Abstract
The Mediterranean fruit fly (Ceratitis capitata) is a cosmopolitan pest of hundreds of species of commercial and wild fruits. It is considered a major economic pest of commercial fruits in the world. Adult Mediterranean fruit flies feed on all sorts of protein sources, including animal excreta, in order to develop eggs. After reaching sexual maturity and copulating, female flies lay eggs in fruit by puncturing the skin with their ovipositors and injecting batches of eggs into the wounds. In view of the increase in food-borne illnesses associated with consumption of fresh produce and unpasteurized fruit juices, we investigated the potential of Mediterranean fruit fly to serve as a vector for transmission of human pathogens to fruits. Addition of green fluorescent protein (GFP)-tagged Escherichia coli to a Mediterranean fruit fly feeding solution resulted in a dose-dependent increase in the fly's bacterial load. Flies exposed to fecal material enriched with GFP-tagged E. coli were similarly contaminated and were capable of transmitting E. coli to intact apples in a cage model system. Washing contaminated apples with tap water did not eliminate the E. coli. Flies inoculated with E. coli harbored the bacteria for up to 7 days following contamination. Fluorescence microscopy demonstrated that the majority of fluorescent bacteria were confined along the pseudotrachea in the labelum edge of the fly proboscis. Wild flies captured at various geographic locations were found to carry coliforms, and in some cases presumptive identification of E. coli was made. These findings support the hypothesis that the common Mediterranean fruit fly is a potential vector of human pathogens to fruits.
Outbreaks of bacterial diseases associated with the consumption of fresh produce, such as lettuce, apple cider, unpasteurized apple juice, and alfalfa sprouts, have been reported with increasing frequency during the last decade (reviewed in references 3 and 17). Although some produce-associated outbreaks may be due to cross-contamination from meat products, others more likely reflect direct contamination in the field with residues from feces of wild or domestic animals (1, 5, 7). Direct contact with manure-contaminated soil or dust might be a source of preharvest contamination. Indirect sources of contamination could also be the trophic interactions between fruits and plant foragers like birds, mammals, and insects.
The association between bacteria and fruit flies has usually been mentioned in relation to the source of attractants or in relation to symbiosis, which is important to the nutrition of the insects. Fruit flies have seldom been referred to as vectors of plant or human disease. The exceptions to this include the study of Cayol et al. (4), which showed the potential capacity of the Mediterranean fruit fly (Ceratitis capitata) to transmit plant disease, and more recently, the study of Janisiewicz et al. (12), which showed the possible involvement of the vinegar fly (Drosophila melanogaster) in the transmission of pathogenic bacteria to postharvest wounded apples.
The ability of the Mediterranean fruit fly to serve as a vector for food-borne pathogens has not been reported previously, although several features of this insect suggest its potential as a vector. The Mediterranean fruit fly is a generalist cosmopolitan pest that infests more than 200 species of commercial and wild fruits (6, 10, 15). Like most flies, fruit flies must feed on protein in order to develop eggs. The protein sources for the Mediterranean fruit fly include rotting fruits and fecal material (FM) (9, 14, 21). Most fruit flies locate protein food sources through attraction to ammonium-releasing substances (16). This biological phenomenon was used by Piñero et al. (18) to develop an inexpensive attractant made of human urine and chicken feces for monitoring of fruit flies by resource-poor fruit farmers. After reaching sexual maturity and copulating (in the summer, approximately 10 days after eclosion), female flies lay eggs in fruit by puncturing the skin of the fruit with their ovipositors about 1 to 2 mm deep and injecting batches of eggs into the wounds. First-instar larvae hatch from the eggs 2 to 3 days later, and two more instars feed on the fruit tissue. Fully grown third-instar larvae exit from the fruit, crawl and jump to the ground, and dig a few centimeters into the soil to pupate. After approximately 10 days (in the summer), newly emerged flies come of the ground, starting the cycle again. In a tropical setting, the Mediterranean fruit fly has the potential to have more than 10 generations per year.
The attraction of the Mediterranean fruit fly to a variety of fecal material and its foraging on this material as a nitrogen source, in conjunction with its egg-laying activity in a variety of fruits and its extensive prevalence in numerous agricultural regions in the world, suggested that this fly potentially is a vector for feces-borne pathogens. In the present study we investigated the capacity of this fly to transmit Escherichia coli from contaminated fecal material to intact apples.
MATERIALS AND METHODS
Bacteria and bacteriological determination.
E. coli strain TG1 (Amersham Biosciences, United Kingdom), which expresses green fluorescence protein (GFP) on a high-copy-number plasmid (a gift from I. Benhar, Tel-Aviv University) was used in this study. When this organism is grown on a Trypticase soy agar plate, fluorescence is easily observed under long-wavelength UV light. For each experiment, bacteria were grown in tryptic soy broth supplemented with 100 μg/ml ampicillin for 20 h at 37°C and washed twice with double-distilled water (DDW). A number of CFU were added to the feeding solution, as described below.
Contamination of flies by GFP-tagged E. coli was determined as follows. Twenty flies were put into a 15-ml tube containing 2 ml phosphate-buffered saline, pH 7.4, and vortexed (Vortex-Genie 2) three times at maximal power for 30 s. One hundred microliters of the solution and 10-fold serial dilutions were spread plated on Trypticase soy agar containing ampicillin. The plates were incubated for 24 h at 37°C, and the number of ampicillin-resistant GFP-expressing bacteria was recorded.
Preparation of flies.
Mediterranean fruit fly pupae (strain Sade) were obtained from the laboratory colony of the Institute of Biological Control (Israel). Approximately 100 puparia 2 days before adult eclosion were placed for emergence in a petri dish in a Perspex insect cage (40 by 30 by 30 cm) and supplied with water and with a 20% sucrose solution. All feeding materials consisted of 10 ml of solution soaked into cotton wool in a petri dish to prevent drowning of the feeding flies. Maturation of insects and experiments were conducted in a temperature-controlled room at 27°C ± 2°C.
Contamination of Mediterranean fruit flies with E. coli present in the feeding solution.
Adult flies (ca. 2 days old) that were previously maintained on a 20% sucrose solution were starved for 8 h and then exposed to a 20% sucrose solution containing a predetermined concentration of bacteria (6, 7, 8, or 9 log10 cells per ml). In some experiments, the feeding solution was replaced with FM enriched with GFP-tagged E. coli. The FM solution was prepared by vigorously mixing 4 g of fresh human feces with 36 ml of DDW containing 2 × 109 CFU GFP-tagged E. coli per ml. All feeding solutions (sucrose and FM) were introduced into the cages by using soaked cotton wool, and the flies (100 flies per cage) were allowed free access to the solutions for 20 h. After this, 10 males and 10 females were randomly sampled from each concentration and cage (one cage per concentration), pooled by sex, and subjected to bacteriological determination, as described above. For bacterial survival experiments, following exposure of the flies to E. coli, the contaminated solution was removed from the cage, and regular (noncontaminated) food was reintroduced. Flies were sampled, as described above, at time zero (control, before exposure) and 1, 2, 3, 6, and 7 days after the beginning of exposure, and the number of GFP-tagged E. coli was determined, as described above. The experiment was conducted with three replicate cages.
Contamination of apples by Mediterranean fruit flies.
The experimental design of the model system followed essentially the design of the transmission experiments conducted by Janisiewicz et al. (12). Adult flies were fed for 3 days after eclosion on protein-hydrolyzed yeast and sucrose (1:3) “cake” to stimulate egg development. The cake was prepared by mixing the ingredients and allowing the formation of a compact mass through hygroscopic absorption of ambient humidity. The flies then were starved for 20 h to stimulate hunger and exposed either to FM alone or to FM enriched with GFP-tagged bacteria. After 1 h, one previously washed apple (cv. Starking; weight, 110 to 190 g) was introduced into each cage for 15 h. After this, the fruits were carefully removed from the cages to avoid cross-contamination and put individually into sterile stomacher bags. The bags were shaken in 250 ml DDW in an orbital shaker at 180 rpm for 20 min, and samples were taken for bacterial enumeration, as described above. The presence of at least 1 GFP-expressing CFU indicated apple contamination. Contaminated fruits typically harbored 10 to 103 E. coli cells per g. To verify that no mechanical contamination occurred during handling of the FM solution and apples, control experiments were performed with apples and E. coli-enriched FM but without flies. Each experiment was conducted with three replicate cages and repeated three times on different dates. Thus, the entire set of experiments consisted of nine exposed apples and nine control apples.
To check the effect of the “common” home-style fruit decontamination treatment on bacterial persistence, a similar set of experiments was performed. However, in these experiments apples removed from cages were hand washed under running tap water for 30 s before they were subjected to bacteriologic analysis.
Field survey.
Wild Mediterranean fruit flies were collected in three geographic locations in central Israel during March and April 2004 using McPhail traps loaded with Biolure (trimethylamine-putrecine-ammonium acetate; Suterra, Bend, Oreg.). The locations included Beth-Dagan (10 km east of Tel-Aviv), Rehovot (20 km southeast of Tel-Aviv), and Kfar-Saba (20 km northeast of Tel-Aviv) and represented two types of ecosystems (Table 1). The traps were left in the field for up to 2 weeks, and the flies were aseptically removed from the traps and brought to the laboratory. To estimate the level of coliforms and E. coli, all flies collected in a single trap were homogenized in a tube containing Luria broth, and samples were spread plated, as described above, on both violet red-bile agar and Chromcult TBX (tryptone bile x-glucuronide) agar (Merck). The plates were incubated at 37°C for 24 to 48 h, and pink to purple colonies (diameter, >0.5 mm) on violet red-bile agar plates were considered to be coliforms, while blue-green colonies grown on Chromcult agar were presumptively identified as E. coli. Luria broth tubes containing flies (after vortexing) were further incubated at 30°C for 18 h to recover possible injured bacteria. Samples that were negative for coliforms or E. coli in the direct plating test were retested for the presence of these bacteria following the enrichment step.
TABLE 1.
Presence of coliforms and E. coli in wild Mediterranean fruit fliesa
| Sampling location | Ecosystem type | No. of flies in sample | No. of coliforms (CFU/fly) | No. of E. coli (CFU/fly)b |
|---|---|---|---|---|
| Beth-Dagan 1 | Mixed citrus orchard | 16 | 1.3 × 104 | |
| Beth-Dagan 2 | Mixed citrus orchard | 23 | 4.9 × 104 | 1.1 × 104 |
| Kfar-Saba | Home gardens with bearing fruit trees in rural areas | 3 | ||
| Rehovot | Home gardens with bearing fruit trees in urban areas | 4 | —c | —c |
Mediterranean fruit fly samples were obtained from different geographic locations in central Israel and different ecosystems. Flies samples were collected with McPhail traps loaded with Biolure. The traps were exposed for 2 weeks during March and April 2004.
Presumptive identification as E. coli.
Bacteria were detected following enrichment.
Statistical analysis.
The relationship between the bacterial loads in feed and bacterial contamination in flies was investigated using linear regression analysis (22). The relationship between the E. coli contamination of apples when they were exposed to flies and feces and the E. coli contamination of apples when they were exposed to feces but no flies (control) was inferred by analysis of frequencies from a test of independence (after Yates' correction) (22). Similarly, a test of independence (after Yates' correction) was used to infer the relationship between the bacterial contamination of apples after they were washed in the presence of flies and the bacterial contamination of apples after they were washed in the absence of flies. The effect of food substrate (sucrose or FM) on the ability of male and female Mediterranean fruit flies to acquire bacteria was analyzed with a two-way analysis of variance (22) after log10 transformation
RESULTS
Contamination of Mediterranean fruit flies with E. coli.
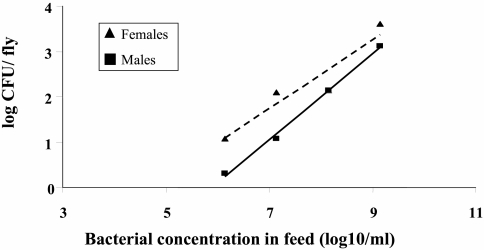
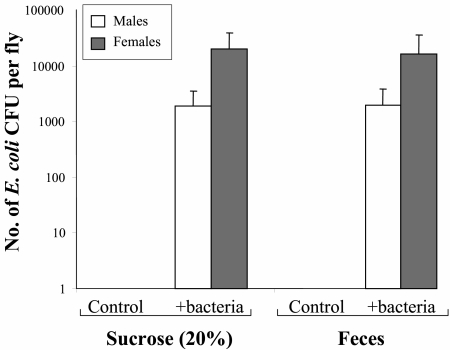
The results show that GFP-tagged E. coli cells are readily transmitted to Mediterranean fruit flies. The average log10 number of bacteria per fly increased linearly with the log10 amount of bacteria in feed (for males, F = 17.7 and P < 0.05; for females, F = 547.1 and P < 0.05) (Fig. 1). The contamination of females (b = 1.18, t = 4.21, P < 0.05) was slightly greater than that of males (b = 1.05, t = 23.4, P < 0.05). After 24 h, the average number of bacteria per fly when the flies were exposed to FM was similar (F = 0.001, P > 0.9) to the average number of bacteria per fly observed when the flies were exposed to the contaminated sucrose solution (Fig. 2). There were no statistical differences between males and females (F = 3.17, P > 0.1); however, there was a tendency of females to acquire larger numbers of bacteria in the two substrates.
FIG. 1.
Acquisition of GFP-tagged E. coli by Mediterranean fruit flies exposed to contaminated feed. Flies were exposed for 24 h to a 20% sucrose solution supplemented with different numbers of tagged E. coli cells. Male and female flies were collected separately, and the number of Ampr, GFP-expressing bacteria in 20 flies was determined.
FIG. 2.
Mediterranean fruit flies acquire E. coli from contaminated fecal material. Flies were exposed to a 20% sucrose solution or fecal material enriched with 2 × 109 CFU/ml GFP-labeled E. coli for 20 h. The numbers of labeled bacteria in males and females were determined. Flies could efficiently acquire E. coli from both the sucrose solution and fecal material in similar numbers. Bacteriological determinations were performed with batches of 10 males and 10 females per replicate (three replicates per treatment).
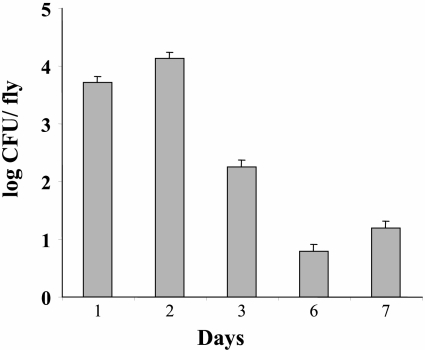
Survival of E. coli on Mediterranean fruit flies.
Flies continued to harbor living E. coli for at least 7 days following the beginning of exposure (Fig. 3). No E. coli cells were detected in samples taken at time zero (before exposure).
FIG. 3.
Survival of E. coli on contaminated Mediterranean fruit flies. Flies were exposed to a sucrose solution supplemented with 4.6 × 109 CFU/ml of E. coli for 20 h. After this, a sample of 10 to 20 flies was collected each day from the cage and subjected to microbiological analysis. Day 1 refers to the 24 h after the beginning of exposure. No E. coli was detected on preexposure flies.
Transmission of E. coli to intact apples by contaminated flies.
The presence of FM and Mediterranean fruit flies in a cage resulted in a high contamination rate. Six of nine apples were found to be contaminated with the specific GFP-tagged E. coli strain. Washing the fruits with tap water did not prevent apples from maintaining the bacterial contamination; four of nine apples were found to harbor E. coli after they were washed with tap water.
In control fly-free cages containing apples and FM enriched with E. coli, no contamination of the fruits was detected, supporting the hypothesis regarding the role of Mediterranean fruit flies in transmitting the bacteria (e.g., contamination was significantly dependent on the presence of flies in the cage; χ2 = 4.18 and P < 0.05 in experiments in which the fruit was not washed and χ2 = 2.89 and P = 0.09 in experiments in which the fruit was washed with tap water).
Wild Mediterranean fruit flies carry coliforms and E. coli.
Three of four samples of wild flies, collected during the end of March and the beginning of April, were found to harbor coliforms, either by direct plating or following enrichment (Table 1). The number of coliforms ranged from 1.3 × 104 to 4.9 × 104 cells. One of the samples, which contained 23 flies, also harbored a significant number of presumptive E. coli cells (1.1 × 104 CFU/fly). In another sample composed of four flies, presumptive E. coli was detected only after enrichment.
Microscopic examination of contaminated Mediterranean fruit flies.
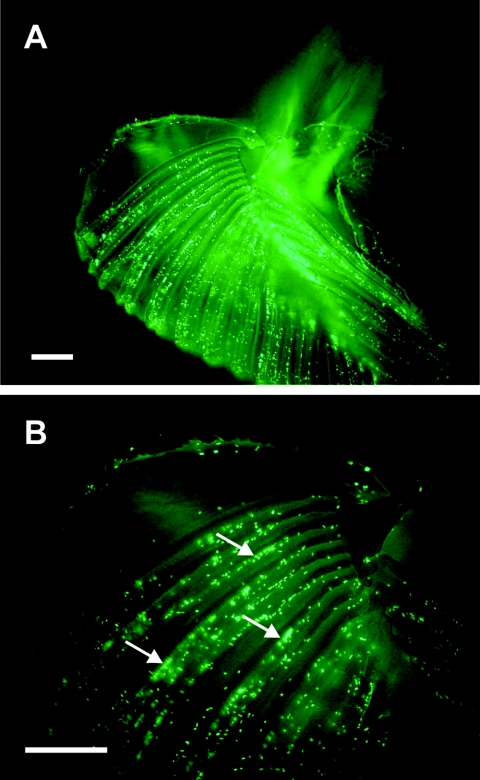
Flies which were exposed to 2 × 109 GFP-expressing bacteria in the sucrose feed solution for 24 h were dissected and observed under a fluorescence microscope (Leica model DMLB) equipped with a charge-coupled device camera (Leica model DC200). Fluorescent bacteria were detected in the flies' labelum, specifically along the pseudotrachea (Fig. 4), but not in other organs, including the ovipositor, other mouthparts, and tarsomeres.
FIG. 4.
Visualization of E. coli in the Mediterranean fruit fly by fluorescence microscopy. Flies were fed a 20% sucrose solution supplemented with 109 CFU/ml GFP-expressing E. coli. (A) Micrograph of the labelum under UV light, with bacteria clearly present in the pseudotrachea of the labelum (arrows). (B) Fine structure of the labelum with associated fluorescent bacteria at a higher magnification. Bars, 100 μm.
DISCUSSION
Feces have been suspected as sources of pathogens on contaminated fruits, vegetables, or minimally processed produce that have subsequently been associated with or confirmed as causes of human disease outbreaks (2). Insects are a recognized contributing epidemiological factor in the spread of food-borne pathogens, especially E. coli, Listeria, Salmonella, and Shigella. Houseflies, for example, were shown to be potential vectors of E. coli O157:H7 (11, 13). The vinegar fly (D. melanogaster) was also implicated in the transmission of pathogenic E. coli to wounded apple tissues under laboratory conditions (12). Yet the involvement of the Mediterranean fruit fly in transmission of human pathogens to fruits has not been investigated previously. Potentially, there are a number of reasons why this pest and other fruit flies should be of concern as potential vectors. The Mediterranean fruit fly, like most fruit flies, forages for food on nitrogenous sources, such as fecal material (14, 19). This natural food source often contains pathogens. For example, Robacker et al. (20) isolated a pathogenic bacterium (Staphylococcus aureus) from the mouthparts of a female Mexican fruit fly (Anastrepha ludens). Moreover, different strains of E. coli (including strains typical of warm-blooded animal feces) have been isolated from the apple maggot (Rhagoletis pomonella) (14). The last finding reinforces the potential risk for transmission of human diseases by fruit flies foraging for proteinaceous material and supports the hypothesis that other fruit-foraging insect species have the potential to serve as vectors in the contamination of fresh produce.
While the vinegar fly (D. melanogaster) was shown to transmit E. coli O157:H7 to wounds on apple fruit (12), the present study, in which a similar experimental system was used, showed that the Mediterranean fruit fly might also act as a vector for transmitting bacteria to intact apples. This model system, which facilitates bacterium-fly-apple interactions, simulates the reported acquisition of microorganisms by fruit flies feeding on fecal material in nature (14). Microscopic analysis suggested that the main organ involved in bacterial uptake is the fly's mouthparts. The mouthparts may also be the main vehicle for the contamination of fruits, since fruit flies forage for fruit juices on the surface of fruits (8). An alternative mechanism of fruit contamination may be through the introduction of pathogenic bacteria underneath the intact fruit skin by the mechanical activity of the female ovipositor during egg laying. Fruit fly eggs derived from flies feeding on a GFP-tagged E. coli contaminated sucrose solution were associated with fluorescent bacteria (Sela and Nestel, unpublished data). The finding that washing E. coli-contaminated apples under flowing tap water did not remove bacteria further supports this route of transmission.
It is noteworthy that investigation of the association between E. coli O157:H7 and houseflies showed that a large number of the bacteria adhered to the surface of the housefly mouthparts and actively proliferated in the minute spaces of the labelum. The ingested bacteria were excreted continuously for at least 3 days after feeding (13). It has not been established yet whether E. coli can also grow within the Mediterranean fruit fly or whether the fly merely serves as a mechanical vector. However, survival studies showed that Mediterranean fruit flies continued to harbor viable GFP-tagged E. coli for at least 7 days following contamination. The results of a restricted field survey demonstrated that wild flies do carry coliforms and that some even harbor presumptive E. coli during a period (spring) that the Mediterranean fruit fly population is still low. These findings highlight the potential of the fly to carry human pathogens and to serve as a vector for transmission of food-borne diseases. Our findings strengthen the need for further investigations to evaluate the actual epidemiological potential of the Mediterranean fruit fly to disseminate human pathogens. Control measures to reduce fruit fly populations should include cultural control, as well as strict sanitation measures both within and surrounding orchards. These measures are expected to eliminate the risk of disseminating bacterial pathogens to commercially grown fruits.
Acknowledgments
We are grateful to Yoav Gazit (The Institute for Biological Control, Beth-Dagan, Israel) for supplying the flies and for microscopic analyses. We also thank Zvi Mendel (Department of Entomology, The Volcani Center) for helpful discussions and three anonymous reviewers whose comments greatly improved the manuscript.
This work was partially supported by an intramural grant from The Volcani Center to S. Sela.
REFERENCES
- 1.Besser, R. E., S. M. Lett, J. T. Weber, M. P. Doyle, T. J. Barrett, J. G. Wells, and P. M. Griffin. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217-2220. [PubMed] [Google Scholar]
- 2.Beuchat, L. R. 1996. Pathogenic microorganisms associated with fresh produce. J. Food Prot. 59:204-206. [DOI] [PubMed] [Google Scholar]
- 3.Beuchat, L. R., and J.-H. Ryu. 1997. Produce handling and processing practices. Emerg. Infect. Dis. 3:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cayol, J. P., R. Causse, C. Louis, and J. Barthes. 1994. Medfly Ceratitis capitata Wiedemann (Dipt., Trypetidae) as a rot vector in laboratory conditions. J. Appl. Entomol. 117:338-343. [Google Scholar]
- 5.Cody, S. H., M. K. Glynn, J. A. Farrar, K. L. Cairns, P. M. Griffin, J. Kobayashi, M. Fyfe, R. Hoffman, A. S. King, J. H. Lewis, B. Swaminathan, R. G. Bryant, and D. J. Vugia. 1999. An outbreak of Escherichia coli O157:H7 infection from unpasteurized commercial apple juice. Ann. Intern. Med. 130:202-209. [DOI] [PubMed] [Google Scholar]
- 6.Enkerlin, W., and J. Mumford. 1997. Economic evaluation of three alternative methods for control of the Mediterranean fruit fly (Diptera: Tephritidae) in Israel, Palestinian Territories, and Jordan. J. Econ. Entomol. 90:1066-1072. [Google Scholar]
- 7.Goverd, K. A., F. W. Beech, R. P. Hobbs, and R. Shannon. 1979. The occurrence and survival of coliforms and salmonellas in apple juice and cider. J. Appl. Bacteriol. 46:521-530. [DOI] [PubMed] [Google Scholar]
- 8.Hendrichs, J., B. I. Katsoyannos, D. R. Papaj, and R. J. Prokopy. 1991. Sex differences in movement between natural feeding and mating sites and tradeoffs between food consumption, success and predator evasion in Mediterranean fruit flies (Diptera: Tephritidae). Oecolgia 86:223-231. [DOI] [PubMed] [Google Scholar]
- 9.Hendrichs, J., B. I. Katsoyannos, and R. J. Prokopy. 1993. Bird feces in the nutrition of adult Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) in nature. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 8:703-707. [Google Scholar]
- 10.Hendrichs, J., A. S. Robinson, J. P. Cayol, and W. Enkerlin. 2002. Medfly areawide sterile insect technique programmes for prevention, suppression or eradication: the importance of mating behavior studies. Fla. Entomol. 85:1-13. [Google Scholar]
- 11.Iwasa, M., S. Makino, H. Asakura, H. Kobori, and Y. Morimoto. 1999. Detection of Escherichia coli O157:H7 from Musca domestica (Diptera: Muscidae) at a cattle farm in Japan. J. Med. Entomol. 36:108-112. [DOI] [PubMed] [Google Scholar]
- 12.Janisiewicz, W. J., W. S. Conway, M. W. Brown, G. M. Sapers, P. Fratamico, and R. L. Buchanan. 1999. Fate of Escherichia coli O157:H7 on fresh cut apple tissue and its potential for transmission by fruit flies. Appl. Environ. Microbiol. 65:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi, M., T. Sasaki, N. Saito, K. Tamura, K. Suzuki, H. Watanabe, and N. Agui. 1999. Houseflies: not simple mechanical vectors of enterohemorrhagic Escherichia coli O157:H7. Am. J. Trop. Med. Hyg. 61:625-629. [DOI] [PubMed] [Google Scholar]
- 14.Lauzon, C. R. 2003. Symbiotic relationships of tephritids, p. 115-129. In K. Bourtzis and T. A. Miller (ed.), Insect symbiosis. CRC Press, Boca Raton, Fla.
- 15.Liquido, N. J., R. T. Cunningham, and S. Nakagawa. 1990. Hosts plants of the Mediterranean fruit fly (Diptera: Tephritidae) on the Islands of Hawaii (1949-1985 survey). J. Econ. Entomol. 83:1863-1868. [Google Scholar]
- 16.Mazor, M., S. Gothilf, and R. Galun. 1987. The role of ammonia in the attraction of females of the Mediterranean fruit fly to protein hydrolysate baits. Entomol. Exp. Appl. 43:25-29. [Google Scholar]
- 17.Parish, M. E. 1997. Public health and unpasteurized fruit juices. Crit. Rev. Microbiol. 23:109-119. [DOI] [PubMed] [Google Scholar]
- 18.Piñero, J., M. Aluja, A. Vazquez, M. Equihua, and J. Varon. 2003. Human urine and chicken feces as fruit fly (Diptera: Tephritidae) attractants for resource-poor fruit growers. J. Econ. Entomol. 96:334-340. [PubMed] [Google Scholar]
- 19.Prokopy, R. J., C. L. Hsu, and R. I. Vargas. 1993. Effect of source and condition of animal excrement on attractiveness to adults of Ceratitis capitata (Diptera: Tephritidae). Environ. Entomol. 22:453-458. [Google Scholar]
- 20.Robacker, D. C., J. A. Garcia, A. J. Martinez, and M. G. Kaufman. 1991. Strain of Staphylococcus attractive to laboratory strain Anastrepha ludens (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 84:555-559. [Google Scholar]
- 21.Robacker, D. C., J. A. Garcia, and R. J. Bartelt. 2000. Volatiles from duck feces attractive to Mexican fruit fly. J. Chem. Ecol. 26:1849-1867. [Google Scholar]
- 22.Sokal, R. R., and F. J. Rohlf. 1981. Biometry. W.H. Freeman and Company, New York, N.Y.