Abstract
Retail organic (n = 198) and conventional (n = 61) chickens were analyzed. Most organic (76%) and conventional (74%) chickens were contaminated with campylobacters. Salmonellae were recovered from 61% of organic and 44% of conventional chickens. All Salmonella enterica serovar Typhimurium isolates from conventional chickens were resistant to five or more antimicrobials, whereas most S. enterica serovar Typhimurium isolates (79%) from organic chickens were susceptible to 17 antimicrobials tested.
Campylobacters and salmonellae are common bacterial pathogens associated with human gastroenteritis (14). Contaminated raw or undercooked poultry is particularly important in transmitting campylobacters and salmonellae (8, 11). Consumers and farmers have been increasingly interested in organic food products. The number of organic livestock increased sharply during the last decade, and the market for organic meat products is expected to grow considerably (9). Although the growth of the organic food market has been fueled by consumers' perception of organic products as healthier and safer (19), organic livestock production is not designed to reduce pathogen loads in food animals (3, 20). Organic meat production involves potentially higher microbiological safety risks due to raising animals outdoors, the use of slow-growing breeds, the prohibition of antimicrobial use, and the use of very small slaughtering facilities (3, 19). However, little is known about the microbiological status of organic animal products. In a Danish study, campylobacters were found in all of 22 organic broiler flocks compared to one-third of 79 conventional flocks, and most Campylobacter isolates (>90%) from the organic and conventional flocks were susceptible to antimicrobials (10). Sato et al. (18) reported no significant difference in the campylobacter prevalence and antimicrobial resistance in organic and conventional dairy herds in Wisconsin. However, two isolates from the conventional herds were resistant to ciprofloxacin.
The objectives of this study were to determine the prevalence of campylobacters and salmonellae in retail organic chickens in order to evaluate the microbiological safety of organic chicken relative to meat derived from conventionally raised chickens and to characterize the bacteria and their antimicrobial susceptibility.
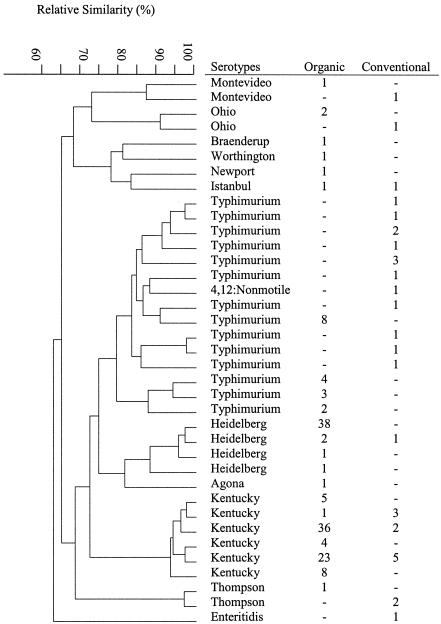
Organic (198 samples) and conventional (61 samples) chicken carcasses were randomly collected from three organic and three conventional retail stores, respectively, in Maryland between September 2002 and August 2003. Four organic and six conventional chicken brands were included in the sampling. Campylobacters and salmonellae were isolated from chickens using previously described methods (23, 24). Three presumptive Campylobacter or Salmonella isolates from each positive sample were selected and confirmed by morphological and microscope examination and PCR assays (12, 13, 17). Salmonella serotypes were determined at the U.S. Department of Agriculture National Veterinary Service Laboratories in Ames, Iowa. Approximately 76% of the organic and 74% of the conventional chicken samples were positive for campylobacters (Table 1). Similar numbers of the organic chicken samples were contaminated with Campylobacter coli (47%) and Campylobacter jejuni (45%). Conventional chickens were more commonly contaminated with C. jejuni (62%) than C. coli (40%). A number of the samples (10% organic and 13% conventional chickens) were contaminated with two or more Campylobacter species. Salmonellae were isolated from 61% of the organic and 44% of the conventional chickens (Table 1). Eleven serotypes were identified among the salmonellae from the organic chickens; Kentucky, Heidelberg, and Typhimurium were the top three serotypes (Fig. 1). Typhimurium and Kentucky were the predominant serotypes (78%) among eight serotypes of salmonellae from the organic chickens (Fig. 1).
TABLE 1.
Contamination with Salmonella and Campylobacter bacteria in organically and conventionally produced chicken samples
| Bacteria | No. (%) of contaminated samplesa
|
|
|---|---|---|
| Organic (n = 198) | Conventional (n = 61) | |
| Campylobacters | 150 (76) | 45 (74) |
| C. jejuni | 68 (45) | 28 (62) |
| C. coli | 71 (47) | 18 (40) |
| Other campylobacters | 31 (20) | 7 (16) |
| Salmonellae | 121 (61) | 27 (44) |
| Serovar Kentucky | 72 (59) | 10 (37) |
| Serovar Heidelberg | 40 (33) | 1 (4) |
| Serovar Typhimurium | 20 (17) | 12 (44) |
| Other salmonellae | 11 (9) | 6 (2) |
Twenty-seven samples contained two or more Campylobacter species, and 21 samples had two or more Salmonella serotypes.
FIG. 1.
Dendrogram of PFGE of Salmonella isolates recovered from organically and conventionally produced chicken samples. The tree of relative genetic similarity was constructed based on the neighbor-joining method; scale at 100 means identical. The numbers under columns organic and conventional are the numbers of chicken samples from which each serotype was recovered.
Pulsed-field gel electrophoresis (PFGE) with XbaI digestion was performed to determine genomic DNA fingerprinting profiles of Salmonella isolates (355 from organic and 74 from conventional chickens) using the procedures described previously (23). Thirty-seven distinct PFGE patterns were identified (Fig. 1); 14 were found only in conventional chicken samples, 18 were only in organic chicken samples, and 5 were in both conventional and organic chickens. No identical pattern was shared among Salmonella enterica serovar Typhimurium isolates from organic versus conventional chickens.
Antimicrobial resistance of bacterial pathogens has become a significant concern for public health. Antimicrobial resistance in campylobacters and salmonellae isolated from both food and clinical sources appears to be increasing in many countries. Nachamkin et al. (15) reported a dramatic increase (from 8.3% in 1996 to 40.5% in 2001) in fluoroquinolone-resistant C. jejuni in Pennsylvania. Our previous findings revealed that a common resistance among campylobacters from retail chicken and turkey was to tetracycline (82%), followed by erythromycin (54%), nalidixic acid (41%), and ciprofloxacin (35%) (6). The rise of antimicrobial-resistant salmonellae is attributed in part to the clonal spread of multidrug-resistant varieties, including S. enterica serovar Typhimurium DT104 (21). In Great Britain, the reported rates of antimicrobial-resistant S. enterica serovar Typhimurium more than doubled between 1981 and 1989 (22). In the United States, resistance to tetracycline has increased from 9% in 1980 to 24% in 1990 and ampicillin resistance increased from 10% to 14% (2, 7). Resistance to fluoroquinolones increased from 0.4% in 1996 to 1% in 2001, and resistance to ceftriaxone increased over 20-fold, from 0.1% in 1996 to 2% in 2001. We reported that many salmonellae from ground meats were resistant to tetracycline (80%), streptomycin (73%), and sulfamethoxazole (69%) (23). Ceftiofur- and ceftriaxone-resistant salmonellae were also isolated from ground turkey, chicken, and beef. These data have significant public health implications since fluoroquinolones and ceftriaxone are the most commonly used antimicrobial agents for the treatment of invasive Salmonella infections in adults and children, respectively (1, 4).
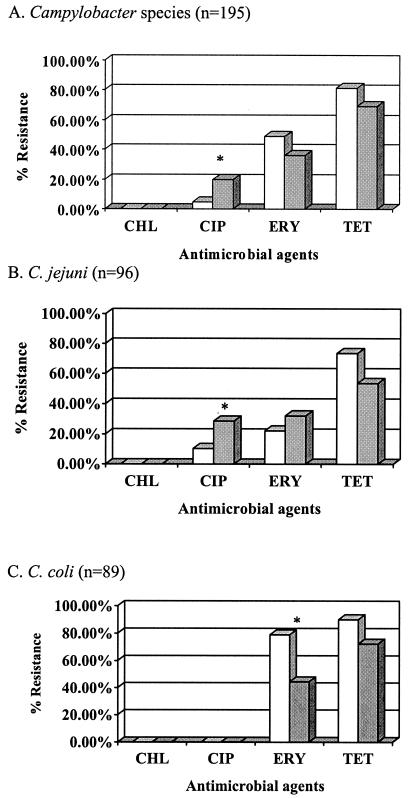
In the present study, we used agar dilution and the Sensititre system as described previously (5, 23) to determine antimicrobial susceptibility of campylobacters and salmonellae, respectively. The antimicrobials and resistance breakpoints used in the study are presented in Table 2. All campylobacters were susceptible to chloramphenicol (Fig. 2). Resistance to tetracycline was most common (78%), followed by resistance to erythromycin (46%) and ciprofloxacin (8%). More isolates from the conventional chickens (20%) were resistant to ciprofloxacin than those from the organic chickens (5%). Rates of resistance to erythromycin and tetracycline were higher in organic chicken isolates (49% and 81%, respectively) than conventional chicken isolates (36% and 69%, respectively).
TABLE 2.
Antimicrobials, dilution ranges, and resistance breakpoints used in antimicrobial susceptibility tests for campylobacters and salmonellaea
| Antimicrobial | Dilution range tested (μg/ml) | Resistance breakpoint (μg/ml) |
|---|---|---|
| Campylobacters | ||
| Chloramphenicol | 0.25-128 | ≥32 |
| Ciprofloxacin | 0.03-16 | ≥4 |
| Erythromycin | 0.125-64 | ≥8 |
| Tetracycline | 0.125-64 | ≥16 |
| Salmonellae | ||
| Amikacin | 4-32 | ≥64 |
| Amoxicillin-clavulanic acid | 0.5-32 | ≥32 |
| Ampicillin | 2-32 | ≥32 |
| Apramycin | 2-32 | ≥32 |
| Ceftiofur | 0.5-16 | ≥8 |
| Ceftriaxone | 0.25-64 | ≥32 |
| Cephalothin | 1-32 | ≥32 |
| Chloramphenicol | 4-32 | ≥32 |
| Ciprofloxacin | 0.015-4 | ≥4 |
| Florfenicol | 2-16 | |
| Gentamicin | 0.25-16 | ≥16 |
| Kanamycin | 16-64 | ≥64 |
| Nalidixic acid | 4-256 | ≥32 |
| Streptomycin | 32-256 | ≥64 |
| Sulfamethoxazole | 128-512 | ≥512 |
| Tetracycline | 4-32 | ≥16 |
| Trimethoprim-sulfamethoxazole | 0.12-2/4-76 | ≥4/76 |
Antimicrobial susceptibility testing was performed according to NCCLS guidelines (16). C. jejuni ATCC 33560 was used as the quality control organism for campylobacters, whereas Escherichia coli ATCC 25922, E. coli ATCC 35218, Enterococcus faecalis ATCC 29212, Staphylococcus aureus ATCC 29213, and Pseudomonas aeruginosa ATCC 27853 were used for salmonellae.
FIG. 2.
Antimicrobial resistance of campylobacters isolated from 150 organic (cross-hatch bars) and 45 conventional (solid bars) chickens. Abbreviations: CHL, chloramphenicol; CIP, ciprofloxacin; ERY, erythromycin; TET, tetracycline. An asterisk indicates a significant difference.
The antimicrobial resistance profiles of the Salmonella isolates differed among the serotypes and also depended upon the sources from which they were recovered. S. enterica serovars Typhimurium and Kentucky were more resistant to antimicrobials than S. enterica serovar Heidelberg. S. enterica serovar Typhimurium isolates recovered from 79% of the organic chicken samples were susceptible to all 17 antimicrobials tested (Table 3). In contrast, all 12 S. enterica serovar Typhimurium isolates from conventional chicken samples were resistant to five to seven different antimicrobials (Table 3). However, one serovar Heidelberg and one serovar Newport isolate from the organic chickens were resistant to nine antimicrobials, including amoxicillin-clavulanic acid, ampicillin, cefoxitin, ceftiofur, cephalothin, chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline. Regardless of the source, the isolates of serovars Ohio, Agona, Braenderup, Thompson, Worthington, and Montevideo were susceptible to all antimicrobials tested.
TABLE 3.
Antimicrobial resistance of S. enterica serovars Kentucky and Typhimurium isolated from organic and conventional chicken samples
| Antimicrobial resistance profilea | Serovar Kentucky
|
Serovar Typhimurium
|
||
|---|---|---|---|---|
| Organic (n = 72) | Conventional (n = 10) | Organic (n = 19) | Conventional (n = 12) | |
| AUG-AMP-CEP-FOX-STR-TET-TIO | 2 (3)b | 2 (17) | ||
| AUG-AMP-CEP-FOX-TET-TIO | 1 (10) | 2 (17) | ||
| AUG-AMP-CEP-FOX-TIO | 1 (1) | 1 (10) | 8 (67) | |
| GEN-SMX-STR-TET | 5 (7) | 1 (5) | ||
| KAN-STR-TET | 1 (1) | |||
| STR-TET | 40 (56) | 6 (60) | 1 (5) | |
| KAN-NAL | 1 (5) | |||
| TET | 1 (1) | |||
| NAL | 1 (5) | |||
| Susceptible to all tested antimicrobials | 23 (32) | 2 (20) | 15 (79) | 0 (0) |
AUG, amoxacillin-clavulanic acid; AMP, ampicillin; CEP, cephalothin; TIO, ceftiofur; FOX, cefoxitin; GEN, gentamicin; KAN, kanamycin; SMX, sulfamethoxazole; STR, streptomycin; TET, tetracycline; NAL, nalidixic acid.
The number (percentage) of isolates is shown.
Our results revealed that the prevalence and antimicrobial susceptibility of campylobacters and salmonellae varied among different animal production systems. While organic chickens were more frequently contaminated with campylobacters and salmonellae, the pathogens from organic animal production were more susceptible to certain antimicrobials. However, findings based on the data from one study may be inconclusive. Baseline data on the microbiological status of organic products are needed to ensure the safety of food supplies as sales of organic foods are expected to increase.
Acknowledgments
We are indebted to Patrick McDermott from the Division of Animal and Food Microbiology, Center for Veterinary Medicine, Food and Drug Administration, for assistance with and comments on the preparation of this article.
REFERENCES
- 1.Angulo, F. J., K. R. Johnson, R. V. Tauxe, and M. L. Cohen. 2000. Origins and consequences of antimicrobial-resistant nontyphoidal Salmonella: implications for the use of fluoroquinolones in food animals. Microb. Drug Resist. 6:77-83. [DOI] [PubMed] [Google Scholar]
- 2.Besser, T. E., C. C. Gay, J. M. Gay, D. D. Hancock, D. Rice, L. C. Pritchett, and E. D. Erickson. 1997. Salmonellosis associated with S typhimurium DT104 in the USA. Vet. Rec. 140:75. [PubMed] [Google Scholar]
- 3.Engvall, A. 2001. May organically farmed animals pose a risk for Campylobacter infections in humans? Acta Vet. Scand. Suppl. 95:85-87. [PubMed] [Google Scholar]
- 4.Fey, P. D., T. J. Safranek, M. E. Rupp, E. F. Dunne, E. Ribot, P. C. Iwen, P. A. Bradford, F. J. Angulo, and S. H. Hinrichs. 2000. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. N. Engl. J. Med. 342:1242-1249. [DOI] [PubMed] [Google Scholar]
- 5.Ge, B., S. Bodeis, R. D. Walker, D. G. White, S. Zhao, P. F. McDermott, and J. Meng. 2002. Comparison of the Etest and agar dilution for in vitro antimicrobial susceptibility testing of Campylobacter. J. Antimicrob. Chemother. 50:487-494. [DOI] [PubMed] [Google Scholar]
- 6.Ge, B., D. G. White, P. F. McDermott, W. Girard, S. Zhao, S. Hubert, and J. Meng. 2003. Antimicrobial-resistant Campylobacter species from retail raw meats. Appl. Environ. Microbiol. 69:3005-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glynn, M. K., C. Bopp, W. Dewitt, P. Dabney, M. Mokhtar, and F. J. Angulo. 1998. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N. Engl. J. Med. 338:1333-1338. [DOI] [PubMed] [Google Scholar]
- 8.Gomez, T. M., Y. Motarjemi, S. Miyagawa, F. K. Kaferstein, and K. Stohr. 1997. Foodborne salmonellosis. World Health Stat. Q. 50:81-89. [PubMed] [Google Scholar]
- 9.Greene, C. R. 2001. U.S. organic farming emerges in the 1990s: adoption of certified systems. U.S. Department of Agriculture, Washington, D.C.
- 10.Heuer, O. E., K. Pedersen, J. S. Andersen, and M. Madsen. 2001. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol. 33:269-274. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs-Reitsma, W. 2000. Campylobacter in the food supply, p. 467-482. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 12.Linton, D., A. J. Lawson, R. J. Owen, and J. Stanley. 1997. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J. Clin. Microbiol. 35:2568-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malorny, B., J. Hoorfar, C. Bunge, and R. Helmuth. 2003. Multicenter validation of the analytical accuracy of Salmonella PCR: towards an international standard. Appl. Environ. Microbiol. 69:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachamkin, I., H. Ung, and M. Li. 2002. Increasing fluoroquinolone resistance in Campylobacter jejuni, Pennsylvania, USA, 1982-2001. Emerg. Infect. Dis. 8:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals—approved standards M31-A, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Rahn, K., S. A. De Grandis, R. C. Clarke, S. A. McEwen, J. E. Galan, C. Ginocchio, R. Curtiss III, and C. L. Gyles. 1992. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol. Cell. Probes 6:271-279. [DOI] [PubMed] [Google Scholar]
- 18.Sato, K., P. C. Bartlett, J. B. Kaneene, and F. P. Downes. 2004. Comparison of prevalence and antimicrobial susceptibilities of Campylobacter sp. isolates from organic and conventional dairy herds in Wisconsin. Appl. Environ. Microbiol. 70:1442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundrum, A. 2001. Organic livestock farming. A critical review. Livest. Prod. Sci. 67:207-215. [Google Scholar]
- 20.Thamsborg, S. M. 2001. Organic farming in the Nordic countries—animal health and production. Acta Vet. Scand. Suppl. 95:7-15. [PubMed] [Google Scholar]
- 21.Threlfall, E. J., J. A. Frost, L. R. Ward, and B. Rowe. 1996. Increasing spectrum of resistance in multiresistant Salmonella typhimurium. Lancet 347:1053-1054. [DOI] [PubMed] [Google Scholar]
- 22.Threlfall, E. J., L. R. Ward, J. A. Frost, and G. A. Willshaw. 2000. The emergence and spread of antibiotic resistance in food-borne bacteria. Int. J. Food Microbiol. 62:1-5. [DOI] [PubMed] [Google Scholar]
- 23.White, D. G., S. Zhao, R. Sudler, S. Ayers, S. Friedman, S. Chen, P. F. McDermott, S. McDermott, D. D. Wagner, and J. Meng. 2001. The isolation of antibiotic-resistant Salmonella from retail ground meats. N. Engl. J. Med. 345:1147-1154. [DOI] [PubMed] [Google Scholar]
- 24.Zhao, C., B. Ge, J. De Villena, R. Sudler, E. Yeh, S. Zhao, D. G. White, D. Wagner, and J. Meng. 2001. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the greater Washington, D.C., area. Appl. Environ. Microbiol. 67:5431-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]