similar to those observed for the free enzyme. Moreover, only in the case of corn fiber activity did XynA-mini-CbpA5&6 complexes display high activity (1.9-fold), whereas XynA-mini-CbpA1&2 had less impact (1.3-fold) on the same substrate.
TABLE 5.
Influence of the type of hemicellulosic substrate on the activity of XynA and EngL in the free state or complexed onto mini-CbpA1&2 and mini-CbpA5&6
| Substrate | Enzyme | Free enzyme (μM) | Mini-CbpA1&2
|
Mini-CbpA5&6
|
||
|---|---|---|---|---|---|---|
| Amt (μM)a | SFb | Amt (μM)a | SFb | |||
| Corn stem | EngL | 24 (1.6) | 45 (1.7) | 1.9 | 39 (0.9) | 1.7 |
| XynA | 43 (0.9) | 87 (3.0) | 2.0 | 53 (0.9) | 1.2 | |
| Corn fiber | EngL | 15 (1.6) | 30 (1.0) | 2.0 | 31 (1.1) | 2.1 |
| XynA | 15 (0.8) | 19 (1.6) | 1.3 | 29 (2.7) | 1.9 | |
| CAX | EngL | 47 (2.9) | 100 (5.0) | 2.2 | 181 (5.4) | 3.9 |
| XynA | 55 (2.7) | 86 (1.6) | 1.6 | 79 (3.0) | 1.4 | |
| CFG | EngL | 26 (3.4) | 56 (0.1) | 2.1 | 41 (1.5) | 1.6 |
| XynA | 23 (1.5) | 55 (2.3) | 2.3 | 38 (1.0) | 1.6 | |
The amount of released reducing sugars from each substrate (0.5%) at 37°C for 20 h is given. The contents of the substrates are: corn stem, 40% cellulose and 20% arabinoxylan; corn fiber, 15% cellulose and 40% arabinoxylan; CAX, 21% cellulose and 68% arabinoxylan; and CFG, 2% cellulose and 85% arabinoxylan. All enzymes and mini-scaffolding proteins were in equimolar amounts at 0.25 μM. The number in parentheses indicates standard deviation.
SF is as described in Table 4.
The sequential studies of the resultant mini-cellulosomes were also investigated using the CAX substrate. The results are shown in Table 6. The synergism degree was higher when the degradation was initiated by the mini-cellulosome containing XynA than in the case where EngL-mini-CbpA was first to act. Interestingly, these results are remarkably in agreement with those described above in the case of the free enzyme sequential reactions (Table 3), supporting the fact that the xylanolytic enzymes represent potential rate-limiting enzymes in hemicellulose degradation.
TABLE 6.
Sequential reactions against CAX by XynA and EngL attached to mini-CbpAa
| Added enzyme(s)
|
Liberated reducing sugar (μM) | Synergy | |
|---|---|---|---|
| In first reaction (time of reaction) | In second reaction (time of reaction) | ||
| XynA-mini-CbpA1&2 (15 h) | EngL-mini-CbpA1&2 (5 h) | 108 (5.0) | 1.4 |
| EngL-mini-CbpA1&2 (15 h) | XynA-mini-CbpA1&2 (5 h) | 97 (2.1) | 1.1 |
| XynA-mini-CbpA5&6 (15 h) | EngL-mini-CbpA5&6 (5 h) | 163 (0.9) | 1.3 |
| EngL-mini-CbpA5&6 (15 h) | XynA-mini-CbpA5&6 (5 h) | 144 (1.1) | 1 |
| Control reactions | |||
| XynA-mini-CbpA1&2 (5 h) | 24 (2.0) | ||
| XynA-mini-CbpA1&2 (20 h) | 24 (1.0) | ||
| XynA-mini-CbpA5&6 (5 h) | 19 (0.3) | ||
| XynA-mini-CbpA5&6 (20 h) | 21 (0.4) | ||
| EngL-mini-CbpA1&2 (5 h) | 52 (1.7) | ||
| EngL-mini-CbpA1&2 (20 h) | 60 (0.9) | ||
| EngL-mini-CbpA5&6 (5 h) | 105 (2.0) | ||
| EngL-mini-CbpA5&6 (20 h) | 134 (3.7) | ||
CAX (0.5%) was degraded by 0.1 μM EngL and XynA at 37°C for 20 h. Mini-scaffolding proteins (mini-CbpA1&2 or mini-CbpA5&6) were at 0.1 μM. The second enzyme was added immediately after the end of the incubation period of the first reaction. The number in parentheses indicates standard deviation.
DISCUSSION
With the goal of gaining a better insight into the native plant degradation process, synergy studies between endoglucanases, exoglucanases, and hemicellulases from many microorganisms have been extensively documented (4, 19, 27-29, 33, 38, 44-46). Indeed, due to the heterogeneity in the composition and the structure of the plant cell wall, a wide range of enzymes is required for the biodegradation of these polysaccharides. The mechanism of synergism is still not well understood. However, as Schwarz discussed (33), the synergism between hydrolytic enzymes can be explained by differences between cellulases and hemicellulases in terms of the mode of action, the binding affinity to cellodextrins, the velocity of adsorption or desorption, the sliding behavior along the substrate, the protein-protein interaction, or the size of the products. Thus, in order to understand enzymatic hydrolysis of hemicellulosic substrates, a hemicellulase preparation having C. cellulovorans xylanolytic enzymes has been developed. Arabinoxylan is one of the major hemicelluloses, and its hydrolysis is an important prerequisite for improved degradation of hemicelluloses. The present study has further investigated the individual and combined efficiencies of three different enzymes, cellulolytic EngL and hemicellulolytic XynA and ArfA, in the degradation of the arabinoxylan substrates. We observed an increase in the amount of reducing sugar released by the addition of XynA and ArfA (compared to the sum of the individual activities of the corresponding enzymes) in simultaneous reaction mixtures containing either corn stem powder, corn fiber, or CAX. These results were confirmed from the sequential addition of xylanase and α-l-arabinofuranosidase showing also a strong cooperative action on CAX degradation. Similar strong synergistic effects were observed between α-l-arabinofuranosidase and xylanases from Aspergillus for wheat arabinoxylan degradation (44). Understanding the mechanism of enzymatic cellulose hydrolysis requires knowledge of the action mode of enzymes. α-l-Arabinofuranosidase (ArfA), which attacks mainly α-l-arabinosyl side chains of xylans, exhibits also low xylanase activity (21). We assumed that the synergism observed on CAX degradation could be due to various mechanisms that could account for the diverse specificity and function of the xylan-degrading enzymes. The existence of enzymes from different families of glycosyl hydrolases should allow an increased accessibility of the substrate through hydrolysis sites on the substrate surface.
On the other hand, since the rates of synergism from the sequential reaction on CAX between XynA and ArfA are similar regardless of the sequence of enzyme action, it is difficult to determine whether both enzymes cooperate in a sequential or a simultaneous way. However, based on the arabinoxylan structure, ArfA activity should be a crucial first step to allow efficient removal of side chains followed by xylanase activity for further degradation. This assumption was reported in previous work from Thermonospora fusca, where α-arabinofuranosidase was shown to represent a potential rate-limiting enzyme in xylan degradation, particularly for corn fiber, corn stover, and rice straw (31). In addition, xylanolytic enzymes produced from Penicillium sp. have been shown to interact cooperatively and sequentially in the hydrolysis of oat spelt in the following order: α-l-arabinofuranosidase→→xylanase→→β-xylosidase (30).
We also observed that no or insignificant synergism was observed when cellulases (EngL and EngE) were used as the first enzyme rather than hemicellulases (XynA and ArfA). These results suggest that only hemicellulases can independently modify the substrate to increase digestibility of CAX. This action by XynA and ArfA is consistent with converting arabinoxylan-hindered cellulose into a more suitable modified substrate. Theoretically, side chain-cleaving enzymes remove the side substituent groups of heteroxylans, and then, after that treatment, cellulose is exposed for further hydrolytic attack by cellulases. In conclusion, the results indicate the greater susceptibility of substrate to hemicellulase activity which is amplified by the cooperative action of xylanase and arabinofuranosidase, as shown in Table 3. In this study, we assume, as reported from Penicillium (30), that the hemicellulosic substrate was attacked by xylanolytic and cellulosic enzymes cooperatively and/or sequentially in the following order: α-l-arabinofuranosidase→→endo-β-xylanase→→cellulases.
In addition to synergism, the organization of cellulolytic and xylanolytic enzymes in mini-cellulosomes was also investigated for their cellulolytic and hemicellulolytic activity. The results indicated that when EngL or XynA was bound to the mini-CbpA's (mini-CbpA1&2 or mini-CbpA5&6), the complexes compared to the corresponding free enzymes exhibited, in most cases, an increase of activity against cellulose or hemicellulose. The increase of the activity observed after the binding of the free enzyme to the mini-CbpA may be due to the conformation changes of the participating components, thereby allowing the enzyme more freedom to distribute on the preferred substrate sites. On the other hand, our results suggest, as reported by Fierobe et al. (16, 17), that interactions between cellulosomal enzymes and the nine cohesins from C. cellulovorans CbpA displayed a diversity. Depending on the carbon source of the C. cellulovorans growth, specific dockerin-cohesin interactions might take place for cellulosome formation. Since the enzymatic hydrolysis by mini-cellulosomes (mini-CbpA1&2 and mini-CbpA5&6) is not in an equal manner, and these data were confirmed by the interaction Western blot analyses, the dockerin-cohesin interaction in C. cellulovorans may be more selective than the random one hypothesized for Clostridium thermocellum (2) and for Clostridium cellulolyticum (15), where it was suggested that cohesins recognized all dockerins in an equivalent manner. In conclusion, the present results suggest that the cellulosome complex from C. cellulovorans is not assembled in a random way. This assumption is reinforced by the cellulosome fraction analysis carried out by means of ion-exchange chromatography (R. Koukiekolo, S.-O. Han, and R. H. Doi, unpublished data). In the latter case, it had been shown that cellulosomes, isolated from C. cellulovorans grown on xylan, were fractionated into 7 to 10 high-molecular-weight multiprotein complexes. However, further investigations are still to be defined for a better understanding of the molecular mechanism of recognition and binding of the enzymatic subunits into C. cellulovorans scaffolding protein.
Acknowledgments
We are grateful to David B. Johnston of the U.S. Department of Agriculture for the gift of CAX, corn fiber gum, and corn fiber.
The research was supported by the Research Institute of Innovative Technology for the Earth (RITE), Kyoto, Japan, and grant DE-DDF03-92ER20069 from the U.S. Department of Energy.
REFERENCES
- 1.Aspinall, G. O. 1980. Chemistry of cell wall polysaccharides, p. 473-500. In J. Press (ed.), The biochemistry of plants, vol. 3. Carbohydrates: structure and function. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 2.Bayer, E. A., L. J. W. Shimon, Y. Shoham, and R. Lamed. 1998. Cellulosomes—structure and ultrastructure. J. Struct. Biol. 124:221-234. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, E. A., Y. Shoham, and R. Lamed. 2001. Cellulose-decomposing bacteria and their enzyme systems. .In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed. [Online.] Springer-Verlag, New York, N.Y. http://link.springer-ny.com/link/service/books/10125/.
- 4.Belaich, A., G. Parsiegla, L. Gal, C. Villard, R. Haser, and J.-P. Belaich. 2002. Cel9M, a new family 9 cellulase of the Clostridium cellulolyticum cellulosome. J. Bacteriol. 184:1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boisset, C., S. Armand, S. Drouillard, H. Chanzy, H. Driguez, and B. Henrissat. 1998. Structure-function relationships in cellulases: the enzymatic degradation of insoluble cellulose, p. 124-132. In M. Claeyssens, W. Nerinckx, and K. Piens (ed.), Carbohydrases from Trichoderma reesei and other microorganisms. Royal Society of Chemistry, London, United Kingdom.
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Carpita, N. C. 1996. Structure and biogenesis of the cell wall of grasses. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47:445-476. [DOI] [PubMed] [Google Scholar]
- 8.Carpita, N., M. McCann, and L. R. Griffing. 1996. The plant extracellular matrix: news from the cell's frontier. Plant Cell 8:1451-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi, R. H., and A. Kosugi. 2004. Cellulosome: plant-cell-wall-degrading enzyme complexes. Nat. Rev. Microbiol. 2:541-551. [DOI] [PubMed] [Google Scholar]
- 10.Doi, R. H., A. Kosugi, K. Murashima, Y. Tamura, and S.-O. Han. 2003. Cellulosomes from mesophilic bacteria. J. Bacteriol. 185:5907-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi, R. H., J.-S. Park, C. C. Liu, L. M. Malburg, Y. Tamaru, A. Ichi-ishi, and A. Ibrahim. 1998. Cellulosome and noncellulosomal cellulase of Clostridium cellulovorans. Extremophiles 2:53-60. [DOI] [PubMed] [Google Scholar]
- 12.Doner, L. W., and K. B. Hicks. 1997. Isolation of hemicellulose from corn fiber by alkaline extraction. Cereal Chem. 74:176-181. [Google Scholar]
- 13.Doner, L. W., D. B. Johnston, and V. Singh. 2001. Analysis and properties of arabinoxylans from discrete corn wet-milling fiber fractions. J. Agric. Food Chem. 49:1266-1269. [DOI] [PubMed] [Google Scholar]
- 14.Dygert, S., L. H. Li, R. D. Florida, and J. A. Thoma. 1965. Determination of reducing sugar with improved precision. Anal. Chem. 13:367-374. [DOI] [PubMed] [Google Scholar]
- 15.Fierobe, H.-P., S. Pages, A. Belaich, S. Champ, D. Lexa, and J.-P. Belaich. 1999. Cellulosome from Clostridium cellulolyticum: molecular study of the dockerin/cohesion interaction. Biochemistry 38:12822-12832. [DOI] [PubMed] [Google Scholar]
- 16.Fierobe, H.-P., A. Mechaly, C. Tardif, A. Belaich, R. Lamed, Y. Shoham, J.-P. Belaich, and E. A. Bayer. 2001. Design and production of active cellulosome chimeras. J. Biol. Chem. 276:21257-21261. [DOI] [PubMed] [Google Scholar]
- 17.Fierobe, H.-P., E. A. Bayer, C. Tardif, M. Czjzek, A. Mechaly, A. Belaich, R. Lamed, Y. Shoham, and J.-P. Belaich. 2002. Degradation of cellulose substrates by cellulosome chimeras. J. Biol. Chem. 277:49621-49630. [DOI] [PubMed] [Google Scholar]
- 18.Foong, F., T. Hamamoto, O. Shoseyov, and R. H. Doi. 1991. Nucleotide sequence and characteristics of endoglucanase gene engB from Clostridium cellulovorans. J. Gen. Microbiol. 137:1729-1736. [DOI] [PubMed] [Google Scholar]
- 19.Henrissat, B., H. Driguez, C. Viet, and M. Schulein. 1985. Synergism of cellulases from Trichoderma reesei in the degradation of cellulose. Bio/Technology 3:722-726. [Google Scholar]
- 20.Hespell, R. B. 1998. Extraction and characterization of hemicellulose from the corn fiber produced by corn wet-milling processes. J. Agric. Food Chem. 46:2615-2619. [Google Scholar]
- 21.Kosugi, A., K. Murashima, and R. H. Doi. 2002. Characterization of noncellulosomal subunits, ArfA and BgaA, from Clostridium cellulovorans that cooperate with the cellulosome in plant cell wall degradation. J. Bacteriol. 184:6859-6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosugi, A., K. Murashima, and R. H. Doi. 2002. Xylanase and acetyl esterase activities of XynA, a key subunit of the Clostridium cellulovorans cellulosome for xylan degradation. Appl. Environ. Microbiol. 68:6399-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosugi, A., K. Murashima, Y. Tamaru, and R. H. Doi. 2002. Cell-surface-anchoring role of N-terminal surface layer homology domains of Clostridium cellulovorans EngE. J. Bacteriol. 184:884-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Lamed, R., and E. A. Bayer. 1988. The cellulosome of Clostridium thermocellum. Adv. Appl. Microbiol. 33:1-46. [Google Scholar]
- 26.Liu, C. C., and R. H. Doi. 1998. Properties of exgS, a gene for a major subunit of the Clostridium cellulovorans cellulosome. Gene 211:39-47. [DOI] [PubMed] [Google Scholar]
- 27.Murashima, K., A. Kosugi, and R. H. Doi. 2002. Synergistic effects on crystalline cellulose degradation between cellulosomal cellulases from Clostridium cellulovorans. J. Bacteriol. 184:5088-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murashima, K., A. Kosugi, and R. H. Doi. 2003. Synergistic effects of cellulosomal xylanase and cellulases from Clostridium cellulovorans on plant cell wall degradation. J. Bacteriol. 185:1518-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nidetzky, B., W. Steiner, and M. Claeyssens. 1995. Synergistic interaction of cellulases from Trichoderma reesei during cellulose degradation, p. 90-112. In J. N. Saddler and M. E. Himmel (ed.), Enzymatic degradation of insoluble carbohydrates. American Chemical Society Symposium Series 618. American Chemical Society, Washington, D.C.
- 30.Rahman, A. K. M., N. Sugitani, M. Hatsu, and K. Takamizawa. 2003. A role of xylanase, α-l-arabinofuranosidase, and xylosidase in xylan degradation. Can. J. Microbiol. 49:58-64. [DOI] [PubMed] [Google Scholar]
- 31.Saha, B. C., and R. J. Bothast. 1999. Pretreatment and enzymatic saccharification of corn fiber. Appl. Biochem. Biotechnol. 76:65-77. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Schwarz, W. H. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl. Microbiol. Biotechnol. 56:634-649. [DOI] [PubMed] [Google Scholar]
- 34.Shoham, Y., R. Lamed, and E. A. Bayer. 1999. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 7:275-281. [DOI] [PubMed] [Google Scholar]
- 35.Shoseyov, O., and R. H. Doi. 1990. Essential 170-kDa subunit for degradation of crystalline cellulose of Clostridium cellulovorans cellulase. Proc. Natl. Acad. Sci. USA 87:2192-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoseyov, O., M. M. Takagi, M. A. Goldstein, and R. H. Doi. 1992. Primary sequence analysis of Clostridium cellulovorans cellulose binding protein A. Proc. Natl. Acad. Sci. USA 89:3483-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sleat, R., R. A. Mah, and R. Robinson. 1984. Isolation and characterization of an anaerobic, cellulolytic bacterium, Clostridium cellulovorans sp. nov. Appl. Environ. Microbiol. 48:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorensen, H. R., A. S. Meyer, and S. Pedersen. 2003. Enzymatic hydrolysis of water wheat arabinoxylan. 1. Synergy between α-l-arabinofuranosidases, endo-1,4-β-xylanases, and β-xylosidase activities. Biotechnol. Bioeng. 81:726-731. [DOI] [PubMed] [Google Scholar]
- 39.Takagi, M., S. Hashida, M. A. Goldstein, and R. H. Doi. 1993. The hydrophobic repeated domain of the Clostridium cellulovorans cellulose-binding protein (CbpA) has specific interactions with endoglucanases. J. Bacteriol. 175:7119-7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamaru, Y., and R. H. Doi. 2000. The engL gene cluster of Clostridium cellulovorans contains a gene for cellulosomal ManA. J. Bacteriol. 182:244-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamaru, Y., and R. H. Doi. 2001. Pectate lyase A, an enzymatic subunit of the Clostridium cellulovorans cellulosome. Proc. Natl. Acad. Sci. USA 98:4125-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamaru, Y., S. Karita, A. Ibrahim, H. Chan, and R. H. Doi. 2000. A large gene cluster for the Clostridium cellulovorans cellulosome. J. Bacteriol. 182:5906-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teunissen, M. J., and H. J. M. Op den Camp. 1993. Anaerobic fungi and their cellulolytic and xylanolytic enzymes. Antonie Leeuwenhoek 63:63-76. [DOI] [PubMed] [Google Scholar]
- 44.Vries, R. P., H. C. M. Kester, C. H. Poulsen, J. A. E. Benen, and J. Visser. 2000. Synergy between enzymes from Aspergillus involved in the degradation of plant cell wall polysaccharides. Carbohydr. Res. 327:401-410. [DOI] [PubMed] [Google Scholar]
- 45.Wilson, D. B. 2004. Studies of Thermobifida fusca plant cell wall degrading enzymes. Chem. Rec. 4:72-82. [DOI] [PubMed] [Google Scholar]
- 46.Wood, T. M., S. I. McCrae, and K. M. Bhat. 1989. The mechanism of fungal cellulase action. Synergism between enzyme components of Penicillium pinophilum cellulase in solubilizing hydrogen bond-ordered cellulose. Biochem. J. 260:37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]