Abstract
The alkane-degrading, sulfate-reducing bacterium Desulfatibacillum aliphaticivorans strain CV2803T, recently isolated from marine sediments, was investigated for n-alkane metabolism. The total cellular fatty acids of this strain had predominantly odd numbers of carbon atoms (C odd) when the strain was grown on a C-odd alkane (pentadecane) and even numbers of carbon atoms (C even) when it was grown on a C-even alkane (hexadecane). Detailed analyses of those fatty acids by gas chromatography/mass spectrometry allowed us to identify saturated 2-, 4-, 6-, and 8-methyl- and monounsaturated 6-methyl-branched fatty acids, with chain lengths that specifically correlated with those of the alkane. Growth of D. aliphaticivorans on perdeuterated hexadecane demonstrated that those methyl-branched fatty acids were directly derived from the substrate. In addition, cultures on pentadecane and hexadecane produced (1-methyltetradecyl)succinate and (1-methylpentadecyl)succinate, respectively. These results indicate that D. aliphaticivorans strain CV2803T oxidizes n-alkanes into fatty acids anaerobically, via the addition of fumarate at C-2. Based on our observations and on literature data, a pathway for anaerobic n-alkane metabolism by D. aliphaticivorans is proposed. This involves the transformation of the initial alkylsuccinate into a 4-methyl-branched fatty acid which, in addition to catabolic reactions, can alternatively undergo chain elongation and desaturation to form storage fatty acids.
The biodegradation of alkanes under aerobic conditions has been studied intensively, and the mechanisms of initial activation have been carefully described. The initial attack requires a mono- or dioxygenase enzyme and the presence of molecular oxygen (18). The demonstration of hydrocarbon biodegradation in the absence of molecular oxygen is more recent. Studies have reported the biodegradation of n-alkanes under nitrate-reducing (3, 9), sulfate-reducing (1, 4, 23), and methanogenic (29) conditions, but limited information is available so far on the degrading organisms and the mechanisms of degradation (11, 24, 27). Rabus et al. (17) have demonstrated that the initial oxidation of n-hexane by a denitrifying bacterium, strain HxN1, involves the addition of fumarate at C-2 of the alkane to form a substituted alkylsuccinate. The latter intermediate was shown to be further mineralized via a rearrangement of the carbon skeleton and conversion into specific methyl-branched fatty acids (28). Similarly, a sulfate-reducing enrichment culture, grown on n-dodecane, formed (1-methylundecyl)succinate by the addition of fumarate at the subterminal carbon of the alkane (14). However, neither the complete degradation pathways nor the organisms responsible for the degradation were described. On the other hand, two phylogenetically different sulfate reducers, strains Hxd3 (1, 25) and AK-01 (23), which are able to oxidize alkanes into carbon dioxide under anaerobic conditions, were studied for n-alkane metabolism. These strains utilize alkanes with chain lengths from C12 to C18 and C13 to C18, respectively, but exhibit different mechanisms of anaerobic alkane degradation. While strain Hxd3 attacks alkanes by carboxylation at the C-3 position and removes two adjacent terminal carbon atoms (25), the initial attack by strain AK-01 involves carbon addition at C-2 and is not effected by carboxylation (24).
In this report, we present the results of an investigation on n-alkane metabolism by the sulfate-reducing bacterium Desulfatibacillum aliphaticivorans strain CV2803T. This strain, which was recently isolated from marine sediments, is able to oxidize alkanes from C13 to C18 completely into carbon dioxide during sulfate reduction (6). It was proposed as the type strain of a novel species in a new genus of the family Desulfobacteraceae (class δ-Proteobacteria). The strain is phylogenetically related to other unnamed alkane-oxidizing strains, such as strain AK-01 (23, 24). Following previously reported analytical methods (24, 28), we studied the total cellular fatty acids of D. aliphaticivorans grown on n-alkanes or perdeuterated hexadecane. We demonstrated that this organism oxidizes alkanes into fatty acids by the same fumarate addition mechanism as the one involved in the denitrifying strain HxN1 (28). We propose a pathway for anaerobic n-alkane metabolism by D. aliphaticivorans in which, in parallel with catabolic reactions, the 4-methyl-branched fatty acids derived from the transformation of the initial alkylsuccinate can alternatively undergo chain elongation and desaturation to form storage fatty acids.
MATERIALS AND METHODS
Source of bacterium and cultivation.
Desulfatibacillum aliphaticivorans strain CV2803T was isolated from hydrocarbon-polluted Mediterranean (Gulf of Fos, France) marine sediments (6). It was cultivated in 500 ml of defined anoxic sulfate-reducing medium as described previously (7). Cultures were carried out with pentadecane (1.3 mM) or hexadecane (1.25 mM) as the organic substrate. Cultures with perdeuterated hexadecane (C16D34; Eurisotop Laboratories) were performed by mixing equal volumes of unlabeled and labeled hexadecane (1.25 mM final concentration). Cultures in the presence of [13C]bicarbonate (Aldrich Chemical Co.) were performed with the medium buffered with morpholinepropanesulfonic acid (3 g liter−1; pH 7.5) instead of bicarbonate buffer and prepared under argon. [13C]bicarbonate and hexadecane were added at concentrations of 5 mM and 1.25 mM, respectively. Sterile controls were carried out under the same conditions, and the inoculum was autoclaved before use. Cultures were analyzed after 3 months of incubation at 30°C.
Extraction and analysis of total cellular fatty acids.
Cells were collected by filtration through glass microfiber filters (grade GF/B; Whatman). Filters were saponified with 1 N KOH in CH3OH-H2O (1:1, vol/vol; reflux, 2 h). After extraction of the neutral lipids from the basic solution (n-hexane; three times with 30 ml), acids were extracted using dichloromethane (three times with 30 ml) following the addition of 2 N HCl (pH 1). The combined organic extracts were dried over Na2SO4, concentrated by rotary evaporation, and evaporated to dryness under nitrogen. Fatty acids were either silylated by reaction with bis-trimethylsilyl-trifluoroacetanamide in pyridine (10) or methylated with 1% sulfuric acid in methanol (5). Assignment of the methyl branch and double-bond positions in branched and/or unsaturated fatty acids was based on the formation of pyrrolidide derivatives according to Christie (5). The positions of the double bonds were further confirmed by stereospecific oxidation with OsO4 and subsequent trimethylsilylation as described by Rontani (19).
Fatty acids were identified with an HP5890 series II Plus gas chromatograph connected to an HP5972 mass spectrometer. The gas chromatograph was equipped with a fused silica capillary column (30 m by 0.25 mm inside diameter) coated with SolGel-1 (film thickness, 0.25 μm). The oven temperature was programmed from 60 to 130°C at 30°C min−1 and then at 4°C min−1 to 300°C, at which it was held for 10 min. The carrier gas (He) was maintained at 104 × 103 Pa until the end of the temperature program and was then programmed from 104 × 103 to 150 × 103 Pa at 4 × 103 Pa min−1.
Nomenclature for fatty acids.
The fatty acid nomenclature recommended by the IUPAC-IUB (13) was adopted in this study. An n-saturated octadecanoic acid is designated as 18:0, with the first number representing the number of carbon atoms in the acyl group and the second number representing the number of double bonds present. A branched fatty acid, such as 4-methyloctadecanoic acid, is designated 4-Me-18:0, and a monounsaturated branched fatty acid, such as 6-methyl-nonadec-5-enoic acid, is designated 6-Me-19:1Δ5. i- and a- refer to iso- and anteiso- fatty acids, respectively.
RESULTS
Fatty acid composition of Desulfatibacillum aliphaticivorans strain CV2803T.
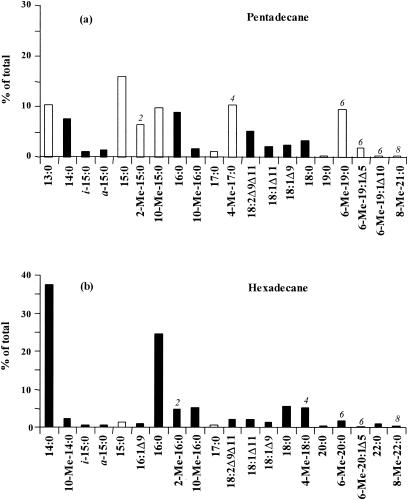
The cellular fatty acid composition of strain CV2803T grown on pentadecane and hexadecane is depicted in Fig. 1. When pentadecane was used as the growth substrate, fatty acids predominantly had odd numbers of carbon atoms (C-odd fatty acids), whereas fatty acids with even numbers of carbon atoms (C-even fatty acids) predominated after growth on hexadecane. As already reported for other sulfate reducers (1, 24, 25), the chain length of the alkane substrate strongly influenced those of the cellular fatty acids of D. aliphaticivorans.
FIG. 1.
Relative abundances of the total cellular fatty acids of D. aliphaticivorans strain CV2803T grown on pentadecane (a) and hexadecane (b). Only those which comprise more than 0.5% are presented. The 2-methyl, 4-methyl, 6-methyl, and 8-methyl fatty acids are denoted by the numbers 2, 4, 6, and 8, respectively. Black bars represent C-even fatty acids, and white bars represent C-odd fatty acids.
The detailed fatty acid composition of the strain grown on n-alkanes was further analyzed by gas chromatography-mass spectrometry (GC-MS). Linear saturated fatty acids were accompanied by branched (iso-, anteiso-, and 10-Me-) or unsaturated (e.g., 18:1Δ11 and 18:1Δ9) fatty acids commonly encountered in bacteria (15, 26) and by saturated monomethyl-branched fatty acids with a methyl group located at the C-2, C-4, C-6, or C-8 position (Fig. 1). The last-named fatty acids were detected neither in the sterile controls nor after growth on other organic substrates such as n-alkenes or n-fatty acids (data not shown). The identification of the methyl-branched fatty acids was based on their relative retention times (Fig. 2) and on the interpretation of their mass spectra, which show specific ions due to cleavage α to the branched carbon atoms (28). The position of the methyl branch was further confirmed either by comparison of the mass spectra with those already reported for similar fatty acids (24) or by the analysis of their pyrrolidide derivatives (5, 7). The chain lengths of the monomethyl-branched fatty acids were specifically correlated with those of the alkane substrates (Fig. 1). 2-Me-15:0, 4-Me-17:0, 6-Me-19:0, and 8-Me-21:0 were detected only after growth on pentadecane (Fig. 1a), and 2-Me-16:0, 4-Me-18:0, 6-Me-20:0, and 8-Me-22:0 were detected only after growth on hexadecane (Fig. 1b).
FIG. 2.
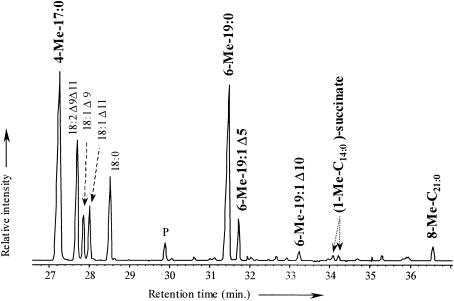
Partial total-ion chromatogram showing the presence of saturated and monounsaturated methyl-branched fatty acids and of alkylsuccinates in cultures of D. aliphaticivorans strain CV2803T grown on pentadecane.
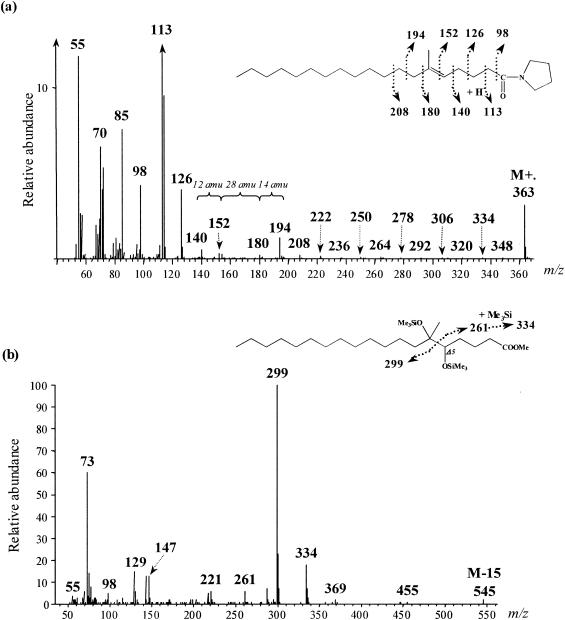
For each alkane substrate, a compound eluting just after the 6-methyl-branched fatty acid and showing a molecular ion (M+·) 2 atomic-mass units (amu) lower than this one was detected. This suggested the presence of monounsaturated homologues of the 6-Me-19:0 and 6-Me-20:0 fatty acids in cultures grown on pentadecane and hexadecane, respectively. The positions of the methyl branch and of the double bond in these compounds were determined from the mass spectra of their pyrrolidide derivatives (Fig. 3a). Unsaturation and branching positions can be identified by fragments with intervals of 12 and 28 amu, respectively, instead of the regular 14 amu (Fig. 3a). The position of the double bond was further confirmed by stereospecific oxidation with OsO4 (Fig. 3b). In the case of a monounsaturated fatty acid, this yields the corresponding vicinal dihydroxy derivative which, after trimethylsilylation, is well suited for mass spectrometric determination of the original double-bond position (19). The α-cleavage product ions of the vicinal bis(trimethylsilyl)-ether derivatives (at m/z 261, 299, and 334 for 6-Me-19:1 [Fig. 3b]) are in agreement with a double bond at C-5 in both 6-Me-19:1 and 6-Me-20:1 fatty acids. These monounsaturated methyl-branched fatty acids were likely formed by desaturation of the corresponding saturated homologues. It should be noted that, during growth on pentadecane, another minor monounsaturated 6-Me-19:1 fatty acid with the double bond located at C-10 was detected (Fig. 1a and 2a). These results suggest the presence of Δ5 and Δ10 desaturases in D. aliphaticivorans strain CV2803T.
FIG. 3.
Mass spectra of 6-methyl-nonadec-5-enoylpyrrolidide (a) and 5,6-bis(trimethylsilyloxy)-6-methyl-nonadecanoic acid trimethylsilyl ester (b) formed after the derivatization of 6-methyl-nonadec-5-enoic acid.
Identification of alkyl succinates.
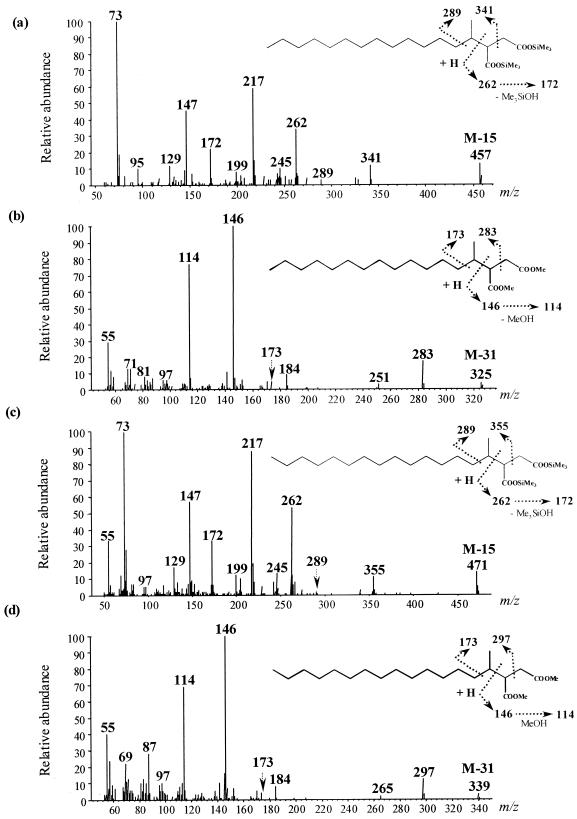
Owing to the formation of 2-Me- and 4-Me-branched fatty acids from an initial alkylsuccinate intermediate during the anaerobic degradation of n-hexane by a denitrifying bacterium, strain HxN1 (28), we searched for the presence of similar succinates among the total cellular fatty acids of D. aliphaticivorans grown on n-alkanes. (1-Methyltetradecyl)succinate and (1-methylpentadecyl)succinate were detected after growth on pentadecane and hexadecane, respectively. The identification of those alkylsuccinates was based on the interpretation of the mass spectra of the trimethylsilyl (TMS) and methyl-esterified derivatives (Fig. 4) and on the comparison between these mass spectral data and those previously reported for alkane-derived succinic acids (14, 17).
FIG. 4.
Mass spectra of the silylated (a) and methylated (b) (1-methyltetradecyl)succinic acid from cultures of D. aliphaticivorans strain CV2803T grown on pentadecane and of the silylated (c) and methylated (d) (1-methylpentadecyl)succinic acid from cultures grown on hexadecane.
For both types of derivatization, the ion with the largest mass in the spectrum is not the molecular ion (M+·) of the metabolite. In the case of TMS derivatives (Fig. 4a and c), it corresponds to the (M-15)+· ion, which results from the loss of a methyl group from one of the TMS substituents. For methyl-esterified derivatives (Fig. 4b and d), it corresponds to the (M-31)+· ion, which results from the loss of a methoxy group from one of the methyl-esterified carboxylic group. Prominent ions at m/z 262 for TMS derivatives, and at m/z 146 for methyl-esterified derivatives, come from the McLafferty rearrangement of the metabolites during GC-MS analysis and indicate the presence of a succinyl moiety in the molecules. These ions lose 90 and 32 amu, respectively (corresponding to the loss of HOSi[CH3]3 and HOCH3, respectively) to give the resulting ions at m/z 172 and 114 (Fig. 4). In the mass spectra of the TMS derivatives, the presence of two silylated groups is indicated by ions at m/z 73 and 147. The abundant ions at m/z 341 and 355 for TMS derivatives and at m/z 283 and 297 for methyl-esterified derivatives result from a cleavage α to the tertiary carbon from the succinic moiety. The detected alkylsuccinates correspond to the addition of the alkane substrate (pentadecane or hexadecane) to one of the ethylenic carbons of fumarate. The attachment of the succinyl moiety at C-2 of the alkane is assumed from the weak but definite and specific ions at m/z 289 for TMS derivatives and at m/z 173 for methyl-esterified derivatives (Fig. 4). These ions result from cleavage α to the branched carbon atom on the alkyl chain. This subterminal attachment of the succinyl moiety is further supported by the detection, during GC-MS analysis, of two equally abundant TMS derivatives for each alkylsuccinate (see Fig. 2 for pentadecane). In each case, both peaks gave nearly identical mass spectra, so only one is presented in Fig. 4. The presence of two chiral carbon atoms (each in the R or S configuration) in the alkylsuccinates implies the existence of two pairs of enantiomers and thus of two diastereoisomers which are likely to be resolved by GC-MS analysis (14). On the contrary, the addition of fumarate at the terminal carbon of the alkane (C-1 instead of C-2) should lead to the formation of only one chiral carbon in the succinyl moiety. In this case, there should be no possibility for diastereoisomer formation, and both alkylsuccinates should appear as single GC peaks. However, it should be noted that, contrary to the TMS derivatives, the methyl-esterified (1-methyltetradecyl)succinate and (1-methylpentadecyl)succinate appeared as single GC peaks. This was likely due to the inability of the GC-MS analysis to resolve the diastereoisomers when they were methyl esterified. These differences in GC resolution, between that of TMS and that of methyl-esterified derivatives of alkylsuccinates, have already been observed for dodecylsuccinic acids (14).
Identification of perdeuterated hexadecane-derived fatty acids.
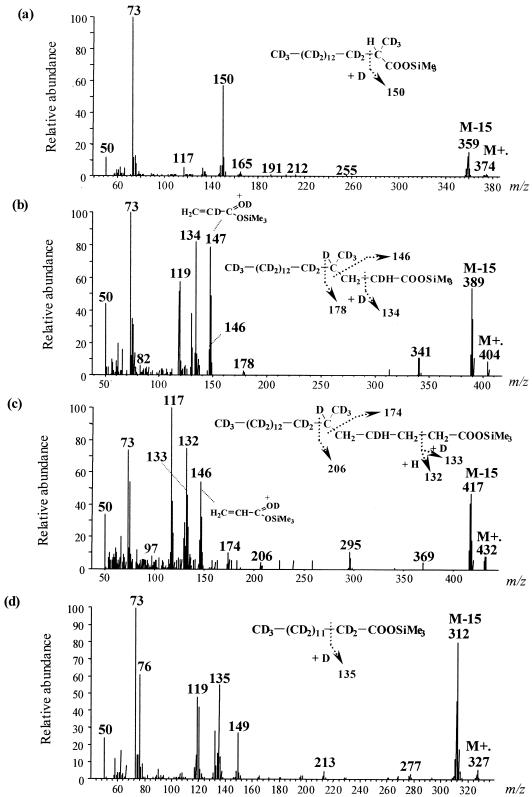
Growth of D. aliphaticivorans on D34-n-hexadecane yielded linear and methyl-branched deuterated fatty acids (Fig. 5), demonstrating that these fatty acids were derived from the alkane substrate. Traces of deuterated alkylsuccinates were detected using mass spectrometry (14), but clear mass spectra could not be satisfactorily recorded. Although labeling experiments were performed using a mixture of unlabeled and perdeuterated hexadecane, fatty acids formed from D34-n-hexadecane appeared completely separated from those resulting from the unlabeled substrate during GC-MS analyses. However, isotopomers which differed by one or two deuterium atoms were formed but did not yield distinct GC peaks. Structural identification of the main isotopomers was achieved by careful selection of mass spectra, using mass chromatograms of selected ions (Fig. 5).
FIG. 5.
Mass spectra and tentative structures of (a) D32-2-Me-16:0, (b) D34-4-Me-18:0, (c) D34-6-Me-20:0, and (d) D27-14:0 fatty acid trimethylsilyl esters from cultures of D. aliphaticivorans strain CV2803T grown on a mixture of unlabeled hexadecane and D34-hexadecane.
Figure 5a shows the mass spectrum of the TMS derivative of the deuterated 2-Me-16:0 fatty acid. The molecular ion (m/z 374) and the ion corresponding to the loss of one methyl from the TMS group (M-15 at m/z 359) indicate the presence of 32 deuterium atoms in the molecule. The ion fragment at m/z 150 corresponds to the McLafferty rearrangement. The corresponding ion in the unlabeled analogue is downshifted by 4 amu (m/z 146; data not shown), indicating the presence of four deuterium atoms in the labeled ion. The reagent used for TMS derivatization was not deuterated, and one deuterium atom would come from the McLafferty rearrangement (16), which indicates the presence of three deuterium atoms at C-2 and/or at the 2-methyl group. Since the initial activation of the alkane appears at C-2 (as determined from the structures of the alkylsuccinates [Fig. 4]), the 2-methyl group of the D32-2-Me-16:0 fatty acid corresponds to the original terminal carbon of D34-n-hexadecane and, thus, likely contains three deuterium atoms (Fig. 5a).
Mass spectral analysis of the deuterated 4-Me-18:0 fatty acid (Fig. 5b) shows that it contains a total of 34 deuterium atoms together with three hydrogen atoms located between C-1 and C-4. Assuming the presence of three deuterium atoms at the 4-methyl group (see above), the presence of one deuterium atom at C-2 and one at C-4 (position γ from the carbonyl group) can be deduced from the McLafferty ion at m/z 134. This ion appears at m/z 132 for the unlabeled analogue. The ion at m/z 146 formed by cleavage α to the branched carbon (Fig. 5b) thus indicates the presence of one hydrogen atom at C-2 and of two others at C-3. This is further supported by the prominent ion at m/z 147 (which appears at m/z 145 for the unlabeled analogue), formed by γ cleavage of the TMS group (20).
Similar analysis of the mass spectrum of the deuterated 6-Me-20:0 fatty acid (Fig. 5c) indicates the presence of 34 deuterium atoms together with 7 hydrogen atoms located within C-1 to C-6. The ion at m/z 146 resulting from γ cleavage shows the presence of two hydrogen atoms at C-2 and of two others at C-3, whereas the presence of one deuterium atom and one hydrogen atom at C-4 is suggested from the McLafferty ions at m/z 132 and 133. This fatty acid was likely formed by the addition of an unlabeled C-2 unit to the D34-4-Me-18:0 fatty acid. In addition to the D34-6-Me-20:0 fatty acid, a Δ5-unsaturated D32-6-Me-20:0 fatty acid was detected (data not shown), confirming the results of the unlabeled experiment.
Examination of the mass spectrum of the deuterated 14:0 fatty acid indicates that it is fully deuterated (Fig. 5d), which suggests that it was formed by β oxidation (loss of a C-2 unit) of the D32-2-Me-16:0 fatty acid.
Fatty acids formed in the presence of [13C]bicarbonate.
The fatty acids formed in cultures of D. aliphaticivorans grown on unlabeled alkane in the presence of [13C]bicarbonate were similar to those formed in the presence of unlabeled bicarbonate. Mass spectral analysis of the methyl-branched fatty acids formed in the presence of [13C]bicarbonate showed that they were not 13C labeled, indicating that the incorporation of carbon in the original alkane does not proceed by the addition of an inorganic carboxyl group (24).
DISCUSSION
The biodegradation of n-alkanes by pure sulfate-reducing bacteria was previously investigated in strains Pnd3 (1), Hxd3 (1, 25), and AK-01 (24) through the analysis of cellular fatty acids. Like Desulfatibacillum aliphaticivorans, strains Pnd3 and AK-01 formed C-odd fatty acids from C-odd n-alkanes and C-even fatty acids from C-even n-alkanes, whereas strain Hxd3 transformed C-odd n-alkanes to C-even fatty acids and vice versa. Labeling experiments have further demonstrated that n-alkane activation in strain AK-01 occurs through the addition of carbon at C-2 to yield methyl-branched fatty acids and that this process is not effected by carboxylation (24), in contrast to strain Hxd3, which oxidizes alkanes by carboxylation at C-3 (25). Phylogenetic analyses showed that D. aliphaticivorans is closely related to strains AK-01 and Pnd3, which may represent other species in the same genus (6). These similarities support the idea that reactions of n-alkane metabolism in strains AK-01 and Pnd3 are similar to those in D. aliphaticivorans.
The present results demonstrate that D. aliphaticivorans strain CV2803T oxidizes n-alkanes to fatty acids via an initial activation reaction at C-2, similarly to strain AK-01 (24). Such selectivity for the subterminal carbon may be due to the conformational structure of the degrading enzyme (24). Based on previous reports (14, 17, 28) and on the detection of alkylsuccinates among the total cellular fatty acids of D. aliphaticivorans (Fig. 4), it is reasonable to foresee that these alkylsuccinates constitute the first intermediates of n-alkane metabolism and the precursors for (methyl-branched) fatty acid synthesis. The possibility that strain AK-01 utilizes the same fumarate addition mechanism to degrade n-alkane was previously suggested (25, 28). The results from our [13C]bicarbonate experiment demonstrated that initial activation of the alkane by carboxylation (25) does not appear to occur in D. aliphaticivorans.
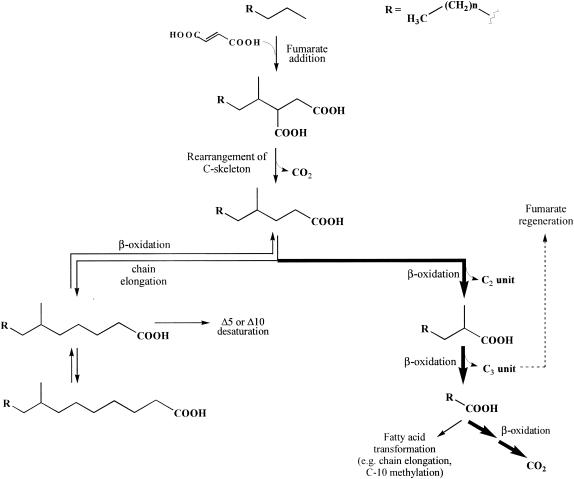
A pathway for anaerobic n-alkane metabolism by D. aliphaticivorans strain CV2803T is proposed in Fig. 6. The catabolic reactions depicted are based on those proposed by Wilkes et al. (28) for the degradation of n-hexane by the denitrifying bacterium strain HxN1. As already pointed out by those authors, although the metabolites were detected as free carboxylic acids, many of them are certainly present in vivo as their coenzyme A (CoA) thioesters (which can be hydrolyzed during the analytical procedure used). Following the addition of a molecule of fumarate at position C-2 of the alkane, the formed alkylsuccinic acid likely undergoes a rearrangement of the carbon skeleton to form a (2-methyl-alkyl)malonate, which is subsequently decarboxylated to a 4-Me-branched fatty acid. This rearrangement can be compared with that observed in Propionibacterium, which transforms succinyl CoA to propionyl CoA via methylmalonyl CoA (8, 28). Alternative hypothetical pathways for the degradation of the alkylsuccinates have been discussed previously and can reasonably be disproved (28). The 4-Me-branched fatty acid can be further degraded to the corresponding 2-Me-branched fatty acid by β oxidation (Fig. 6). The intermediate metabolites expected to be involved in this sequence [i.e., (E)-Δ2-unsaturated 4-Me, 3-hydroxy-4-Me, and 3-oxo-4-Me fatty acids] were not detected among the total fatty acids of D. aliphaticivorans. Surprisingly, (Z)- and (E)-Δ3 unsaturated 4-Me isomers were observed in some cultures (data not shown). The formation and possible role of these fatty acids are not clearly understood, but it seems that their formation is favored during the β oxidation of fatty acids branched at C-4. Indeed, similar compounds were observed during the aerobic degradation of some isoprenoid compounds by marine bacteria (21) and during the anaerobic degradation of n-hexane by the denitrifying bacterium strain HxN1 (28). The 2-Me-branched fatty acid can be further transformed to a linear fatty acid by a second round of β oxidation (Fig. 6). The detection of trace amounts of 2-Me-15:1Δ2 (3) fatty acid, after growth of D. aliphaticivorans on pentadecane, provided evidence for this sequence (data not shown). During this sequence, a C-3 unit, which can be used for the regeneration of fumarate and the subsequent activation of another molecule of substrate, is released (Fig. 6) (28). The linear fatty acid formed is then mineralized to CO2 or, alternatively, transformed by chain elongation and C-10 methylation (Fig. 6).
FIG. 6.
Proposed pathway for anaerobic n-alkane metabolism by the sulfate-reducing bacterium D. aliphaticivorans strain CV2803T (bold arrows indicate the major pathway).
It should be emphasized that during the growth of D. aliphaticivorans on D34-hexadecane, the first degradation steps (i.e., the addition of fumarate and the formation of a 4-Me fatty acid) involved the migration of one deuterium atom from C-2 of the labeled alkane towards the succinate moiety, as demonstrated by the detection of the D34-4-Me-18:0 and D34-6-Me-20:0 fatty acids (Fig. 5b and c). Such a deuterium transfer has already been shown to occur in anaerobes during the initial addition of fumarate to D14-n-hexane (17) and methyl-D3-toluene (2). These studies further demonstrate that the deuterium atom abstracted from the initial hydrocarbon (to enable formation of the new C—C bond) is rebound at C-2 of the succinate moiety (if the addition occurs at C-3). This could not be verified in the present case due to our inability to obtain a clear mass spectrum of deuterated alkylsuccinate. However, if a similar mechanism occurred in D. aliphaticivorans, this might indicate that no further migration of the deuterium atom occurred along the succinate moiety during the rearrangement of the carbon skeleton and subsequent decarboxylation, contrary to the observation of Wilkes et al. (28). On the other hand, the formation of D32-2-Me-16:0 from D34-4-Me-18:0 may suggest the exchange of one deuterium atom with an external hydrogen atom during the first sequence of β oxidation. This observation is consistent with the formation of D12-2-Me-6:0 fatty acid from D14-hexane by the denitrifying bacterium HxN1 (28) and the formation of D30-2-Me-15:0 fatty acid from D32-pentadecane by the sulfate-reducing strain AK-01 (24). The mechanism involved in this deuterium/hydrogen exchange remains unknown so far. It may be, however, that this reaction occurs artificially during basic or acidic analytical treatments used for fatty acid extraction, owing to the ease with which a proton α to a carbonyl group undergoes such an exchange (22). In this specific case, the exchange is further favored by the tertiary nature of the carbon.
The detection of saturated 6-Me- and 8-Me-branched fatty acids and of Δ5 and Δ10 monounsaturated 6-Me homologues in D. aliphaticivorans grown on n-alkanes (Fig. 1 to 3) allowed the completion of the metabolic pathway with additional anabolic reactions (Fig. 6). The 4-Me fatty acid formed from the initial alkylsuccinate can alternatively be elongated to a 6-Me fatty acid, which, in turn, can be either desaturated at C-5 (or to a lesser extent at C-10) or further elongated to an 8-Me fatty acid (Fig. 6). These anabolic reactions were confirmed by the experiment performed with perdeuterated hexadecane. Δ5 and Δ10 desaturases are commonly encountered in bacteria (12), and their presence in D. aliphaticivorans is not surprising.
Acknowledgments
This work was funded by the Centre National de la Recherche Scientifique (CNRS) and Elf Society through the research group HYCAR (no. 1123) and by the MATBIOPOL European Project (contract no. EVK3-CT-1999-00010).
We thank J.-F. Rontani for helpful discussions and comments and L. Casalot for careful reading of the English.
REFERENCES
- 1.Aeckersberg, F., F. A. Rainey, and F. Widdel. 1998. Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch. Microbiol. 170:361-369. [DOI] [PubMed] [Google Scholar]
- 2.Beller, H. R., and A. M. Spormann. 1998. Analysis of the novel benzylsuccinate synthase reaction for anaerobic toluene activation based on structural studies of the product. J. Bacteriol. 180:5454-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bregnard, T. P. A., P. Höhener, A. Häner, and J. Zeyer. 1996. Degradation of weathered diesel fuel by microorganisms from a contaminated aquifer in aerobic and anaerobic microcosms. Environ. Toxicol. Chem. 15:299-307. [Google Scholar]
- 4.Caldwell, M. E., R. M. Garrett, R. C. Prince, and J. M. Suflita. 1998. Anaerobic biodegradation of long-chain n-alkanes under sulfate-reducing conditions. Environ. Sci. Technol. 32:2191-2195. [Google Scholar]
- 5.Christie, W. W. 1989. The analysis of fatty acids, p. 64-184. In Gas chromatography and lipids: a practical guide. The Oily Press, Dundee, Scotland.
- 6.Cravo-Laureau, C., R. Matheron, J.-L. Cayol, C. Joulian, and A. Hirschler-Réa. 2004. Desulfatibacillum aliphaticivorans gen. nov., sp. nov., an n-alkane- and n-alkene-degrading sulfate-reducing bacterium. Int. J. Syst. Evol. Microbiol. 54:77-83. [DOI] [PubMed] [Google Scholar]
- 7.Cravo-Laureau, C., A. Hirschler-Réa, R. Matheron, and V. Grossi. 2004. Growth and cellular fatty-acid composition of a sulphate-reducing bacterium, Desulfatibacillum aliphaticivorans strain CV2803T, grown on n-alkenes. C. R. Biol. 327:687-694. [DOI] [PubMed] [Google Scholar]
- 8.Cummins, C. S., and J. L. Johnson. 1992. The genus Propionibacterium, p. 834-849. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, vol. 1. Springer, New York, N.Y. [Google Scholar]
- 9.Ehrenreich, P., A. Behrends, J. Harder, and F. Widdel. 2000. Anaerobic oxidation of alkanes by newly isolated denitrifying bacteria. Arch. Microbiol. 173:58-64. [DOI] [PubMed] [Google Scholar]
- 10.Grossi, V., S. Caradec, and F. Gilbert. 2003. Burial and reactivity of sedimentary microalgal lipids in bioturbated Mediterranean coastal sediments. Mar. Chem. 81:57-69. [Google Scholar]
- 11.Harayama, S., Y. Kasai, and A. Hara. 2004. Microbial communities in oil-contaminated seawater. Curr. Opin. Biotechnol. 15:205-214. [DOI] [PubMed] [Google Scholar]
- 12.Harwood, J. L., and N. J. Russell. 1984. Lipids in plants and microbes. George Allen & Unwin, London, United Kingdom.
- 13.IUPAC-IUB Commission on Biochemical Nomenclature. 1978. The nomenclature of lipids. Biochem. J. 171:21-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kropp, K. G., I. A. Davidova, and J. M. Suflita. 2000. Anaerobic oxidation of n-dodecane by an addition reaction in a sulfate-reducing bacterial enrichment culture. Appl. Environ. Microbiol. 66:5393-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackie, R. I., B. A. White, and M. P. Bryant. 1991. Lipid metabolism in anaerobic ecosystems. Crit. Rev. Microbiol. 17:449-479. [DOI] [PubMed] [Google Scholar]
- 16.McLafferty, F. W., and F. Tureček. 1993. Interpretation of mass spectra, 4th ed. University Science Books, Sausalito, Calif.
- 17.Rabus, R., H. Wilkes, A. Behrends, A. Armstroff, T. Fischer, A. J. Pierik, and F. Widdel. 2001. Anaerobic initial reaction of n-alkanes in a denitrifying bacterium: evidence for (1-methylpentyl)succinate as initial product and for involvement of an organic radical in n-hexane metabolism. J. Bacteriol. 183:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reineke, W. 2001. Aerobic and anaerobic biodegradation potentials of microorganisms, p. 1-161. In B. Beek (ed.), The handbook of environmental chemistry: biodegradation and persistence, vol. 2. Springer, New York, N.Y. [Google Scholar]
- 19.Rontani, J.-F. 1998. Electron ionization mass spectrometric determination of double bond position in mono-unsaturated α,β- and β,γ-isomeric isoprenoid acids. Rapid Commun. Mass Spectrom. 12:961-967. [Google Scholar]
- 20.Rontani, J.-F., and C. Aubert. 2004. Trimethylsilyl transfer during electron ionisation mass spectral fragmentation of some ω-hydroxycarboxylic and ω-dicarboxylic acid trimethylsilyl derivatives and the effect of chain length. Rapid Commun. Mass Spectrom. 18:1889-1895. [DOI] [PubMed] [Google Scholar]
- 21.Rontani, J.-F., P. C. Bonin, and J. K. Volkman. 1999. Production of wax esters during aerobic growth of marine bacteria on isoprenoid compounds. Appl. Environ. Microbiol. 65:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, M. B., and J. March. 2001. Aliphatic electrophilic substitution, p. 759-849. In March's advanced organic chemistry: reactions, mechanisms, and structure, 5th ed. Wiley-Interscience, New York, N.Y.
- 23.So, C. M., and L. Y. Young. 1999. Isolation and characterization of a sulfate-reducing bacterium that anaerobically degrades alkanes. Appl. Environ. Microbiol. 65:2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.So, C. M., and L. Y. Young. 1999. Initial reactions in anaerobic alkane degradation by a sulfate reducer, strain AK-01. Appl. Environ. Microbiol. 65:5532-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.So, C. M., C. D. Phelps, and L. Y. Young. 2003. Anaerobic transformation of alkanes to fatty acids by a sulfate-reducing bacterium, strain Hxd3. Appl. Environ. Microbiol. 69:3892-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vainshtein, M., H. Hippe, and R. M. Kroppenstedt. 1992. Cellular fatty acids of Desulfovibrio species and its use in classification of sulfate-reducing bacteria. Syst. Appl. Microbiol. 15:554-556. [Google Scholar]
- 27.Widdel, F., and R. Rabus. 2001. Anaerobic biodegradation of saturated and aromatic hydrocarbons. Curr. Opin. Biotechnol. 12:259-276. [DOI] [PubMed] [Google Scholar]
- 28.Wilkes, H., R. Rabus, T. Fischer, A. Armstroff, A. Behrends, and F. Widdel. 2002. Anaerobic degradation of n-hexane in a denitrifying bacterium: further degradation of the initial intermediate (1-methylpentyl)succinate via C-skeleton rearrangement. Arch. Microbiol. 177:235-243. [DOI] [PubMed] [Google Scholar]
- 29.Zengler, K., H. H. Richnow, R. Rosselló-Mora, W. Michaelis, and F. Widdel. 1999. Methane formation from long-chain alkanes by anaerobic microorganisms. Nature 401:266-269. [DOI] [PubMed] [Google Scholar]