Abstract
We previously demonstrated that xanthobaccin A from the rhizoplane bacterium Lysobacter sp. strain SB-K88 suppresses damping-off disease caused by Pythium sp. in sugar beet. In this study we focused on modes of Lysobacter sp. strain SB-K88 root colonization and antibiosis of the bacterium against Aphanomyces cochlioides, a pathogen of damping-off disease. Scanning electron microscopic analysis of 2-week-old sugar beet seedlings from seeds previously inoculated with SB-K88 revealed dense colonization on the root surfaces and a characteristic perpendicular pattern of Lysobacter colonization possibly generated via development of polar, brush-like fimbriae. In colonized regions a semitransparent film apparently enveloping the root and microcolonies were observed on the root surface. This Lysobacter strain also efficiently colonized the roots of several plants, including spinach, tomato, Arabidopsis thaliana, and Amaranthus gangeticus. Plants grown from both sugar beet and spinach seeds that were previously treated with Lysobacter sp. strain SB-K88 displayed significant resistance to the damping-off disease triggered by A. cochlioides. Interestingly, zoospores of A. cochlioides became immotile within 1 min after exposure to a SB-K88 cell suspension, a cell-free supernatant of SB-K88, or pure xanthobaccin A (MIC, 0.01 μg/ml). In all cases, lysis followed within 30 min in the presence of the inhibiting factor(s). Our data indicate that Lysobacter sp. strain SB-K88 has a direct inhibitory effect on A. cochlioides, suppressing damping-off disease. Furthermore, this inhibitory effect of Lysobacter sp. strain SB-K88 is likely due to a combination of antibiosis and characteristic biofilm formation at the rhizoplane of the host plant.
Members of the Peronosporomycetes (previously classified as Oomycetes) (7), specifically genera such as Phytophthora, Pythium, and Aphanomyces, are related to brown algae and diatoms yet are also devastating pathogens that affect many economically important crops (7, 23, 34). Control of these pathogenic soilborne Peronosporomycetes is very difficult, as they are resistant to many fungicides (34). Therefore, novel approaches are needed to develop a biologically rational method to control these notorious plant parasites (13, 34). Use of biological control strategies, such as introduction of bacterial or other rhizosphere microbial antagonists to suppress the root-infecting Peronosporomycetes, is a rapidly growing area of research (11, 20, 24, 34).
A relevant example of a potential target for biocontrol is the characteristic preinfection stages of Aphanomyces cochlioides, the pathogen associated with damping-off in sugar beet and spinach. Damping-off is a disease of seedlings that causes them to rot at the soil level and fall over. Once liberated from the mycelium, the zoospores of A. cochlioides (see Fig. 6A) locate host roots via perception of cochliophilin A (5-hydroxy-6,7-methylenedioxyflavone), a host-specific flavonoid signal released from the roots. Zoospores are biflagellate, motile, asexual spores of the Peronosporomycetes (14). Once they arrive at the host surface, they become immobilized by shedding flagella and are transformed into cystospores. The cystospores then germinate to form germ tubes and invade the root tissues directly or via appressoria (14, 15, 17). This sequence is extremely rapid and leads to infection within 30 to 40 min after the zoospores arrive at the host surface (13, 15). Thus, zoospore taxis is an essential part of the preinfection process and is therefore a potential target for controlling diseases caused by such soilborne zoosporogenic phytopathogens (16, 18).
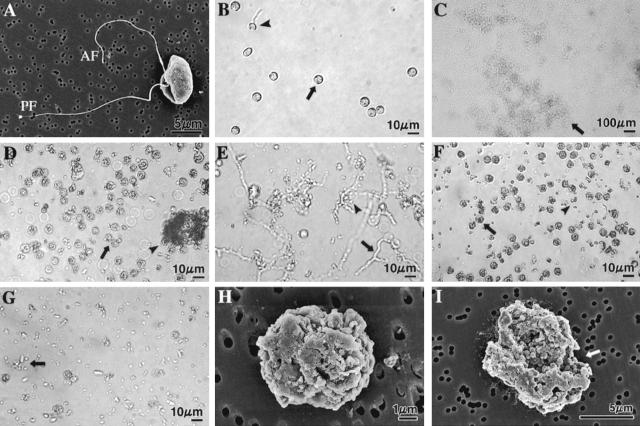
FIG. 6.
Light (B to G) and SEM (H and I) micrographs showing A. cochlioides zoospore-lytic activity of the freeze-dried culture supernatant, EtOAc- and water-soluble fractions of the culture supernatant, and pure xanthobaccin A. The micrographs in panels B to G were taken after 3 h of treatment by focusing on the bottom of a petri dish with a digital camera connected to the microscope (for details see Materials and Methods). Crude extracts or pure xanthobaccin A (dissolved in small quantities of DMSO) at the concentrations tested immediately caused inhibition of the motility of zoospores (see Table 1 and Materials and Method for details of the bioassay method). The halted zoospores rapidly settled to the bottom of the dish and then started to burst or lyse. The final concentration of DMSO in the aqueous zoospore suspension was maintained at less than 1% in all treatments. DMSO alone (final concentration, 1%) was used as the negative control and caused no lysis of zoospores. Each experiment was replicated at least five times, and representative micrographs are shown. (A) SEM micrograph of a biflagellate A. cochlioides zoospore (untreated control). AF, anterior flagellum; PF, posterior flagellum. (B) No lysis in the control dish (1% DMSO). A small portion (10 to 15%) of the motile zoospores in the control dish were stopped and changed into round cystospores (arrow) after 3 h and then settled to the bottom of the dish; 5 to 8% of these cystospores germinated (arrowhead) and formed germ tubes. No motile zoospores were observed in aqueous medium because the photograph was taken by focusing on bottom of the dish. (C) Complete lysis of all halted zoospores by freeze-dried culture supernatant (500 μg/ml). The arrow indicates lysed material. (D) All spores were granulated or lysed (arrow) by the EtOAc-soluble fraction (100 μg/ml). Some lysed material aggregated (arrowhead). (E) Water-soluble fraction (500 μg/ml) initially induced germination of cystospores (arrow and arrowhead) within 1 h, and then (3 h) all spores and germ tubes were partially lysed (arrow and arrowhead). (F) Xanthobaccin A (1 μg/ml) caused granulation (arrowhead) and lysis (arrowhead) of all spores. (G) Complete lysis of zoospores (arrow) by xanthobaccin A (1 μg/ml). (H and I) Scanning electron micrographs of granulated, cracked, and lysed (arrow in I) A. cochlioides zoospores exposed to 1 ppm xanthobaccin A for 30 min.
Biological control by using antagonistic microorganisms has been investigated as one of biorational means of controlling the diseases caused by A. cochlioides (11, 24, 35). For instance, Homma et al. isolated a number of bacterial strains that were antagonistic to the development of damping-off diseases from the rhizoplane of sugar beet host plants grown in a field heavily infested with Polymyxa betae (a vector of a viral pathogen of sugar beet) in Hokkaido, Japan (11). One of these isolates, strain SB-K88, has shown promise as a control agent for root rot and damping-off diseases (24). This bacterium was tentatively identified as a strain of Stenotrophomonas sp. or Xanthomonas sp. on the basis of traditional morphological and biochemical tests (11, 24). However, recent analysis of the 16S rRNA genes and other traits of SB-K88 revealed that it is instead a strain of the gliding bacterium Lysobacter sp. (32).
Members of the genus Lysobacter are typically found in soil and water habitats and are characterized by gliding motility and the ability to lyse other microorganisms, including fungi and nematodes (4). One member of this genus, Lysobacter enzymogenes (previously identified as Stenotrophomonas maltophilia strain C3) (37-40), has recently been reported to be a field-effective bacterial biocontrol agent that exhibits activity against a wide range of fungal diseases, such as Bipolaris leaf spot (caused by Bipolaris sorokiniana) and brown patch (caused by Rhizoctonia solani) in turfgrass (37, 38). Also, Lysobacter spp. can colonize plant surfaces after artificial inoculation. This evidence suggests that members of this genus are potential biocontrol agents for use against soilborne plant pathogens (4, 8, 21). However, very few attempts have been made to characterize these newly identified, potential biocontrol agents. Hence, little information is available concerning the modes of disease suppression and the colonization behavior of Lysobacter spp. on plant surfaces.
The mycelial growth-inhibiting compounds produced by Lysobacter sp. strain SB-K88 were identified as three metabolites, xanthobaccins A, B, and C (24). Seed inoculation with live bacteria and seed inoculation with isolated xanthobaccin A (recognized as a quantitatively and qualitatively major metabolite) both suppressed damping-off disease in sugar beet grown in Pythium sp.-infested soil (24). However, so far no information is available concerning the effects of xanthobaccin A on the infecting agents (i.e., zoospores of any soilborne member of the Peronosporomycetes). The method by which Lysobacter sp. strain SB-K88 colonizes roots of host plants that suffer from damping-off disease also has not been established. Therefore, a clear understanding of the mechanisms governing the interactions among the host plant, SB-K88, and the Peronosporomycete pathogen is needed to enable practical use of this bacterium as a biocontrol agent. Thus, the objectives of the present work were to (i) investigate the modes of attachment and patterns of Lysobacter sp. strain SB-K88 colonization on plant roots growing from inoculated seeds, as well as on the surface of A. cochlioides; (ii) test the effects of living bacteria and their secondary metabolites on A. cochlioides hyphae and zoospores; and (iii) evaluate the ability of SB-K88 to control A. cochlioides pathogenesis in sugar beet and spinach.
MATERIALS AND METHODS
Strain SB-K88 and xanthobaccin A.
Bacterial strain SB-K88 was isolated from the fibrous roots of sugar beet (11) and was tentatively identified as Stenotrophomonas sp. (24) based on its morphological and biochemical characteristics. Recently, we identified this strain as a member of Lysobacter sp. based on 16S rRNA gene sequencing data (accession no. AB190258) and other traits (gliding motility, lytic activity, and morphology of the cell) (32); the results of this analysis will be reported elsewhere. Strain SB-K88 was stored in sterilized 20% (vol/vol) glycerol at −82°C until use.
A tetramic acid-containing macrolactam, the xanthobaccin A used in this study, was isolated and purified from the culture supernatant of strain SB-K88 by chromatographic techniques (24). For bioassays, 0.5 mg of xanthobaccin A was dissolved in a small quantity of dimethyl sulfoxide (DMSO) (solvent) and then serially diluted with sterilized distilled water to obtain a range of final concentrations (1000, 100, 10, and 1 μg/ml) for both the zoospore bioassay and the scanning electron microscopy (SEM) study (15, 16, 18). The final DMSO concentration was maintained at less than 1% in the aqueous zoospore suspensions (ca. 105 zoospores/ml). A negative control with 1% DMSO did not have any effect on the motility and viability of zoospores.
Culture conditions and extraction.
Lysobacter sp. strain SB-K88 was cultured in a 500-ml flask containing 200 ml of a nutrient solution at 25°C for 15 days with shaking at 100 rpm. Each liter of the medium contained 3.0 g K2HPO4, 1.0 g NaH2PO4, 1.0 g NH4Cl, 0.3g MgSO4 · 7H2O, 0.15 g KCl, 0.01 g CaCl2, 0.0025 g FeSO4 · 7H2O, and 5.0 g saccharose. The culture fluid was centrifuged at 8,000 × g for 15 min at 5°C, and then the supernatant was freeze-dried. The residue was dissolved in a small amount of H2O, extracted with excess ethyl acetate (EtOAc), and concentrated in vacuo. All fractions (crude freeze-dried material and EtOAc and water-soluble fractions) were subjected to the zoospore bioassay. The bacterial pellets were washed three times with sterilized phosphate buffer (8 mM, pH 7.2) and used for seed coating and bioassays.
Thin-layer chromatography with Silica Gel 60 F254 plates (thickness, 0.25 and 0.50 mm; Merck) and with CHCl3-methanol-H2O (65:25:4) as the developing solvent was used to detect xanthobaccin A (Rf, 0.49) in the freeze-dried material and extracts of the culture supernatant of SB-K88.
Bioassay.
A. cochlioides AC-5, which was isolated from the soil of a sugar beet field, was a gift from R. Yokosawa. It was routinely grown on corn meal agar (Difco) in a glass petri dish (inside diameter, 9 cm), and zoospores were produced by using protocols described by Islam et al. (17). Zoospore bioassays with living bacteria, freeze-dried material, EtOAc-soluble material, water-soluble material, and pure xanthobaccin A were carried out by a homogeneous solution method as described previously (16, 18).
Seed coating, zoospore inoculation, and disease intensity.
Surface-sterilized seeds (Daigaku-Noen, Tokyo, Japan) of sugar beet cultivar Abendrot, Arabidopsis thaliana Ws (N915), Amaranthus gangeticus L. cv. Altapati, and spinach cultivar Tonic were coated with SB-K88 (ca. 108 CFU/seed) (24) and grown in 0.3% gellan gum containing 0.2× Hoagland's S medium in either test tubes or sterilized (160°C for 4 h) soil in 36-cell small plastic packs (cell size, 4.5 by 4.5 by 4.5 cm). On day 12 of cultivation (23°C, 16 h of light and 8 h of darkness), each seedling was inoculated with 1 ml of an A. cochlioides zoospore suspension (aqueous) containing 1 × 104, 1 × 103, 1 × 102, or no zoospores. Flooded conditions were maintained immediately after zoospore inoculation in order to enhance the infection process. To compare the ability of SB-K88 to control disease with the ability of a conventional fungicide to control disease, seeds were treated with hymexazol (Tachigaren; Hokkai Sankyo, Hokkaido, Japan) at a level of 7.5 g/kg seeds. Data for the frequency of healthy seedlings were recorded 2 weeks after zoospore inoculation. Seedlings that did not have black and shrunken or dark, slender, thread-like hypocotyls or roots were considered healthy seedlings. Each treatment was replicated three times.
TEM.
To study the ultrastructure of SB-K88 fimbriae, bacterial cells were cultured in the nutrient solution described above without shaking for 48 h at 27°C. Exactly 100 μl of a 10-fold-diluted (with sterilized water) bacterial suspension was collected in an Eppendorf tube and fixed with 2% glutaraldehyde (TAAB, Aldermaston, Berkshire, United Kingdom) for 30 min. The specimen was then prepared for transmission electron microscopy (TEM) studies as described previously (14).
A mechanical stimulus resulting from vortex mixing for 25 to 30 s caused 100% encystment of the zoospores. To study the ultrastructure and colonization of SB-K88 on mechanically induced cystospores, a 1-ml aqueous suspension of SB-K88 cells (104 CFU/ml) was added to an equal volume of A. cochlioides cystospores (ca. 105 spores/ml) in an Eppendorf tube and allowed to interact for 1 h. The sample was then fixed with 2% glutaraldehyde for 30 min and postfixed with osmium tetroxide (10 g/liter) for 2 h at 4°C. Then the sample was dehydrated with a graded acetone series, embedded in Epon 812 (electron microscopy grade; TAAB), and polymerized at 60°C for 24 h. Ultrathin sections (thickness, 100 nm) were cut with a diamond knife (SK1045; Sumitomo Electric Industries, Tokyo, Japan) and stained with 2% uranyl acetate for 5 min. Sections were washed with phosphate-buffered saline and then briefly stained with lead citrate for 3 min and observed with an Hitachi H-800 transmission electron microscope with an accelerating voltage of 75 kV. Four specimens were examined for both affected and untreated control hyphae.
SEM of zoospore lysis and root and hyphal colonization by SB-K88.
Appropriate amounts of a xanthobaccin A suspension were directly added to aliquots of an aqueous zoospore suspension on a SEMpore membrane (pore size, 0.6 μm; JEOL), yielding a final volume of 400 μl. The final concentrations of xanthobaccin A and DMSO in the zoospore suspensions were 0.1 μg/ml and 0.1%, respectively. After 30 min of treatment, the specimens were fixed with 2% buffered glutaraldehyde and subjected to further processing in preparation for SEM as described previously (16).
To observe proliferation of bacteria on seed surfaces, surface-sterilized seeds of sugar beet and spinach were immersed in a nutrient-free SB-K88 aqueous suspension for 15 min and then incubated for 0 and 48 h on soaked (with sterilized distilled water) filter paper in a glass petri dish (inside diameter, 9 cm). After incubation for a set time, the seeds were fixed with 2% glutaraldehyde overnight, and the remaining preparation procedures for the SEM study were carried out as described previously (12, 14, 17).
To study colonization of bacteria on plant surfaces, 2-week-old seedlings were carefully removed from a soft gel composed of gellan gum or from soil and gently washed six times with sterilized phosphate buffer (8 mM, pH 7.2). Roots, stems, and leaves of these seedlings were then separated using a sharp blade and fixed with 2% glutaraldehyde overnight. The remaining preparation procedures for an SEM study were carried out as described previously (12, 14).
To investigate colonization of A. cochlioides hyphae by SB-K88, 6-mm-diameter blocks of mycelia in agar were transferred from a petri dish containing corn meal agar to a 3-cm-inside-diameter petri dish on day 6 of cultivation. A bacterial suspension (2 ml; ca. 104 CFU/ml) was directly added to the mycelial blocks and allowed to interact with the hyphae for 2 h. The mycelial agar blocks were gently washed with sterilized deionized water three times and then fixed with 2% glutaraldehyde in phosphate buffer (8 mM, pH 7.2) for 3 h. The remaining preparation procedures for SEM observation were performed as described previously (17, 18). The experiment was replicated three times.
RESULTS
Growth inhibition and hyphal morphological alterations in A. cochlioides.
The results of a dual-culture assay on potato dextrose agar (PDA) demonstrated that A. cochlioides mycelial growth is inhibited by coculture with Lysobacter sp. strain SB-K88 (Fig. 1A and B). Microscopic observation of A. cochlioides hyphae growing close to Lysobacter colonies revealed alterations in the hyphal morphology, including excessive branching, irregular swelling, curling of hyphal tips, and loss of apical growth (Fig. 1D). At a later stage of growth, SB-K88 exhibited gliding motility on PDA, as shown in Fig. 1B. This type of motility is an identifying characteristic of Lysobacter species (4).
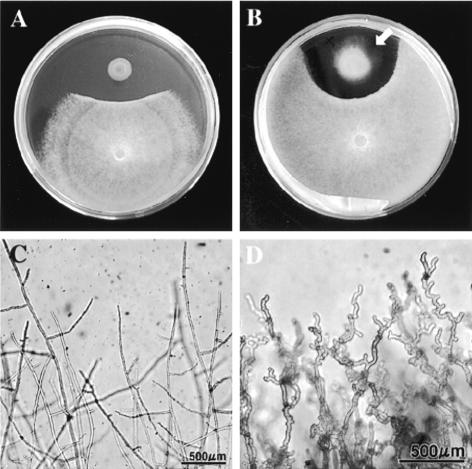
FIG. 1.
In vitro interactions between Lysobacter sp. strain SB-K88 and A. cochlioides AC-5 in a dual culture on PDA. (A) Inhibition of AC-5 mycelial growth in the presence of SB-K88 (arrow; 4 days). (B) Gliding motility of SB-K88 (arrow) on PDA (10 days) and changes in the hyphal density at the edge of the AC-5 colony. (C) Normal hyphal growth in a control. (D) Curly growth of AC-5 hyphae approaching an SB-K88 colony. The photographs in panels A and B were taken with a digital camera (CAMEDIA C-3040 zoom; Olympus Optical Co. Ltd.), and the micrographs in panels C and D were taken with the same digital camera connected to a light microscope (IX70-S1F2; Olympus).
We used SEM to further investigate the SB-K88-induced morphological changes in A. cochlioides hyphae (Fig. 2). The results of this analysis confirmed that affected hyphae commonly exhibited a higher degree of branching, reduced polar growth, and irregular swelling (Fig. 2B and C). Interestingly, A. cochlioides hyphae developing at a distance from the bacterial colony displayed an overlapping growth habit (Fig. 2D). At more advanced stages of interactions with the bacterium, cytoplasmic extrusion (Fig. 2C) and necrosis of distant hyphae were observed. Similar effects on A. cochlioides growth and hyphal morphology were observed when the pathogen was cultured in the presence of SB-K88 culture supernatant or xanthobaccin A in a paper disk bioassay on PDA (data not shown).
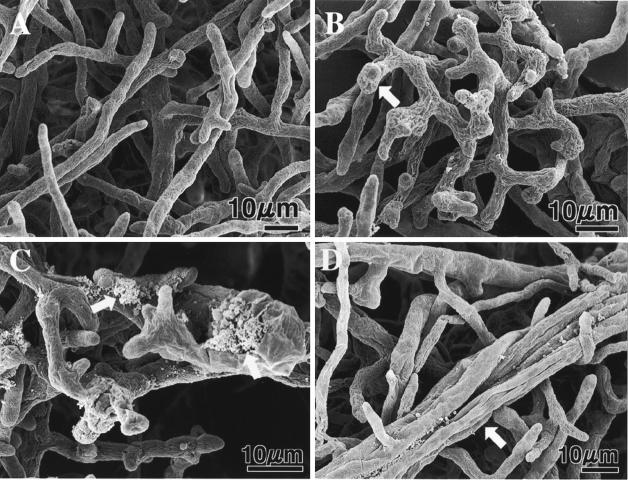
FIG. 2.
Scanning electron micrographs of A. cochlioides mycelial samples interacting (5 days) with Lysobacter sp. strain SB-K88 and an untreated control. For the SEM study, small blocks (diameter, 6 mm) of affected mycelia (approaching a bacterial colony) in agar were transferred from a petri dish (inside diameter, 9 cm) containing corn meal agar to a 3-cm-inside-diameter petri dish on day 6 of cultivation and then fixed with 2% glutaraldehyde in phosphate buffer (8 mM, pH 7.2) for 3 h. Other preparation procedures for microscopy were similar to those described previously (14, 17, 18). (A) Normal growth in the absence of SB-K88 (control). (B) Bulbous structures (arrow) and curly growth in the presence of SB-K88. (C) Cytoplasmic extrusion from the hyphae (arrows). (D) Overlapping growth of mycelia (arrow).
Proliferation of bacteria on the seed coat.
Using dilution plating and SEM, we determined the number of Lysobacter sp. strain SB-K88 cells present on spinach and sugar beet seed coats incubated for 0 and 48 h (Fig. 3). We found that during the first 48 h after inoculation, robust (twofold) proliferation of the bacteria occurred on the seed coats of both plant species. The results of a seed coating experiment indicated that more bacteria remained attached to the spinach seed coats (Fig. 3A and B) than to the sugar beet seed coats (Fig. 3C and D).
FIG. 3.
Scanning electron micrographs showing seed coats of spinach (A and B) and sugar beet (C and D) at zero time (A and C) and 48 h (B and D) after inoculation of seeds with Lysobacter sp. strain SB-K88 cells (see Materials and Methods for details). The numbers of bacteria per 100 μm2 of seed coat determined by SEM were 69 ± 8 cells (zero time) and 158 ± 11 cells (48 h) for spinach and 22 ± 4 cells (zero time) and 53 ± 9 cells (48 h) for sugar beet. These values are averages ± standard errors of five replications.
Characteristic attachment and colonization of Lysobacter sp. strain SB-K88 on plant tissue.
We visualized Lysobacter sp. strain SB-K88 by TEM. We observed very long (∼6-μm), brush-like, fragile fimbriae at one pole of the sessile bacterial rod (1.5 to 2.0 μm) (Fig. 4A). This structural feature is characteristic of bacteria exhibiting gliding motility (31). Additionally, the fimbriae were observed at both poles of dividing bacterial cells. Somewhat predictably, bacteria grown in shaking culture conditions or in prolonged liquid culture displayed missing or broken fimbriae.
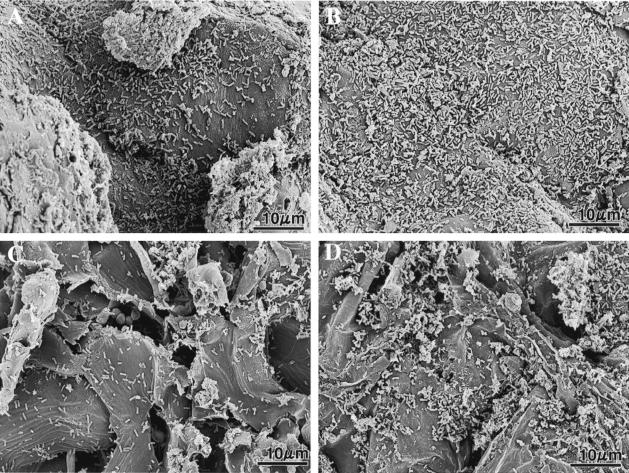
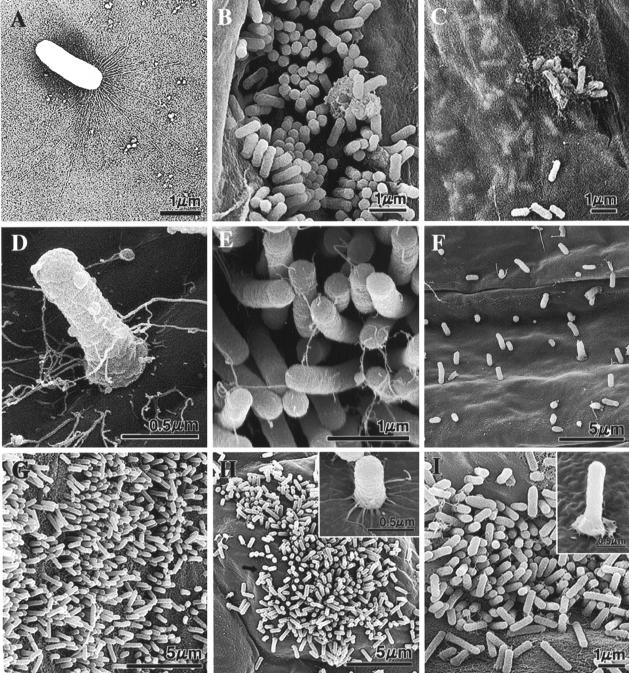
FIG. 4.
TEM (A) and SEM (B to I) micrographs illustrating the morphology of Lysobacter sp. strain SB-K88 (A) and colonization of SB-K88 (B to I) on plant surfaces upon inoculation of seeds and seedlings grown in the gellan gum-based medium (B to D and G to I) or soil (F). (A) TEM micrograph of a sessile SB-K88 bacterial cell having large, brush-like fimbriae at one end. (B) Colonization on sugar beet root by perpendicular attachment. (C) Bacterial biofilm that developed under a semitransparent film of sugar beet root mucigel. (D) Typical perpendicular attachment of a bacterial cell to a sugar beet cotyledon. (E) High-density perpendicular attachment and colonization on the sugar beet leaf surface after immersion into an SB-K88 bacterial suspension (ca. 105 CFU/ml). (F) Colonization of sugar beet root. (G) Colonization of tomato root. (H) Colonization of A. thaliana leaf. (I) Colonization of A. thaliana root. Each experiment was repeated three times, and representative micrographs are shown (see Materials and Methods for details).
To examine the ability of Lysobacter sp. strain SB-K88 to colonize plant roots, we inoculated seeds of various plant species with an aqueous suspension of bacterial cells (ca. 108 CFU/seed) and then incubated them in a test tube (length, 18 cm; inside diameter, 1.5 cm) containing 0.2× Hoagland's solution with gellan gum. Using SEM, we observed that for seedlings grown from seeds previously inoculated with SB-K88, the bacteria vigorously colonized and attached in a perpendicular fashion to the roots of both A. cochlioides hosts, including sugar beet (Fig. 4B to F) and spinach (data not shown), and nonhost plants, such as tomato (Fig. 4G), Arabidopsis thaliana (Fig. 4H and I), and Amaranthus gangeticus (data not shown) grown in this system. SB-K88 was equally capable of colonizing the same plant species grown in small plastic cells containing a soil medium (Fig. 4F).
In the case of sugar beet, we found that (i) SB-K88 densely colonized both the root and cotyledon surfaces in a perpendicular fashion, presumably employing polar fimbriae (Fig. 4B and D); (ii) a semitransparent film appeared to enclose the root surface, and microcolonies were present on the root itself (Fig. 4C); and (iii) microcolonies were localized primarily at the junctions between the primary and secondary roots (Fig. 4B), with bacterial numbers declining from the root base to the root tip (data not shown). Interestingly, spinach and some of the other plant species examined in this study exhibited characteristic colonization by SB-K88, yet no semitransparent bacterial film formed on the root surfaces in these cases (data not shown). We also found that while the surfaces of main and lateral roots were heavily colonized, the root hairs were practically free from the bacterium.
Figure 4F shows colonization of the root surface of soil-grown sugar beet by SB-K88. The relatively low density of the bacterium on the root surface might have been due to loss from the vigorous washing that was used to remove soil from the sample. To clarify this aspect of SB-K88 colonization, we treated both root tips and leaf disks of 2-week-old sugar beet seedlings by immersion in an SB-K88 suspension (105 CFU/ml) for 1 h, and this was followed by fixation with 2% glutaraldehyde and visualization by SEM. We then observed the same perpendicular attachment pattern, but the SB-K88 colonization was denser at both the rhizoplane and the phylloplane than it was in soil-grown samples (Fig. 4E). This might have been due to the direct contact of the high-density bacteria with host plant surfaces in the aqueous medium.
For every plant species, for plants that grew from seeds that were previously inoculated with SB-K88 colonization of the phylloplane appeared to be less robust than colonization of the rhizoplane for seedlings grown in gellan gum-based medium. In these samples, SB-K88 colonization was localized mainly on and around the stomata (Fig. 4H) of the leaves. For the plants studied, the highest level of SB-K88 root colonization was observed with sugar beet, followed by tomato, spinach, A. thaliana, and A. gangeticus.
Perpendicular attachment of Lysobacter sp. strain SB-K88 to A. cochlioides AC-5.
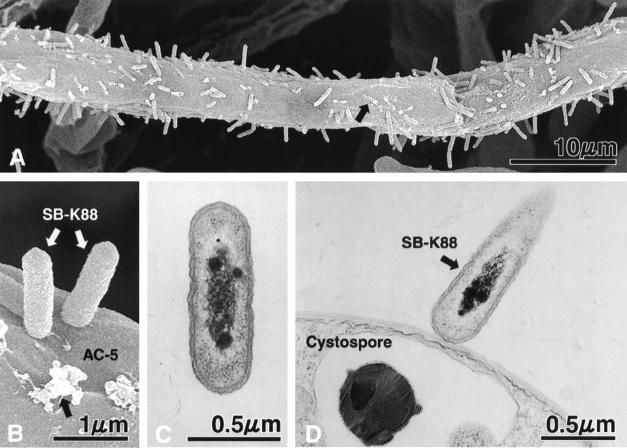
To determine if SB-K88 can also colonize AC-5 in a perpendicular fashion, we immersed a small block of PDA containing freshly grown hyphae of A. cochlioides into a low-density SB-K88 suspension for 2 h. Interestingly, SEM revealed that SB-K88 displayed perpendicular attachment and dense colonization on the hyphoplane (surface of hyphae) of A. cochlioides (Fig. 5A and B) similar to the colonization patterns observed on plant surfaces. In the case of A. cochlioides, however, we frequently noted the presence of unidentifiable granular deposits on the surface of the hyphae (Fig. 5A and B) coincident with SB-K88 colonization. No such granular deposits were observed on the surfaces of control hyphae (Fig. 2A). Moreover, SB-K88 displayed similar perpendicular attachment to the surface of A. cochlioides cystospores when a low-density SB-K88 suspension was introduced into and allowed to interact with a suspension of mechanically induced (by vortexing an aqueous zoospore suspension for 25 to 30 s) A. cochlioides cystospores for 60 min (Fig. 5D). The longitudinal TEM section of SB-K88 in Fig. 5C shows the ultrastructure of the bacterium.
FIG. 5.
SEM (A and B) and TEM (C and D) micrographs showing perpendicular attachment of Lysobacter sp. strain SB-K88 to a hypha (A and B) and a cystospore (D) of A. cochlioides (for details see Materials and Methods) and a longitudinal section of SB-K88 (C). The black arrows in panels A and B indicate unidentified granular deposits on the surface of the A. cochlioides hyphae colonized by SB-K88. No such granular deposits were observed on the surfaces of untreated control hyphae (see Fig. 2A). Each experiment was repeated at least three times.
Inhibition of motility and lysis of A. cochlioides zoospores.
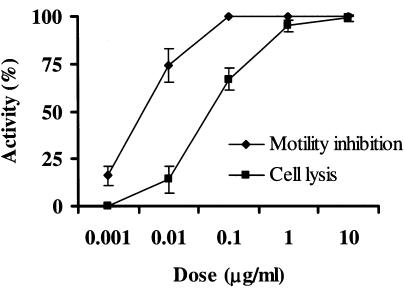
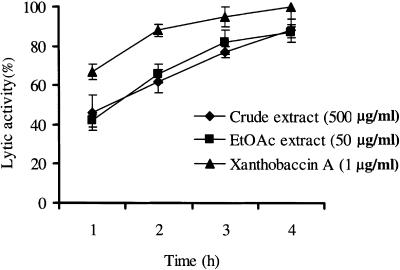
Exposure to either a cell suspension (ca. 109 CFU/ml) or the cell-free culture supernatant (MIC, 100 μg/ml) of SB-K88 caused the motility of zoospores to cease within 1 min. Such a treatment also resulted in a change in the zoospores morphology from a reniform-ovate shape (Fig. 6A) to a round shape, and the cellular contents had a granular appearance. Ultimately, these conditions led to lysis of the majority of zoospores within 30 to 60 min (Table 1 and Fig. 6). A crude EtOAc-soluble fraction (MIC, 10 μg/ml) of the freeze-dried culture supernatant or pure xanthobaccin A (0.1 μg/ml) caused almost identical inhibition of motility (100%), followed by lysis of the zoospores in a dose-dependent manner (Table 1 and Fig. 6 and 7). The lytic activity increased over time, plateauing at 4 h after addition of the freeze-dried SB-K88 culture supernatant, EtOAc-soluble fraction, or xanthobaccin A (Fig. 8). Thin-layer chromatography analysis and a bioassay of the EtOAc-soluble fraction and the freeze-dried SB-K88 culture supernatant revealed that SB-K88-derived xanthobaccin A was primarily responsible for mycelial growth inhibition and zoospore lysis. Significantly, when treated with high doses of freeze-dried culture supernatant, zoospores were completely lysed (no trace remained) (Fig. 6C).
TABLE 1.
Motility-inhibiting and lytic activities of xanthobaccin A and extracts of SB-K88 culture supernatant with zoospores of A. cochlioides
| Extract or compound | Dose (μg/ml) | Activity (%)a
|
||
|---|---|---|---|---|
| Motility inhibition | Cell lysis | Germination | ||
| Freeze-dried culture supernatant | 1,000 | 100 ± 0 | 100 ± 0 | 0 ± 0 |
| 100 | 100 ± 0 | 27 ± 5 | 3 ± 1 | |
| 10 | 66 ± 5 | 5 ± 2 | 40 ± 5 | |
| 1 | 21 ± 7 | 0 ± 0 | 62 ± 8 | |
| EtOAc-soluble fraction | 100 | 100 ± 0 | 100 ± 0 | 0 ± 0 |
| 10 | 100 ± 0 | 22 ± 7 | 3 ± 1 | |
| 1 | 100 ± 0 | 6 ± 4 | 13 ± 2 | |
| 0.1 | 43 ± 9 | 0 ± 0 | 35 ± 4 | |
| Water-soluble fraction | 1,000 | 100 ± 0 | 60 ± 9 | 30 ± 8 |
| 100 | 100 ± 0 | 21 ± 10 | 60 ± 6 | |
| 10 | 90 ± 5 | 8 ± 4 | 83 ± 2 | |
| 1 | 68 ± 9 | 0 ± 0 | 95 ± 6 | |
| Xanthobaccin A | 10 | 100 ± 0 | 100 ± 0 | 0 ± 0 |
| 1 | 100 ± 0 | 95 ± 3 | 0 ± 0 | |
| 0.1 | 100 ± 0 | 67 ± 6 | 0 ± 0 | |
| 0.01 | 74 ± 9 | 14 ± 7 | 10 ± 0 | |
| 0.001 | 16 ± 5 | 0 ± 0 | 49 ± 2 | |
| Control | 17 ± 4 | 0 ± 0 | 8 ± 5 | |
Activity was recorded after 3 h of incubation of zoospores with extract or xanthobaccin A at about 22°C. Xanthobaccin A and extracts were first dissolved in small quantities of DMSO and then diluted with distilled water. Appropriate amounts of a sample suspension were directly added to a zoospore suspension (ca. 105 spores/ml) in one Nunc Multidish (Nunc) to obtain a final volume of 300 μl and gently mixed well with a glass rod. The final concentration of DMSO in the zoospore suspension was less than 1% in all treatments; 1% DMSO alone was completely inactive in the assays for motility and the viability of zoospores. A zoospore suspension in separate dishes (300 μl) was used as a control. Crude extracts or pure xanthobaccin A immediately caused inhibition of motility of zoospores. The halted zoospores rapidly settled to the bottom of the dish and then started to lyse or burst. The number of settled and lysed spores was determined microscopically (magnification, ×20). The percentage of halted and lysed zoospores was calculated as described previously (16). The data are averages ± standard errors of at least three replications for each dose of xanthobaccin A or extract.
FIG. 7.
Inhibition of motility and lysis of A. cochlioides zoospores treated with various doses of xanthobaccin A. Xanthobaccin A was first dissolved in a small quantity of DMSO and then serially diluted with distilled water. Appropriate amounts of a sample suspension were added to the aqueous zoospore suspension. The final DMSO concentration was always less than 1% in the zoospore suspension. The inhibition of motility and lysis of zoospores were observed microscopically (magnification, ×20) (for details see Table 1) 3 h after the treatment with xanthobaccin A, and the percentages of activity were calculated as described previously (16). DMSO alone (final concentration in the zoospore suspension, 1%) was used as the control and did not have any effect on the motility and lysis of the zoospores. The data are the averages ± standard errors of at least three replications for each dose of xanthobaccin A.
FIG. 8.
Time course of lytic activity of the crude freeze-dried culture supernatant, the EtOAc-soluble fraction, and xanthobaccin A against A. cochlioides zoospores. Xanthobaccin A or extracts of the culture supernatant were first dissolved in a small quantity of DMSO and then serially diluted with distilled water. Appropriate amounts of a sample suspension were added to the aqueous zoospore suspension. The final DMSO concentration was always less than 1% in the zoospore suspension. The time course of lysis of zoospores was observed microscopically (magnification, ×20) (for details see Table 1) for up to 4 h after the treatment, and the percentages of activity were calculated as described previously (16). DMSO alone (final concentration in the zoospore suspension, 1%) was used as the control and did not cause any lysis of the zoospores. The data are the averages ± standard errors of at least three replications for each dose of xanthobaccin A.
Disease suppression activity.
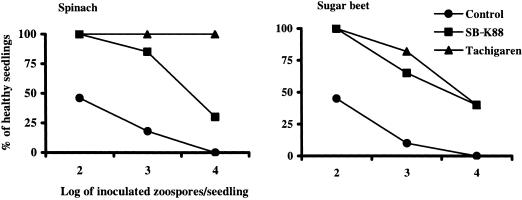
Both sugar beet and spinach seedlings grown from seeds treated with SB-K88 (ca. 108 CFU/seed) remarkably suppressed damping-off disease (65% healthy sugar beet plants; 85% healthy spinach plants) when the seedlings were artificially challenged with A. cochlioides zoospores (103 zoospores/seedling) (Fig. 9). Compared with SB-K88, the commercial fungicide Tachigaren had a slightly stronger disease suppression effect in both spinach and sugar beet, especially at the higher doses of zoospores.
FIG. 9.
Damping-off disease suppression by Lysobacter sp. strain SB-K88 in spinach and sugar beet. Five surface-sterilized seeds coated with nutrient-free SB-K88 cells (ca. 108 CFU/seed) or fungicide (Tachigaren; 7.5 g/kg seeds) were sown in 36-cell plastic packs containing sterilized soil. On day 12 of cultivation, each seedling was inoculated with an appropriate number of zoospores (for details see Materials and Methods). The percentage of healthy seedlings was recorded 2 weeks after zoospore inoculation. Seedlings that did not have black and shrunken or dark, slender hypocotyls or roots were considered healthy seedlings. Seedlings obtained from surface-sterilized seeds (no treatment with bacteria or fungicide) were considered controls. The values are averages of three replications.
DISCUSSION
A hallmark of Lysobacter sp. strain SB-K88 is its perpendicular attachment to plant cells, as well as to surfaces of A. cochlioides hyphae and cystospores (Fig. 4 and 5). SB-K88 displayed another defining characteristic, the development of brush-like fimbriae at one end of each bacterial cell (Fig. 4A). Since fimbriae are known to function as attachment organs, allowing bacteria to adhere to hard surfaces, SB-K88's polar fimbriae may help the cells attach perpendicular to plant cell walls. Although prior studies have described perpendicular attachment of the plant-pathogenic Pseudomonas aeruginosa strain PA14 and Ralstonia solanacearum to Arabidopsis mesophyll cell walls (27) and to tobacco tissue culture cells (33), respectively, most plant-associated (pathogenic or nonpathogenic) bacteria do not appear to attach to plant cell walls in this manner (3, 12). Perpendicular binding of the opportunistic bacterial pathogen P. aeruginosa to the opportunistic pathogenic fungus Candida albicans has also been demonstrated by Hogan and Kolter (10).
In the case of the human opportunistic pathogen P. aeruginosa PA14, the bacterial cells orient themselves perpendicular to the outer surface of mesophyll cell walls. Next, the PA14 cells generate circular perforations, whose diameter is approximately equal to the diameter of P. aeruginosa, in these mesophyll cell walls (27). We could not determine from the current study if SB-K88 is capable of penetrating A. cochlioides hyphae or spores, as we observed specimens after only 2 h of interaction with the phytopathogen. Further studies are needed to determine whether Lysobacter spp. can penetrate the hyphae or spores of Peronosporomycetes or other fungi by utilizing their lytic antibiotics and/or enzymes.
To the best of our knowledge, this is the first report to describe perpendicular attachment of a biocontrol bacterium to both plant and hyphal cell walls. Biofilm formation accompanying this perpendicular mode of attachment in theory could support a larger population of rod-shaped bacteria on a given plant surface. Also, establishing a clearer understanding of SB-K88's mechanism of perpendicular attachment to plant and hyphal surfaces may be important in realizing its potential as a biocontrol agent. Determining whether this phenomenon is characteristic of the genus Lysobacter in general may also help workers find other useful species.
Our in vitro and in vivo experiments demonstrated that Lysobacter can colonize both leaves and roots of plants via perpendicular attachment in a single layer (Fig. 4). However, we observed markedly reduced bacterial colony formation on the leaves of plants grown from SB-K88-coated seeds compared with the roots. We made the noteworthy observation that SB-K88 itself developed a biofilm beneath a semitransparent film, most likely composed of root mucigel at the root surface of sugar beet (Fig. 4C). No such root mucigel film was observed on uninoculated sugar beet plants (data not shown). However, we did not find any similar biofilm on the roots of other plants tested, indicating that this film might originate from the host plant rather the bacteria. A similar phenomenon was observed in an SEM study conducted by investigators examining Pseudomonas fluorescens strain WCS365 colonization on tomato roots (3). Effective colonization of both foliar and subterranean plant parts by L. enzymogenes has also been observed (32).
The following are some important aspects of the Lysobacter sp. strain SB-K88 spatial-temporal behavior on sugar beet. (i) Upon colonization, the bacterial cells proliferate on the seed coat, utilizing nutrients provided by the seed coat and/or seed exudates (Fig. 3). (ii) Individual cells colonize the root base and over time spread gradually toward the tip. (iii) An increased number of microcolonies appear, mainly in the junctions between epidermal cells and at the root base. And (iv) root hairs remain completely free from bacterial colonization. The sites and patterns of colonization by rhizosphere bacteria have been described in previous studies (3). Formation of microcolonies at the junctions between epidermal cells reflects the availability of nutrients on the root surface, as these junctions are considered to be leaky for root exudates (3).
Another novel finding of the present study involves SB-K88-mediated antagonistic activity against A. cochlioides zoospore motility, leading to subsequent lysis. Our results indicated that this antibiosis is due to production of the bacterial antibiotic xanthobaccin A by SB-K88 (Table 1 and Fig. 7 and 8). We found that the MIC of xanthobaccin A for inhibition of motility and lysis of A. cochlioides zoospores is approximately 0.01 μg/ml, which is within a reasonable range for an antimicrobial agent and may meet the requirements for application in rhizosphere settings (1). In an experiment involving SB-K88-inoculated sugar beet seeds that were germinated and grown under hydroponic conditions, the level of xanthobaccin A in the rhizosphere was estimated to be 3 μg per plant (24). Thus, it is possible that at the root surface the levels of dissolved xanthobaccin A are substantially higher than the MIC for A. cochlioides zoospore motility and survival in vitro, suggesting that the biocontrol exhibited against this pathogen is linked to production of a Lysobacter sp. strain SB-K88-derived antibiotic at the root surface. Lytic activity against other microorganisms is a generic characteristic of Lysobacter spp. (4). However, this is the first report to describe inhibition of Aphanomyces zoospore motility followed by lysis by the bacterial metabolite xanthobaccin A. Recently, Folman et al. also found that culture filtrates of L. enzymogenes strain 3.1T8 caused rapid immobilization of zoospores and inhibited germination of cysts of Pythium aphanidermatum, a cucumber root and crown rot pathogen (8).
Zoosporicidal activity of cyclic lipopeptide surfactants produced by the biocontrol bacterium P. fluorescens has been reported for multiple members of the Peronosporomycetes, including Pythium species, Albugo candida, and Phytophthora infestans (6). Similarly, rhamnolipids, another class of effective zoospore-lytic biosurfactants produced by P. aeruginosa, were demonstrated to be highly effective against plant pathogens, including P. aphanidermatum, Plasmopara latucae-radicis, and Phytophthora capsici (22).
In the present study, the excessive branching and deformation of A. cochlioides hyphae resulting from SB-K88 antibiotic activity occurred not only at the hyphal tips but also in regions distal to the tips. In the latter area, some granular cytoplasmic extrusions developed, followed by necrosis of the hyphae (Fig. 2). The major metabolite of SB-K88, xanthobaccin A, inhibited the growth of mycelia of a wide range of fungi and Peronosporomycetes (24). Although the exact xanthobaccin A mode of action is not clear at present, our results suggest that the xanthobaccin A produced by SB-K88 plays a role in inhibiting the growth and altering the hyphal morphology of A. cochlioides. Excessive branching of the hyphae has also been reported in different fungi and Peronosporomycetes following treatment with ergosterol biosynthesis-inhibiting fungicides (19). Sisler and Ragsdale speculated that such excessive hyphal branching may be due to changes in the activity of wall synthesis enzymes (30).
Our observations provide convincing evidence that Lysobacter sp. has a direct inhibitory effect on A. cochlioides growth and development, suppressing damping-off diseases in sugar beet and spinach (Fig. 9). This activity is attributable to a combination of antibiosis (Table 1 and Fig. 6) and vigorous root colonization via perpendicular attachment (Fig. 4). In a related TEM study, we observed remarkable ultrastructural alterations, including accumulation of excess lipid bodies and degeneration and necrosis of the cytoplasm, in hyphae affected by SB-K88 (unpublished data). The cell walls of hyphae and membranes of Peronosporomycetes zoospores are composed of β-1,3- and β-1,6-glucans and cellulose. Lysobacter species are known to produce several antibiotics (2, 8, 9, 25, 28) and antimicrobial enzymes, including β-1,3-glucanase (26, 39, 40). Therefore, our results which demonstrated that there was A. cochlioides zoospore lysis and hyphal necrosis attributable to SB-K88 antibiotic activity do not exclude a possible contribution by lytic enzymes acting in concert with xanthobaccin A.
The concept that environmental sensing mechanisms modulate production of many antifungal factors is well understood (5, 29, 36). Nakayama et al. reported that the amount of xanthobaccin A produced per SB-K88 cell was approximately 1 × 104- to 1 × 105-fold larger in the rhizoplane near attached SB-K88 than in liquid cultured cells (24). Further research is required to elucidate the mechanisms underlying sensing of the rhizoplane environment and simultaneous production of xanthobaccin A by Lysobacter sp. strain SB-K88. Since areas of the plant exhibiting high bacterial densities and the presence of microcolonies (Fig. 4B and C) are among the most vulnerable sites for attack by soilborne Peronosporomycetes, we suggest that the formation of microcolonies by perpendicular attachment of bacterial cells is crucial step in biocontrol by Lysobacter species.
Acknowledgments
We thank R. Yokosawa (Health Science University of Hokkaido, Hokkaido, Japan) and Y. Homma and T. Nakayama (National Agricultural Research Center for Hokkaido Region, Sapporo, Japan) for their kind gifts of A. cochlioides AC-5 and Lysobacter sp. strain SB-K88, respectively. We also thank Yuriko Aoyama (Laboratory of Electronic Microscopy, Hokkaido University, Hokkaido, Japan) for her assistance during the SEM study.
Financial support and a postdoctoral fellowship (to M.T.I) from the Japan Society for the Promotion of Science and financial support provided by grants-in-aid for scientific research (grant 16208032 to Y.H. and grant 14206013 to S.T.) from the Ministry of Education, Science, Sports, Culture, and Technology of Japan are also very much appreciated.
REFERENCES
- 1.Bais, H. P., R. Fall, and J. M. Vivanco. 2004. Biocontrol of Bacillus subtilis against infection of Arabidopsis root by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 134:307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner, D. P., J. O'Sullivan, S. K. Tanaka, J. M. Clark, and R. R. Whitney. 1988. Lysobactin, a novel antibacterial agent produced by Lysobacter sp. II. Biological properties. J. Antibiot. 41:1745-1751. [DOI] [PubMed] [Google Scholar]
- 3.Chin-A-Woeng, T. F. C., W. D. Priester, A. J. V. D. Bij, and B. J. J. Lugtenburg. 1997. Description of the colonization of a gnotobiotic tomato rhizosphere by Pseudomonas fluorescens biocontrol strain WCS365, using scanning electron microscopy. Mol. Plant-Microbe Interact. 10:79-86. [Google Scholar]
- 4.Christensen, P., and F. D. Cook. 1978. Lysobacter, a new genus of non-fruiting, gliding bacteria with high base ratio. Int. J. Syst. Bacteriol. 28:367-393. [Google Scholar]
- 5.Cook, R. J., L. S. Thomashow, D. M. Weller, D. Fujimoto, M. Mazzola, G. Bangera, and D.-S. Kim. 1995. Molecular mechanisms of defense by rhizobacteria against root disease. Proc. Natl. Acad. Sci. USA 92:4197-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza, J. T., M. de Boer, P. de Waard, T. A. van Beek, and J. M. Raaijmakers. 2003. Biochemical, genetic, and zoosporicidal properties of cyclic lipopeptide surfactants produced by Pseudomonas fluorescens. Appl. Environ. Microbiol. 69:7161-7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dick, M. W. 2001. The Peronosporomycetes, p. 39-72. In D. J. McLaughlin, E. G. McLaughlin, and P. A. Lemke (ed.), The Mycota VII, part A. Systematics and evolution. Springer-Verlag, Berlin, Heidelberg, Germany.
- 8.Folman, L. B., J. Postma, and J. A. van Veen. 2003. Characterization of Lysobacter enzymogenes (Christensen and Cook, 1978) strain 3.1T8, a powerful antagonist of fungal disease of cucumber. Microbiol. Res. 158:107-115. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume, H., S. Hirosawa, R. Sawa, Y. Muraoka, D. Ikeda, H. Naganawa, and I. Masayuki. 2004. Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. II. Structure elucidation. J. Antibiot. 57:52-58. [DOI] [PubMed] [Google Scholar]
- 10.Hogan, D. A., and R. Kolter. 2002. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229-2232. [DOI] [PubMed] [Google Scholar]
- 11.Homma, Y., H. Uchino, K. Kanzawa, T. Nakayama, and M. Sayama. 1993. Suppression of sugar beet damping-off and production of antagonistic substances by strains of rhizobacteria. Ann. Phytopathol. Soc. Jpn. 59:282. [Google Scholar]
- 12.Hoo, H., Y. Hashidoko, M. T. Islam, and S. Tahara. 2004. Requirement of relatively high threshold level of Mg2+ for cell growth of a rhizoplane bacterium, Sphingomonas yanoikuyae EC-S001. Appl. Environ. Microbiol. 70:5214-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam, M. T., and S. Tahara. 2001. Chemotaxis of fungal zoospores, with special reference to Aphanomyces cochlioides. Biosci. Biotechnol. Biochem. 65:1933-1948. [DOI] [PubMed] [Google Scholar]
- 14.Islam, M. T., T. Ito, and S. Tahara. 2001. Morphological studies on zoospores of Aphanomyces cochlioides and changes during interaction with host materials. J. Gen. Plant Pathol. 67:255-261. [Google Scholar]
- 15.Islam, M. T., T. Ito, and S. Tahara. 2002. Microscopic studies on attachment and differentiation of zoospores of the phytopathogenic fungus Aphanomyces cochlioides. J. Gen. Plant Pathol. 68:111-117. [Google Scholar]
- 16.Islam, M. T., T. Ito, M. Sakasai, and S. Tahara. 2002. Zoosporicidal activity of polyflavonoid tannin identified in Lannea coromandelica stem bark against phytopathogenic oomycete Aphanomyces cochlioides. J. Agric. Food Chem. 50:6697-6703. [DOI] [PubMed] [Google Scholar]
- 17.Islam, M. T., T. Ito, and S. Tahara. 2003. Host-specific plant signal and G-protein activator, mastoparan, trigger differentiation of zoospores of the phytopathogenic oomycete Aphanomyces cochlioides. Plant Soil 255:131-142. [Google Scholar]
- 18.Islam, M. T., T. Ito, and S. Tahara. 2004. Interruption of the homing events of phytopathogenic Aphanomyces cochlioides zoospores by secondary metabolites from nonhost Amaranthus gangeticus. J. Pestic. Sci. 29:6-14. [Google Scholar]
- 19.Kang, Z., L. Huang, U. Kreig, A. Mauler-Machnik, and H. Buchenauer. 2001. Effects of tubeconazole on morphology, structure, cell wall components and trichothecene production of Fusarium culmurum in vitro. Pest Manag. Sci. 57:491-500. [DOI] [PubMed] [Google Scholar]
- 20.Kilic-Ekici, O., and G. Y. Yuen. 2003. Induced resistance as a mechanism of biological control by Lysobacter enzymogenes strain C3. Phytopathology 93:1103-1110. [DOI] [PubMed] [Google Scholar]
- 21.Kilic-Ekici, O., and G. Y. Yuen. 2004. Comparison of strains of Lysobacter enzymogenes and PGPR for induction of resistance against Bipolaris sorokiniana in tall fescue. Biol. Control 30:446-455. [Google Scholar]
- 22.Kim, B. S., J. Y. Lee, and B. K. Hwang. 2000. In vivo control and in vitro antifungal activity of rhamnolipid B, a glycolipid antibiotic, against Phytophthora capsici and Colletotrichum orbiculare. Pest Manag. Sci. 56:1029-1035. [Google Scholar]
- 23.Money, N. P. 2001. Reverend Berkeley's devil. Nature 411:644-645. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama, T., Y. Homma, Y. Hashidoko, J. Mizutani, and S. Tahara. 1999. Possible role of xanthobaccins produced by Stenotrophomonas sp. strain SB-K88 in suppression of sugar beet damping-off disease. Appl. Environ. Microbiol. 65:4334-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Sullivan, J., J. E. McCullough, A. A. Tymiak, D. R. Kirsch, W. H. Trejo, and P. A. Principe. 1988. Lysobactin, a novel antibacterial agent produced by Lysobacter sp. I. Taxonomy, isolation and partial characterization. J. Antibiot. 41:1740-1744. [DOI] [PubMed] [Google Scholar]
- 26.Palumbo, J. D., R. F. Sullivan, and D. Y. Kobayashi. 2003. Molecular characterization and expression in Escherichia coli of three β-1,3-glucanase genes for Lysobacter enzymogenes strain N4-7. J. Bacteriol. 185:4362-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plotnikova, J. M., L. G. Rahme, and F. M. Ausubel. 2000. Pathogenesis of the human opportunistic pathogen Pseudomonas aeruginosa PA14 in Arabidopsis. Plant Physiol. 124:1766-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roh, J. W., J. H. Bang, and D. H. Nam. 1992. Nutritional requirements of Lysobacter lactamgenus for the production of cephabacins. Biotechnol. Lett. 14:455-460. [Google Scholar]
- 29.Salmond, G. P. C., W. B. Bycroft, G. S. A. B. Stewart, and P. Williams. 1995. The bacteria ‘enigma’: cracking the code of cell-cell communication. Mol. Microbiol. 16:615-624. [DOI] [PubMed] [Google Scholar]
- 30.Sisler, H. D., and N. N. Ragsdale. 1984. Biochemical and cellular aspects of antifungal action of ergosterol biosynthesis inhibitors, p. 257-281. In A. P. J. Trinci and J. F. Riley (ed.), Mode of action of antifungal agents. Cambridge University Press, Cambridge, United Kingdom.
- 31.Spormann, A. M. 1999. Gliding motility in bacteria: insights from studies of Myxococcus xanthus. Microbiol. Mol. Biol. Rev. 63:621-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan, R. F., M. A. Holtman, G. J. Zylstra, J. F. White, Jr., and D. Y. Kobayashi. 2003. Taxonomic positioning of two biological control agents for plant diseases as Lysobacter enzymogenes based on phylogenetic analysis of 16S rDNA, fatty acid composition and phenotypic characteristics. J. Appl. Microbiol. 94:1079-1086. [DOI] [PubMed] [Google Scholar]
- 33.Van Gijsegem, F., J. Vasse, J. C. Camus, M. Marenda, and C. Baucher. 2000. Ralstonia solanacearum produces Hrp-dependent pili that are required for PopA secretion but not for attachment of bacteria to plant cells. Mol. Microbiol. 36:249-260. [DOI] [PubMed] [Google Scholar]
- 34.West, P. V., A. A. Appiah, and N. A. R. Gow. 2003. Advances in research on oomycete root pathogens. Physiol. Mol. Plant Pathol. 62:99-113. [Google Scholar]
- 35.Williams, G. E., and M. J. C. Asher. 1996. Selection of rhizobacteria for the control of Pythium ultimum and Aphanomyces cochlioides on sugar-beet seedlings. Crop Prot. 15:479-486. [Google Scholar]
- 36.Wood, D. W., and L. S. Pierson. 1996. The phzI gene of Pseudomonas aureofaciens 30-84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene 168:49-53. [DOI] [PubMed] [Google Scholar]
- 37.Yuen, G. Y., and Z. Zhang. 2001. Control of brown patch disease using the bacterium Stenotrophomonas maltophilia strain C3 and culture fluid. Int. Turfgrass Soc. Res. J. 9:742-747. [Google Scholar]
- 38.Zhang, Z., and G. Y. Yuen. 1999. Biological control of Bipolaris sorokiniana on tall fescue by Stenotrophomonas maltophilia C3. Phytopathology 89:817-822. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, Z., and G. Y. Yuen. 2000. The role of chitinase production by Stenotrophomonas maltophilia C3 in biological control of Bipolaris sorokiniana. Phytopathology 90:384-389. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, Z., G. Y. Yuen, G. Sarath, and A. Penheiter. 2001. Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology 91:204-211. [DOI] [PubMed] [Google Scholar]