Abstract
The ABC transporter (TliDEF) from Pseudomonas fluorescens SIK W1, which mediated the secretion of a thermostable lipase (TliA) into the extracellular space in Escherichia coli, was engineered using directed evolution (error-prone PCR) to improve its secretion efficiency. TliD mutants with increased secretion efficiency were identified by coexpressing the mutated tliD library with the wild-type tliA lipase in E. coli and by screening the library with a tributyrin-emulsified indicator plate assay and a microtiter plate-based assay. Four selected mutants from one round of error-prone PCR mutagenesis, T6, T8, T24, and T35, showed 3.2-, 2.6-, 2.9-, and 3.0-fold increases in the level of secretion of TliA lipase, respectively, but had almost the same level of expression of TliD in the membrane as the strain with the wild-type TliDEF transporter. These results indicated that the improved secretion of TliA lipase was mediated by the transporter mutations. Each mutant had a single amino acid change in the predicted cytoplasmic regions in the membrane domain of TliD, implying that the corresponding region of TliD was important for the improved and successful secretion of the target protein. We therefore concluded that the efficiency of secretion of a heterologous protein in E. coli can be enhanced by in vitro engineering of the ABC transporter.
Gram-negative bacteria use several strategies to secrete proteins across the inner and outer membranes into the extracellular environment. So far, five classes of secretion pathways have been identified in these bacteria (33). The type I secretion pathway (ABC transporter) examined in this study is rather different from the widespread type II secretion pathway. Proteins secreted by the type I pathway lack an amino-terminal (N-terminal) signal sequence. They cross both membranes without a periplasmic intermediate. The target proteins have an uncleaved carboxy-terminal (C-terminal) signal sequence containing several repeats of the glycine-rich sequence GGXGXD (12, 30) and a predicted amphipathic α-helix region (17, 26). The ABC transporter consists of three components, the ATP binding cassette (ABC) protein, the membrane fusion protein (MFP), and the outer membrane protein (OMP) (5). The ABC protein is an inner membrane protein composed of an N-terminal membrane domain containing six to eight transmembrane segments (14, 39) and a C-terminal ATPase domain (27). The ABC protein belongs to the well-characterized ABC protein superfamily, which includes eukaryotic and prokaryotic proteins related to the import or export of a wide variety of substrates, such as ions, antibiotics, sugars, amino acids, oligosaccharides, peptides, and proteins (21). It recognizes the C-terminal signal sequence of the target protein and supplies energy from ATP hydrolysis for secretion of the target protein (11, 25). MFP is exposed mainly to the periplasm and has one transmembrane segment anchored in the inner membrane (35). MFP connects the ABC protein and OMP during formation of the transport complex (23, 40). OMP is an outer membrane porin protein that forms a tunnel across the periplasm and the outer membrane (28).
Escherichia coli has long been the most versatile host for the production of recombinant proteins (3, 32). Generally, during cultivation of E. coli there are three compartments for accumulation of recombinant proteins. Secretion of recombinant proteins into the extracellular space has some advantages over production in the other compartments, the cytoplasm and the periplasm. First, it does not result in the formation of inclusion bodies and the proteolytic degradation of recombinant proteins (32). Second, it simplifies separation and purification of recombinant proteins (32). Third, it allows continuous production of recombinant proteins since cells do not have to be lysed for recovery of the proteins (6, 24, 32). Moreover, the ABC transporter has additional advantages over other secretion pathways. Compared with other secretion machineries, the ABC transporter machinery consists of only three protein components, and thus its complexity is relatively low and it can be engineered and modified by molecular evolutionary approaches (19). Because the target proteins secreted by the ABC transporter have a distinct C-terminal signal sequence, their secretion generally does not interfere with the endogenous Sec-dependent export pathway concerned with the export of essential periplasmic and outer membrane proteins (13). Therefore, the ABC transporter has been used to secrete a number of recombinant proteins (6, 7, 22), such as the single-chain Fv antibody (13), vaccine antigen (15, 16, 36), and human interleukin (18), etc.
To make the ABC transporter a more efficient system for production of recombinant proteins, it is essential to enhance its secretion efficiency. To our knowledge, there have been no reports on the enhancement of the efficiency of secretion of the target protein by engineering the ability of the ABC transporter to secrete. We previously cloned all genes encoding the ABC transporter and showed that this transporter could mediate the extracellular secretion of TliA in recombinant E. coli (1). Here, we describe the use of directed evolution (29, 38) (error-prone PCR) to create TliD variants exhibiting an increased level of secretion of the heterologous TliA lipase in E. coli.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
E. coli XL10-Gold {Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB lacIqZΔ15 Tn10 (Tetr) Tn5 (Kanr) Amy]} (Stratagene, San Diego, Calif.) was used as a host strain for DNA manipulation and gene expression. Plasmids pACYC184 (New England Biolabs, Beverly, Mass.) and pKK223-3 (Amersham Pharmacia. Piscataway, N.J.) were used as vectors. Plasmids pACYC184 and pKK223-3 were used for construction of pABCSK-ACYC harboring ABC transporter-encoding genes (i.e., a gene cassette composed of tliD, tliE, and tliF from Pseudomonas fluorescens SIK W1) and pTliA-PKK harboring a lipase-encoding gene (tliA from P. fluorescens SIK W1), respectively. Luria-Bertani (LB) medium was used for growth of recombinant E. coli. When necessary, ampicillin (50 μg/ml) and chloramphenicol (34 μg/ml) were added to the growth media.
DNA manipulation and sequencing.
Standard recombinant DNA manipulation techniques were used for isolation of plasmids, restriction endonuclease digestion, ligation, transformation into E. coli, agarose gel electrophoresis of DNA, and purification of DNA fragments. All restriction enzymes, DNA-modifying enzymes, and related reagents used for DNA manipulation were purchased from Takara Shuzo (Shiga, Japan), Solgent (Daejeon, Korea), or Sigma (St. Louis, Mo.). DNA sequences were determined by cycle sequencing with an ABI PRISM BigDye primer cycle sequencing kit with AmpliTaq DNA polymerase (Perkin-Elmer, Foster City, Calif.).
Construction of plasmids.
PCR amplification of the tliA gene was performed with pTOTAL, which contained all four tli genes (tliD, tliE, tliF, and tliA), as the template DNA (1) and with two primers flanked by EcoRI or HindIII restriction sites (5′-ACAGAATTCATGGGTGTATTTGACTACAAG-3′ and 5′-CAGAAGCTTCATGAACCGCCGATAATCCGT-3′). The amplified PCR products were digested with EcoRI and HindIII and then ligated with the same enzyme-digested pKK223-3 plasmid, resulting in plasmid pTliA-PKK. In order to facilitate the introduction of random mutations into only the tliD gene, plasmid pABCSK-ACYC was prepared by inserting SacI and KpnI restriction sites at the 5′ and 3′ ends of the tliD gene of pABC-ACYC (1), respectively, by using the overlapping PCR technique (34).
Random mutagenesis and mutant library construction.
Random mutagenesis of the tliD gene was carried out by performing mutagenic PCR (10) using pABCSK-ACYC as the template. Two oligonucleotides, 5′-GGTCTAGATTTCAGTGCAATTTATCTCTTCAA-3′ and 5′-ATTGGATCCCCGCCTGCTCAC-3′, were used as the forward and reverse primers, respectively. In order to obtain the desired level of mutation (0.5 to 2 nucleotide substitutions per 1 kb of gene), the conditions used for PCR random mutagenesis were optimized; a 100-μl reaction mixture contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 7 mM MgCl2, 0.2 mM dATP, 0.2 mM dGTP, 1 mM dCTP, 1 mM dTTP, 25 pmol of each oligonucleotide primer, 5 ng of template, and 5 U of Taq polymerase (Solgent, Daejeon, Korea). The PCR was performed with an automatic thermal cycler (Bio-Rad, Hercules, Calif.) for 30 cycles consisting of 94°C for 1 min, 58°C for 1 min, and 72°C for 3 min. The mutagenic PCR products were digested with SacI and KpnI and were gel purified by using a QIAGEN kit (QIAGEN, Hilden, Germany). The purified PCR products were ligated with the same enzyme-digested pABCSK-ACYC. E. coli cells harboring pTliA-PKK (E. coli/pTliA-PKK) were transformed with the resulting ligated DNA and were plated onto LB agar plates containing 3% (vol/vol) tributyrin (LB-TB plates) with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), 50 μg/ml ampicillin, and 34 μg/ml chloramphenicol.
Screening procedure.
E. coli cells harboring a randomly mutated tliD gene with wild-type tliE, tliF, and tliA genes were incubated on LB-TB plates at 25°C. The level of lipase activity secreted by TliDEF variants was semiquantitatively estimated by comparing the sizes of clear halos surrounding the lipase-secreting colonies. While E. coli cells expressing only the tliA gene could not form any clear halos, E. coli coexpressing the tliA and tliDEF genes did form clear halos on LB-TB plates. To guarantee more precise and more reproducible screening for the extracellular lipase activities, it is important to ensure consistent thickness and flatness of LB-TB plates. Twenty-five milliliters of tributyrin-emulsified LB agar was used for each 83-mm petri dish, and the plates were laid on a flat surface until the agar became hard. The colonies that formed larger halos among all the members of the tliD library were selected. The selected colonies were transferred to 1 ml LB medium in each well of 96-well deep plates and incubated at 25°C for 18 h. After incubation for 18 h, the cells were induced with 1 mM IPTG and then incubated at 25°C for 12 h. Cell growth was monitored by measuring the absorbance at 600 nm with a microplate reader (Bio-Rad). Cells were centrifuged at 4°C at 5,000 × g for 30 min, and the lipase activity in the culture supernatant of each culture was assayed using p-nitrophenyl palmitate (pNPP). The lipase activity was normalized to unit cell density. All experiments were carried out in triplicate.
Preparation of cell fractions.
The recombinant E. coli cells were cultured at 25°C in 100 ml LB medium. The cells were harvested at the stationary phase (optical density at 600 nm [OD600], 4) and centrifuged at 4°C at 13,000 × g for 10 min. The culture supernatants were concentrated fivefold using a Centricon (Millipore, Bedford, Mass.) for immunoblot analysis. In order to prepare cell lysates for assays of the intracellular lipase activity of the recombinant E. coli cells, cells at an OD600 equivalent of 0.15 were lysed by addition of 50 μl turbolytic solution (Genofocus, Daejeon, Korea), followed by incubation at 25°C for 30 min. One OD600 equivalent was the amount of cells or culture supernatant corresponding to 1 ml of cell culture at an OD600 of 1. For preparation of crude membrane fractions, the cells were disrupted with a French press (American Instrument Company, Silver Spring, Md.) and crude membrane pellets were collected by centrifugation at 4°C at 100,000 × g for 30 min.
Immunoblot analysis.
For analysis of the level of secretion of TliA in the recombinant E. coli cells, the culture supernatants of recombinant E. coli cells were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and then were electrophoretically transferred onto a nitrocellulose membrane. TliA was detected with anti-TliA antiserum as described previously (1). The level of expression of the TliDEF transporter in the recombinant E. coli cells was estimated by measuring the TliD protein level by immunoblot analysis using the anti-TliD antiserum. The glutathione S-transferase (Amersham Pharmacia) gene and the six-histidine tag-encoding sequence were fused at the 5′ and 3′ ends of the tliD gene, respectively. The fusion gene was expressed in E. coli and was purified with nickel-nitrilotriacetic acid agarose resin (QIAGEN). The antiserum against the TliD protein was obtained by injecting a mouse with the fused TliD protein in Freund's complete adjuvant (20). Membrane fractions of the recombinant E. coli cells were subjected to SDS-PAGE and then were electrophoretically transferred onto a nitrocellulose membrane. TliD was detected with anti-TliD antiserum. The densities of signals on immunoblots were quantified with a GS710 calibrated imaging densitometer (Bio-Rad).
Assay of lipase activity.
Lipase activity was assayed quantitatively by a spectrophotometric method using pNPP as a substrate (9). The pNPP was dissolved in acetonitrile at a concentration of 10 mM. Ethanol and 50 mM Tris-HCl (pH 8.5) buffer were subsequently added to this solution, in which the final ratio of acetonitrile to ethanol to Tris-HCl buffer was 1:4:95 (vol/vol/vol). Fifty microliters of culture supernatant or cell lysate was added to 200 μl of the pNPP solution and incubated at 45°C for 20 min, and the lipase activity was assayed by measuring the absorbance at 405 nm (the extinction coefficient of p-nitrophenol [ɛ] was 18.1 cm2/μmol) with a microplate reader (Bio-Rad). One unit of enzyme activity was defined as the amount of enzyme that released 1 μmol of p-nitrophenol per min.
Assay of protease activity.
Protease activity was measured by a slight modification of the method of Braun and Schmitz (8). Two hundred microliters of culture supernatant was added to 600 μl of a substrate solution containing 2.4% (wt/vol) azocasein in 50 mM potassium phosphate buffer (pH 7.5), and the mixture was incubated at 37°C for 30 min. The enzyme reaction was stopped by adding 600 μl of 10% trichloroacetic acid, and the mixture was allowed to stand for 1 h at 25°C. After centrifugation at 13,000 × g for 10 min, 800 μl of supernatant was collected and added to 300 μl of 10 N NaOH. The absorbance at 420 nm was measured with a spectrophotometer (Shimadzu, Kyoto, Japan). One unit of enzyme activity was defined as the amount of enzyme required to increase the absorbance per min by 1 under the experimental conditions.
RESULTS
Isolation of mutant ABC transporters exhibiting increased secretion of lipase.
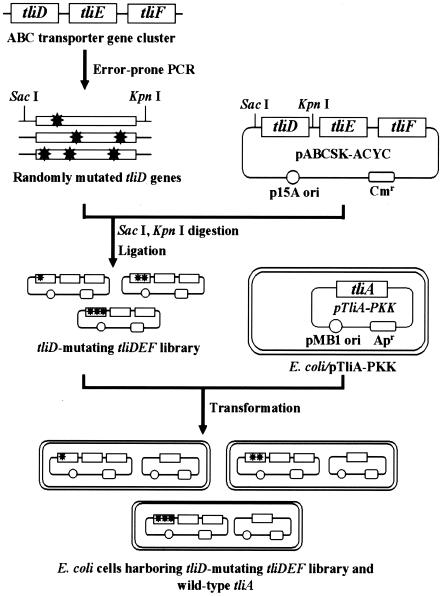
As a strategy to obtain ABC transporter variants showing increased efficiency in the secretion of recombinant proteins, we applied the directed evolution techniques to the E. coli system composed of the TliDEF transporter and the TliA lipase from P. fluorescens. In fact, we previously found that the TliDEF transporter can successfully function extracellularly to produce the TliA lipase from E. coli and Pseudomonas hosts (1, 2). One of the three component proteins of the ABC transporter, the ABC protein (TliD), plays an important role in secretion of target protein by recognizing the signal sequence of the target protein and subsequently supplying energy from ATP hydrolysis (11, 25). In addition, the binding of the ABC protein to the signal sequence of target proteins induces the sequential assembly of MFP and OMP (31), and the assembly of these components subsequently initiates the secretion of the target protein into extracellular space (4, 37). As a result, the interactions between the ABC protein and the target protein are the most decisive steps in successful secretion of a target protein. In this study, therefore, random point mutations were introduced only into the tliD gene by error-prone PCR, and the library of tliD variants was coexpressed with wild-type tliA in E. coli (Fig. 1).
FIG. 1.
Schematic representation of the experimental procedures for constructing a library of ABC transporter mutants and coexpressing the library with the target lipase (tliA) gene. The randomly mutated ABC protein-encoding gene (tliD), a component of the ABC transporter-encoding gene cluster (tliDEF) of P. fluorescens, was inserted into SacI- and KpnI-digested pABCSK-ACYC, yielding a library of tliD-mutated tliDEF variants. The wild-type tliA gene encoding the target protein of the wild-type ABC transporter was coexpressed with the library in E. coli. The extracellular TliA activity secreted due to the action of each ABC transporter variant was screened by the lipase activity-based screening methods using LB-TB indicator plates and a 96-well plate pNPP assay. p15A ori, p15A origin; pMB1 ori, pMB1 origin; Cmr, chloramphenicol resistance gene; Apr, ampicillin resistance gene. Asterisks indicate the locations of mutations.
Plasmid pABCSK-ACYC containing the tliDEF genes was used as a template DNA in the first mutagenic PCR to introduce random point mutations only into the TliD-encoding region. E. coli cells carrying the tliA gene on a compatible plasmid, pTliA-PKK, were transformed with the resultant library of tliD variants. For library preselection, the cells were grown on semiquantitative indicator plates (LB-TB plates), and the capacities of different tliD variants to secrete the target lipase were estimated. From approximately 10,000 colonies, we selected 200 colonies that formed relatively larger halos from all members of the tliD library. The colonies selected were transferred to 1 ml LB medium in each well of 96-well deep plates and cultured at 25°C. The extracellular lipase activity of each clone was measured and compared with that of the clone harboring the wild-type tliD gene. While 20% of the clones showed lipase activity comparable to that of clones harboring the wild-type tliD gene, 50% of the clones showed an increase in the extracellular lipase activity of about 30 to 100% and 10% of the clones showed an increase in the extracellular lipase activity of more than 100%. The remaining 20% of the clones showed a decrease in the extracellular lipase activity of up to 50% (data not shown). Four of the clones showing the highest extracellular lipase activity were isolated. Plasmids encoding each mutant protein were isolated and pooled (equal amounts of all plasmids). We used this plasmid mixture as a template DNA in the second round of mutagenesis in order to further improve the efficiency of secretion. After the second round of PCR mutagenesis and two-step screening of 10,000 clones, however, no clones that showed higher extracellular lipase activity were identified. Consequently, we selected four clones from the first round of mutagenesis for further characterization. The plasmids of these clones were designated pT7, pT8, pT24, and pT35 (Fig. 2).
FIG. 2.
TliD variants on an activity indicator plate used in the first step of the two-step screening procedure. Semiquantitative estimation of the extracellular secretion of target lipase (TliA) was performed by comparing the sizes of lipolytic clear halos. After E. coli cells containing each TliD variant isolated from the first round of mutagenesis and screening were incubated on the LB-TB plate at 25°C for 48 h, they produced larger halos than the wild-type TliDEF. Colony 1, E. coli(pTliA-PKK, pACYC184) (negative control); colony 2, E. coli(pTliA-PKK, pABCSK-ACYC) (wild-type tliDEF gene); colony 3, E. coli(pTliA-PKK, pT6); colony 4, E. coli (pTliA-PKK, pT8); colony 5, E. coli (pTliA-PKK, pT24); colony 6, E. coli (pTliA-PKK, pT35).
Characterization of selected ABC transporter variants.
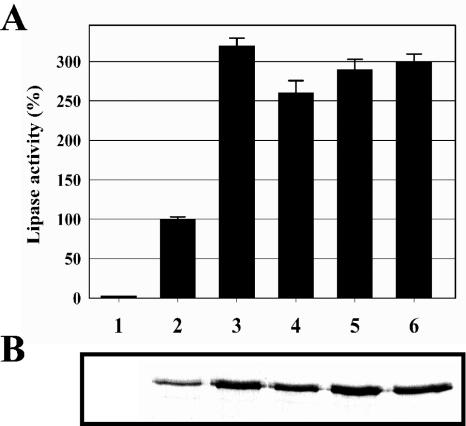
E. coli/pTliA-PKK clones harboring the wild-type and mutant tliD genes were cultured in 100 ml LB medium at 25°C. The extracellular and intracellular lipase activities of each clone were assayed with culture supernatant and cell lysate, respectively (Table 1). Compared with the wild-type TliDEF, the selected TliD variants, T6, T8, T24, and T35, showed 3.2-, 2.6-, 2.9-, and 3.0-fold increases in the level of secretion of TliA, respectively (Fig. 3A). Immunoblot analysis was also used to estimate the level of secretion of TliA in the culture supernatant of each clone. As shown in Fig. 3B, extracellular TliA secretion by the E. coli/pTliA-PKK clones expressing the mutant tliD genes was significantly increased. Based on the results shown in Fig. 3, introduction of a random mutation into the gene encoding the ABC protein TliD, a constituent of the ABC transporter cluster, could increase the level of secretion of TliA, a target protein of the ABC transporter cluster, in a recombinant E. coli host. Therefore, we concluded that the ABC transporter machinery, in particular the ABC protein, could be successfully engineered by directed evolution for improved secretion of a target protein.
TABLE 1.
Secretion of lipase by recombinant E. coli(pTliA-PKK) cells harboring the wild-type and mutant tliD genesa
| Plasmids | Lipase activity (U ml−1 OD600−1)b
|
Secretion efficiency (%)c | |
|---|---|---|---|
| Cell lysate | Culture supernatant | ||
| pTliA-PKK + pACYC184 | 9.6 ± 0.1 | NDd | 0 |
| pTliA-PKK + pABCSK-ACYC | 11.6 ± 0.1 | 2.8 ± 0.1 | 19.4 ± 0.1 |
| pTliA-PKK + pT6 | 5.7 ± 0.2 | 9.1 ± 0.3 | 61.5 ± 0.6 |
| pTliA-PKK + pT8 | 6.1 ± 0.2 | 7.3 ± 0.4 | 54.4 ± 2.2 |
| pTliA-PKK + pT24 | 5.8 ± 0.1 | 8.2 ± 0.4 | 58.5 ± 1.6 |
| pTliA-PKK + pT35 | 5.9 ± 0.3 | 8.5 ± 0.3 | 59.0 ± 2.1 |
E. coli cells harboring the plasmids were grown at 25°C for 30 h in LB medium. The cells were harvested at the stationary phase (OD600, 4).
All experiments were carried out in triplicate.
Secretion efficiency = lipase activity in culture supernatant/(lipase activity in culture supernatant + lipase activity in cell lysate) × 100.
ND, not detected.
FIG. 3.
Comparison of the amounts of lipase secreted by E. coli/pTliA-PKK cells harboring the wild-type and mutant tliD genes. (A) Relative activity of the lipase in culture supernatant. E. coli cells harboring each plasmid were grown at 25°C for 30 h in LB medium. The lipase activities were expressed as percentages of the activity produced by E. coli (pTliA-PKK, pABCSK-ACYC). (B) Immunoblot analysis of the lipase in culture supernatant. Culture supernatant at an OD600 equivalent of 0.36 was subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and analyzed by immunoblot analysis using anti-TliA antiserum. Lane 1, pTliA-PKK plus pACYC184; lane 2, pTliA-PKK plus pABCSK-ACYC; lane 3, pTliA-PKK plus pT6; lane 4, pTliA-PKK plus pT8; lane 5, pTliA-PKK plus pT24; lane 6, pTliA-PKK plus pT35.
In order to determine whether the improved ability to secrete the target protein TliA was due to a difference in the level of expression of the TliD transporter in the membrane of the E. coli host, an immunoblot analysis of the TliD protein was performed with membrane fractions of E. coli harboring the wild-type and mutant tliD genes (Fig. 4). The levels of expression of TliD in the membrane fraction were almost the same for all clones tested. Introduction of a mutation into the tliD gene, therefore, did not affect the level of expression of the TliD transporter in E. coli. This result indicates that the increased level of secretion of TliA was not due to the increased expression of TliD variants in the E. coli host but was due to enhancement of the variants' ability to secrete.
FIG. 4.
Comparison of levels of TliD expression in the membrane fraction of E. coli/pTliA-PKK cells harboring the wild-type and mutant tliD genes. E. coli cells harboring each plasmid were grown at 25°C for 30 h in LB medium. Membrane fractions at an OD600 equivalent of 1.2 were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and analyzed by immunoblot analysis using anti-TliD antiserum. Lane 1, pTliA-PKK plus pACYC184; lane 2, pTliA-PKK plus pABCSK-ACYC; lane 3, pTliA-PKK plus pT6; lane 4, pTliA-PKK plus pT8; lane 5, pTliA-PKK plus pT24; lane 6, pTliA-PKK plus pT35.
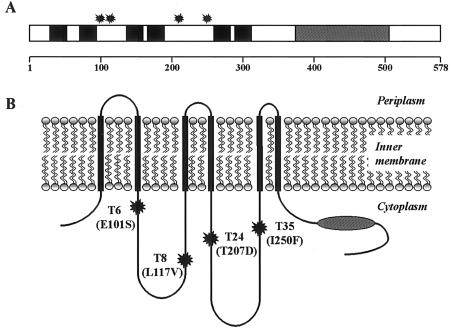
Each of the four TliD variants had a single point mutation leading to one amino acid change in TliD. All the mutations were located in the predicted cytoplasmic regions in the membrane domain of TliD (Fig. 5). This result also indicates that the corresponding region of TliD may be the important site for affecting secretion of TliA into extracellular space.
FIG. 5.
Location of mutations in the TliD-encoding region of selected TliDEF mutants. (A) Mutations in TliD in the primary structure of TliD. The bar represents the 578 amino acids of the TliD-encoding region, and the line at the bottom indicates the relative positions. The solid boxes represent the transmembrane segments, and the shaded box indicates the ATP binding region. The asterisks indicate the locations of mutations. (B) Location of mutations in the secondary structure of TliD in the inner membrane of E. coli. The secondary structure of TliD was predicted by the SOSUI program (http://sosui.proteome.bio.tuat.ac.jp). The shaded oval indicates the ATP binding region.
DISCUSSION
The goal of this work was to enhance the ability of the ABC transporter to secrete its target protein. In this study, we demonstrated that introduction of a mutation only into the tliD gene encoding one of the three component proteins of the TliDEF transporter could increase the ability of this transporter to secrete. To isolate TliDEF variants in order to increase the amount of lipase secreted, we performed a two-step activity-based screening procedure involving an LB-TB indicator plate and a 96-well microtiter plate. First, a semiquantitative analysis of the lipase secreted on LB-TB plates by a number of TliDEF variants was carried out by comparing the sizes of clear halos. To make the size of a clear halo proportional to the amount of lipase secreted, we ensured consistent thickness and flatness of LB-TB indicator plates and determined the optimum incubation times (40 to 48 h) at which most of the library members exhibited a suitable range of halo sizes. Under the conditions used in this first screening step, we could reduce the number of candidate clones from approximately 10,000 to 200 by a 2% ratio. When the extracellular lipase activity of each clone was assayed in the second screening step based on the 96-well plate format, approximately 60% of the 200 clones showed an increase in extracellular lipase activity compared to the clones expressing wild-type TliD. This result indicates that LB-TB plate-based visual screening was effective for reducing the number of clones to be tested with the 96-well plate-based lipase assay.
Three factors are considered the important determinants of efficient secretion by an ABC transporter: (i) the intracellular amount of target proteins, which is mainly influenced by the total expression level, (ii) the total amount of ABC transporter in the membrane of E. coli, and (iii) the ability of the ABC transporter to secrete. In order to improve the last factor, tliA and tliDEF were subcloned into different plasmids, pKK223-3, which is a medium-copy-number plasmid under the control of the inducible tac promoter, and pACYC184, which is a low-copy-number plasmid under the control of a constitutive tet promoter, respectively. In this manner, the expression of the target protein in each member of the E. coli library could be maintained at levels much higher than those observed with the ABC transporter, and therefore, an increased ability of the ABC transporter that could continuously secrete the excess amount of target protein was successfully identified.
The DNA sequence analysis revealed that only single amino acid substitutions occurred in all the mutants selected and that they were located in the predicted cytoplasmic regions in the membrane domain of TliD. Meanwhile, when an additional mutation was introduced into the clones from the first round during the second round of mutagenesis and screening, we did not obtain any clones that showed higher extracellular lipase activity. Thus, it is certain that just one amino acid change in TliD of the TliDEF transporter can improve the ability of the TliDEF transporter to secrete the TliA target protein. Our results are comparable with the results of complementation mutagenesis of transport-deficient E. coli hemolysin A (HlyA) mutants (41). The secretion of HlyA is naturally mediated through an E. coli ABC transporter comprised of HlyB (ABC protein), HlyD (MFP), and TolC (OMP) (6). HlyB variants, which could secrete the C-terminal signal sequence-defective HlyA mutant, also had single point mutations that changed one amino acid of the predicted cytoplasmic regions in the membrane domain of HlyB. Therefore, we believe that all the TliD mutations resulted in more efficient interaction of TliD variants with the C-terminal signal sequence of TliA and thus facilitated the secretion of TliA. However, the detailed mechanism and why mutation of TliD increased the ability of the TliDEF transporter to secrete TliA are still unclear.
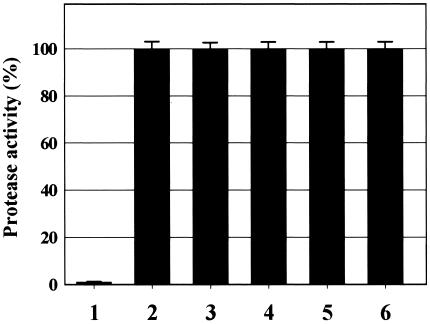
In order to determine the effect of our TliDEF mutants on the efficiency of secretion of another target protein, we attempted to use TliDEF mutants to secrete the protease PrtA, which was encoded upstream of the tliDEF genes in the natural microbial host, P. fluorescens SIK W1 (1). The primary structure of the C-terminal signal sequences of PrtA bears little similarity to that of TliA; nevertheless, PrtA is secreted through the wild-type TliDEF transporter with an efficiency comparable to that of TliA secretion. When the amount of PrtA secreted by each of the TliDEF mutants was examined, the results showed that none of the TliDEF mutants exhibited increased secretion of PrtA (Fig. 6). The fact that the mutations that resulted in the increase in lipase secretion did not influence the secretion of PrtA indicates that the TliDEF transporter recognizes the signal sequences of TliA and PrtA in different ways.
FIG. 6.
Comparison of the amounts of secreted protease from E. coli/pTliA-PKK cells harboring the wild-type and mutant tliD genes: relative activity of the protease in culture supernatant. E. coli cells harboring each plasmid were grown at 25°C for 30 h in LB medium. The protease activities were expressed as percentages of the activity produced by E. coli(pTliA-PKK, pABCSK-ACYC). Bar 1, pTliA-PKK plus pACYC184; bar 2, pTliA-PKK plus pABCSK-ACYC; bar 3, pTliA-PKK plus pT6; bar 4, pTliA-PKK plus pT8; bar 5, pTliA-PKK plus pT24; bar 6, pTliA-PKK plus pT35.
The ABC transporter has received some biotechnological attention as a system for production of recombinant proteins in E. coli. First, it is important to increase the efficiency of secretion of the target protein (for example, by improving the ability of the ABC transporter to secrete). In addition, increasing the amount of the functional ABC transporter located in the cellular membrane can be another way to increase the efficiency of secretion of a target protein. Based on our results, we concluded that the efficiency of secretion of the target protein was successfully increased by enhancing the ability of the ABC transporter to secrete through directed evolution techniques and not by increasing the amount of the functional ABC transporter. Therefore, we are now attempting to find an appropriate coexpression system to maximize the secretion of a functional target protein by modulating the level of ABC transporter expression in the membrane of E. coli.
In addition to the extracellular production of useful proteins in E. coli, the protein secretion system using the ABC transporter should also be a very efficient E. coli expression vector system for high-throughput screening. Foreign protein variants which are fused with a C-terminal signal sequence suited for the ABC transporter machinery can be secreted into the extracellular medium, although not all of them fold correctly into their functional forms. If a protein that has been difficult to screen in other expression systems can be secreted by the ABC transporter, it can be used more easily for high-throughput screening for directed evolution work. Now we are also investigating the usefulness of the ABC transporter system for activity-based high-throughput screening of a number of foreign protein variants.
Acknowledgments
This work was supported in part by the 21C Frontier Microbial Genomics and Application Center Program, Ministry of Science & Technology (grant MG05-0103-3-0), Republic of Korea.
REFERENCES
- 1.Ahn, J. H., J. G. Pan, and J. S. Rhee. 1999. Identification of the tliDEF ABC transporter specific for lipase in Pseudomonas fluorescens SIK W1. J. Bacteriol. 181:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, J. H., J. G. Pan, and J. S. Rhee. 2001. Homologous expression of the lipase and ABC transporter gene cluster, tliDEFA, enhances lipase secretion in Pseudomonas spp. Appl. Environ. Microbiol. 67:5506-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baneyx, F. 1999. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 10:411-421. [DOI] [PubMed] [Google Scholar]
- 4.Binet, R., and C. Wandersman. 1995. Protein secretion by hybrid bacterial ABC-transporters: specific functions of the membrane ATPase and the membrane fusion protein. EMBO J. 14:2298-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binet, R., S. Letoffe, J. M. Ghigo, P. Delepelaire, and C. Wandersman. 1997. Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene 192:7-11. [DOI] [PubMed] [Google Scholar]
- 6.Blight, M. A., and I. B. Holland. 1994. Heterologous protein secretion and the versatile Escherichia coli haemolysin translocator. Trends Biotechnol. 12:450-455. [DOI] [PubMed] [Google Scholar]
- 7.Blight, M. A., C. Cheravaux, and I. B. Holland. 1994. Protein secretion pathway in Escherichia coli. Curr. Opin. Biotechnol. 5:468-474. [DOI] [PubMed] [Google Scholar]
- 8.Braun, V., and G. Schmitz. 1980. Excretion of a protease by Serratia marcescens. Arch. Microbiol. 124:55-61. [DOI] [PubMed] [Google Scholar]
- 9.Bulow, L., and K. Mosbach. 1987. The expression in Escherichia coli of a polymeric gene coding for a esterase mimic catalyzing the hydrolysis of p-nitrophenyl esters. FEBS Lett. 210:147-152. [DOI] [PubMed] [Google Scholar]
- 10.Cadwell, R. C., and G. F. Joyce. 1992. Randomization of genes by PCR mutagenesis. PCR Methods Appl. 2:28-33. [DOI] [PubMed] [Google Scholar]
- 11.Delepelaire, P. 1994. PrtD, the integral membrane ATP-binding cassette component of the Erwinia chrysanthemi metalloprotease secretion system, exhibits a secretion signal-regulated ATPase activity. J. Biol. Chem. 269:27952-27957. [PubMed] [Google Scholar]
- 12.Duong, F., C. Soscia, A. Lazdunski, and M. Murgier. 1994. The Pseudomonas fluorescens lipase has a C-terminal secretion signal and is secreted by a three-component bacterial ABC-exporter system. Mol. Microbiol. 11:1117-1126. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez, L. A., I. Sola, L. Enjuanes, and V. de Lorenzo. 2000. Specific secretion of active single-chain Fv antibodies into the supernatants of Escherichia coli cultures by use of the hemolysin system. Appl. Environ. Microbiol. 66:5024-5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentschev, I., and W. Goebel. 1992. Topological and functional studies on HlyB of Escherichia coli. Mol. Gen. Genet. 232:40-48. [DOI] [PubMed] [Google Scholar]
- 15.Gentschev, I., H. Mollenkopf, Z. Sokolovic, J. Hess, S. H. E. Kaufmann, and W. Goebel. 1996. Development of antigen-delivery systems, based on the Escherichia coli hemolysin secretion pathway. Gene 179:133-140. [DOI] [PubMed] [Google Scholar]
- 16.Gentschev, I., G. Dietrich, and W. Goebel. 2002. The Escherichia coli α-hemolysin secretion system and its use in vaccine development. Trends Microbiol. 10:39-45. [DOI] [PubMed] [Google Scholar]
- 17.Ghigo, J. M., and C. Wandersman. 1992. Cloning, nucleotide sequence and characterization of the gene encoding the Erwinia chrysanthemi B374 PrtA metalloprotease: a third metalloprotease secreted via a C-terminal secretion signal. Mol. Gen. Genet. 236:135-144. [DOI] [PubMed] [Google Scholar]
- 18.Hahn, H. P., C. Hess, J. Gabelsberger, H. Domdey, and B. U. von Specht. 1998. A Salmonella typhimurium strain genetically engineered to secrete effectively a bioactive human interleukin (hIL)-6 via the Escherichia coli hemolysin secretion apparatus. FEMS Immunol. Med. Microbiol. 20:111-119. [DOI] [PubMed] [Google Scholar]
- 19.Hahn, H. P., and B. U. von Specht. 2003. Secretory delivery of recombinant proteins in attenuated Salmonella strains: potential and limitations of type I protein transporters. FEMS Immunol. Med. Microbiol. 37:87-98. [DOI] [PubMed] [Google Scholar]
- 20.Harlow, E., and D. Lane. 1988. Antibody: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 22.Idei, A., H. Matsumae, E. Kawai, R. Yoshioka, T. Shibatani, H. Akatsuka, and K. Omori. 2002. Utilization of ATP-binding cassette exporter for hyperproduction of an exoprotein: construction of lipase-hyperproducing recombinant strains of Serratia marcescens. Appl. Microbiol. Biotechnol. 58:322-329. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, J. M., and G. M. Church. 1999. Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J. Mol. Biol. 287:695-715. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J. K., E. L. Iannotti, and R. Bajpal. 1999. Extractive recovery of products from fermentation broth. Biotechnol. Bioprocess Eng. 4:1-11. [Google Scholar]
- 25.Koronakis, E., C. Hughes, I. Milisav, and V. Koronakis. 1995. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol. Microbiol. 16:87-96. [DOI] [PubMed] [Google Scholar]
- 26.Koronakis, V., E. Koronakis, and C. Hughes. 1989. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 8:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koronakis, V., C. Hughes, and E. Koronakis. 1993. ATPase activity and ATP/ADP-induced conformational change in the soluble domain of the bacterial protein translocator HlyB. Mol. Microbiol. 8:1163-1175. [DOI] [PubMed] [Google Scholar]
- 28.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 29.Kuchner, O., and F. H. Arnold. 1997. Directed evolution of enzyme catalysts. Trends Biotechnol. 15:523-530. [DOI] [PubMed] [Google Scholar]
- 30.Letoffe, S., and C. Wandersman. 1992. Secretion of CyaA-PrtB and HlyA-PrtB fusion proteins in Escherichia coli: involvement of the glycine-rich repeat domain of Erwinia chrysanthemi protease B. J. Bacteriol. 174:4920-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letoffe, S., P. Delepelaire, and C. Wandersman. 1996. Protein secretion in gram-negative bacteria: assembly of the three components of ABC protein-mediated exporters is ordered and promoted by substrate binding. EMBO J. 15:5804-5811. [PMC free article] [PubMed] [Google Scholar]
- 32.Makrides, S. C. 1996. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol. Rev. 60:512-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omori, K., and A. Idei. 2003. Gram-negative bacterial ATP-binding cassette protein exporter family and diverse secretory proteins. J. Biosci. Bioeng. 95:1-12. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Schulein, R., I. Gentschev, H. Mollenkopf, and W. Goebel. 1992. A topological model for the haemolysin translocator protein HlyD. Mol. Gen. Genet. 234:155-163. [DOI] [PubMed] [Google Scholar]
- 36.Spreng, S., G. Dietrich, W. Goebel, and I. Gentschev. 1999. The Escherichia coli haemolysin secretion apparatus: a potential universal antigen delivery system in Gram-negative bacterial vaccine carriers. Mol. Microbiol. 31:1596-1598. [DOI] [PubMed] [Google Scholar]
- 37.Thanabalu, T., E. Koronakis, C. Hughes, and V. Koronakis. 1998. Substrate-induced assembly of a contiguous channel for protein export from Escherichia coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 17:6487-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobin, M. B., C. Gustafsson, and G. W. Huisman. 2000. Directed evolution: the ‘rational’ basis for ‘irrational’ design. Curr. Opin. Struct. Biol. 10:421-427. [DOI] [PubMed] [Google Scholar]
- 39.Wang, R. C., S. J. Seror, M. Blight, J. M. Pratt, J. K. Broome-Smith, and I. B. Holland. 1991. Analysis of the membrane organization of an Escherichia coli protein translocator, HlyB, a member of a large family of prokaryote and eukaryote surface transport proteins. J. Mol. Biol. 217:441-454. [DOI] [PubMed] [Google Scholar]
- 40.Zgurskaya, H. I., and H. Nikaido. 2000. Multidrug resistance mechanisms: drug efflux across two membranes. Mol. Microbiol. 37:219-225. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, F., J. A. Sheps, and V. Ling. 1993. Complementation of transport-deficient mutants of Escherichia coli alpha-hemolysin by second-site mutations in the transporter hemolysin B. J. Biol. Chem. 268:19889-19895. [PubMed] [Google Scholar]