Abstract
A bacterial community from Danish agricultural soil was enriched with linuron [N-(3,4-dichlorophenyl)-N′-methoxy-N′-methylurea] as the sole carbon and nitrogen source. The community mineralized [ring-U-14C]linuron completely to 14CO2 and 14C-biomass. Denaturing gradient gel electrophoresis analysis and cultivation revealed that a Variovorax sp. was responsible for the mineralization activity.
The phenylurea herbicide linuron [N-(3,4-dichlorophenyl)-N′-methoxy-N′-methylurea] is used worldwide in the conventional production of corn, cereals, vegetables, and fruit. The rates of dissipation in agricultural soils determined by laboratory and field experiments are highly variable, with values ranging from days to several years (2, 8, 9, 11, 16, 23). Linuron is frequently detected in surface and ground waters near or below areas with intensive use, and in one extreme case, linuron was detected in a drinking-water well in concentrations up to 2,800 μg liter−1 (2). Unfortunately, linuron and some of its metabolites are suspected of being endocrine disruptors (12) and of having toxic effects on various aquatic and soil organisms (2, 22), which has stimulated research aimed at studying linuron-mineralizing microorganisms from agricultural soils.
Mixed bacterial cultures able to mineralize linuron have been derived from extensively treated British and Belgian agricultural soils (4, 7, 15). Similar enrichments based on related phenylurea herbicides (1, 3, 6, 19) suggest that this group can serve as carbon and nitrogen sources for bacterial metabolism in agricultural soils. Several attempts to cultivate phenylurea-metabolizing soil bacteria from degradative enrichment cultures, however, were unsuccessful (e.g., studies described in references 7, 15, and 18), and the active bacteria seem reluctant to grow on agar media. Recently, however, the first linuron-mineralizing bacterium, Variovorax sp. strain WDL1 (4), was isolated from previously treated Belgian agricultural soil. Strain WDL1 appeared to be an ineffective linuron degrader in pure culture and dependent on four other consortium members (4). The phenomenon of synergistic bacterial interactions has also been described for the herbicide isoproturon (19, 20). An extensively linuron-treated Danish agricultural field harboring a potential for rapid linuron mineralization was located among three investigated fields. The objective of this study was to obtain linuron-mineralizing enrichment cultures and pinpoint degradative microorganisms by molecular and cultivation-based techniques.
Linuron-mineralizing enrichment cultures.
Bacterial communities capable of rapid linuron mineralization from Danish agricultural soil were enriched (14) by inoculating 5 g (wet weight) soil into sterilized 100-ml glass flasks containing 25 ml of an autoclaved mineral salt solution (MS) (17) with 10 mg liter−1 linuron as the sole source of carbon, nitrogen, and energy. Linuron was added from 10,000-mg liter−1 stock solutions in high-pressure liquid chromatography-grade acetonitrile, and the solvent was evaporated before the addition of the MS. Following inoculation, the flasks were equipped with a 10-ml glass tube containing 2 ml 0.5 M NaOH to trap 14CO2 produced from mineralization of 120,000 dpm [phenyl-U-14C]linuron (16.24 mCi mmol−1; radiochemical purity, >98%; International Isotope, München, Germany) and sealed with airtight glass stoppers before incubation in the dark at 20°C. After a sampling, the NaOH was mixed with 10 ml Wallac OptiPhase HiSafe 3 scintillation cocktail (Turku, Finland), and the cells were counted in a Wallac 1409 liquid scintillation counter. The enrichments were subcultured six times by transferring 1 ml to fresh 24 ml MS containing linuron over a period of 4 months, in which the last three subculturings were in MS with a linuron concentration of 100 mg liter−1. The enrichments were stored in 30% glycerol at −80°C, and before each experiment, the glycerol was removed by centrifugation and the cells were washed twice in MS before inoculation.
Linuron mineralization by bacterial community Sp8-3.
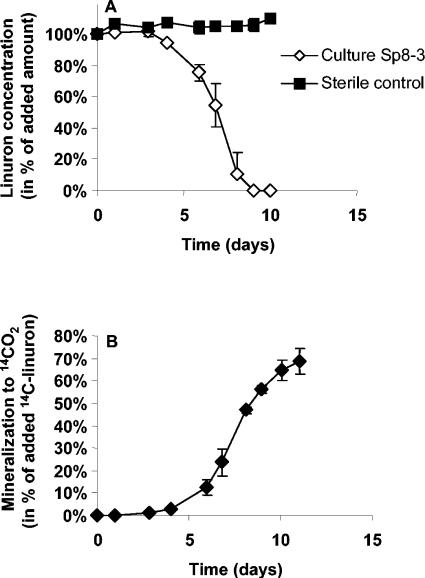
One enrichment culture, designated Sp8-3, mineralized [14C]linuron, with approximately 60 to 70% metabolized to 14CO2 within 10 days (Fig. 1). Linuron mineralization in concentrations of 10, 50, 100, 250, 500, and 1,000 mg liter−1 was verified. High turbidity and extensive cell flocculation were pronounced with the high linuron concentrations. Unless otherwise stated, a linuron concentration of 50 mg liter−1 was used. High-pressure liquid chromatography analysis, using the method described by Juhler et al. (10), revealed no linuron, N-(3,4-dichlorophenyl)-N-methylurea, N-(3,4-dichlorophenyl)urea, or unknown peaks at the end of the experiment. Transient amounts of a metabolite with the same retention time as 3,4-dichloroaniline were detected during linuron mineralization. This metabolite was detected from days 1 to 8 with a maximum concentration of 2.4% of the initial linuron amount, and it was never detected in the sterile controls.
FIG. 1.
Degradation and mineralization of linuron (50.0 mg liter−1) by bacterial community Sp8-3. Two parallel sets of flasks were used for measuring the degradation of linuron (A) and the mineralization of [14C]linuron to 14CO2 (B), respectively. The data are mean values (n = 3). The bars indicate the standard deviations.
PCR and RT-PCR DGGE fingerprinting of community Sp8-3.
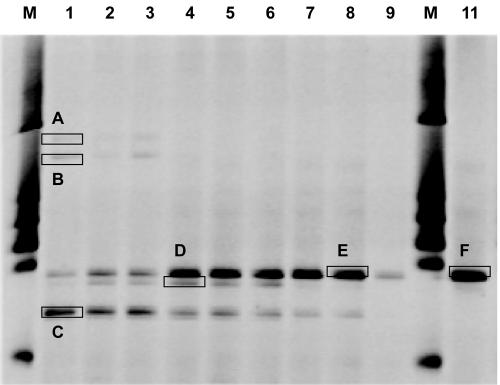
The denaturing gradient gel electrophoresis (DGGE) fingerprinting enabled us to pinpoint Sp8-3 members benefiting from linuron mineralization, facilitating easier cultivation and selection of appropriate strains for detailed analysis. The community structure of Sp8-3 (Fig. 2) was determined during linuron mineralization by DNA extraction (FastDNA; Qbiogene Inc., Carlsbad, CA) and analysis of PCR-amplified 16S rRNA genes from cells from 1.0 ml culture. The metabolically active Sp8-3 members were studied by RNA extraction (FastRNA Pro Blue kit; Qbiogene Inc.) from cells obtained from 2.0 ml subjected to reverse transcription (RT) by using the ThermoScrip RT-PCR system (Invitrogen, Carlsbad, CA) after DNase treatment. Reverse-transcribed cDNA and DNA extracted directly from Sp8-3 were amplified using Bacteria-specific forward primer PRBA338f with a 40-bp clamp and the reverse primer PRUN518r (13) targeting the V3 16S rRNA gene region. The products were separated by DGGE as described by Muyzer et al. (13) with the modifications presented by Thirup et al. (21). Bands of interest were excised and left for two days in Milli-Q water. A 1-μl subsample was reamplified, and the PCR products were purified with the QIAquick PCR purification kit (QIAGEN Ltd., VWR International A/S, Denmark) and subsequently sequenced. Four to five different DGGE bands were detected at day 8 (Fig. 2, lanes 1 through 3) after mineralization of 10 to 30% of the [14C]linuron to 14CO2. Following mineralization of 65 to 72% of the [14C]linuron to 14CO2 at day 15 (Table 1), one DGGE band from 16S rRNA genes had increased in intensity (Fig. 2, lanes 4 through 6). Parallel DGGE analysis of RT 16S rRNA extracted at day 15 revealed a dominant band (Fig. 2, lanes 7 through 9) with a migration pattern similar to that of the dominant 16S rRNA gene-based DGGE band (Fig. 2, lanes 4 through 6). The RNA band intensity obtained from replicate number 3 (Fig. 2, lane 9) was lower than those of bands from replicates 1 and 2 (lanes 7 and 8). The linuron mineralization in replicate 3 had reached the maximal 14CO2 production before mineralization of the other two replicates, and at day 15, replicate 3 had shown no mineralization activity for possibly up to five days (data not shown). This is in accordance with the fact that rRNA is labile and thereby degrades faster than DNA that may prevail after cell death. The estimation of 14C-labeled biomass by centrifugation (14,100 × g, 20 min, 20°C) of 2 ml Sp8-3 at day 15, followed by MS washing, dissolution of the pellet in methanol, and scintillation counting, suggested that 9 to 19% of the 14C from linuron had been incorporated into bacterial biomass (Table 1). Replicate 3 had a smaller amount of 14C-biomass, which could indicate cell death.
FIG. 2.
DGGE analysis of the bacterial community in the linuron-degrading enrichment culture Sp8-3. Profiles of the PCR-amplified 16S rRNA genes (lanes 1 through 6) and of the reverse-transcribed 16S rRNA (lanes 7 through 9). Lanes M show size markers. Lanes 1 through 3 correspond to 16S rRNA genes from replicates 1 through 3 extracted at day 8 (Fig. 1); lanes 4 through 6 correspond to 16S rRNA genes from replicates 1 through 3 extracted at day 15 (Fig. 1); lanes 7 through 9 correspond to reverse-transcribed 16S rRNA from replicates 1 through 3 extracted at day 15; and lane 11 represents 16S rRNA genes from the strain SRS16 isolated from Sp8-3. The DGGE bands (A to F) marked by open boxes were excised and sequenced.
TABLE 1.
14C distribution following mineralization of [14C]linuron by community Sp8-3a
| Culture Sp8-3 |
14C recovery (%)
|
|||
|---|---|---|---|---|
| 14CO2 | 14C in liquid phase | 14C-biomass | Total recovery | |
| Replicate 1 | 72.3 | 8.7 | 18.6 | 99.6 |
| Replicate 2 | 64.9 | 9.8 | 19.2 | 93.8 |
| Replicate 3 | 72.1 | 16.4 | 9.4 | 97.8 |
Initial linuron concentration was 50 mg liter−1. Results for three replicate flasks, serving as the basis for the DGGE profiles presented in Fig. 2, are shown individually. The data were obtained after 15 days of incubation.
The DGGE-based characterization of Sp8-3 indicates that the mineralization activity is primarily related to the metabolism performed by one single bacterium represented by band E (Fig. 2) with similarities to Variovorax spp. (Table 2). However, the presence of bands C and D at day 15 may suggest the involvement of secondary strains in linuron mineralization. No sequencing was possible with band D due to lack of success in purifying the PCR products. Bands A and B had homologies with various Pseudomonas spp. from soil and were not detected at day 15 or in the RNA-based DGGE. Band C also had homology with soil-derived Pseudomonas spp., and in contrast to bands A and B, it also appeared after 15 days of incubation. Additionally, a weak band resembling band C was detected in the RNA-based DGGE analysis.
TABLE 2.
Phylogenetic affiliation of members of the linuron-degrading bacterial community Sp8-3 based on 16S rRNA gene sequence from DGGE bands (A through E) and isolated strains (SRS16, SRS17, and SRS18)
| DGGE band(s) or isolate | Length of sequence (bp) | Phylogenetic affiliation | GenBank accession no. | Similarity (%) |
|---|---|---|---|---|
| Bands | ||||
| A | 199 | Pseudomonas sp. strain 19 | AY044091 | 98 |
| Pseudomonas borealis GOBB3-203 | AF321013 | 98 | ||
| B | 208 | Pseudomonas sp. strain B65 | AF332541 | 98 |
| Pseudomonas corrugata SB4 | AY050495 | 98 | ||
| C | 205 | Pseudomonas fluorescens DP20 | AY538264 | 100 |
| Pseudomonas sp. strain Dp2 | AF288736 | 100 | ||
| E and F | 208 | Variovorax sp. strain WDL1 | AF538929 | 98 |
| Variovorax sp. strain MB16 | AB013416 | 98 | ||
| Strains | ||||
| SRS16 | 566 | Variovorax sp. strain HW1 | D89026 | 99 |
| Variovorax sp. strain WDL1 | AF538929 | 99 | ||
| SRS17 | 575 | Variovorax sp. strain HW1 | D89026 | 99 |
| Variovorax sp. strain WDL1 | AF538929 | 99 | ||
| SRS18 | 561 | Flavobacterium ferrugineum ATCC 13524 | M62798 | 98 |
Isolation of Variovorax sp. strain SRS16—the key member of community Sp8-3.
Dejonghe et al. (4) described a linuron-metabolizing enrichment subcultured for 25 months, preceded by failed attempts to isolate the degradative members (7), before isolation of the only currently cultured linuron-mineralizing bacterium, Variovorax sp. strain WDL1. Cultivation of the key microorganisms from Sp8-3 was attempted following approximately 4 months of enrichments. Plating of Sp8-3 microorganisms on different agars, including R2A (Difco Laboratories, Detroit, MI) and a linuron-based agar, based on MS with Agar Noble (Difco) and 50 mg liter−1 linuron, revealed different colony morphologies. No growth on the linuron agar was observed following 30 days. The three most dominant colony types on R2A were streaked onto fresh agar three times to ensure purity before further studies. The isolates were designated SRS16, SRS17, and SRS18. DNA-based DGGE band F derived from strain SRS16 had a migration similar to that of the dominant RNA- and DNA-based DGGE bands from Sp8-3 detected at day 15 (Fig. 2). Partial 16S rRNA gene sequence analysis of strain SRS16 confirmed the phylogenetic relationship with different Variovorax spp., and the highest similarity was to the linuron-degrading Variovorax sp. strain WDL1 (Table 2). Strain SRS17 had 100% 16S rRNA gene homology to SRS16. Strain SRS18 was related to different Flavobacterium spp. (Table 2), and no mineralization or degradation of linuron was detected. Strain SRS18 was not included in the DGGE fingerprinting, and it may represent band D, from which no sequencing was possible. Alternatively, Sp8-3 may be more complex than suggested by the DGGE fingerprinting.
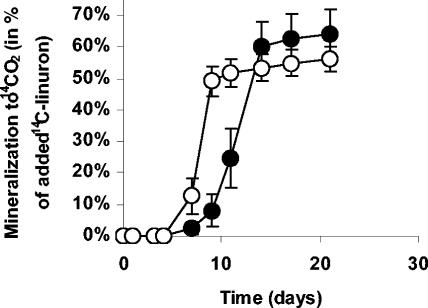
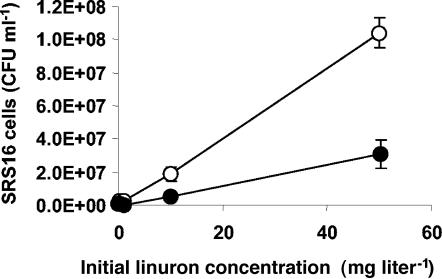
Variovorax sp. strain SRS16 was screened for linuron mineralization and confirmed as a linuron-mineralizing bacterium (Fig. 3) able to use the herbicide as a carbon, nitrogen, and energy source (Fig. 4). Linuron mineralization by SRS16 was tested and verified in concentrations of 0.1, 0.2, 1.1, 10.1, 50.0, 100.0, and 1,000.0 mg liter−1. The pattern of mineralization presented in Fig. 3 with 50 mg liter−1 is representative for the tested concentration range. Supplementing MS with 1 g liter−1 succinate as a carbon source with linuron as the sole nitrogen source increased the biomass production (Fig. 4), but extensive flocculation obstructed accurate growth measurements at the highest concentrations. Estimating 14C-biomass at day 21 following mineralization of 50 mg liter−1 linuron revealed 10 to 30% of the 14C associated with washed SRS16 cells, and drop-plating showed 10.4 × 107 ± 0.9 × 107 cells ml−1 in MS with succinate compared to 3.1 × 107 ± 0.9 × 107 cells ml−1 in MS (Fig. 4). In contrast to results from previous studies with an isoproturon-mineralizing isolate (1), no sharp pH dependence on the mineralization activity was detectable in a pH range of 6.5 to 7.5 (data not shown). Only low concentrations of 3,4-dichloroaniline, similar to those obtained by measurements with Sp8-3, were detected during linuron metabolism by SRS16.
FIG. 3.
Mineralization of linuron (50.0 mg liter−1) by Variovorax sp. strain SRS16 after inoculation with 105 cells ml−1 in MS (•) or in MS with 1 g liter−1 succinate (○). The data are mean values (n = 3). The bars indicate the standard deviations.
FIG. 4.
Cell densities of Variovorax sp. strain SRS16 after mineralization of linuron (0.1, 0.2, 1.1, 10.1, or 50.0 mg liter−1) following incubation in MS (•) or in MS with 1 g liter−1 succinate (○) for 21 days. The data are mean values (n = 3). The bars indicate the standard deviations.
Enrichment culture Sp8-3 seems to be a simple community with one primary degrader. The linuron mineralization rates in the exponential phase were similar for strain SRS16 in pure culture and the mixed culture Sp8-3 (Fig. 1 and 3). However, the lag phases before the onset of the exponential mineralization phase differed, and the mineralization of linuron therefore took approximately 5 days longer with strain SRS16 in pure culture. This might reflect that the initial size of the SRS16 population within Sp8-3 is smaller than the 105 cells ml−1 used in the pure culture experiments, as the lag phase can be reduced by increasing the inoculum size of SRS16. All evidence suggests that Variovorax sp. strain SRS16 is the primary degrader, and in contrast to the first described linuron-mineralizing bacterium, Variovorax sp. strain WDL1 (4), SRS16 appears to be independent of other bacteria when performing linuron mineralization. Another difference is the amount of 3,4-dichloroaniline occurring during linuron degradation, whereas strain WDL1, in contrast to strain SRS16, accumulates up to approximately 40% (of the initial linuron concentration) (4). The phylogenetic similarities between SRS16 and WDL1 suggest that these strains represent one Variovorax sp. or, alternatively, a group of closely related Variovorax spp., having a natural ability to utilize linuron as a carbon, nitrogen, and energy source. The inherent biases associated with enrichment procedures are known to significantly change the diversities of degraders (5), and it is therefore possible that these Variovorax spp. are well adapted for proliferation in the enrichment procedures, enabling them to outcompete other linuron-degrading microorganisms occurring in soils. Further studies will aim at characterizing the active members of the remaining enrichment cultures. Additionally, a broader screening of different European agricultural soils having a potential for mineralization of linuron will be initiated.
Nucleotide sequence accession numbers.
Variovorax sp. strain SRS16 was deposited at Institut Pasteur Collection and given accession number CIP108393. Sequences for SRS16 and SRS18 were given GenBank database accession numbers AY621157 and AY621158, respectively. Other accession numbers are provided in Table 2.
Acknowledgments
This work was supported by the Danish Technical Research Council, talent grant 26-04-0051 (funding for S.R.S.), and the Danish Agricultural and Veterinary Research Council through the SOUND project.
We thank Mette Andersen for skillful technical assistance and Patricia Simpson for excellent help during the writing of the manuscript.
REFERENCES
- 1.Bending, G. D., S. D. Lincoln, S. R. Sørensen, J. A. W. Morgan, J. Aamand, and A. Walker. 2003. In-field spatial variability in the degradation of the phenyl-urea herbicide isoproturon is the result of interactions between degradative Sphingomonas spp. and soil pH. Appl. Environ. Microbiol. 69:827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caux, P.-Y., R. A. Kent, G. T. Fan, and C. Grande. 1998. Canadian water quality guidelines for linuron. Environ. Water Qual. 13:1-41. [Google Scholar]
- 3.Cullington, J. E., and A. Walker. 1999. Rapid biodegradation of diuron and other phenylurea herbicides by a soil bacterium. Soil Biol. Biochem. 31:677-686. [Google Scholar]
- 4.Dejonghe, W., E. Berteloot, J. Goris, N. Boon, K. Crul, S. Maertens, M. Höfte, P. D. Vos, W. Verstraete, and E. M. Top. 2003. Synergistic degradation of linuron by a bacterial consortium and isolation of a single linuron-degrading Variovorax strain. Appl. Environ. Microbiol. 69:1532-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunbar, J., S. White, and L. Forney. 1997. Genetic diversity through the looking glass: effect of enrichment bias. Appl. Environ. Microbiol. 63:1326-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Deeb, B. A., S. M. Soltan, A. M. Ali, and K. A. Ali. 2000. Detoxication of the herbicide diuron by Pseudomonas sp. Folia Microbiol. 45:211-216. [DOI] [PubMed] [Google Scholar]
- 7.El-Fantroussi, S. 2000. Enrichment and molecular characterization of a bacterial culture that degrades methoxy-methyl urea herbicides and their aniline derivatives. Appl. Environ. Microbiol. 66:5110-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fryer, J. D., and K. Kirkland. 1970. Field experiments to investigate long-term effects of repeated applications of MCPA, tri-allate, simazine and linuron: reports after 6 years. Water Res. 10:133-158. [Google Scholar]
- 9.Glad, G., L. Nilsson, T. Popoff, O. Theander, and N. T. L. Torstensson. 1980. Performance of linuron in four Swedish soil types. Swed. J. Agric. Res. 10:133-137. [Google Scholar]
- 10.Juhler, R. K., S. R. Sørensen, and L. Larsen. 2001. Analysing transformation products of herbicide residues in environmental samples. Water Res. 35:1371-1378. [DOI] [PubMed] [Google Scholar]
- 11.Kempson-Jones, G., and R. J. Hance. 1979. Kinetics of linuron and metribuzin degradation in soil. Pestic. Sci. 10:449-454. [Google Scholar]
- 12.Lintelmann, J., A. Katayama, N. Kurihara, L. Shore, and A. Wenzel. 2003. Endocrine disruptors in the environment—IUPAC technical report. Pure Appl. Chem. 75:631-681. [Google Scholar]
- 13.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen, J., J. Aamand, P. Rosenberg, O. S. Jacobsen, and S. R. Sørensen. Spatial variability in the mineralization of the phenylurea herbicide linuron within a previously treated agricultural field: multivariate correlation to simple soil parameters. Pest Manag. Sci., in press. doi: 10.1002/ps.1041. [DOI] [PubMed]
- 15.Roberts, S. J., A. Walker, N. R. Parekh, S. J. Welch, and M. J. Waddington. 1993. Studies on a mixed bacterial culture from soil which degrades the herbicide linuron. Pestic. Sci. 39:71-78. [Google Scholar]
- 16.Smith, A. E., and G. S. Emmond. 1975. Persistence of linuron in Saskatchewan soils. Can. J. Soil Sci. 55:145-148. [Google Scholar]
- 17.Sørensen, S. R., and J. Aamand. 2003. Rapid mineralization of the herbicide isoproturon in soil from a previously treated Danish agricultural field. Pest Manag. Sci. 59:1118-1124. [DOI] [PubMed] [Google Scholar]
- 18.Sørensen, S. R., and J. Aamand. 2001. Biodegradation of the phenylurea herbicide isoproturon and its metabolites in agricultural soils. Biodegradation 12:69-77. [DOI] [PubMed] [Google Scholar]
- 19.Sørensen, S. R., Z. Ronen, and J. Aamand. 2001. Isolation from agricultural soil and characterization of a Sphingomonas sp. able to mineralize the phenylurea herbicide isoproturon. Appl. Environ. Microbiol. 67:5403-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sørensen, S. R., Z. Ronen, and J. Aamand. 2002. Growth in coculture stimulates metabolism of the phenylurea herbicide isoproturon by Sphingomonas sp. strain SRS2. Appl. Environ. Microbiol. 68:3478-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thirup, L., K. Johnsen, V. Torsvik, N. H. Spliid, and C. S. Jacobsen. 2001. Effects of fenpropimorph on bacteria and fungi during decomposition of barley roots. Soil Biol. Biochem. 33:1517-1524. [Google Scholar]
- 22.Tixier, C., P. Bogaerts, M. Sancelme, F. Bonnemoy, L. Twagilimana, A. Cuer, J. Bohatier, and H. Veschambre. 2000. Fungal biodegradation of a phenylurea herbicide, diuron structure and toxicity of metabolites. Pest Manag. Sci. 56:455-462. [Google Scholar]
- 23.Torstensson, L., and J. Stenström. 1990. Persistence of herbicides in forest nursery soils. Scand. J. For. Res. 5:457-469. [Google Scholar]