Abstract
The goal of these studies was to determine how sorption by humic acids affected the bioavailability of polynuclear aromatic hydrocarbons (PAHs) to PAH-degrading microbes. Micellar solutions of humic acid were used as sorbents, and phenanthrene was used as a model PAH. Enrichments from PAH-contaminated soils established with nonsorbed phenanthrene yielded a total of 25 different isolates representing a diversity of bacterial phylotypes. In contrast, only three strains of Burkholderia spp. and one strain each of Delftia sp. and Sphingomonas sp. were isolated from enrichments with humic acid-sorbed phenanthrene (HASP). Using [14C]phenanthrene as a radiotracer, we verified that only HASP isolates were capable of mineralizing HASP, a phenotype hence termed “competence.” Competence was an all-or-nothing phenotype: noncompetent strains showed no detectable phenanthrene mineralization in HASP cultures, but levels of phenanthrene mineralization effected by competent strains in HASP and NSP cultures were not significantly different. Levels and rates of phenanthrene mineralization exceeded those predicted to be supported solely by the metabolism of phenanthrene in the aqueous phase of HASP cultures. Thus, competent strains were able to directly access phenanthrene sorbed by the humic acids and did not rely on desorption for substrate uptake. To the best of our knowledge, this is the first report of (i) a selective interaction between aerobic bacteria and humic acid molecules and (ii) differential bioavailability to bacteria of PAHs sorbed to a natural biogeopolymer.
Polycyclic aromatic hydrocarbons (PAHs) are persistent, widespread environmental pollutants (10, 11, 46). A potentially important process for remediation of PAH-contaminated soil or sediment is biodegradation. A diversity of bacteria that grow on and degrade a variety of PAHs have been isolated (54). From the study of these organisms, catabolic pathways have been deduced, and a number of the genes encoding catabolic enzymes, particularly dioxygenases, have been identified (23, 28, 36, 40, 47, 50, 58). However, relatively little is known about the occurrence or type of microbial adaptations that might mitigate the low bioavailability of PAHs, which is considered to be the most important factor limiting PAH biodegradation (11, 41, 46).
A bioavailable compound occurs in a physiochemical state accessible to microbes. Conventionally, a PAH or other substrate is considered bioavailable if it is dissolved in the aqueous phase and nonbioavailable if it is associated with a sorbent (46). However, kinetic analysis of sorbed substrate mineralization (i.e., release of substrate-carbon as CO2) by organisms in soil or bioreactors has indicated that, in some cases, mineralization rates exceed those of desorption (15, 34, 35, 48). These findings were interpreted to indicate that some organisms interact with the environmental sorbent in a way that creates a pathway for substrate uptake that circumvents desorption to the bulk aqueous solution or increases mass transfer rates from the sorbent to the cell (34, 35, 57).
Microbes with abilities to gain access to sorbed substrates might occupy an important niche in the biodegradation of PAH, and isolation of such organisms has recently been investigated. Recognizing that the traditional enrichment system of adding PAH crystals in an aqueous culture is likely inadequate for selective culture of such organisms, systems in which selection is imposed for organisms capable of growing on a PAH (or other hydrophobic compound) sorbed to synthetic resins or membranes have been used (6, 13, 14, 48). From these systems, bacteria which differ from organisms isolated by the traditional approach in that they are different groups of species and/or more effective in the degradation of sorbed PAH have been isolated (6, 13, 14, 48). These findings support the hypothesis that a unique microbial population may adapt to and mediate degradation of sorbed compounds. It is unknown, however, if microbes may adapt to interact with heterogeneous natural materials (e.g., soil organic matter), which serve as the primary environmental sorbents for PAH, in ways that facilitate the uptake of PAH sorbed by these materials. Both mineral and organic constituents of soil and sediment may act as sorbents, but the latter typically play the major role. The organic phase is complex and may be composed of pollutants (e.g., non-aqueous-phase liquids) as well as a variety of natural materials. Among the latter, humic substances, which are complex macromolecules derived from the microbial degradation of plant, animal, and microbial residues, are predominant. The chemistry of humic substances and sorption of hydrophobic compounds, such as PAH, resemble a partitioning process between an aqueous solution and an organic phase (9, 10, 25, 55). Desorption of humic-substance-sorbed compounds is typically considered necessary for entry into the bioavailable phase and subsequent biodegradation (46). However, little is known about how microbes interact with humic sorbents or how cell-humic-substance interactions might affect bioavailability.
Humic substances are the most abundant form of organic matter in terrestrial ecosystems (43), and microbial interactions with these materials may be fundamental in many ways. In anaerobic environments, humic acids (HA) are utilized as electron acceptors or electron shuttles by a variety of bacteria (12, 24, 29). With aerobes, exposure to humic materials has been shown to have various effects on metabolic activity in general and on the biodegradation of organic pollutant compounds in particular. For example, the growth of heterotrophic and nitrifying bacteria was reported to be stimulated by the addition of humic acids to culture media, and in both cases, the effect was attributed to the alteration of membrane characteristics such that nutrient uptake was enhanced (49, 52). Exposure to humic acids improved the survival of Arthrobacter crystallopoietes by unknown mechanisms (22). Degradation of homo- and heterocyclic aromatic compounds was stimulated by humic acids, an effect attributed to enhanced uptake rates (31). However, Shimp and Pfaender (42) observed a diminished capacity of mixed bacterial cultures to degrade phenolic compounds following exposure to humic acids and speculated that the latter elicited cell surface alterations that hindered phenol uptake. Exposure to humic acids has also been shown to stimulate PAH degradation, an effect postulated to reflect (i) an enhanced solubilization of PAH by humic acids (21) or (ii) the concentration of PAH on the surfaces of humic acids (33).
In the present study, enrichment cultures and mineralization tests were done in which micellar solutions of humic acids were used as the sorbent and phenanthrene was used as the sorbate. Micellar humic acid solutions are stable colloidal dispersions of humic molecular aggregates. The term “micellar” is used here because, although the occurrence of micelle-like structures is debated (44, 53, 55), humic acid solutions display characteristics consistent with the occurrence of a critical micelle concentration, as is observed with simpler amphiphiles. Also like simple amphiphiles, at concentrations above the critical micelle concentration, humic acid micellar solutions can solubilize relatively large amounts of hydrophobic chemicals (16, 55, 59). As a model system to explore microbial interactions with natural sorbents, we viewed micellar HA solutions as useful in that they possess some of the complexity of humic substances that have a major role in PAH sorption in the environment. The solubilization capacity of the micellar HA solutions and their liquid character also facilitated their use as a sorbent in enrichment cultures or activity tests.
We viewed the use of HA solutions as a means to address a basic question: can microbes adapt to interact with humic substances in ways that facilitate the uptake of humic-substance-sorbed compounds such as PAH? Our hypothesis was that the HA-sorbed phenanthrene would present a large pool of substrate that would be bioavailable only to certain strains adapted to interact with the HA in a manner that permitted access to the sorbed compound.
MATERIALS AND METHODS
Humic acid preparation.
The HA used was a preparation commercially available through Aldrich Chemicals (Milwaukee, WI). For use in enrichment cultures, the HA was purified using a simplified version of the protocol outlined by the International Humic Substance Society (IHSS) (45). Briefly, HA (1 g) was dissolved in 10 ml of 0.1 N NaOH. This solution was then acidified to pH 1.0 with 6 N HCl to flocculate the HA and dissolve oxide minerals. The acidified HA solution stood quiescently overnight, and then the precipitated HA was recovered by centrifugation (10,000 × g, 15 min). To dissolve silicate minerals, the HA was resuspended in a solution of 0.1 M HCl and 0.3 M hydrofluoric acid and incubated overnight with mixing, and then the particulate material was recovered by centrifugation (10,000 × g, 15 min). The HCl-hydrofluoric acid treatment was done a total of three times, and the resulting solution was transferred to dialysis tubing (molecular weight cutoff [MWCO], 5,000; snakeskin dialysis tubing; Pierce, Rockford, IL) and the tubing placed in distilled water. Distilled water was exchanged daily until the pH and electrical conductivity of the water did not change after overnight dialysis. The HA preparation was freeze-dried. Prior to use, samples of the freeze-dried HA preparation were plated on 1/10 nutrient broth to verify that there was no microbial contamination.
Humic acid preparations used in mineralization tests were subjected to additional purification. First, KCl (final concentration, 300 mM) was added to the initial solution of HA in NaOH and the flocculated suspended solids removed by centrifugation (10,000 × g, 15 min). Second, after precipitation with 6 N HCl, the samples were dissolved in 0.1 N KOH to coagulate any remaining undissolved minerals. Third, dialysis was done with a 10,000-MWCO membrane. With these modifications, the cleanup procedure followed the complete IHSS protocol (45). Experiments showed that the HA prepared by the simplified IHSS protocol contained substances labile to mineralization by phenanthrene degraders. In initial tests, production of carbon dioxide from labile humic acid-carbon interfered with mineralization measurements of humic acid-sorbed [14C]phenanthrene; purification by the complete IHSS protocol eliminated this interference.
Equilibrium dialysis.
Radiolabeled phenanthrene ([9-14C]phenanthrene, 2.04 GBq mmol−1; purity, 99.6%; Sigma, St. Louis, MO) and nonlabeled phenanthrene were mixed in hexane to give a working stock of 25 μg total phenanthrene μl−1 and a specific activity of 7.4 Bq μg−1. An aliquot (40 μl) of the working stock was added to a 12-ml chamber of an acrylic Spectra/Por MacroDialyzer (Spectrum, Rancho Dominguez, CA) containing mineral salts medium (MSM) (19). The total amount of phenanthrene added to the aqueous-phase chamber was ca. 83-fold in excess of its water solubility level. The other chamber, containing 2% (wt/vol) HA in MSM, was separated from the first chamber by a Spectra/Por-regenerated cellulose dialysis membrane (MWCO, 1,000; Spectrum). This HA concentration represented the upper range of HA concentrations previously reported to enhance the solubility of hydrophobic compounds (7, 8). Both chambers contained stir bars, and the apparatus was incubated on a stir plate for 250 h. Aliquots (100 μl) taken from the HA chamber were added to 10 ml of Harvey Cocktail (R. J. Harvey Instrument Co., Hillsdale, NJ) and counted on a Rackbeta model 1209 scintillation counter (LKB Wallac, San Fransisco, CA). When HA appeared to be saturated with 14C, 13 aliquots were taken from the HA chamber for determination of 14C activity and calculation of 95% confidence intervals for phenanthrene sorption.
The rate and amount of phenanthrene desorbing from the HA were also determined by equilibrium dialysis. The apparatus described above was used, and the 2% (wt/vol) HA chamber was spiked with the [14C]phenanthrene mixture. The starting concentration of phenanthrene in the 2% (wt/vol) HA chamber was 32 μg ml−1, which approximated the lower 95% confidence interval for phenanthrene sorbed to HA. The aqueous phase was sampled for 14C activity immediately after assembly of the dialysis unit. When the 14C activity in the aqueous chamber was in equilibrium with the HA chamber, 16 samples were taken to establish 95% confidence intervals for the amount of phenanthrene desorbed.
Soil samples.
PAH-contaminated soil was acquired from sites of former coal gasification plants in Ashland, Chippewa Falls, and Eau Claire, Wisconsin. A soil borer was used to take samples from 0- to 50-cm depths, and samples were placed on ice for transport. Samples of a fourth soil were acquired from a former coal gasification plant in Iowa City, Iowa. All soil samples were refrigerated at 2°C until used in enrichment experiments.
Isolation of phenanthrene degraders.
Paired enrichments were done to compare the types and characteristics of phenanthrene degraders isolated via a conventional approach with nonsorbed phenanthrene (NSP) to those enriched with HA-sorbed phenanthrene (HASP). The HASP enrichment cultures contained 32 μg phenanthrene ml−1 dissolved in 2% (wt/vol) HA-MSM. A common, homogenized inoculum was prepared by adding 2 g of each soil to 50 ml of MSM containing 10 mg phenanthrene. After 1 week of incubation, aliquots (500 μl) of this solution were inoculated into NSP and HASP cultures. The NSP and HASP cultures were incubated for 1 week, and 5% (vol/vol) aliquots were transferred to fresh NSP or HASP medium. An HA enrichment blank (HAB) was established using the soil from Chippewa Falls as the inoculum; the HAB contained 2% (wt/vol) HA in MSM but no added PAH. HAB enrichments were not established with the other three soils. This was because the amount of HA needed for each culture was relatively large, and the processing of HA for use in cultures was a time-consuming process; other experiments were deemed to have greater priority for use of this limiting resource. After three transfers, 10−5 dilutions of enrichment cultures were spread onto MSM noble agar plates coated with phenanthrene (27). When zones of clearing were observed, colonies were picked and restreaked onto MSM phenanthrene plates. Single colonies showing zones of clearing were picked and inoculated into liquid MSM, with phenanthrene being supplied in the crystalline form as the sole carbon and energy source to verify utilization of phenanthrene.
Outside sources of phenanthrene-degrading bacterial cultures.
Sphingomonas aromaticivorans F199 was acquired from the American Type Culture Collection (strain ATCC 700278), and Sphingomonas sp. strain P5-2 was kindly provided by M. Alexander (Cornell University).
Phenanthrene mineralization in HASP and NSP cultures.
Two systems were used to conduct paired tests to determine an isolate's ability to grow on and mineralize phenanthrene in HASP and NSP cultures. The first system was a large-volume radiorespirometry device that was suited for time point studies with a limited number of isolates. The second was a small-volume system that facilitated replicated screening of large numbers of isolates for the mineralization endpoints.
The large-volume system was used to test cultures isolated from the HASP enrichments: Sphingomonas sp. strain 12H6; Burkholderia sp. strains Ch1-1, Ch3-5, and Eh1-1; and Delftia sp. strain Eh2-1. The NSP-acquired isolate, Burkholderia sp. strain Cs1-4, was also included in these tests because based on 16S rRNA analysis, it was closely related to the three HASP-enriched strains of Burkholderia sp. listed above.
For the time point study, the large-volume radiorespirometry vessel was similar to that described by Reid et al. (38) and consisted of a 250-ml glass screw-top flask, a Teflon-lined top with an alligator clip to hold the alkali trap, and a 20-ml scintillation vial as the alkali trap. The traps contained 1.5 ml of 2 N NaOH. All reaction mixtures contained 70 μg of phenanthrene (42.6 Bq μg−1) in 5 ml MSM or in 5 ml of 2% (wt/vol) HA-MSM, which had mixed for at least 7 days on a stir plate. Results from equilibrium dialysis tests (see below) indicated that a 2% (wt/vol) HA solution could sorb 15 μg ml−1 phenanthrene in 3 days and a maximum of 32 μg ml−1 of phenanthrene in 7 days. Thus, the concentration of phenanthrene added to the 2% (wt/vol) HA-MSM solution (14 μg ml−1) was about half the HA sorption capacity, and for which 7 days was more than sufficient for full sorption to occur. To each flask, 500 μl of mid-log-phase phenanthrene-grown culture was added to give an initial cell density in the cultures of 104 CFU ml−1. Burkholderia sp. strain Eh1-1 was also tested at an initial density of 102 CFU ml−1. Flasks were then incubated with shaking, and the NaOH traps were periodically replaced during the 14-day incubation period. The 14C activity in the traps was counted directly after the addition of 9 ml ScintSafe Plus 50% scintillation cocktail (Fisher Scientific, Pittsburgh, PA). At the end of the experiment, 10 μl of culture fluid was added to 9 ml of scintillation cocktail and counted to determine 14C mass balances. The aqueous phase would have included 14C-labeled cells in suspension and water-soluble 14C metabolites produced from [14C]phenanthrene, as well as nonmetabolized parent compound.
The radiorespirometry apparatus used for endpoint screening of the entire culture collection was a 20-ml scintillation vial, into which was inserted a 15-mm by 45-mm vial containing 750 μl of 2 N NaOH. Each NSP mineralization test established in these contained 7 μg of phenanthrene (42.6 Bq μg−1) in 500 μl of MSM, while each HASP test culture contained 7 μg of phenanthrene (42.6 Bq μg−1) in 500 μl of 2% (wt/vol) HA in MSM. For the latter, phenanthrene was added at least 7 days in advance of the study, and the solutions were mixed on a stir plate. Cultures of all isolates were grown on 10 mg phenanthrene in 25 ml MSM. Aliquots (1.5 ml) of log-phase cultures for each strain were centrifuged, the supernatants were discarded, and the cell pellets were frozen at −20°C for later use in mineralization tests (approximately 1 to 3 weeks). Cell pellets were resuspended in 200 μl of 13 mM phosphate buffer (pH 7), and 20 μl (106 to 107 CFU ml−1) of each strain was added to replicate vessels. Scintillation vials were capped and then incubated for 7 days with shaking. The NaOH traps were then removed, and 9 ml ScintSafe Plus 50% scintillation cocktail was added to the trap. For the NSP systems, 9 ml scintillation cocktail was directly added to the reaction vial.
BOX-PCR genomic fingerprinting.
Each reaction mixture contained Gitschier reaction buffer [16.6 mM (NH4)2SO4; 67 mM Tris-HCl, pH 8.8; 6.7 mM MgCl2; 6.5 μM EDTA, pH 8.8; and 30 mM β-mercaptoethanol], 1.25 mM deoxynucleoside triphosphates, 0.16 mg ml−1 bovine serum albumin, 10% dimethyl sulfoxide, 1.4 μM BOXA1R primer (51), 2 U of Taq DNA polymerase, and 1 μl of genomic DNA. Reaction mixtures were brought to 25 μl with distilled water. The thermal cycling program was 95°C for 2 min followed by 31 cycles of 94°C for 3 s, 92°C for 30 s, 50°C for 1 min, and 65°C for 8 min and a final extension step of 65°C for 8 min. PCR products were separated by electrophoresis at 75 V for 2 h in 1.5% agarose gels containing 3 μg ml−1 ethidium bromide and 1× Tris-acetate-EDTA containing 3 μg ml−1 ethidium bromide.
Sequencing and analysis of 16S rRNA genes.
Amplification by PCR of the 16S rRNA gene was done with the bacterial primer set 27f and 1492r (3). The thermal cycling parameters were a 5-min hot start at 94°C, followed by 30 cycles of denaturation for 1 min at 94°C, annealing at 50°C for 30 s, and extension for 2 min at 72°C, with a final extension of 5 min at 72°C. Each 50-μl reaction mixture included 5 μl Taq buffer B (Promega, Madison, WI), 2.5 mM MgCl2, 0.4 μM each primer, 200 μM deoxynucleoside triphosphates, 2 U of Taq (Promega), and 1 to 3 μl genomic DNA. For organisms with high G+C contents (e.g., Mycobacterium spp.) 5% (vol/vol) dimethyl sulfoxide was included in the PCR. Prior to sequencing, the PCR product was purified using a Qiaquick PCR purification kit (QIAGEN, Valencia, CA). Sequencing reactions with primers 27f, 1492r, and 338f (5) were done using the ABI PRISM BigDye terminator chemistry (PE Applied Biosystems). Reaction products were analyzed by an ABI PRISM 373 DNA sequencer (PE Applied Biosystems) at the University of Wisconsin—Madison Biotechnology Center. Phylogenetic assignments were made by BLAST-N (4) analysis of nucleotide sequences. It should be noted that, while the GenBank matches were, in many cases, to organisms named to the species level (Table 1), proper assignment of the isolates to species would require confirmatory tests. In the absence of these data, all discussion regarding isolates acquired in the present study will be limited to the genus level.
TABLE 1.
Summary of phenanthrene degraders isolated in these studies
| Sitea | Strain | Enrichment approach(s) | No. of isolatesb | Closest GenBank match to 16S rRNA sequence
|
16S rRNA gene GenBank accession no. | ||
|---|---|---|---|---|---|---|---|
| Strain or species | Accession no. | % Identity | |||||
| A | As1-2 | NSP | 1 | Delftia acidovorans MBIC 1305 | AB020186.1 | 99.9 | AY367024 |
| A | As1-3 | NSP | 2 | Sphingomonas sp. strain JS1 | SSP427917 | 98.6 | AY367025 |
| A | As1-5 | NSP | 1 | Sphingomonas sp. strain MBIC3020 | AB025279.1 | 98.4 | AY367026 |
| A | As1-6 | NSP | 1 | Delftia acidovorans strain WDL34 | AF538930.1 | 96.0 | AY367031 |
| A | As2-1 | NSP | 1 | Sphingomonas sp. strain JS1 | SSP427917 | 98.3 | AY367027 |
| A | As2-3 | NSP | 1 | Sphingomonas sp. strain ATCC 31555 | AF503280.1 | 97.5 | AY367032 |
| A | As2-4 | NSP | 1 | Comamonas testosteroni WDL7 | AF538933.1 | 95.4 | AY367033 |
| A | As3-4 | NSP | 1 | Delftia acidovorans MBIC 1305 | AB020186.1 | 100 | AY367028 |
| C | Ch1-1 | HASP | 5 | Burkholderia sp. strain S1-17 | AF448032 | 99.9 | AY367011 |
| C | Cs1-4 | NSP | 1 | Burkholderia sp. strain S1-17 | AF448032 | 99.9 | AY367009 |
| C | Cs1-5 | NSP | 1 | Comamonas testosteroni ZD4-1 | AY125951.1 | 99.8 | AY367038 |
| C | Cs3-2 | NSP | 1 | Stenotrophomonas maltophilia LMG11087 | SMRR11087 | 92.1 | AY367034 |
| C | Ch3-5 | HASP | 1 | Burkholderia sp. strain S1-17 | AF448032 | 99.9 | AY367010 |
| C | Chnp3-1 | HAB | 3 | Oxalobacter sp. strain p8E | PR0496038 | 97.1 | AY367035 |
| C | Chnp3-4 | HAB | 1 | Pseudomonas sp. strain V-07-1 | AY136543.1 | 92.8 | AY367036 |
| C | Chnp3-5 | HAB | 1 | Herbaspirillum sp. strain BA17 | AF364861 | 97.2 | AY367037 |
| E | Eh1-1 | HASP, NSP | 4 | Burkholderia sp. strain S4.11 | AF247495 | 99.9 | AY367008 |
| E | Eh2-1 | HASP | 2 | Delftia acidovorans MBIC 1305 | AB020186.1 | 99.8 | AY367007 |
| E | Es2-1 | NSP | 1 | Oxalobacter sp. strain p8E | PR0496038 | 97.1 | AY367029 |
| E | Es2-5 | NSP | 1 | Stenotrophomonas maltophilia LMG10857 | SMA131117 | 100 | AY367030 |
| I | 12H6 | HASP, NSP | 9 | Sphingomonas paucimobilis EPA505 | SPEPA505 | 99.9 | AY367017 |
| I | Mc1 | NSP | 2 | Mycobacterium fortuitum ATCC 6841 | MFO16SRN | 98.7 | AY367018 |
| I | Mc2 | NSP | 2 | Mycobacterium sp. strain SM7.6.1 | AF247497 | 97.2 | AY367019 |
| I | Mc3 | NSP | 2 | Mycobacterium sp. strain LB208 | MSP245704 | 99.5 | AY367020 |
| I | Mc4 | NSP | 2 | Mycobacterium sp. strain LB208 | MSP245704 | 99.4 | AY367021 |
| I | Mc5 | NSP | 2 | Mycobacterium sp. strain CH-1 | AF054278 | 99.1 | AY367022 |
| I | Mc6 | NSP | 2 | Mycobacterium sp. strain SM7.6.1 | AF247497 | 97.1 | AY367023 |
| I | Ps1 | NSP | 1 | Pseudomonas corrugata ND9L | AF348508 | 99.8 | AY367013 |
| I | Sg5 | NSP | 2 | Sphingomonas paucimobilis EPA505 | SPEPA505 | 99.9 | AY367015 |
| I | Sg6 | NSP | 4 | Sphingomonas paucimobilis EPA505 | SPEPA505 | 99.9 | AY367016 |
| I | Um1 | NSP | 6 | Ultramicrobacterium strain ND5 | AB008506 | 99.6 | AY367012 |
| I | Xan | NSP | 3 | Xanthobacter agilis SA35 | XA16RR | 97.5 | AY367014 |
Soil origins were as follows: Iowa (I); Ashland, WI (A); Chippewa Falls, WI (C); and Eau Claire, WI (E).
Number of isolates represented by each strain as determined by BOX-PCR analysis.
Nucleotide sequence accession numbers.
Sequence data obtained in this study from 16S rRNA gene analysis were cataloged in GenBank under the accession numbers listed in Table 1.
RESULTS AND DISCUSSION
Characterization of micellar HA solutions for phenanthrene sorption capacity, the phenanthrene partitioning coefficient, and aqueous-phase phenanthrene concentrations.
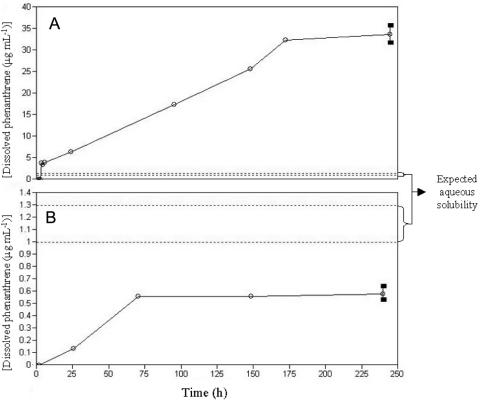
In the equilibrium dialysis experiment, HA sorption of phenanthrene (aqueous solubility, ∼1 μg ml−1) reached a plateau after about 175 h (Fig. 1A). The apparent aqueous concentration of phenanthrene associated with the HA (HA sorption capacity) was 34 ± 2 μg ml−1 (95% confidence level). The enhancement in phenanthrene solubility was of the same order of magnitude as that reported for solubilization of other hydrophobic compounds (water solubility, ≤5 μg liter−1) in humic acid solutions (16, 55). Desorption reached an apparent equilibrium after ca. 70 h (Fig. 1B), after which the average aqueous-phase concentration of phenanthrene was 0.58 ± 0.06 μg ml−1 (95% confidence level).
FIG. 1.
Equilibrium dialysis analysis of sorption (A) and desorption (B) of phenanthrene by a 2% (wt/vol) humic acid solution. Open circles represent data points. The two broken lines indicate the upper and lower values reported in the literature for the aqueous solubility of phenanthrene. Error bars (filled rectangles) at endpoints represent 95% confidence intervals.
A full appreciation of the results discussed below regarding mineralization of phenanthrene in HASP cultures requires an understanding of the partitioning of phenanthrene between the aqueous (bioavailable) phase and the humic phase. The phenanthrene partition coefficient (KP) can be estimated from both the sorption and desorption experiments. The former of these (sorption coefficient, KP,sorption) can be calculated using data from the equilibrium dialysis test and the following equations:
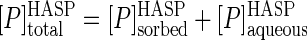 |
(1) |
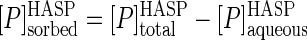 |
(2) |
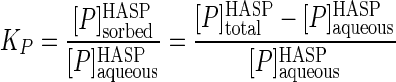 |
(3) |
where [P]sorbedHASP, [P]aqueousHASP, and [P]totalHASP are the phenanthrene concentrations in the sorbed phase, the aqueous phase, and the two phases combined, respectively, in the HASP culture. The last term was experimentally determined in the equilibrium dialysis test, and using this value and assuming 1 μg ml−1 for the aqueous solubility of phenanthrene, equation 3 becomes:
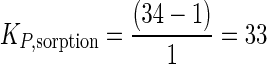 |
(4) |
The phenanthrene desorption coefficient (KP,desorption) can also be calculated using equations 1 to 3. Note that [P]sorbedHASP is calculated using the mass of phenanthrene in the 12-ml chamber containing the HA solution only, while [P]aqueousHASP includes the mass in the aqueous phases of both dialysis chambers. To calculate these terms, we start with the total amount of phenanthrene added in the HA solution: 384 μg. Next we consider that at equilibrium a total of 13.92 μg had desorbed ([P]aqueousHASP = 0.58 μg ml−1 in the two 12-ml chambers). Thus, the mass of sorbed phenanthrene remaining in the 12 ml of HA solution was 370.08 μg ([P]sorbedHASP = 30.84 μg ml−1). Placing this value into equation 3 gives
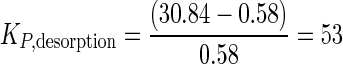 |
(5) |
The partition coefficients determined from the two experiments were similar but not identical: we assume that that the true sorption coefficient is in the range of 30 to 50. However, to gain a conservative estimate of [P]aqueousHASP (i.e., the traditionally defined bioavailable phenanthrene concentration) in the HASP cultures, we will use the lower KP value of 33. In these cultures, [P]totalHASP was 14 μg ml−1, and rearranging equation 3 gives
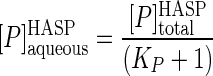 |
(6) |
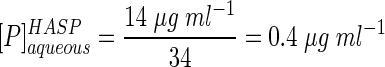 |
(7) |
The value of 0.4 μg ml−1 represents the initial concentration of phenanthrene in the aqueous phase. The total amount of bioavailable phenanthrene includes the initial amount in the aqueous phase along with that which desorbed during the course of the experiment. From the slope of the desorption curve, the estimated rate of phenanthrene diffusion from the humic phase was ca. 0.007 μg h−1 1 (e.g., 0.4 μg ml−1 in 48 h). The actual desorption rate in HASP cultures tested in the experiments reported below (Fig. 2; Table 2) would be less than 0.007 μg ml−1 h−1 because sorbed phenanthrene levels in HASP cultures were 50% lower than the levels used in the desorption experiments (Fig. 1B): 14 versus 32 μg ml−1, respectively. Nevertheless, we can estimate that in the HASP mineralization studies (see below), the total amount of phenanthrene accessible through the aqueous phase in 48 h (i.e., the period in the time course study in which mineralization reached maximum levels [see below]) would have ranged from 0.6 to 0.8 μg ml−1. Thus, desorption combined with the initial phenanthrene levels would give bioavailable phenanthrene levels ranging from 4 to 6% of [P]totalHASP.
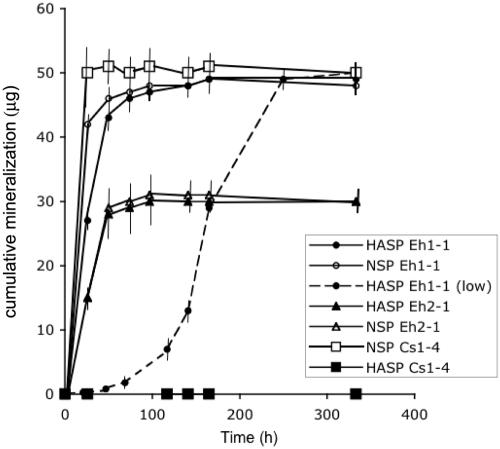
FIG. 2.
Time course of phenanthrene mineralization in NSP (open symbols) and HASP (filled symbols) cultures. Results are shown for Burkholderia sp. strain Eh1-1 (circles), Burkholderia sp. strain Cs1-4 (squares), and Delftia sp. strain Eh2-1 (triangles). All cultures were started with an initial inoculum of 107 CFU except that identified as HASP Eh1-1 (low), which was inoculated with 104 CFU. Bars indicate standard deviations of duplicated measures and do not appear where these were smaller than the symbol plotted.
TABLE 2.
Phenanthrene mineralization in NSP and HASP cultures during a 7-day incubationa
| Strainc | Enrichment approachd | % 14Cb
|
|||||
|---|---|---|---|---|---|---|---|
| NSP
|
HASP
|
||||||
| CO2 | Solution | Total | CO2 | Solution | Total | ||
| Ch1-1 | HASP | 70 ± 0 | 24 ± 0 | 98 ± 1 | 67 ± 16* | 49 ± 19 | 115 ± 3 |
| Ch3-5 | HASP | 75 ± 1 | 16 ± 4 | 91 ± 1 | 76 ± 6* | 37 ± 7 | 113 ± 8 |
| Eh1-1 | HASP | 67 ± 3 | 26 ± 4 | 94 ± 3 | 77 ± 5* | 31 ± 6 | 108 ± 9 |
| Eh2-1 | HASP | 28 ± 7 | 57 ± 2 | 86 ± 7 | 38 ± 10* | 72 ± 12 | 110 ± 15 |
| Chnp3-1 | HAB | 59 ± 1 | 33 ± 1 | 92 ± 1 | 1 ± 0 | 102 ± 2 | 103 ± 3 |
| Chnp3-4 | HAB | 56 | 31 | 87 | 1 ± 0 | 98 ± 0 | 98 ± 0 |
| Chnp3-5 | HAB | 61 ± 0 | 26 ± 7 | 87 ± 6 | 1 ± 0 | 104 ± 4 | 105 ± 4 |
| As1-2 | NSP | 66 | 27 | 93 | 1 ± 0 | 106 ± 2 | 106 ± 2 |
| As1-3 | NSP | 18 ± 1 | 65 ± 5 | 82 ± 3 | 1 ± 0 | 103 ± 5 | 104 ± 5 |
| As1-5 | NSP | 76 | 19 | 95 | 1 ± 0 | 104 ± 7 | 105 ± 7 |
| As1-6 | NSP | 34 | 61 | 86 | 1 ± 0 | 110 ± 4 | 111 ± 4 |
| As2-1 | NSP | 15 | 69 | 84 | 1 ± 0 | 107 ± 0 | 108 ± 0 |
| As2-4 | NSP | 64 | 27 | 91 | 1 ± 0 | 103 ± 1 | 103 ± 1 |
| As3-4 | NSP | 70 ± 1 | 26 ± 1 | 96 ± 1 | 3 ± 0 | 106 ± 0 | 109 ± 1 |
| Cs1-4 | NSP | 65 ± 1 | 28 ± 2 | 93 ± 3 | 2 ± 0 | 104 ± 1 | 105 ± 1 |
| Cs3-2 | NSP | 68 ± 0 | 22 ± 1 | 90 ± 1 | 1 ± 0 | 106 ± 1 | 107 ± 1 |
| Es2-1 | NSP | 24 | 45 | 70 | 4 ± 7 | 94 ± 8 | 99 ± 7 |
| Mc1 | NSP | 63 ± 1 | 29 ± 1 | 91 ± 1 | 2 ± 1 | 90 ± 1 | 92 ± 2 |
| Mc2 | NSP | 33 ± 14 | 38 ± 5 | 71 ± 8 | 3 ± 3 | 92 ± 2 | 95 ± 6 |
| Mc4 | NSP | 18 ± 8 | 45 ± 22 | 63 ± 12 | 2 ± 2 | 90 ± 5 | 93 ± 3 |
| Mc5 | NSP | 59 | 23 | 81 | 1 ± 1 | 100 ± 17 | 102 ± 16 |
| Mc6 | NSP | 69 ± 4 | 25 ± 3 | 93 ± 1 | 1 ± 0 | 108 ± 4 | 108 ± 4 |
| Sg5 | NSP | 71 | 21 | 93 | 1 ± 0 | 104 ± 6 | 104 ± 6 |
| Sg6 | NSP | 67 ± 1 | 26 ± 1 | 93 ± 1 | 1 ± 0 | 107 ± 4 | 108 ± 4 |
| Um1 | NSP | 62 ± 1 | 28 ± 2 | 90 ± 3 | 1 ± 0 | 89 ± 5 | 89 ± 5 |
| P5-2 | Outside | 57 ± 13 | 23 ± 5 | 81 ± 8 | 1 ± 0 | 68 ± 6 | 70 ± 5 |
| F199 | Outside | 63 ± 4 | 28 ± 4 | 91 ± 0 | 4 ± 5 | 72 ± 17 | 77 ± 12 |
| Abiotic | NA | 5 ± 3 | 61 ± 11 | 66 ± 10 | 1 ± 0 | 98 ± 7 | 99 ± 7 |
NSP contained a total of 14 μg ml−1 phenanthrene in MSM. HASP contained a total of 14 μg ml−1 phenanthrene in MSM with 2% (wt/vol) humic acid.
% 14C, percentage of total added 14C recovered in the indicated fraction. For HASP isolates, values are means from four replicates ± standard deviations. For all other isolates, values are means from duplicated tests ± standard deviations. Some NSP tests had poor mass balances (<70%); these were excluded from the table, and results from the single remaining test are presented without a standard deviation. All NSP percent CO2 means were significantly different from the abiotic control (Dunnett's method; α = 0.05). None of the HASP percent CO2 means were significantly different from the abiotic control (Dunnett's method; α = 0.05). Asterisks indicate that NSP percent CO2 means were significantly different from those for the abiotic control (Dunnett's method; α = 0.05). Percent CO2 means in NSP and HASP cultures were not significantly different (Wilcoxon rank sum test; α = 0.05).
See Table 1 for further information on strain identities. Strains listed in Table 1 but not included in the above data set because of inactive inocula were Cs1-5, MC3, Ps1, Es2-1, 12H6, As2-1, Es2-5, and Xan. Abiotic, noninoculated controls of NSP and HASP cultures.
“Outside” indicates an isolate acquired from an outside source. NA, not applicable.
Thus, in the HASP cultures, for phenanthrene mineralization levels to significantly exceed 7 to 8% in 48 h, an isolate would need to access phenanthrene sorbed by the humic acids and indicate that some or all of the humic acid-sorbed phenanthrene was directly bioavailable (i.e., substrate uptake not dependent on desorption).
Phenanthrene degrader isolation and identification.
A total of 68 phenanthrene degraders were isolated from the four soils (Table 1). Banding patterns (genomic fingerprints) generated from BOX-PCR were reproducible to the extent that two isolates could be distinguished as unique strains based on the presence or absence of a single band. At this level of resolution, we could identify unique isolates as well as redundant isolates (clones) of the same strain. Thus, based on BOX-PCR analysis, the total number of unique phenanthrene-degrading isolates recovered from all four soils by the HAB, NSP, and HASP systems was 3, 25, and 5, respectively.
The NSP approach yielded isolates from all soils (Table 1), which represented a diversity of phylotypes including a variety of gram-negative (Burkholderia, Comamonas, Delftia, Herbaspirillum, Oxalobacter, Pseudomonas, Sphingomonas, Stenotrophomonas, ultramicrobacterium, and Xanthobacter spp.) and gram-positive (Mycobacterium spp.) bacteria. These findings are similar to those of many previous investigators (1, 2, 18, 20, 26, 32, 37, 39, 56, 60). However, with the HASP system, isolates were obtained from the Chippewa Falls, Eau Claire, and Iowa City soils, but not from Ashland (Table 1). Isolation of bacteria from HAB cultures showed that HA prepared for use in enrichments could serve as a carbon source. The cumulative dilution during HAB enrichments was greater than a millionfold, so there would be little carryover of carbon from the soil inoculum. Plate culture examination verified that the HA was sterile; thus, HAB isolates were not HA-introduced contaminants. Thus, we hypothesize that HAB isolates were cultured on HA during enrichment and then selected on plates for their ability to utilize phenanthrene.
Comparative analysis of HASP and NSP mineralization: identification of isolates with the “competence” phenotype.
In the time point tests, the patterns and levels of NSP mineralization were similar for all strains, and maximum levels of mineralization were reached within 2 days of incubation (Fig. 2). Delftia sp. strain Eh2-1 differed in that 14CO2 production reached a level that was about half of that produced by the other strains (Fig. 2): this likely reflects differences between the strains in growth yields. Mineralization of HASP, however, was observed only with four of the five HASP isolates: Burkholderia sp. strains Eh1-1, Eh2-1, Ch3-5, and Ch1-1. For these isolates, the time frame and endpoints of HASP and NSP mineralization were the same (Fig. 2). Thus, sorption by HA did not significantly slow the process of phenanthrene mineralization by these strains. Diluting the initial inoculum of strain Eh1-1 lengthened the period needed to attain maximum levels of HASP mineralization, but not the endpoint (Fig. 2). The dilution effect and the shape of the resulting mineralization curve would be consistent with phenanthrene mineralization in HASP cultures as a growth-linked process. The lack of HASP mineralization by Burkholderia sp. strain Cs1-4 and Sphingomonas sp. strain 12H6 could not be attributed to differences in inoculant density (all inoculants were of similar cell densities [see Materials and Methods]) or, as evidenced by the high levels of NSP mineralization by these strains, inactive inoculant.
In the endpoint screening experiment, all isolates mineralized phenanthrene in the NSP cultures (Table 2). However, HASP mineralization was again detected with only four of the five HASP enrichment isolates: Burkholderia sp. strains Eh1-1, Ch3-5, and Ch1-1 and Delftia sp. strain Eh2-1 (Table 2). As with the time point experiment, all isolates were tested at similar initial inoculum densities, and all strains reported in Table 2 showed relatively high activity in NSP cultures. So, again, effects of low inoculum density or activity could be eliminated as factors to which the inability of most isolates to mineralize HASP could be ascribed.
Neither of the outside isolates displayed competence. One of these (S. aromaticivorans F199) was isolated by a traditional enrichment approach, while Sphingomonas sp. strain P5-2 was isolated by selection for growth on phenanthrene sorbed to polyacrylic beads (48). It is noteworthy that, when synthetic resins have been used as PAH sorbents to enrich PAH degraders, mycobacteria typically predominate the isolate collection (6, 13, 14). However, none of the mycobacteria isolated in the present study were competent. Adaptation of mycobacteria to grow on resin-sorbed PAH is often attributed to their hydrophobic cell surface. Thus, the lack of competence by mycobacteria may suggest that a hydrophobic cell surface does not facilitate access to humic acid-sorbed PAH.
Some replicate NSP cultures were omitted from Table 2 because of poor mass balances, which most likely resulted from volatile losses of phenanthrene. In HASP cultures, these losses would be minimized by sorption of phenanthrene to the humic acids. While volatile losses of phenanthrene in some replicate NSP cultures reduced the accuracy by which NSP mineralization endpoints were determined, it did not change the most important conclusion to be drawn from this comparative test: most strains enriched by the traditional approach (i.e., in NSP cultures) were incapable of mineralizing HASP. The single exception was Burkholderia sp. strain Eh1-1, clones of which were independently isolated from the HASP and NSP approaches (Table 1). The clones had identical BOX-PCR patterns and effected similar levels of HASP mineralization. The latter finding was significant because it showed that the HASP mineralization phenotype was not an artifact of enrichment in HASP cultures. That is, the characteristics that enabled HASP mineralization by certain strains occurred naturally in these organisms.
Isolates able to mineralize HASP are hereafter referred to as possessing the “competence” phenotype. The levels of HASP mineralization effected by these organisms (38 to 76% of [P]totalHASP) (Table 2; Fig. 2) far exceeded the amount estimated to be bioavailable in the aqueous phase alone (7 to 8% of [P]totalHASP [see above]). These data indicate that a key attribute of competence was the ability to access phenanthrene in the sorbed state. Thus, for competent isolates, bioavailable phenanthrene included some or all of that sorbed by the humic acids.
Competence was an all-or-nothing phenotype; strains were either competent and showed high levels of HASP mineralization or noncompetent and showed no significant HASP mineralization. While the cellular characteristic(s) enabling competence is as yet unknown, the presence/absence nature of the phenotype suggests that it was not attributable solely or primarily to characteristics that vary across a continuum (e.g., permeability or cell surface charge). The process underlying competence appeared similar to “direct bioavailability,” which referred to the bacterial uptake of a surfactant-sorbed substrate directly from the cores of surfactant micelles (17). Direct bioavailability was suggested based on mineralization kinetics of phenanthrene sorbed by micellar solutions of synthetic, nonionic surfactants (17). The mechanism by which direct bioavailability occurred was not determined, but it was proposed that uptake occurred from a layer of surfactant hemi-micelles that formed on the cell surface (17). A similar process could be envisioned to underlay competence.
For the noncompetent strains, the lack of HASP mineralization did not reflect the toxicity of the HA solutions, as these organisms grew well in MSM-2% HA containing phenanthrene in excess of the HA sorption capacity or in tryptic soy broth containing 2% HA (data not shown). Thus, phenanthrene degradation by noncompetent strains in HASP cultures was likely limited by mass transfer of phenanthrene from the HA sorbent to the aqueous phase. It was noteworthy that none of the HAB isolates were competent. This may indicate that selection for the phenotype required some incentive to interact with humic acids (e.g., to access growth substrate sorbed by the humic acids) and that exposure to humic acids alone did not apply sufficient selective pressure. Noncompetence of the HASP isolate 12H6 suggested that it was enriched from the Iowa City soil by a mechanism similar to that of the HAB isolates. The lack of recovery of competent strains from the Iowa City and Ashland soils could indicate either that they were absent from these soils or that some aspect of the HASP enrichment system was inadequate to allow their recovery. Further studies would be needed to resolve this issue.
Summary and conclusions.
These studies demonstrated that sorption of phenanthrene to humic acids created a barrier to bioavailability that was breached only by a specific group of competent bacteria. Providing HASP as a carbon source enriched for these organisms, presumably by selecting for strains capable of interacting with humic acids in such a way as to gain access to the sorbed phenanthrene. While the nature of these attributes is unknown, to the best of our knowledge, this is the first report of a selective interaction between aerobic bacteria and humic acid molecules. Further studies are needed to identify the physical basis for competence.
Previous investigators demonstrated bacterial adaptation to degrade PAH sorbed to synthetic resins (6, 13, 14, 48). Exposure to humic acids has also been shown to stimulate PAH degradation (21, 33). The present work has shown that bacteria may also adapt to interact with humic acids, such that PAH sorbed by these molecules is bioavailable. Furthermore, sorption to colloidal humics is an important environmental pathway for PAH transport (30), and the present studies open the possibility that certain types of PAH-degrading bacteria might preferentially interact with these colloids and consequently affect the fate and behavior of this mobile pool of PAH. Given that humic acids are the most prevalent form of terrestrial and aquatic organic matter, there may be many interactions with competent microbes that we do not yet recognize.
There are many differences between natural environmental sorbents and all of the surrogates that have been explored so far, and it remains to be determined if characteristics of surrogate-adapted bacteria can facilitate access to PAH sorbed by the environmental sorbents. However, it is clear that the majority of PAH degraders isolated by conventional enrichments with PAH supplied as crystals in aqueous solutions probably lack characteristics that enable interactions with sorbents and, as such, may be of questionable environmental significance. The potential for effective interaction with environmental sorbents warrants attention as a primary characteristic to be considered in the selection and study of organisms that degrade PAH or other hydrophobic compounds.
Acknowledgments
This work was funded by the UW—Madison College of Agricultural and Life Sciences (Hatch projects WIS04289, WIS04371, and WIS04891 to W.J.H.).
We thank Jack Eslien, Chris Saari, and Jamie Dunn of the Wisconsin Department of Natural Resources for providing access to sites for soil sampling. We also thank Michael Stanchack for assisting with data collection and an anonymous reviewer for many constructive comments on the manuscript.
REFERENCES
- 1.Ahn, Y., J. Sanseverino, and G. S. Sayler. 1999. Analyses of polycyclic aromatic hydrocarbon-degrading bacteria isolated from contaminated soils. Biodegradation 10:149-157. [DOI] [PubMed] [Google Scholar]
- 2.Aitken, M. D., W. T. Stringfellow, R. D. Nagel, C. Kazunga, and S. H. Chen. 1998. Characteristics of phenanthrene-degrading bacteria isolated from soils contaminated with polycyclic aromatic hydrocarbons. Can. J. Microbiol. 44:743-752. [PubMed] [Google Scholar]
- 3.Akkermans, A. D. L., J. D. Van Elsas, and F. J. De Bruijn. 1996. Molecular microbial ecology manual. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 4.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. H. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial-cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastiaens, L., D. Springael, P. Wattiau, H. Harms, R. deWachter, H. Verachtert, and L. Diels. 2000. Isolation of adherent polycyclic aromatic hydrocarbon (PAH)-degrading bacteria using PAH-sorbing carriers. Appl. Environ. Microbiol. 66:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien, Y. Y., and W. F. Bleam. 1997. Fluorine-19 nuclear magnetic resonance study of atrazine in humic and sodium dodecyl sulfate micelles swollen by polar and nonpolar solvents. Langmuir 13:5283-5288. [Google Scholar]
- 8.Chien, Y.-Y., E. G. Kim, and W. F. Bleam. 1997. Paramagnetic relaxation of atrazine solubilized by humic micellar solutions. Environ. Sci. Technol. 31:3204-3208. [Google Scholar]
- 9.Chiou, C. T., L. J. Peters, and V. H. Freed. 1979. A physical concept of soil-water equilibria for nonionic organic compounds. Science 206:831-832. [DOI] [PubMed] [Google Scholar]
- 10.Chiou, C. T., P. E. Porter, and D. W. Schmedding. 1983. Partition equilibria of non-ionic organic compounds between soil organic matter and water. Environ. Sci. Technol. 17:227-231. [DOI] [PubMed] [Google Scholar]
- 11.Dua, M., A. Singh, N. Sethunathan, and A. K. Johri. 2002. Biotechnology and bioremediation: successes and limitations. Appl. Microbiol. Biotechnol. 59:143-152. [DOI] [PubMed] [Google Scholar]
- 12.Finneran, K. T., H. M. Forbush, C. V. G. VanPraagh, and D. R. Lovley. 2002. Desulfitobacterium metallireducens sp. nov., an anaerobic bacterium that couples growth to the reduction of metals and humic acids as well as chlorinated compounds. Int. J. Syst. Evol. Microbiol. 52:1929-1935. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich, M., R. J. Grosser, E. A. Kern, W. P. Inskeep, and D. M. Ward. 2000. Effect of model sorptive phases on phenanthrene biodegradation: molecular analysis of enrichments and isolates suggests selection based on bioavailability. Appl. Environ. Microbiol. 66:2703-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosser, R. J., M. Friedrich, D. M. Ward, and W. P. Inskeep. 2000. Effect of model sorptive phases on phenanthrene biodegradation: different enrichment conditions influence bioavailability and selection of phenanthrene-degrading isolates. Appl. Environ. Microbiol. 66:2695-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerin, W. F., and S. A. Boyd. 1997. Bioavailability of naphthalene associated with natural and synthetic sorbents. Water Res. 31:1504-1512. [Google Scholar]
- 16.Guetzloff, T. F., and J. A. Rice. 1994. Does humic acid form a micelle? Sci. Total. Environ. 152:31-35. [Google Scholar]
- 17.Guha, S., and P. Jaffe. 1996. Bioavailability of hydrophobic compounds partitioned into the micellar phase of nonionic surfactants. Environ. Sci. Technol. 30:1382-1391. [Google Scholar]
- 18.Habe, H., and T. Omori. 2003. Genetics of polycyclic aromatic hydrocarbon metabolism in diverse aerobic bacteria. Biosci. Biotechnol. Biochem. 67:225-243. [DOI] [PubMed] [Google Scholar]
- 19.Hickey, W. J., and D. D. Focht. 1990. Degradation of mono-, di-, and trihalogenated benzoic acids by Pseudomonas aeruginosa JB2. Appl. Environ. Microbiol. 56:3842-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho, Y., M. Jackson, Y. Yang, J. G. Mueller, and P. H. Pritchard. 2000. Characterization of fluoranthene- and pyrene-degrading bacteria isolated from PAH-contaminated soils and sediments. J. Ind. Microbiol. Biotechnol. 24:100-112. [Google Scholar]
- 21.Holman, H. Y. N., K. Nieman, D. L. Sorensen, C. D. Miller, M. C. Martin, T. Borch, W. R. McKinney, and R. C. Sims. 2002. Catalysis of PAH biodegradation by humic acid shown in synchrotron infrared studies. Environ. Sci. Technol. 36:1276-1280. [DOI] [PubMed] [Google Scholar]
- 22.Hwang, S., and R. L. Tate. 1997. Humic acid effects on 2-hydroxypyridine metabolism by starving Arthrobacter crystallopoietes cells. Biol. Fertil. Soils 25:36-40. [Google Scholar]
- 23.Kahng, H. Y. 2002. Cellular responses of Pseudomonas sp. KK1 to two-ring polycyclic aromatic hydrocarbon, naphthalene. J. Microbiol. 40:38-42. [Google Scholar]
- 24.Kappler, A., M. Benz, B. Schink, and A. Brune. 2004. Electron shuttling via humic acids in microbial iron(III) reduction in a freshwater sediment. FEMS Microbiol. Ecol. 47:85-92. [DOI] [PubMed] [Google Scholar]
- 25.Karickhoff, S. W., D. S. Brown, and T. A. Scott. 1979. Sorption of hydrophobic pollutants on natural sediments water pollution. Water Res. 13:241-248. [Google Scholar]
- 26.Kastner, M., M. Breuerjammali, and B. Mahro. 1994. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic-hydrocarbons (PAH). Appl. Microbiol. Biotechnol. 41:267-273. [Google Scholar]
- 27.Kiyohara, H., K. Nagao, and K. Yana. 1982. Rapid screen for bacteria degrading water-insoluble, solid hydrocarbons on agar plates. Appl. Environ. Microbiol. 43:454-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd-Jones, G., A. D. Laurie, D. W. F. Hunter, and R. Fraser. 1999. Analysis of catabolic genes for naphthalene and phenanthrene degradation in contaminated New Zealand soils. FEMS Microbiol. Ecol. 29:69-79. [Google Scholar]
- 29.Luijten, M., S. A. B. Weelink, B. Godschalk, A. A. M. Langenhoff, M. H. A. van Eekert, G. Schraa, and A. J. M. Stams. 2004. Anaerobic reduction and oxidation of quinone moieties and the reduction of oxidized metals by halorespiring and related organisms. FEMS Microbiol. Ecol. 49:145-150. [DOI] [PubMed] [Google Scholar]
- 30.MacKay, A. A., and P. M. Gschwend. 2001. Enhanced concentrations of PAHs in groundwater at a coal tar site. Environ. Sci. Technol. 35:1320-1328. [DOI] [PubMed] [Google Scholar]
- 31.Meredith, C. E., and M. Radosevich. 1998. Bacterial degradation of homo- and heterocyclic aromatic compounds in the presence of soluble/colloidal humic acid. J. Environ. Sci. Health Part B 33:17-36. [DOI] [PubMed] [Google Scholar]
- 32.Mueller, J. G., R. Devereux, D. L. Santavy, S. E. Lantz, S. G. Willis, and P. H. Pritchard. 1997. Phylogenetic and physiological comparisons of PAH-degrading bacteria from geographically diverse soils. Antonie Leeuwenhoek 71:329-343. [DOI] [PubMed] [Google Scholar]
- 33.Ortega-Calvo, J.-J., and C. Saiz-Jimenez. 1998. Effect of humic fractions and clay on biodegradation of phenanthrene by a Pseudomonas fluorescens strain isolated from soil. Appl. Environ. Microbiol. 64:3123-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park, J. H., X. D. Zhao, and T. C. Voice. 2001. Biodegradation of non-desorbable naphthalene in soils. Environ. Sci. Technol. 35:2734-2740. [DOI] [PubMed] [Google Scholar]
- 35.Park, J. H., X. D. Zhao, and T. C. Voice. 2002. Development of a kinetic basis for bioavailability of sorbed naphthalene in soil slurries. Water Res. 36:1620-1628. [DOI] [PubMed] [Google Scholar]
- 36.Pieper, D. H., and W. Reineke. 2000. Engineering bacteria for bioremediation. Curr. Opin. Biotechnol. 11:262-270. [DOI] [PubMed] [Google Scholar]
- 37.Puntus, I. F., A. E. Filonov, I. A. Kosheleva, R. R. Gayazov, A. V. Karpov, and A. M. Boronin. 1997. Isolation and characterization of microorganisms degrading polycyclic aromatic hydrocarbons. Microbiology 66:222-225. [Google Scholar]
- 38.Reid, B. J., C. J. A. MacLeod, P. H. Lee, A. W. J. Morriss, J. D. Stokes, and K. T. Semple. 2001. A simple C14-respirometric method for assessing microbial catabolic potential and contaminant bioavailability. FEMS Microbiol. Lett. 196:141-146. [DOI] [PubMed] [Google Scholar]
- 39.Saadoun, I. 2002. Isolation and characterization of bacteria from crude petroleum oil contaminated soil and their potential to degrade diesel fuel. J. Basic Microbiol. 42:420-428. [DOI] [PubMed] [Google Scholar]
- 40.Saito, A., T. Iwabuchi, and S. Harayama. 2000. A novel phenanthrene dioxygenase from Nocardioides sp. strain KP7: expression in Escherichia coli. J. Bacteriol. 182:2134-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samanta, S. K., O. V. Singh, and R. K. Jain. 2002. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol. 20:243-248. [DOI] [PubMed] [Google Scholar]
- 42.Shimp, R., and F. K. Pfaender. 1985. Influence of naturally occurring humic acids on biodegradation of monosubstituted phenols by aquatic bacteria. Appl. Environ. Microbiol. 49:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevenson, F. J. 1982. Humus chemistry: genesis, composition, reactions. Wiley, New York, N.Y.
- 44.Swift, R. S. 1999. Macromolecular properties of soil humic substances: fact, fiction, and opinion. Soil Sci. 164:790-802. [Google Scholar]
- 45.Swift, R. S. 1996. Organic matter characterization, p. 1018-1020. In D. L. Sparks et al. (ed.), Methods of soil analysis. Part 3. Chemical methods, vol. 5. Soil Science Society of America, Madison, Wis. [Google Scholar]
- 46.Tabak, H. H., J. M. Lazorchak, L. Lei, A. P. Khodadoust, J. E. Antia, R. Bagchi, and M. T. Suidan. 2003. Studies on bioremediation of polycyclic aromatic hydrocarbon-contaminated sediments: bioavailability, biodegradability, and toxicity issues. Environ. Toxicol. Chem. 22:473-482. [PubMed] [Google Scholar]
- 47.Takizawa, N., T. Iida, T. Sawada, K. Yamauchi, Y. W. Wang, M. Fukuda, and H. Kiyohara. 1999. Nucleotide sequences and characterization of genes encoding naphthalene upper pathway of Pseudomonas aeruginosa PaK1 and Pseudomonas putida OUS82. J. Biosci. Bioeng. 87:721-731. [DOI] [PubMed] [Google Scholar]
- 48.Tang, W. C., J. C. White, and M. Alexander. 1998. Utilization of sorbed compounds by microorganisms specifically isolated for that purpose. Appl. Microbiol. Biotechnol. 49:117-121. [DOI] [PubMed] [Google Scholar]
- 49.Vallini, G., A. Pera, M. Agnolucci, and M. M. Valdrighi. 1997. Humic acids stimulate growth and activity of in vitro tested axenic cultures of soil autotrophic nitrifying bacteria. Biol. Fertil. Soils 24:243-248. [Google Scholar]
- 50.van Herwijnen, R., D. Springael, P. Slot, H. A. J. Govers, and J. R. Parsons. 2003. Degradation of anthracene by Mycobacterium sp. strain LB501T proceeds via a novel pathway, through o-phthalic acid. Appl. Environ. Microbiol. 69:186-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Versalovic, J., M. Schneider, F. J. De Bruijn, and J. R. Lupski. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 5:25-40. [Google Scholar]
- 52.Visser, S. A. 1985. Effect of humic acids on numbers and activities of microorganisms within physiological groups. Org. Geochem. 8:81-85. [Google Scholar]
- 53.von Wandruszka, R. 1998. The micellar model of humic acid: evidence from pyrene fluorescence measurements. Soil Sci. 163:921-930. [Google Scholar]
- 54.Watanabe, K. 2001. Microorganisms relevant to bioremediation. Curr. Opin. Biotechnol. 12:237-241. [DOI] [PubMed] [Google Scholar]
- 55.Wershaw, R. L., P. J. Burcar, and M. C. Goldberg. 1969. Interaction of pesticides with natural organic material. Environ. Sci. Technol. 3:271-273. [Google Scholar]
- 56.Widada, J., H. Nojiri, K. Kasuga, T. Yoshida, H. Habe, and T. Omori. 2002. Molecular detection and diversity of polycyclic aromatic hydrocarbon-degrading bacteria isolated from geographically diverse sites. Appl. Microbiol. Biotechnol. 58:202-209. [DOI] [PubMed] [Google Scholar]
- 57.Woo, S. H., J. M. Park, and B. E. Rittmann. 2001. Evaluation of the interaction between biodegradation and sorption of phenanthrene in soil-slurry systems. Biotechnol. Bioeng. 73:12-24. [DOI] [PubMed] [Google Scholar]
- 58.Yang, Y., R. F. Chen, and M. P. Shiaris. 1994. Metabolism of naphthalene, fluorene, and phenanthrene: preliminary characterization of a cloned gene cluster from Pseudomonas putida NCIB 9816. J. Bacteriol. 176:2158-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yonebayashi, K., and T. Hattori. 1987. Surface-active properties of soil humic acids. Sci. Total Environ. 62:55-64. [Google Scholar]
- 60.Zocca, C., S. Di Gregorio, F. Visentini, and G. Vallini. 2004. Biodiversity amongst cultivable polycyclic aromatic hydrocarbon-transforming bacteria isolated from an abandoned industrial site. FEMS Microbiol. Lett. 238:375-382. [DOI] [PubMed] [Google Scholar]