Abstract
Prevention of microbial adhesion and detachment of adhering microorganisms from surfaces is important in many environmental, industrial, and medical applications. Fluid shear is an obvious parameter for stimulating microbial detachment from surfaces, but recently it has been pointed out that a passing air-liquid interface also has potential in stimulating microbial detachment. In the present study, the ability of microbubbles to stimulate detachment of bacterial strains from a glass surface is compared with the effects of fluid flow. Adhesion and detachment of Actinomyces naeslundii T14V-J1, Streptococcus oralis J22, and their coadhering aggregates were studied on glass, mounted in a parallel plate flow chamber. High fluid wall shear rates (11,000 to 16,000 s−1) were established in a laminar flow regime in the absence and presence of microbubbles. Wall shear rates stimulated detachment ranging from 70% to 30% for S. oralis and A. naeslundii, respectively. Coadhering aggregates were detached up to 54%. The presence of microbubbles in the flow increased the detachment of A. naeslundii within 2 min of flow from 40% in the absence of microbubbles to 98%, while detachment of neither S. oralis nor coadhering aggregates was affected by the presence of microbubbles. In summary, extremely high fluid flows can be effective in stimulating microbial detachment, depending on the strain involved. The addition of microbubbles to the flow allows the detachment of tenaciously adhering bacteria not detached by flow alone, but not of adhering coaggregates.
Microorganisms attach to natural and manmade surfaces, such as soil particles, plant fibers, pipelines, ship hulls, orthopedic implants, and contact lenses, as well as to surfaces in the human oral cavity. The formation of a biofilm in the oral cavity involves a sequence of events including initial microbial adhesion, during which individual organisms interact reversibly with oral hard and soft tissues, followed by anchoring through extracellular polysaccharide production, yielding irreversible adhesion and coadhesion of planktonic organisms with already adhering (sessile) ones (4). Coadhesion, a process by which genetically distinct planktonic bacteria attach to already adhering (sessile) bacteria, has been described to occur in the oral cavity, mammalian gut, human urogenital tract, and potable-water-supply systems (6, 17, 18). Coadhesion has been most extensively described for the human oral cavity and occurs between strains such as actinomyces and streptococci, thus contributing to the temporospatial distribution of organisms in oral biofilms (11).
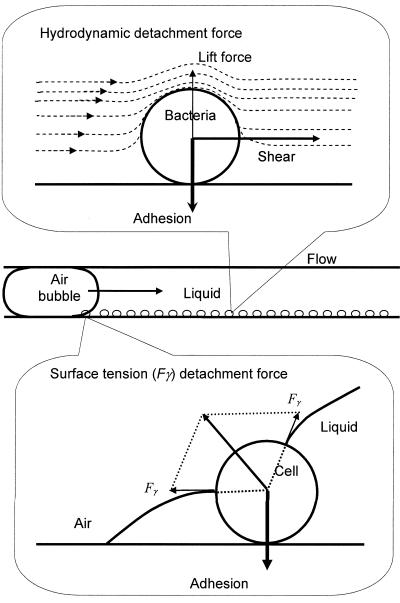
Microbial adhesion in the oral cavity occurs under highly unfavorable conditions, and flow of saliva and movement of the tongue, lips, and cheeks, for instance, cannot prevent adhesion. Similarly, microbial adhesion on ship hulls and in industrial pipelines occurs despite unfavorable hydrodynamic conditions. This indicates that the forces involved in microbial adhesion to surfaces are quite strong. Microorganisms adhere to surfaces through a combination of attractive Lifshitz-van der Waals forces, attractive or repulsive electrostatic interactions, and acid-base forces (2, 19), acting perpendicular to a substratum surface. Thus, it is theoretically ruled out (Fig. 1) that fluid shear, acting parallel to a substratum surface, could cause microbial detachment from surfaces (3, 19), although in practice detachment due to fluid flow has been observed (19). This detachment could be due to rolling phenomena in combination with minor surface roughnesses and chemical heterogeneities.
FIG. 1.
Hydrodynamic detachment force in the form of lift and shear experienced by a microbial cell due to fluid flow, and surface tension detachment force due to the passing air-liquid interface.
Lift forces due to fluid flow do act perpendicular to a surface but are generally considered too weak to cause microbial detachment. Alternatively, passage of an air-liquid interface over a substratum surface causes detachment forces perpendicular to the substratum surface and thus directly opposing the adhesion forces (Fig. 1). A detailed analysis of the surface tension forces causing this detachment was first given in the field of semiconductors (12, 13), and later the method was successfully used to detach colloidal particles (7, 15) and bacteria (8) from surfaces in a parallel plate flow chamber. Surface tension detachment forces range from 10−9 to 10−7 N, depending on the surfaces involved, and are several orders of magnitude larger than gravitational, buoyancy, and hydrodynamic forces acting on microorganisms (15).
Gómez-Suárez et al. (8) reported that the detachment of the oral bacterial strains Actinomyces naeslundii T14V-J1 and Streptococcus oralis J22 from a glass surface due to passage of a single air-liquid interface is more effective at low velocities and high interfacial tensions. Furthermore, bacterial detachment increased when multiple air-liquid interfaces were passed through the flow chamber. For practical applications, however, it would be more advantageous to apply more stable microbubbles than single air-liquid interfaces to stimulate microbial detachment from substratum surfaces. Oral biofilm removal is generally based on the mechanical action of toothbrush bristles, while surface tension forces arising from passing microbubbles have been suggested to be involved in biofilm removal as well (5, 14).
Therefore, the aim of this study was to determine the efficacy of a continuous flow of microbubbles through a parallel plate flow chamber in terms of bacterial detachment and to compare the detachment stimulated by a similar flow of fluid alone. S. oralis J22, a fibrillated, spherical, hydrophilic (water contact angle, 24 degrees; zeta potential, −16 mV) organism, and A. naeslundii T14V-J1, a fimbriated, rod-shaped, hydrophobic (water contact angle, 64 degrees; zeta potential, −12 mV) organism (8), were used for detachment studies. Since these strains constitute a coaggregating pair, detachment of their coadhering aggregates was determined as well in order to establish the influence of flow with and without microbubbles on detachment of larger microbial entities.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and harvesting.
Initial colonizers of tooth surfaces, manifesting coadhesion and coaggregation (11), were chosen for this study. S. oralis J22 was cultured in Todd-Hewitt broth at 37°C in ambient air. A. naeslundii T14V-J1 was cultured in Schaedler's medium supplemented with 0.01 g liter−1 hemin in an anaerobic cabinet (Concept 400; Ruskinn Technology Limited, Leeds, West Yorkshire, United Kingdom) in an atmosphere of 10% H2, 85% N2, and 5% CO2 at 37°C. Single colonies from blood agar plates were precultured in 10 ml of medium for 24 h. This preculture was taken as an inoculum for the main culture grown for 16 h. The cells were harvested by centrifugation for 5 min at 6,500 × g and washed twice with adhesion buffer (2 mM potassium phosphate, 50 mM potassium chloride, and 1 mM calcium chloride at pH 6.8). To break the bacterial aggregates, the harvested cells were sonicated intermittently, while cooling in ice-water, for 30 s at 30 W (Vibra Cell model 375; Sonics and Materials, Dansbury, CT). Microscopically, this duration and power of sonication were found not to cause any cell lysis.
Microbubble generation and characterization.
Bacterial detachment was stimulated by microbubbles, generated using a Braun Oral B Oxyjet irrigator. This apparatus is commercially available for dental care and mixes air into water. It contains a piston pump which sucks the fluid from a reservoir and pushes it through a nozzle, acquiring air through a venture valve on the way. A rotor present in the nozzle rotates due to the fluid flow and breaks down the air-water mixture into microbubbles. A pressure control dial presents the possibility of controlling the fluid pressure, with settings from 1 (gentle) through 5 (strong). A jet switch, present at the nozzle, makes it possible to switch the rotor on or off. Hence, it is possible to achieve an identical flow with or without microbubbles.
The size distribution of microbubbles and the amount of air suspended in the adhesion buffer were determined using the Malvern Mastersizer S (Malvern Instruments Ltd., Malvern, Worcestershire, United Kingdom). A laser beam diffracts due to the presence of microbubbles in its path. The intensity at a particular angle is an indication of the percentage of microbubbles of a particular size, and larger angles correspond to smaller bubbles. The microbubble size distribution was expressed by the surface area possessed by microbubbles of a particular size relative to the surface area of all the suspended microbubbles. The amount of air in suspension was determined from the attenuation of the laser beam at zero diffraction angle and expressed as percentage obscuration.
Saliva collection and preparation.
Human whole saliva from 10 healthy volunteers of both genders was collected into ice-cooled beakers after stimulation by chewing Parafilm, pooled, centrifuged, dialyzed, and lyophilized for storage. For experiments, lyophilized saliva was dissolved at a concentration of 1.5 g ml−1 in adhesion buffer. All volunteers gave their informed consent to saliva donation, in accordance with the rules set out by the Ethics Committee of the University of Groningen.
Parallel plate flow chamber and adhesion protocol.
A parallel plate flow chamber with unidirectional inlet and flow cavity (1) and with dimensions of 7.5 cm by 0.5 cm by 0.06 cm was used to observe bacterial adhesion on the bottom glass plate. The flow chamber has been previously described in detail by Sjollema et al. (20). The glass slide was cleaned by sonication in a 2% solution of surfactant RBS 35 (Fluka Chemie, Buchs, Switzerland), followed by alternate rinsing with methanol and demineralized water. Adhesion and detachment were observed with a charge-coupled device MXR video camera (High Technology, Eindhoven, The Netherlands) mounted on an Olympus BH-2 phase-contrast microscope equipped with a 40× ultralong-working-distance objective (Olympus ULWD-CD Plan 40 PL). The camera was focused at one field of view (0.016 mm2) and images were recorded continuously to monitor bacterial adhesion, while enumeration of adhering organisms was done by image analysis (TEA; Difa, Breda, The Netherlands).
Adhesion of single strains of A. naeslundii T14V-J1 and S. oralis J22 as well as of the coadhering pair (A. naeslundii and S. oralis) was studied, albeit with slightly different protocols.
For single-strain experiments, bacteria were suspended in adhesion buffer to a density of 6 × 108 ml−1. Before each experiment, all the tubes and the flow chamber were filled with buffer and 5 min of perfusion with buffer was applied. Subsequently, the bacterial suspension was passed through the flow chamber until a surface density of 4 × 106 cm−2 was achieved. The flow was then switched again to buffer in order to remove all the unattached bacteria from the chamber.
For coadhesion, A. naeslundii cells were suspended to a density of 108 ml−1 in adhesion buffer and S. oralis cells were suspended to a density of 3 × 108 ml−1 in adhesion buffer supplemented with 1.5 g liter−1 lyophilized human whole saliva. After rinsing of the tubes and flow chamber with buffer for 5 min, a suspension with A. naeslundii was passed through the chamber until a surface coverage of 106 ml−1 was obtained as determined using the image analyzer. Then, flow was again switched to buffer to remove unattached bacteria and coadhesion was initiated by switching the flow to S. oralis suspension for 2 h, yielding adhesion of aggregates differing in size and containing up to 10 or more bacteria per adhering coaggregate.
For adhesion experiments, all the suspensions perfused through the flow chamber at a hydrostatic pressure wall shear rate of 83 s−1 while being recirculated by a roller pump to maintain a constant fluid level in the inlet reservoir.
Detachment protocol.
The nozzle of the microbubble generator was inserted in the upstream tubing of the flow chamber and bubbles, created in adhesion buffer, were taken with the flow into the flow chamber. Out of five possible settings of the Braun Oral B Oxyjet irrigator, only settings 1, 3, and 5, with fluid flow rates of 200, 235, and 300 ml min−1, respectively, were used for detachment experiments, corresponding to Reynolds numbers of 595, 698, and 893, indicating laminar flow and yielding wall shear rates of 11,000, 13,000 and 16,000 s−1, respectively. The detachment experiments were performed with and without microbubbles in the flow, in order to separately illustrate the effects of fluid shear and microbubbles.
Images were recorded at three different areas on the glass surface immediately after adhesion. Fluid flow with or without microbubbles was then introduced to initiate detachment for 2 min. Fluid flow was stopped in order to record images at three different spots and detachment was subsequently continued for another 8 min.
For single-strain experiments, the total number of bacteria in an image were counted and expressed in numbers of bacteria adhering per unit area (bacteria cm−2). Efficacy of the detachment was represented in terms of % removal, which was the percentage of bacteria detached with respect to the initial number of bacteria adhering.
For coadhering aggregates, the number of aggregates of different sizes was counted before and after detachment and expressed per cm−2. Subsequently, their detachment efficacy was expressed in terms of % removal of aggregates of a specific size, as defined above.
All experiments were carried out in triplicate with separately cultured bacteria.
RESULTS
Microbubble characteristics.
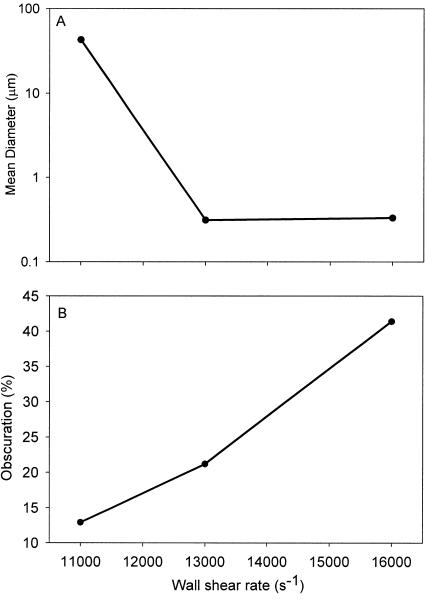
Figure 2A shows an increase in the mean diameter of the microbubbles as the wall shear rate decreases. At wall shear rates of 16,000 and 13,000 s−1 the mean diameter is less than a micrometer, whereas at a wall shear rate of 11,000 s−1 the mean diameter exceeds 10 μm. Figure 2B shows the amount of air in suspension as a function of fluid shear. The amount of air is related to percentage obscuration, i.e., the obstruction offered by the microbubbles to the laser light. The amount of air in suspension increased almost linearly with the fluid flow rate, indicating that more air was suspended in the form of smaller microbubbles with increasing fluid flow.
FIG. 2.
Microbubble characteristics. (A) Mean diameter of the microbubbles as a function of the wall shear rate; (B) amount of air in suspension (percent obscuration) in the form of microbubbles as a function of the wall shear rate.
Adhesion.
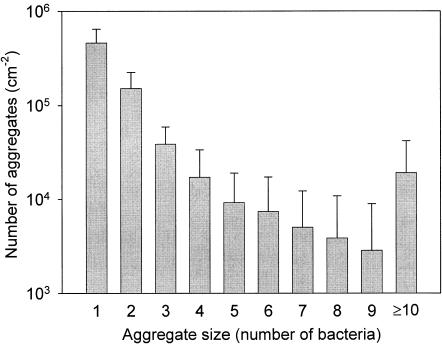
Prior to detachment in the single-strain studies, about 4 × 106 cm−2 A. naeslundii T14V-J1 or S. oralis J22 cells were allowed to adhere on the glass surface, while for coadhesion on average 1.7 × 106 cm−2 bacteria were allowed to adhere, of which 106 cm−2 were A. naeslundii. Figure 3 shows the number distribution for the coadhering aggregates, indicating that their surface density decreases as their size (in terms of number of bacteria) increases.
FIG. 3.
Distribution of aggregate sizes, involving A. naeslundii T14V-J1 and S. oralis J22, coadhering on glass prior to detachment. Error bars denote standard deviation over 18 separate experiments.
Detachment.
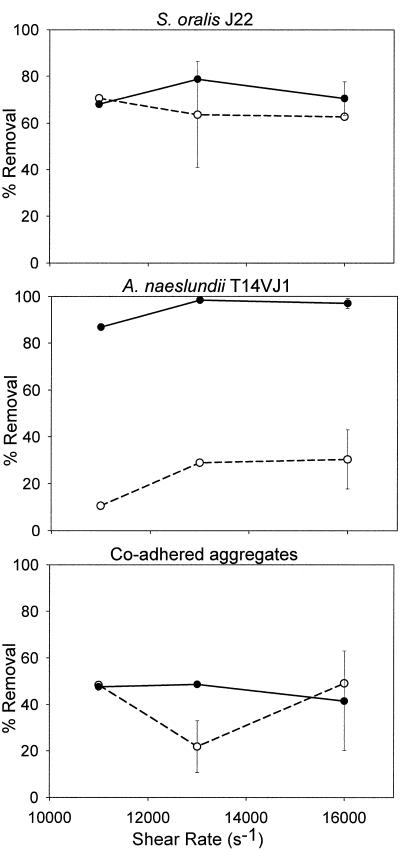
Figure 4 summarizes bacterial detachment from glass after 2 min due to increasing wall shear rate in the presence and absence of microbubbles for the single strains and coadhering aggregates. After 2 min, 60 to 70% of the adhering S. oralis cells were stimulated to detach by the fluid flow, with a minor influence of wall shear rate, while slightly more bacteria (70 to 80%) were removed in the presence of the microbubbles. The detachment of A. naeslundii was strongly dependent on the presence of microbubbles. Only 10 to 30% of the adhering bacteria were removed by fluid flow alone, whereas 85 to 98% of the adhering bacteria were removed in the presence of the microbubbles. The detachment of A. naeslundii increased with increasing wall shear rate up to 13,000 s−1. Fluid flow, on average, was able to detach 50% of the coadhering aggregates, with little or no effect of the presence of microbubbles.
FIG. 4.
Detachment of adhering bacteria and aggregates from glass after 2 min, as stimulated by different wall shear rates in the absence (open symbols with dashed line) or presence (solid symbols with continuous line) of microbubbles. Error bars denote standard deviation over nine separate experiments.
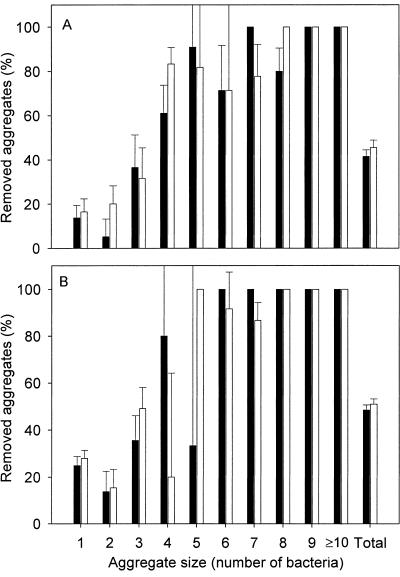
Figure 5 shows that aggregates containing four bacteria or more are most efficiently (>80%) detached, whereas a maximum of 45% detachment is seen for aggregates containing fewer than four bacteria, irrespective of the flow rate used or the presence of microbubbles.
FIG. 5.
Percent removal of aggregates from the glass surface after 2 min (solid bars) and after 10 min (open bars) at a wall shear rate of 16,000 s−1 with microbubbles (A) and at 11,000 s−1 without microbubbles (B) as a function of aggregate size. Error bars denote standard deviations over nine fields of view, equally divided over three separate experiments.
Bacterial detachment occurred predominantly within the first 2 min and increased only slightly over time. In fact, after an additional 8 min, detachment of both S. oralis and A. naeslundii increased, on average, by another 10%, irrespective of the presence or absence of microbubbles.
DISCUSSION
The efficacy of microbial detachment from a substratum surface depends on the magnitude and direction of the detachment forces compared with the adhesive forces between an organism and the substratum surface. In this study, S. oralis J22 could be effectively detached by fluid flow alone, but A. naeslundii T14V-J1 could only be effectively detached in the presence of microbubbles. Since atomic force microscopy has recently indicated (16, 22) that the cohesive forces between different bacteria holding together a coaggregate are generally larger than those mediating their adhesion to a substratum, it is likely to be valid to consider an adhering coaggregate as one bacterial entity of larger size. Detachment of these larger bacterial entities was not stimulated by the presence of microbubbles.
Theoretically, increased fluid flow towards or parallel to a substratum surface results in faster adhesion of microorganisms due to higher mass transport (21). However, when fluid flow exceeds a critical limit, the resulting wall shear rates may become high enough to prevent adhesion or even stimulate detachment (3). For instance, in aqueous suspensions, wall shear rates of 6,000 to 8,000 s−1 were sufficient to prevent adhesion of Pseudomonas fluorescens to stainless steel while a wall shear rate of 12,000 s−1 could detach adhering organisms (19).
The present study demonstrates that fluid shear rates of 11,000 to 16,000 s−1 are effective in detaching a hydrophilic streptococcus but not in detaching a more hydrophobic actinomycete. Since the zeta potentials of both strains are almost identical, the more tenacious adhesion of the actinomycetes can be attributed to their higher hydrophobicity, among other differences between the strains. Thus, cell surface hydrophobicity becomes a factor in bacterial detachment from surfaces as well, in addition to being a known factor in bacterial adhesion (23). Note that this does not rule out a contribution of different cell surface appendages such as fibrils and fimbriae to detachment, as exactly these structures convey the charge and hydrophobic properties to the cell surface through their specific chemistry.
Christersson et al. (3) reported detachment of 70 to 80% of coccoid and rod-shaped oral bacteria from a glass surface with a salivary conditioning film at wall shear rates of 3 to 100 s−1 without observing a difference in the adhesion tenacity of the two types of strains. Although the detachment in the present study was performed at much higher shear rates (11,000 to 16,000 s−1) and from a bare glass surface, a clear difference is seen between A. naeslundii T14V-J1 and S. oralis J22. About 23% of A. naeslundii cells were removed, compared to 66% of S. oralis cells, within 2 min due to fluid shear. This difference in detachment characteristics observed by Christersson et al. (3) and the present study could be a combined effect of differences in fluid shear rates used, absence of a salivary conditioning film in the present study, and usage of the two bacterial species together by Christersson et al. (3), whereas in the present study their detachment characteristics were studied separately.
The efficiency with which microbubbles remove attached bacteria depends upon their collision probability, attachment efficiency, and stability efficiency of the bubble-bacteria aggregate (10). The probability of collision of microbubbles with bacterial cells or entities depends on the cross-sectional area exposed to the flow and is evidently larger for adhering coaggregates than for single bacteria. In the studies by Gómez-Suárez et al. (7-9), the collision efficiency was invariably 1, because air-liquid interfaces fully spanning the width of their parallel plate flow chamber were used. For microbubbles the situation is different, however. For a microbubble to detach an adhering organism, the following events must occur (10): thinning of the liquid film between the bacterial cell and bubble, rupture and formation of a three-phase boundary (9), and expansion of the three-phase boundary to form a stable wetting perimeter. Since both events occur more readily for the hydrophobic A. naeslundii than for the hydrophilic S. oralis, the presence of microbubbles greatly stimulates detachment of tenaciously adhering A. naeslundii cells.
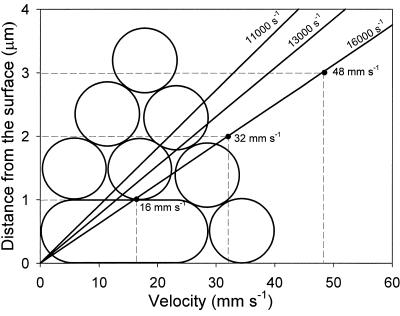
Once the three-phase boundary is formed, the velocity of the interface becomes a determinant for the detachment efficiency, and faster-moving interfaces have been shown to be less effective. Figure 6 presents the velocity profiles above the glass surface at the three fluid flow rates used and the maximum velocity of the fluid carrying the microbubbles over the top of a single adhering bacterium as well as over adhering coaggregates. Larger aggregates experience faster fluid flow than smaller aggregates due to the parabolic flow profile between two parallel plates, and hence fluid flow effectively detaches larger adhering coaggregates. Alternatively, due to the faster fluid flow, the additional detachment of adhering coaggregates as stimulated by microbubbles is less, explaining the observations in Fig. 5.
FIG. 6.
Velocity of the fluid and microbubbles passing over the top of single bacteria and coaggregates of different heights (dashed lines) as a function of distance from the surface. Solid lines show the fluid velocity profile according to parabolic Poiseuille flow at three different shear rates.
Conclusions.
This study demonstrates that high fluid shear rates (11,000 to 16,000 s−1) can be effective in stimulating bacterial detachment, depending on the strain involved. The addition of microbubbles to the flow allows the detachment of tenaciously adhering bacteria not detached by flow alone. Microbubbles become ineffective for larger bacterial entities such as adhering coaggregates. The principles outlined are expected to have general validity for bacterial detachment from surfaces by fluid flow and passing microbubbles.
Acknowledgments
We thank Roy le Clercq and Bart Gottenbos, Philips Research Laboratory, Eindhoven, The Netherlands, for the microbubble size analysis.
REFERENCES
- 1.Bakker, D. P., A. van der Plaats, G. J. Verkerke, H. J. Busscher, and H. C. van der Mei. 2003. Comparison of velocity profiles for different flow chamber designs used in studies of microbial adhesion to surfaces. Appl. Environ. Microbiol. 69:6280-6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos, R., H. C. van der Mei, and H. J. Busscher. 1999. Physico-chemistry of initial microbial adhesive interactions-its mechanisms and methods for study. FEMS Microbiol. Rev. 23:179-230. [DOI] [PubMed] [Google Scholar]
- 3.Christersson, C. E., P.-O. J. Glantz, and R. E. Baier. 1988. Role of temperature and shear forces on microbial detachment. Scand. J. Dent. Res. 96:91-98. [DOI] [PubMed] [Google Scholar]
- 4.Escher, A., and W. G. Characklis. 1990. Modeling the initial events in biofilm accumulation. p. 445-486. In W. G. Characklis and K. C. Marshall (ed.), Biofilms. John Wiley and Sons, New York, NY.
- 5.Farcella, J. A., P. Fernandez, R. D. Gilbert, and M. Cugini. 2000. A randomized, clinical evaluation of the safety and efficacy of a novel oral irrigator. Am. J. Dent. 13:55-58. [PubMed] [Google Scholar]
- 6.Gibbons, R. J., and M. Nygaard. 1970. Interbacterial aggregation of plaque bacteria. Arch. Oral Biol. 15:1397-1400. [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Suárez, C., J. Noordmans, H. C. van der Mei, and H. J. Busscher. 1999. Removal of colloidal particles from quartz collector surfaces as stimulated by the passage of liquid-air interface. Langmuir 15:5123-5127. [Google Scholar]
- 8.Gómez-Suárez, C., H. J. Busscher, and H. C. van der Mei. 2001. Analysis of bacterial detachment from substrate surfaces by the passage of air-liquid interface. Appl. Environ. Microbiol. 67:2531-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gómez-Suárez, C., H. C. van der Mei, and H. J. Busscher. 2001. Air-bubble induced detachment of polystyrene particles with different size from collector surface in a parallel plate flow chamber. Coll. Surf. A 186:211-219. [Google Scholar]
- 10.Hewitt, D., D. Fornasiero, and J. Raltson. 1995. Bubble-particle attachment. J. Chem. Soc. Faraday Trans. 91:1997-2001. [Google Scholar]
- 11.Kolenbrander, P. E. 1988. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu. Rev. Microbiol. 42:627-656. [DOI] [PubMed] [Google Scholar]
- 12.Leenaars, A. F. M. 1988. A new approach to the removal of sub-micron particles from solid (silicon) substrates. p. 361-372 In K. L. Mittal (ed.) Particles on surfaces 1: detection, adhesion and removal, Plenum Press, New York, N.Y.
- 13.Leenaars, A. F. M., and S. B. G. O'Brien. 1989. Particle removal from silicon substrates using surface tension forces. Philips J. Res. 44:183-209. [Google Scholar]
- 14.McInnes, C., and J. W. Pace. 2002. Designing the next generation of a sonic toothbrush. Am. J. Dent. 15:4B-6B. [PubMed] [Google Scholar]
- 15.Noordmans, J., P. J. Wit, H. C. van der Mei, and H. J. Busscher. 1997. Detachment of polystyrene particles from collector surfaces by surface tension forces induced by air-bubble passage through a parallel plate flow chamber. J. Adhesion Sci. Technol. 11:957-969. [Google Scholar]
- 16.Postollec, F., W. Norde, J. de Vries, H. J. Busscher, and H. C. van der Mei. Interaction forces between coaggregating and non-coaggregating oral bacterial pairs. Submitted for publication.
- 17.Reid, G. 1988. Lactobacillus inhibitor production against Escherichia coli and coaggregation ability with uropathogens. Can. J. Microbiol. 34:344-351. [DOI] [PubMed] [Google Scholar]
- 18.Rickard, A. H., P. Gilbert, N. J. High, P. E. Kolenbrander, and P. S. Handley. 2003. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 11:94-100. [DOI] [PubMed] [Google Scholar]
- 19.Rutter, P. R., and B. Vincent. 1988. Attachment mechanisms in the surface growth of microorganisms, p. 87-107 In M. J. Bazin and J. I. Prosser (ed.) Physiological models in microbiology, vol. II. CRC Press, Boca Raton, Fla. [Google Scholar]
- 20.Sjollema, J., H. J. Busscher, and A. H. Weerkamp. 1989. Experimental approaches for studying adhesion of microorganisms to solid substrate: application and mass transport. J. Microbiol. Methods 9:79-90. [Google Scholar]
- 21.Sjollema, J., and H. J. Busscher. 1989. Deposition of polystyrene latex particles towards polymethylmetacrylate in a parallel plate flow chamber. J. Colloid Interface Sci. 132:382-394. [Google Scholar]
- 22.Van Hoogmoed, C. G., R. J. B. Dijkstra, H. C. van der Mei, and H. J. Busscher. Influence of biosurfactant on interaction forces between mutant streptococci and enamel measured by AFM. Submitted for publication. [DOI] [PubMed]
- 23.Van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. B. Zehnder. 1987. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl. Environ. Microbiol. 53:1898-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]