Abstract
Survival in natural bulk soil and colonization of sugar beet seeds and barley straw residues were determined for Pseudomonas sp. strain DSS73 and Tn5 mutants in amsY (encoding a peptide synthetase involved in production of the cyclic lipopeptide amphisin) and gacS (encoding the sensory kinase of the two-component GacA/GacS regulatory system). No differences in survival or growth in response to carbon amendment (citrate) were observed in bulk soil. However, both mutants were impaired in their colonization of sugar beet seeds and barley straw residues by an inoculum established in the bulk soil. The two mutants had comparable colonization phenotypes, suggesting that amphisin production is more important for colonization than other gacS-controlled traits.
Pseudomonas sp. strain DSS73, isolated from sugar beet rhizosphere, produces the 11-amino-acid cyclic lipopeptide (CLP) amphisin, which has antifungal properties and is a biosurfactant important for surface motility (1, 17). DSS73 produces amphisin during exponential growth, and its in vitro synthesis is enhanced by components of sugar beet exudates (9). Amphisin production and the stimulatory effect of seed exudates depend on the GacA/GacS two-component regulatory system (5), which also controls chitinase, protease, and cyanide synthesis in strain DSS73 (9).
No studies have addressed the significance of CLP production for colonization of plant tissues by pseudomonads. However the CLP surfactin produced by Bacillus subtilis is involved in colonization and subsequent biofilm formation on Arabidopsis roots grown in sterilized soil systems (2). In Pseudomonas, the GacA/GacS system controls several traits that are induced by environmental signals. This system has been reported to be nonessential for root colonization by pseudomonads but important for their long-term survival in bulk soil (4, 12, 13). The objective of the present work was to determine the importance the amphisin synthetase (amsY) and sensor kinase (gacS) genes for survival of Pseudomonas sp. strain DSS73 in natural bulk soil and for colonization of sugar beet seeds, as well as dead organic material that may support good survival of Pseudomonas.
Survival in bulk soil.
Pseudomonas sp. strain DSS73 and the Tn5 mutants DSS73-15C2 (amsY) and DSS73-12H8 (gacS) (9) were gfp tagged using a mini-Tn7 system as described by Koch et al. (8). Strain DSS73-MM is a spontaneous GacS mutant of strain DSS73-15C2 (amsY) (9). Washed cells from an overnight culture grown in Davis minimal broth (Difco, Detroit, MI) at 28°C were used to inoculate bulk soil microcosms containing soil with a 15% water content (weight/dry weight). The soil was a sandy loam from the Royal Veterinary and Agricultural University experimental fields with the following characteristics: coarse sand, 69%; silt, 3.3%; clay, 4.0%; organic matter, 4.4% (dry weight); water-holding capacity, 18.9% (dry weight); organic carbon, 9 g kg−1 dry soil; total N, 0.6 g kg−1 dry soil; pH (CaCl2), 6.3 (14, 19). Incubation conditions and cell extraction protocols were as reported by Nielsen and Sørensen (15). All strains were applied to bulk soil at approximately 105 CFU g−1 and tested in independent microcosms. Extraction efficiencies, determined directly after application, were 70 to 100% for all experiments. Survival was determined from CFU counts on LB agar containing kanamycin (25 μg ml−1) and the antifungal compound nystatin (50 μg ml−1) using a 2-day incubation at 28°C. For the gfp-tagged strains [DSS73, DSS73-15C2 (amsY), and DSS73-12H8 (gacS)], the identities of colonies were confirmed by their green fluorescence. Under the experimental conditions employed, the background of colonies formed by indigenous microorganisms was negligible. Cell numbers were log transformed, and significant differences (P ≤ 0.05) between treatments were tested using two-way analysis of variance and least-squares means of pairwise comparisons using the GLM procedure of SAS analyst (SAS Institute Inc., Cary, NC).
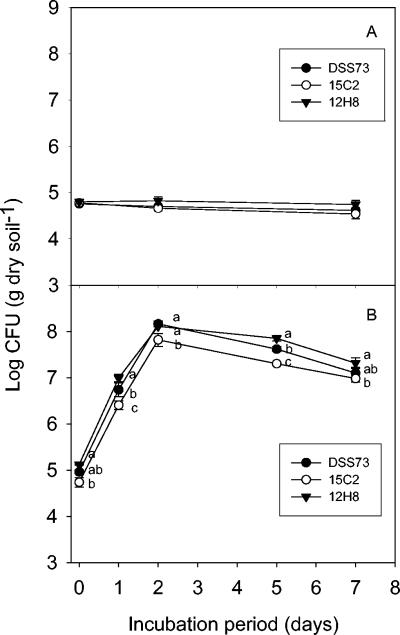
Survival of DSS73, DSS73-15C2 (amsY), and DSS73-12H8 (gacS) was similar during a 7-day incubation period (Fig. 1A). Addition of citrate, 720 μg C g−1, to the soil led to significant and comparable increases in the CFU counts of all three strains (Fig. 1B), considering the slightly lower initial cell density of DSS73-15C2 (amsY) at day 0. The performance of strain DSS73-MM (amsY gacS) was comparable to that of the other strains tested (data not shown). Hence, amsY and gacS were not required to maintain short-term survival (culturability) in the soil, nor were they required for cell growth in response to an added carbon source.
FIG. 1.
(A) Survival of Pseudomonas sp. strain DSS73 wild-type and mutant strains DSS73-15C2 (amsY) and DSS73-12H8 (gacS) added to nonsterile bulk soil. All data are means ± standard deviations (n = 3). No significant difference between inoculation treatments was observed. (B) Cell proliferation of wild-type Pseudomonas sp. strain DSS73 and mutant strains DSS73-15C2 (amsY) and DSS73-12H8 (gacS) in nonsterile bulk soil treated with citrate (720 μg C g−1). All data are means ± standard deviations (n = 3). Statistically significant differences (P ≤ 0.05) between treatments are indicated by the letters a, b, and c for each sampling time.
Colonization of sugar beet seeds and roots.
We investigated the importance of amsY and gacS for colonization of sugar beet seeds. Bacterial cells were inoculated into bulk soil at 105 CFU g−1. Seeds (Madison variety) were then sown into the bulk soil in petri dishes or 50-ml centrifuge tubes to allow root development. After 2 days of incubation, microscopic evaluation of seed colonization by the three strains was performed on a confocal laser scanning microscope (TCS SP2; Leica Microsystems, Wetzlar, Germany). This was equipped with an argon laser, a water immersion objective (63×/1.2 HCX PL APO CS), and a spectral filtering system. Excitation was at 488 nm, and light was detected from 500 to 547 nm. The seeds were mounted in a thin layer of 37°C agar, which solidified immediately after seed application. During sampling, seeds and seed pericarps were collected and bulked for cell extraction as described by Nielsen and Sørensen (15). On days 5 and 7, cells were also extracted from roots, which had been cut off the seeds.
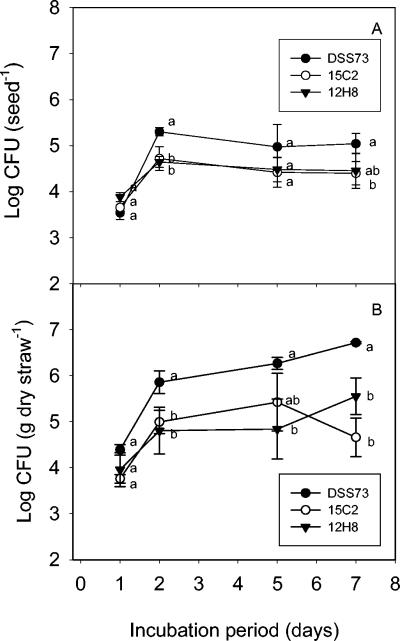
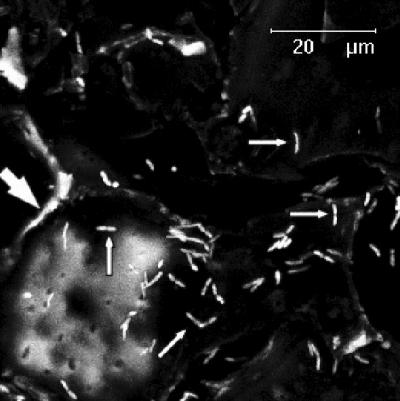
As shown in Fig. 2A, strain DSS73 initially (day 2) established a significantly higher population on the seeds than the two mutant strains DSS73-15C2 (amsY) and DSS73-12H8 (gacS). This difference persisted throughout the experiment, as none of the strains showed a further increase in population size during root appearance (days 4 to 5) and subsequent loss of the pericarp (days 5 to 7). Observations of seed colonization by confocal laser scanning microscopy showed a very heterogeneous distribution of gfp-tagged DSS73 cells on the seed pericarp, with no obvious differences among the three strains. Interestingly, some areas of the very rugged pericarp sustained a high growth activity of the inoculum, as indicated by the numerous dividing cells. Observations of strain DSS73 are shown in Fig. 3. Table 1 shows that both DSS73 and the mutant strains colonized the emerging root; at day 5, the roots were still very small, but at day 7 (when roots were 3 to 5 cm long), strain DSS73 had established a significantly higher population density on the roots than DSS73-15C2 (amsY) or DSS73-12H8 (gacS). In conclusion, colonization of both seeds and roots was impaired in the amsY and gacS mutant strains.
FIG. 2.
(A) Colonization of sugar beet seeds from soil inoculum of wild-type Pseudomonas sp. strain DSS73 and mutant strains DSS73-15C2 (amsY) and DSS73-12H8 (gacS). All data are means ± standard deviations (n = 3). Statistically significant differences (P ≤ 0.05) between treatments are indicated by the letters a and b for each sampling time. (B) Colonization of barley straw from soil inoculum of wild-type Pseudomonas sp. strain DSS73 and mutant strains DSS73-15C2 (amsY) and DSS73-12H8 (gacS). Statistically significant differences (P ≤ 0.05) between treatments are indicated by the letters a and b for each sampling time.
FIG. 3.
Microscopic observations of sugar beet seed colonization after 2 days of incubation showing the very rugged seed pericarp with pericarp cells (large arrow) which in patchy areas sustain many dividing DSS73 cells (small arrows).
TABLE 1.
Sugar beet root colonization from soil inoculum of wild-type Pseudomonas sp. strain DSS73 and mutant strains DSS73-15C2 (amsY) and DSS73-12H8 (gacS) after 5 and 7 days of incubation at 15°Ca
| Incubation period (days) | Log no. of CFU root−1
|
||
|---|---|---|---|
| DSS73 (wild type) | 15C2 (amsY mutant) | 12H8 (gacS mutant) | |
| 5 | 3.7 ± 0.2 (a) | 3.6 ± 0.2 (a) | 3.8 ± 0.1 (a) |
| 7 | 5.2 ± 0.4 (a) | 4.0 ± 0.1 (b) | 4.1 ± 0.1 (b) |
Each sample was a bulk extraction of 10 excised sugar beet roots. All data are means ± standard deviations (n = 3). Statistically significant differences between treatments are indicated by the letters a and b for each sampling time.
Colonization of barley straw.
To assess whether the DSS73-15C2 (amsY) and DSS73-12H8 (gacS) colonization phenotype was unique to sugar beet seeds and roots, where inducing signals from living tissue may be expected, we also studied the colonization of decaying barley straw in soil microcosms. Ground barley straw material (2 mm) was first heat treated at 60°C for 3 days to reduce the background level of kanamycin-resistant microorganisms. The straw was subsequently humidified by adding 6 ml deionized water per g dry straw and left at 5°C for 1 day. Hereafter, an additional 0.5 ml deionized water per g dry straw was added and microcosms were established in square petri dishes (10 by 10 by 1 cm). A compartment measuring 8 by 10 by 1 cm contained bulk soil that had been inoculated with ca. 105 CFU g−1 of the relevant bacterial strains as for the bulk soil experiment. The straw compartment (2 by 10 by 1 cm) was established in direct contact with the soil compartment. During a 7-day incubation period, samples of barley straw at the straw-soil interphase were extracted with 0.9% NaCl and CFU counts obtained as for bulk soil systems.
As shown in Fig. 2B, strain DSS73 soon established a significantly higher population density in the barley straw phase than did both DSS73-15C2 (amsY) and DSS73-12H8 (gacS). The mutant strains showed similar levels of colonization during the 7-day incubation period. The results were thus comparable to those described for the colonization of sugar beet seeds, indicating that amsY and gacS are important to colonization of germinating seeds, as well as decaying straw. For the straw experiment, it should be noted that heat treatment of the straw reduced the indigenous microbial population. Hence, the DSS73 strains faced competition from an unaffected microbial community in the soil into which they were inoculated, whereas they probably faced less competition when they colonized the straw phase.
Proliferation on sugar beet seeds and barley straw.
We speculated that the poorer colonization of sugar beet seeds and barley straw by the mutant strains could be due to impairment of initial establishment of the soil-inoculated cells and/or impairment of the subsequent proliferation on the seeds or straw. To distinguish between these possibilities, we performed a series of experiments where the strains were coated directly onto seeds or barley straw material. For the seed experiment, seeds were immersed for 5 min in washed cultures of the relevant DSS73 strains (approximately 104 CFU ml−1) and sown as described above. In the corresponding straw experiment, each strain was introduced directly onto the straw material at approximately 102 CFU g−1 dry straw.
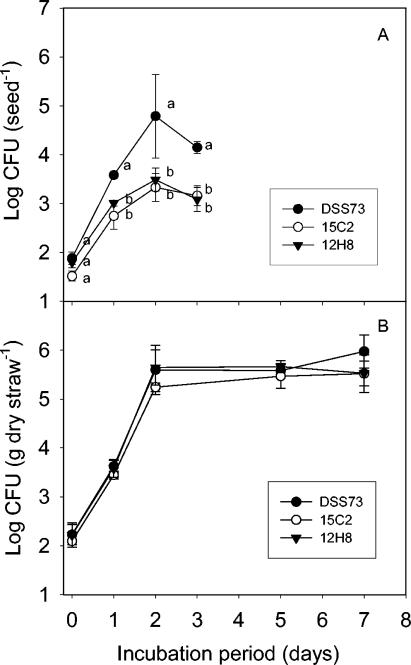
Figure 4A shows that DSS73 established an almost 100-fold higher population density on sugar beet seeds than DSS73-15C2 (amsY) and DSS73-12H8 (gacS) during the first 2 days, indicating that amsY and gacS were important to proliferation of seed-inoculated cells. A test was performed to determine if spontaneous GacA/GacS mutants of strain DSS73 or DSS73-15C2 (amsY) occurred during the course of the experiments. We reisolated DSS73 and DSS73-15C2 (amsY) cells from the seeds at day 2 and observed that these mutants, identified according to the procedure of Koch et al. (9), comprised a negligible fraction of <1% of the reisolated cells.
FIG. 4.
(A) Cell proliferation on sugar beet seeds after direct inoculation onto the seeds of wild-type Pseudomonas sp. strain DSS73 and mutant strains DSS73-15C2 (amsY) and DSS73-12H8 (gacS). All data are means ± standard deviations (n = 3). Statistically significant differences (P ≤ 0.05) between treatments are indicated by the letters a and b for each sampling time. (B) Cell proliferation on barley straw after direct application onto the straw of wild-type Pseudomonas sp. strain DSS73 and mutant strains DSS73-15C2 (amsY) and DSS73-12H8 (gacS). All data are means ± standard deviations (n = 3). No statistically significant difference between inoculation treatments was observed.
As shown in Fig. 4B, all three strains reached comparable population sizes of 105 to 106 CFU g−1 on barley straw after 2 days. In conclusion, neither amsY nor gacS seemed important for cell proliferation on the straw. As noted above, the DSS73 strains faced less severe competition from indigenous microorganisms in the straw phase.
Significance of amsY and gacS for performance of DSS73 in soil and at soil-plant interfaces.
In conclusion, we have shown in this study that the amsY gene required for amphisin production is not important for survival of strain DSS73 in nonsterile bulk soil, in accordance with our earlier study (15), demonstrating that amphisin is not produced in this habitat. Similarly, the regulatory gacS gene is not important for bulk soil survival in the present experiments using a natural sandy loam with low organic content and a neutral pH. Other studies have reported that the GacA/GacS regulatory system is required to maintain the culturability of P. fluorescens. In four Swiss sandy loams, gacA and gacS mutans of P. fluorescens CHA0 were compromised for survival (12, 13). The poorer survival appeared clearly after about 15 days and was more prominent in a conifer forest soil with a low pH and a high organic content than in neutral fallow soils, suggesting that gac-regulated traits may be involved in protection against stress (12, 13). In the present experiments, amendment of bulk soil with citrate stimulated cell proliferation but the growth response of DSS73 did not depend on the amsY or gacS gene.
Our results suggest that the amsY gene is important during two phases of the establishment of Pseudomonas sp. strain DSS73 on sugar beet seeds or decaying barley straw. First, amsY plays a role in the colonization of straw, and possibly of seeds, by cells preinoculated into the bulk soil. We have previously shown that amphisin is required for surface motility by DSS73 in vitro (1). It is therefore plausible that amphisin-supported motility toward the plant material is required during initial establishment by soil-borne DSS73 cells. However, this is most difficult to verify directly in the complex soil environment. Second, the amsY gene is required for cell proliferation on the germinating seeds but not on straw residues. This is in agreement with the stimulation of amphisin synthesis in vitro by components of sugar beet exudates (9) and with the growth-associated amphisin production documented for DSS73 cells in the sugar beet spermosphere (15). It is possible that surface motility promotes the spread of proliferating cells on the seed. Furthermore, a B. subtilis CLP has been implicated in biofilm formation on root surfaces (2). In contrast, recent work addressing the significance of Pseudomonas CLPs for the structure of biofilm formed in vitro has demonstrated that arthrofactin and putisolvin impair biofilm formation and even destroy existing biofilm (10, 18). Pseudomonas inoculants may be a part of biofilms developing on plant roots (16), and it would be of interest to pursue the role of CLPs in this context. However, we have no evidence for biofilm formation in the current experimental systems.
A final possible role for CLPs during colonization and proliferation relates to the biosurfactant properties of amphisin (14), which might relieve a nutrient limitation or a toxic effect exerted by unknown seed components (9).
The similar colonization phenotypes of the DSS73-15C2 (amsY) and DSS73-12H8 (gacS) mutant strains underline the importance of amsY as a colonization gene compared to other Gac-regulated genes in Pseudomonas sp. strain DSS73 (9). The impaired colonization reported here for the DSS73 gacS mutant is more conspicuous than hitherto reported for gac mutants in Pseudomonas sp. Hence, experiments with P. fluorescens CHA0 (13) and P. aureofaciens 30-84 (4) only showed minor differences in the survival of wild-type strains and gac mutants in natural wheat rhizosphere, while Hirano et al. (7) found no effect at all of a gac (lemA) mutation on bean seed colonization by P. syringae pv. syringae in the field.
To our knowledge, no studies have previously addressed the significance of peptide synthetases involved in the production of cyclic lipopeptides, such as amphisin or members of the related viscosin-type CLPs (11), for performance of Pseudomonas spp. in complex plant-soil habitats. The closest related papers report on the inability of a viscosin-deficient P. fluorescens mutant to colonize and spread over hydrophobic broccoli leaf surfaces (3, 6). Hence, the present work presents a novel mechanism important to the efficacy and fate of Pseudomonas sp. inoculants in spermosphere and rhizosphere habitats.
Acknowledgments
This study was supported by the Danish Agricultural and Veterinary Research Council (J. no. 9702796).
We thank Dorte Rasmussen and May-Britt Prahm for excellent technical assistance and Torben Martinussen for guidance in SAS analysis.
REFERENCES
- 1.Andersen, J. B., B. Koch, T. H. Nielsen, D. Sørensen, M. Hansen, O. Nybroe, C. Christophersen, J. Sørensen, S. Molin, and M. Givskov. 2003. Surface motility in Pseudomonas sp. DSS73 is required for efficient biological containment of the root pathogenic microfungi Pythium ultimum and Rhizoctonia solani. Microbiology 149:37-46. [DOI] [PubMed] [Google Scholar]
- 2.Bais, H. P., F. Ray, and J. M. Vivanco. 2004. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 134:304-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, P. G., P. D. Hildebrand, T. C. Ells, and D. Y. Kobayaski. 2001. Evidence and characterization of a gene cluster required for the production of viscosin, a lipopeptide biosurfactant, by a strain of Pseudomonas fluorescens. Can. J. Microbiol. 47:294-301. [DOI] [PubMed] [Google Scholar]
- 4.Chancey, S. T., D. W. Wood, E. A. Pierson, and L. S. Pierson III. 2002. Survival of GacS/GacA mutants of the biological control bacterium Pseudomonas aureofaciens 30-84 in the wheat rhizosphere. Appl. Environ. Microbiol. 68:3308-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heeb, S., and D. Haas. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol. Plant-Microbe Interact. 14:1351-1363. [DOI] [PubMed] [Google Scholar]
- 6.Hildebrand, P. D., P. G. Braun, K. B. McRae, and X. Lu. 1998. Role of the biosurfactant viscosin in broccoli head rot caused by a pectolytic strain of Pseudomonas fluorescens. Can. J. Plant Pathol. 20:296-303. [Google Scholar]
- 7.Hirano, S. S., E. M. Ostertag, S. A. Savage, L. S. Baker, D. K. Willis, and C. D. Upper. 1997. Contribution of the regulatory gene lemA to field fitness of Pseudomonas syringae pv. syringae. Appl. Environ. Microbiol. 63:4304-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch, B., E. J. Jensen, and O. Nybroe. 2001. A panel of Tn7-based vectors for insertion of the gfp marker gene or for delivery of cloned DNA into gram-negative bacteria at a neutral chromosomal site. J. Microbiol. Methods 45:187-195. [DOI] [PubMed] [Google Scholar]
- 9.Koch, B., T. H. Nielsen, D. Sørensen, J. B. Andersen, C. Christophersen, S. Molin, M. Givskov, J. Sørensen, and O. Nybroe. 2002. Lipopeptide production in Pseudomonas sp. strain DSS73 is regulated by components of sugar beet exudates via the Gac two-component regulatory system. Appl. Environ. Microbiol. 68:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuiper, I., E. L. Lagendijk, R. Pickford, J. D. Derrick, G. E. M. Lamers, J. E. Thomas-Oates, B. J. J. Lugtenberg, and G. V. Bloemberg. 2004. Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilm. Mol. Microbiol. 51:97-113. [DOI] [PubMed] [Google Scholar]
- 11.Laycock, M. V., P. D. Hildebrand, P. Thibault, J. A. Walter, and J. L. C. Wright. 1991. Viscosin, a potent peptidolipid biosurfactant and phytopathogenic mediator produced by a pectolytic strain of Pseudomonas fluorescens. J. Agric. Food Chem. 39:483-489. [Google Scholar]
- 12.Mascher, F., Y. Moënne-Loccoz, U. Schnider-Keel, C. Keel, D. Haas, and G. Défago. 2002. Inactivation of the regulatory gene algU or gacA can affect the ability of biocontrol Pseudomonas fluorescens CHA0 to persist as culturable cells in nonsterile soil. Appl. Environ. Microbiol. 68:2085-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natsch, A., C. Keel, H. A. Pfirter, D. Haas, and G. Défago. 1994. Contribution of the global regulator gene gacA to persistence and dissemination of Pseudomonas fluorescens biocontrol strain CHA0 introduced into soil microcosms. Appl. Environ. Microbiol. 60:2553-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen, T. H., D. Sørensen, C. Tobiasen, J. B. Andersen, C. Christophersen, M. Givskov, and J. Sørensen. 2002. Antibiotic and biosurfactant properties of cyclic lipopeptides produced by fluorescent Pseudomonas spp. from sugar beet rhizosphere. Appl. Environ. Microbiol. 68:3416-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen, T. H., and J. Sørensen. 2003. Production of antifungal cyclic lipopeptides from Pseudomonas fluorescens strains in bulk soil and sugar beet rhizosphere. Appl. Environ. Microbiol. 69:861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Normander, B., N. B. Hendriksen, and O. Nybroe. 1999. Green fluorescent protein-marked Pseudomonas fluorescens: localization, viability, and activity in the natural barley rhizosphere. Appl. Environ. Microbiol. 65:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sørensen, D., T. H. Nielsen, C. Christophersen, J. Sørensen, and M. Gajhede. 2001. Cyclic lipoundecapeptide amphisin from Pseudomonas sp. strain DSS73. Acta Crystallogr. C 57:1123-1124. [DOI] [PubMed] [Google Scholar]
- 18.Roongsawang, N., K. Hase, M. Haruki, T. Imanaka, M. Morikawa, and S. Kanaya. 2003. Cloning and characterization of the gene cluster encoding arthrofactin synthetase from Pseudomonas sp. MIS38. Chem. Biol. 10:869-880. [DOI] [PubMed] [Google Scholar]
- 19.Thirup, L., A. Johansen, and A. Winding. 2003. Microbial succession in the rhizosphere of live and decomposing barley roots as affected by the antagonistic strain Pseudomonas fluorescens DR54-BN14 or the fungicide imazalil. FEMS Microbiol. Ecol. 43:383-392. [DOI] [PubMed] [Google Scholar]