Abstract
We have recently developed a gene disruption system for the hyperthermophilic archaeon Thermococcus kodakaraensis by utilizing a pyrF-deficient mutant, KU25, as a host strain and the pyrF gene as a selectable marker. To achieve multiple genetic manipulations for more advanced functional analyses of genes in vivo, it is necessary to establish multiple host-marker systems or to develop a system in which repeated utilization of one marker gene is possible. In this study, we first constructed a new host strain, KU216 (ΔpyrF), by specific and almost complete deletion of endogenous pyrF through homologous recombination. In this refined host, there is no need to consider unknown mutations caused by random mutagenesis, and unlike in the previous host, KU25, there is little, if any, possibility that unintended recombination between the marker gene and the chromosomal allele occurs. Furthermore, a new host-marker combination of a trpE deletant, KW128 (ΔpyrF ΔtrpE::pyrF), and the trpE gene was developed. This system made it possible to isolate transformants through a more simple selection procedure as well as to deduce the transformation efficiency, overcoming practical disadvantages of the first system. The effects of the transformation conditions were also investigated using this system. Finally, we have also established a system in which repeated utilization of the counterselectable pyrF marker is possible through its excision by pop-out recombination. Both endogenous and exogenous sequences could be applied as tandem repeats flanking the marker pyrF for pop-out recombination. A double deletion mutant, KUW1 (ΔpyrF ΔtrpE), constructed with the pop-out strategy, was demonstrated to be a useful host for the dual markers pyrF and trpE. Likewise, a triple deletion mutant, KUWH1 (ΔpyrF ΔtrpE ΔhisD), could also be constructed. The transformation systems developed here now provide the means for extensive genetic studies in this hyperthermophilic archaeon.
Recent phylogenetic analysis of living organisms based on rRNA sequences has indicated that hyperthermophiles occupy the deepest and shortest branches in the phylogenetic tree, postulating that the origin and evolution of biological systems may have derived from hyperthermophiles (25). Studies on the unique properties of hyperthermophiles are expected to provide valuable perspectives on the mechanisms that enable them to survive and grow in extreme environments (26). They are also important as potential resources for highly thermostable enzymes (2, 28). Many members of hyperthermophiles belong to the third domain of life, Archaea, along with halophiles and methanogens. Archaea exhibit a mosaic of features from the other two domains, Bacteria and Eucarya; intriguingly, their components for information processing are more closely related to those in eucaryotes than those in bacteria. From these interests, genome projects of various types of hyperthermophilic archaea have been performed, and complete genome sequences of more than 10 species have been determined. The genomes of hyperthermophilic archaea are rather small, and consequently, they are predicted to possess simplified versions of various biological machineries and metabolisms composed of small sets of genes. This is a great advantage for elucidating the functions of genes identified by genome analyses, and accumulation of this information may ultimately help to understand the basic principles of life.
The progress of research on hyperthermophilic archaea had been constantly hampered by the limitation of available tools for genetic manipulation. In contrast to several genetic methodologies for mesophilic archaea, halophiles (18, 23), and methanogens (12, 23), which are comparable to those for bacteria, manipulative strategies for hyperthermophilic archaea are still at an early stage. In particular, the development of targeted gene disruption in hyperthermophiles had not been achieved, despite the establishment of shuttle vector systems for several strains in the genera Sulfolobus and Pyrococcus (1, 4, 9, 24). As gene disruption is a powerful and direct tool for investigation of in vivo gene functions, development of a gene targeting system would certainly be a major breakthrough in the research on hyperthermophilic archaea.
From this viewpoint, we have recently developed a gene targeting system for Thermococcus kodakaraensis (21), a sulfur-reducing hyperthermophilic archaeon belonging to Thermococcales in Euryarchaeota (5, 16). We successfully disrupted the trpE gene in T. kodakaraensis by homologous recombination utilizing a pyrF-deficient mutant and the pyrF gene as a host strain and a selectable marker, respectively, the first example in hyperthermophiles (21). Gene disruption in the thermoacidophilic archaeon Sulfolobus solfataricus using a lacS marker has also been developed (29). However, in both methods, only one selectable marker gene was available.
In this report, we describe the improvement of the transformation system using the pyrF marker along with the establishment of a new host-marker combination consisting of a trpE deletant and the trpE gene. Moreover, repeated utilization of the pyrF marker was made possible by pop-out excision of the counterselectable marker to achieve multiple genetic manipulations for more advanced functional analyses of genes in vivo.
MATERIALS AND METHODS
Strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. T. kodakaraensis KOD1 and its derivatives were cultivated under strictly anaerobic conditions at 85°C in a rich growth medium (ASW-YT) or a synthetic medium (ASW-AA) (22). The preparation of plate medium and cultivation of the cells on it were performed as described previously (21). Further modifications of the medium for investigation of auxotrophy of mutant strains and selection of transformants are described in the text.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | supE44 ΔlacU169 (Φ80 lacZ ΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relAI | Stratagene (La Jolla, CA) |
| T. kodakaraensis | ||
| KOD1 | Wild type | 5, 16 |
| KU216 | ΔpyrF | This study |
| KW128 | KU216 ΔtrpE::pyrF | This study |
| KH3 | KW128 ΔhisD::trpE | This study |
| KuW1 | KU216 ΔtrpE::3′ region of trpE-pyrF | This study |
| KUW1 | ΔpyrF ΔtrpE | This study |
| KuWH1 | KUW1 ΔhisD::3′ region of hisD-pyrF | This study |
| KUWH1 | ΔpyrF ΔtrpE ΔhisD | This study |
| KuWc1 | KU216 ΔtrpE::2μ′=pyrF-2μ′ | This study |
| KUWc1 | ΔpyrF ΔtrpE::2μ′ | This study |
| KuWcHc1 | KUWc1 ΔhisD::2μ′-pyrF-2μ′ | This study |
| KUWcHc1 | ΔpyrF ΔtrpE::2μ′ ΔhisD::2μ′ | This study |
| Plasmids | ||
| pUC118 | Ampr general cloning vector | Takara Bio (Ohtsu, Japan) |
| pUD2 | pUC118 derivative, pyrF marker cassette (PpyrF::pyrF) | This study |
| pUMT2 | pUC118 derivative; trpE marker cassette (PpyrF::trpE) | 22 |
| pUCMP | pUC118 derivative; 2μ′-pyrF-2μ′ | This study |
| pUDPyrF | pUC118 derivative; ΔpyrF | This study |
| pUDTrpE | pUC118 derivative; ΔtrpE::pyrF | This study |
| pUDHisD | pUC118 derivative; ΔhisD::trpE | This study |
| pUDLysV | pUC118 derivative; ΔlysV::trpE | This study |
| pUDTPOP | pUC118 derivative; ΔtrpE::3′ region of trpE-pyrF | This study |
| pUDHPOP | pUC118 derivative; ΔhisD::3′ region of hisD-pyrF | This study |
| pYES2 | General expression vector for Saccharomyces cerevisiae | Invitrogen (Carlsbad, CA) |
| pUDTPOPC | pUC118 derivative; ΔtrpE::2μ′-pyrF-2μ′ | This study |
| pUDHPOPC | pUC118 derivative; ΔhisD::2μ′-pyrF-2μ′ | This study |
Escherichia coli strain DH5α, used for general DNA manipulation, was routinely cultivated at 37°C in Luria-Bertani (LB) medium (19) and supplemented with 50 μg/ml ampicillin when needed.
General DNA manipulation.
General DNA manipulation was performed as described previously by Sambrook and Russell (19). Genomic DNA of T. kodakaraensis was isolated as described previously (21). PCR was carried out using KOD -Plus- (Toyobo, Osaka, Japan) as a DNA polymerase, and sequences of primers used for PCR in this study are available upon request. When necessary, DNA fragments amplified by PCR were phosphorylated by T4 kinase (Toyobo). Restriction enzymes and modifying enzymes were purchased from Takara Bio (Ohtsu, Japan) or Toyobo. DNA fragments after agarose gel electrophoresis were recovered and purified with GFX PCR DNA and Gel Band Purification kit (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom). Plasmid DNA was isolated using QIAGEN (Hilden, Germany) plasmid kits. DNA sequencing was performed using a BigDye Terminator Cycle Sequencing kit, version 3.0, and a model 3100 capillary sequencer (Applied Biosystems, Foster City, CA).
Construction of disruption vectors.
Construction of marker cassettes and several disruption vectors was carried out as described below. A pyrF marker cassette was constructed by amplification of the putative pyrF promoter-pyrF gene fusion in the plasmid pUD (21) with primers PJPRO-R2/PJPYR-F2, each containing a PvuII restriction site, followed by insertion into pUC118 at the HincII site. From the resulting plasmid, pUD2, the PvuII restriction fragment (763 bp) for use as the marker cassette. Construction of the trpE marker cassette, the putative pyrF promoter-trpE gene fusion flanked by PvuII sites (1,423 bp), has been described previously (22).
Four vectors for disruption of pyrF, trpE, hisD, and lysV genes in T. kodakaraensis through double-crossover homologous recombination (pUDPyrF, pUDTrpE, pUDHisD, and pUDLysV, respectively) were constructed as follows. Four DNA fragments containing the respective target gene together with its flanking regions (about 1,000 bp, except for 732 bp for the 5′-flanking region of pyrF) were amplified from T. kodakaraensis KOD1 genomic DNA using the primer sets PPYR-R/PPYR-F, PTRP-R/PTRP-F, PHISD-R/PHISD-F, and PLYSV-R/PLYSV-F for pUDPyrF, pUDTrpE, pUDHisD, and pUDLysV, respectively. Each amplified DNA fragment was subcloned into pUC118 at the HincII site. The flanking regions of the target gene and the plasmid backbone, excluding the target gene, were then amplified from the respective plasmids using primers PDPYR-R/PDPYR-F, PDTRP-R/PDTRP-F, PDHISD-R/PDHISD-F, and PDLYSV-R/PDLYSV-F, respectively, and the resulting DNA fragments were designated L-PyrF, L-TrpE, L-HisD, and L-LysV, respectively. pUDPyrF (5,010 bp, markerless) (Fig. 1) was obtained by self-ligation of L-PyrF after 5′ phosphorylation of both ends. pUDTrpE (5,925 bp, pyrF marker) (Fig. 1), pUDHisD (6,585 bp, trpE marker) (Fig. 1), and pUDLysV (6,585 bp, trpE marker) were constructed by ligation of the corresponding marker cassette with the fragments L-TrpE, L-HisD, and L-LysV, respectively, with the markers oriented in the same direction as those of the target genes. All plasmids were designed so that upstream and downstream genes, which in some cases overlapped the target gene, were left intact. We also took care not to remove the ribosome-binding sites of downstream genes in order to avoid disturbing their translation. Therefore, in the present study, 5′ regions of trpE (19 bp) and hisD (14 bp) and 3′ regions of pyrF (21 bp) and trpE (8 bp) were preserved after recombination.
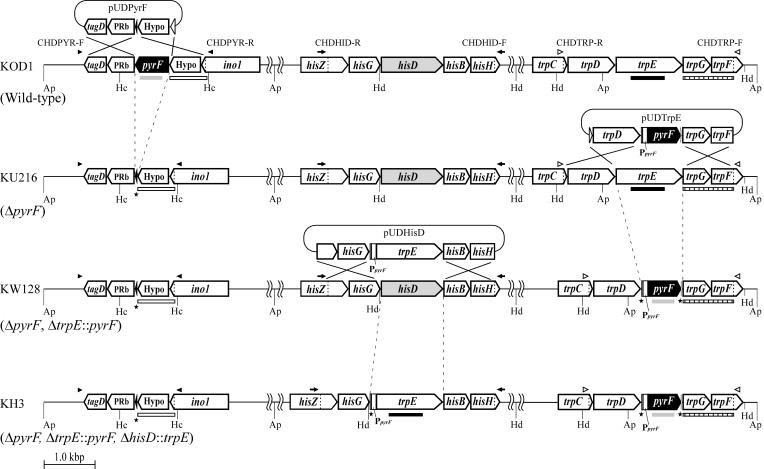
FIG. 1.
Schematic diagram of targeted disruption of pyrF, trpE, and hisD in T. kodakaraensis KOD1, KU216, and KW128 using pUDPyrF, pUDTrpE, and pUDHisD, respectively. Relevant regions of the chromosome are illustrated for (from the top) strains KOD1, KU216, KW128, and KH3. The positions of primer sets used for analyses of targeted disruption of pyrF (CHDPYR-F/CHDPYR-R, closed arrowheads), trpE (CHDTRP-R/CHDTRP-F, open arrowheads), and hisD (CHDHID-R/CHDHID-F, closed arrows) are indicated. The gray, closed, open, and striped boldface bars indicate each region spanned by the pyrF, trpE, pyrF upstream, and trpE downstream probes used in Southern blot analyses, respectively. PpyrF indicates the putative promoter region of the operon containing pyrF. Black stars indicate regions of target genes that were left intact in order to avoid disturbing nearby genes as described in Materials and Methods. Gene name abbreviations: Hypo, hypothetical gene; ino1, myo-inositol-1-phosphate synthase; PRb, predicted RNA-binding protein; tagD, cytidylyltransferase. Restriction site abbreviations: Ap, ApaI; Hc, HincII; Hd, HindIII.
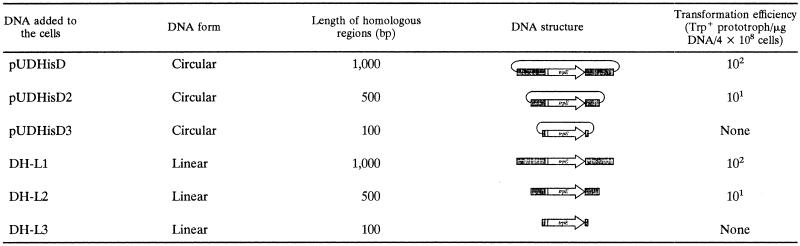
To examine the effect of the lengths of the homologous regions on recombination efficiency at the hisD locus, three linear DNA fragments, DH-L1, DH-L2, and DH-L3, with 1,000 bp, 500 bp, and 100 bp of homologous regions, were amplified from pUDHisD using the primer sets HD-1000R/HD-1000F, HD-500R/HD-500F, and HD-100R/HD-100F, respectively. The fragments DH-L2 and DH-L3 were inserted into pUC118 at the HincII site to obtain the circular disruption plasmids pUDHisD2 and pUDHisD3 harboring 500 bp and 100 bp of homologous regions, respectively.
Construction of pop-out vectors for repeated utilization of pyrF marker.
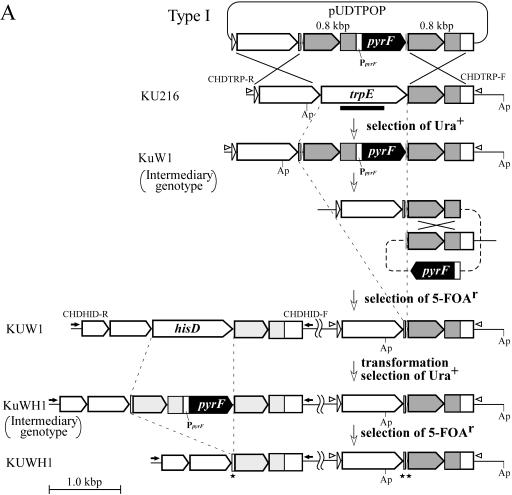
Two types of disruption vectors with tandem repeat regions flanking the pyrF gene were constructed for gene disruption and subsequent excision of the pyrF marker by pop-out recombination. Type I vectors harbor an additional copy of the 3′-flanking region of the respective target genes positioned upstream of the pyrF marker. After the first double-crossover homologous recombination, this additional region leads to a structure in which the marker gene is flanked by two tandem homologous regions. Approximately 0.8 kbp of the 3′-flanking regions of trpE and hisD genes was amplified from T. kodakaraensis genomic DNA using primers DPOPT-R/DPOPT-F and DPOPH-R/DPOPH-F, respectively. The DPOPT-R and DPOPH-R primers and the DPOPT-F and DPOPH-F primers contain SphI and PstI sites, respectively, outside the annealing sequences. Each amplified DNA fragment was digested with SphI and PstI and inserted into pUD2 at the corresponding sites. From each resulting plasmid, the pyrF marker cassette adjacent to the 3′ region of the target gene was excised by HindIII and XbaI digestion, blunted by Blunting High (Toyobo), and then ligated with L-TrpE and L-HisD, respectively. The plasmids harboring the duplicated 3′ regions of trpE and hisD in the same direction were selected to obtain pUDTPOP (see Fig. 4A) and pUDHPOP, respectively.
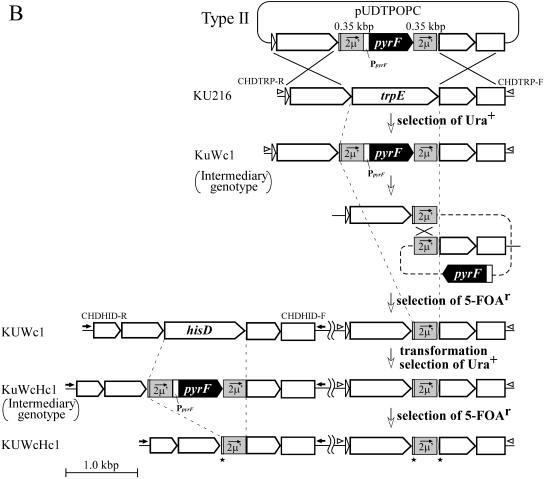
FIG. 4.
Schematic diagram of sequential disruption of trpE and hisD through excision of the pyrF marker by pop-out recombination. (A) Construction of strains KUW1 (ΔpyrF ΔtrpE) and KUWH1 (ΔpyrF ΔtrpE ΔhisD) using type I pop-out vectors harboring tandem repeats of the endogenous 3′ region of the target gene flanking pyrF on both sides. (B) Construction of strains KUWc1 and KUWcHc1 using type II pop-out vectors harboring tandem repeats of the exogenous 2μ′ region flanking pyrF on both sides. The regions shaded in gray indicate the tandem repeat regions in each strategy. Open arrowheads and closed arrows indicate primer sets CHDTRP-R/CHDTRP-F and CHDHID-R/CHDHID-F for analyses of targeted disruption of trpE and hisD, respectively. Restriction site abbreviation: Ap, ApaI. All genes adjacent to the target genes are the same as those mentioned in the legend of Fig. 1.
In type II vectors, we applied a nucleotide sequence derived from exogenous DNA, a portion of the 2μ region (designated as 2μ′) in the yeast-E. coli shuttle vector pYES2, as the tandem repeat region. Two DNA fragments, 0.35 kbp of nucleotide sequences in the 2μ region in pYES2, were amplified using primer sets POPCR-R/POPCR-F, containing XbaI and EcoRI-SmaI sites, respectively, and POPCL-R/POPCL-F, containing SphI-SmaI and PstI sites, respectively. The former and latter DNA fragments were digested with XbaI/EcoRI and Sph1/ PstI, respectively, and then inserted into the corresponding sites in pUD2. The resulting plasmid harboring the pyrF marker flanked by tandem repeats of 2μ′ regions was named pUCMP. The 2μ′-pyrF-2μ′ region can be excised from pUCMP by SmaI as a universal marker cassette for pop-out recombination of the pyrF marker and was used to construct pUDTPOPC for trpE disruption (see Fig. 4B) and pUDHPOPC for hisD disruption by ligation with the fragments L-TrpE and L-HisD, respectively.
Transformation of T. kodakaraensis.
All steps involved in the genetic manipulation of T. kodakaraensis were performed under anaerobic conditions with the exception of centrifugation for cell harvesting. In the case of transformation utilizing the pyrF marker, the CaCl2 method was applied as described previously (21). The pyrF+ strains with uracil prototrophy were selected by cultivation twice in ASW-AA liquid medium prior to the plate cultivation due to the lack of strictness in uracil auxotrophy of the pyrF host on plate medium.
With trpE as a selectable marker, transformation was performed as follows. After cultivation of the host strain in ASW-YT liquid medium, approximately 4 × 108 cells at the late exponential phase were harvested (17,000 × g, 5 min), resuspended in 200 μl of 0.8× ASW medium, and kept on ice for 30 min. Three micrograms of DNA was added into the suspension, and the cells were incubated on ice for 1 h, followed by a heat shock at 85°C for 45 s and further incubation on ice for 10 min. A modified ASW-YT liquid medium (1.3 ml containing 2.0 ml/liter of polysulfide solution [21] instead of elemental sulfur) was added to the transformed cells, and the suspension was incubated at 85°C for 2 h for outgrowth. The cells were then harvested (17,000 × g, 5 min), resuspended in 200 μl of 0.8× ASW, and directly spread onto a selective synthetic ASW-AA plate not containing tryptophan (ASW-AAW−). After cultivation for 5 to 8 days at 85°C, the transformants grown on the plate medium, tryptophan prototrophs, were isolated. Transformation efficiencies were determined by counting colony numbers of tryptophan prototrophs, and all values mentioned in the text are averages of the results of three independent experiments.
Genotypes of transformants obtained in this study were analyzed by PCR using primer sets that anneal outside of the homologous regions, by sequencing of the targeted regions, and/or by Southern hybridization (see below). When necessary, colonies of candidates grown on the selective plate medium were analyzed by colony PCR.
Positive selection of pyrF-deleted strains with 5-FOA.
A pyrF deletion mutant, KU216, and several pyrF pop-out recombinants were positively selected on a synthetic plate medium supplemented with 0.75% 5-fluoroorotic acid (5-FOA) (Wako Pure Chemicals, Osaka, Japan) (8) and 10 μg/ml uracil (Kohjin, Tokyo, Japan) (ASW-AA-FU). In construction of the ΔpyrF strain KU216, T. kodakaraensis KOD1 was cultivated twice in a uracil-free ASW-AA medium to repress growth of spontaneous mutants deficient in pyrF and/or pyrE genes as well as to avoid carryover of uracil. The wild-type cells at the late exponential phase were harvested and transformed with pUDPyrF by the CaCl2 procedure (21). The further transformed cells were grown in ASW-AA liquid medium supplemented with 10 μg/ml uracil to allow the generation of pyrF deletion mutants. Cultures were then spread onto ASW-AA-FU plate medium, and the 5-FOA-resistant mutants were isolated and analyzed.
For repeated utilization of the pyrF marker, excision of the counterselectable pyrF marker inserted within the target locus on the chromosome was achieved through pop-out recombination between tandem repeats flanking the pyrF marker. T. kodakaraensis KU216 was transformed with a pop-out vector, pUDTPOP or pUDTPOPC, by the CaCl2 method. A desired pyrF+ strain was isolated, and the cells grown in 20 ml of ASW-YT medium were inoculated onto ASW-AA-FU plate medium with an appropriate cell density. The resulting 5-FOA-resistant mutants were isolated and analyzed. Frequencies for generation of 5-FOA-resistant mutants were determined by counting colony numbers on ASW-AA-FU plate medium and colony numbers on that without 5-FOA, and all values mentioned in the text are averages of the results of three independent experiments. The double mutants with deletion of both pyrF and trpE were further transformed with pUDHPOP and pUDHPOPC for disruption of the hisD gene to demonstrate further repeated utilization of pyrF marker, according to the procedures mentioned above.
Hybridization analyses.
Southern blot analyses were carried out with 5.0 μg of genomic DNAs digested with appropriate restriction enzymes, and the overall procedures were performed as described previously (22). The primer sets used for preparation of pyrF upstream, pyrF, and trpE downstream probes shown in Fig. 1 were PPYRF-R/PDPYRF-F, PROPYR-R/PROPYR-F, and PDTRP-R/PTRP-F, respectively. The trpE probe was prepared as described previously (22).
RESULTS
Construction of the pyrF deletion mutant.
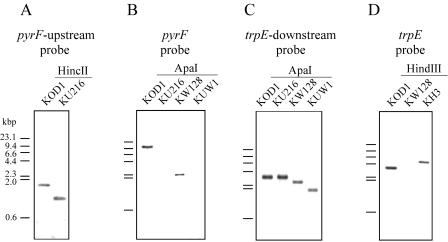
We previously isolated a uracil-auxotrophic PyrF-deficient mutant, KU25, of T. kodakaraensis by UV mutagenesis and then constructed a gene targeting system utilizing this strain and the pyrF gene as a host and a selectable marker, respectively (21). However, the possibility remained that various mutations induced by UV irradiation were present in other genes on the KU25 chromosome. In addition, since the pyrF mutation in KU25 was only a 1-bp deletion, unintended recombination between the marker pyrF gene and the mutated allele on the chromosome could always occur, becoming problematic when the homologous regions of the target gene were shortened to 500 bp (21). To solve these problems, we constructed a new host strain whose endogenous pyrF gene was specifically and almost completely deleted by homologous recombination, as illustrated in Fig. 1. T. kodakaraensis KOD1 was transformed with pUDPyrF as described in Materials and Methods, and several candidates for pyrF deletion were positively selected with 5-FOA. One of the isolates was designated KU216 and was confirmed to require uracil for growth in ASW-AA liquid medium. The genotype of KU216 was determined by PCR using the primer pair CHDPYR-R/CHDPYR-F and Southern blot analyses using a probe of the pyrF upstream region. Both analyses demonstrated that the target region in KU216 was shorter than the native locus in wild-type KOD1 as expected (Fig. 2A and 3A). Moreover, the sole signal detected in the Southern blot analysis of KU216 denied the occurrence of nonhomologous recombination. Sequencing analysis of the target region was also consistent with the ΔpyrF genotype formed by doublecrossover homologous recombination. We further confirmed that the pyrE gene in KU216, whose inactivation is another factor responsible for uracil auxotrophy and 5-FOA resistance, remained intact. These results indicated that the uracil auxotrophy of KU216 was caused solely by the deficiency of pyrF.
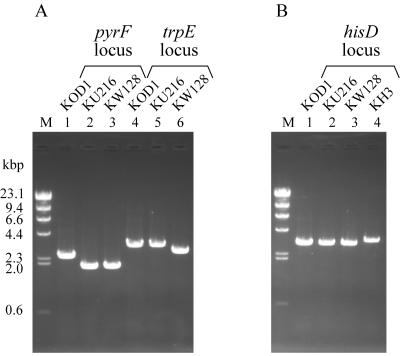
FIG. 2.
PCR analyses of T. kodakaraensis strains KU216 (ΔpyrF), KW128 (ΔpyrF ΔtrpE::pyrF), and KH3 (ΔpyrF ΔtrpE::pyrF ΔhisD::trpE). (A) Amplification of pyrF and trpE loci in strains KOD1, KU216, and KW128 using CHDPYR-R/CHDPYR-F and CHDTRP-R/CHDTRP-F as primer sets, respectively. (B) Amplification of the hisD locus in T. kodakaraensis KOD1, KU216, KW128, and KH3 using CHDHID-R and CHDHID-F as primers. Primers used for these analyses are displayed in Fig. 1. M represents the DNA size marker, HindIII-digested λ DNA.
FIG. 3.
Southern blot analyses of T. kodakaraensis strains KU216 (ΔpyrF), KW128 (ΔpyrF ΔtrpE::pyrF), and KH3 (ΔpyrF ΔtrpE::pyrF ΔhisD::trpE). (A) The pyrF upstream probe was used against genomic DNAs of KOD1 and KU216 digested with HincII. (B) The pyrF probe was used against genomic DNAs of KOD1, KU216, KW128, and KUW1 digested with ApaI. (C) The trpE downstream probe was used against genomic DNAs of KOD1, KU216, KW128, and KUW1 digested with ApaI. (D) The trpE probe was used against genomic DNAs of KOD1, KW128, and KH3 digested with HindIII. The bars on the left side of each panel indicate the mobility of fragments in the DNA size marker, HindIII-digested λ DNA. Regions spanned by probes used for these analyses are displayed in Fig. 1.
Development of an improved transformation system using a trpE marker.
It has been found that pyrF-deficient mutant KU25 (with a point mutation in pyrF) could grow on uracil-free plate medium despite its uracil auxotrophy in the liquid medium, probably due to some pyrimidine-related compounds in the solidifier (21). KU216 (ΔpyrF) also showed the same property. In the use of uracil auxotrophs of this organism as host strains, this property brings about a complicated procedure for isolation of prototrophs after transformation; two rounds of cultivation in uracil-free liquid medium were necessary prior to colony isolation, hampering calculation of transformation efficiency. We therefore attempted to utilize a trpE deletion mutant and the trpE gene as a host strain and a selectable marker, respectively, because the previously constructed trpE deletion mutant, KW4, showed strict tryptophan auxotrophy both in liquid medium and on plate medium (21). As shown in Fig. 1, almost the entire coding region of trpE on the chromosome of KU216 was replaced by the pyrF marker with the CaCl2 method as described for the construction of KW4 (21). Colony PCR analysis after the final plate culture suggested that all of the three uracil prototrophs examined were trpE deletion mutants. One of the isolates was designated KW128, and the expected genotype (ΔpyrF ΔtrpE::pyrF) was confirmed by PCR using CHDTRP-R/CHDTRP-F (Fig. 2A); Southern blot using pyrF, trpE downstream, and trpE probes (Fig. 3B, C, and D, respectively); and sequencing analyses.
We then investigated the capability of strain KW128 and the trpE gene as a host-marker system for transformation of T. kodakaraensis. For this purpose, the hisD gene, encoding histidinol dehydrogenase within a probable histidine biosynthesis operon (his operon) in T. kodakaraensis, was chosen as a target gene to be disrupted. KW128 was transformed with the hisD disruption vector pUDHisD harboring a trpE marker cassette (PpyrF::trpE). After treatment with the plasmid DNA, cells were incubated in rich medium (modified ASW-YT) at 85°C for 2 h, aiming to promote homologous recombination before cultivation on a selective plate medium. The washed cells after outgrowth were directly inoculated onto tryptophan-deficient ASW-AAW− plate medium. As a result, we could obtain tryptophan prototrophs with a transformation efficiency of approximately 1 × 102/μg DNA, while a control experiment without the exogenous DNA gave no tryptophan prototrophs. Colony PCR analysis suggested that 7 out of 10 tryptophan prototrophs examined were hisD deletion mutants. The genotype of one of the isolates, designated KH3, was confirmed to be as expected (ΔpyrF ΔtrpE::pyrF ΔhisD::trpE) by PCR using the primer pair CHDHID-R/CHDHID-F (Fig. 2B), Southern blot using the trpE probe (Fig. 3D), and sequencing analyses. Strain KH3 displayed strict histidine auxotrophy with an inability to grow in ASW-AA liquid medium without histidine (data not shown), indicating that the his operon is actually involved in histidine biosynthesis in T. kodakaraensis. These results demonstrated that the combination of the new host, KW128, and the trpE gene was applicable to the transformation of T. kodakaraensis. This also indicates that the genes downstream of trpE in the trp operon are functioning and that our disruption of trpE did not lead to notable polar effects in this operon. This system enables us to select transformants by a simple procedure without repeated cultivation in liquid medium and to evaluate transformation efficiencies, overcoming the practical disadvantages in the previous system using pyrF as a selectable marker.
We performed further PCR analyses to evaluate the genotype of one of the three Trp prototrophs that were not hisD deletion mutants. PCR analyses indicated the occurrence of single-crossover recombination within the homologous region downstream of hisD, leading to Trp prototrophy. Interestingly, PCR analyses also implied the presence of the original plasmid, pUDHisD, suggesting a spontaneous popping out of the plasmid from the chromosome in these cells.
Effects of transformation conditions and length of homologous regions on transformation efficiency.
With the new system using the trpE marker, we investigated the effects of CaCl2 treatment on the transformation. In the course of the hisD disruption, cells of KW128 were resuspended in transformation buffer containing 80 mM CaCl2 or 0.8× ASW, treated with pUDHisD, and directly inoculated onto ASW-AAW− plate medium. In these experiments, outgrowth of the transformed cells was omitted to avoid precipitation that was probably formed between calcium cations in the transformation buffer and phosphate groups in the outgrowth medium. The numbers of colonies with tryptophan prototrophy grown on the selective plate medium were then counted. Regardless of the presence or absence of CaCl2 treatment, transformation efficiencies were within a similar level of approximately 2 × 102/μg DNA, indicating that the CaCl2 treatment did not have an apparent effect on transformation efficiency. The results also imply that the 2-h outgrowth procedure does not significantly enhance the transformation efficiency.
Next, we investigated the effects of the length of homologous regions that flank the target gene. In the previous gene disruption system using KU25 as the host strain, the use of a linear DNA harboring homologous regions of 500 bp resulted in predominant homologous recombination between the pyrF marker in the exogenous DNA and the mutated allele on the host chromosome instead of the intended recombination (21). In contrast, the new host, KW128, allowed us to evaluate more precisely the effects of length of homologous regions without recombination at the marker (trpE) locus, as the allele on the host chromosome had been almost entirely removed. KW128 was transformed with circular and linear DNAs with 1,000, 500, and 100 bp of homologous regions for the disruption of hisD. As shown in Table 2, DNAs with 1,000-bp homologous regions led to successful homologous recombination regardless of the DNA form. Homologous regions of 500 bp were also sufficient to bring about homologous recombination, although the efficiencies became lower (101/μg DNA). Both circular and linear DNAs with 100-bp homologous regions gave no tryptophan prototrophs, suggesting that this length was too short to promote effective homologous recombination in this organism under the conditions examined.
TABLE 2.
Transformation of T. kodakaraensis using various DNAsa
Transformation was performed without CaCl2, and outgrowth was carried out for 2 h. The order of magnitude of transformation efficiency is the mean obtained from three independent experiments. The circular plasmids were prepared using an E. coli DH5α strain harboring a methylation system, while the linear DNAs were prepared by PCR without methylation.
Repeated utilization of pyrF marker through pop-out recombination.
The use of a single selectable marker often limits genetic modification of the host chromosome to one trial, as the marker remains in the recipient cells, and thus, it can no longer be used for subsequent transformation. Therefore, in addition to developing useful selectable markers, we set out to develop a more versatile system enabling multiple cycles of transformation. This would expand our capabilities in elucidating gene function, enabling us to perform multiple gene disruptions or one gene disruption followed by complementation with an exogenous gene. Based on a strategy described previously for yeast (3), we chose the counterselectable marker pyrF. In this procedure, the pyrF marker that is inserted into the chromosome after the first transformation would undergo excision through pop-out recombination that occurs between tandem repeat regions located on both sides of the marker gene. The resulting markerless transformant can be isolated by positive selection using 5-FOA.
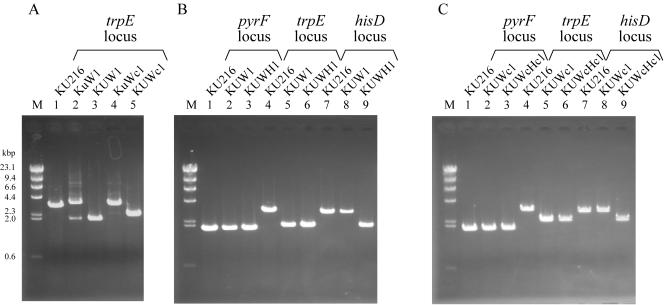
Here, two kinds of vector constructs were adopted for promotion of the pop-out event. In the type I vector, a 3′-flanking region of the target gene was applied as the region repeated in tandem. For gene disruption of trpE and subsequent excision of the pyrF marker, a vector, pUDTPOP, was constructed by inserting a fusion of the trpE 3′ region and the pyrF marker cassette between the homologous regions designed for trpE disruption, as shown in Fig. 4A. In this plasmid, the pyrF marker is directly sandwiched by endogenous 0.8-kbp sequences of the trpE 3′ region. KU216 was transformed with pUDTPOP, and one pyrF+ strain with an intermediary genotype (ΔtrpE::3′-trpE-pyrF), KuW1, could be isolated. PCR analysis of the trpE locus of KuW1 was consistent with replacement of trpE by pyrF along with the additional trpE 3′-flanking region located upstream of the marker (Fig. 5A, main band in lane 2). Subsequent cultivation of KuW1 on the plate medium containing 5-FOA resulted in the generation of 5-FOA-resistant colonies with a frequency of 3 × 10−4. One of the several strains isolated was designated KUW1, and its genotype (ΔpyrF ΔtrpE) was analyzed. First, PCR analysis of the trpE locus led to the amplification of a shorter DNA fragment (Fig. 5A, lane 3). Furthermore, a signal corresponding to a shorter fragment with the trpE downstream probe was detected (Fig. 3C), along with the disappearance of a signal with the pyrF probe (Fig. 3B), in Southern blot analyses. These results clearly indicated pop-out recombination between the tandem repeats. Sequencing analysis also confirmed the intended excision of the pyrF marker. Interestingly, minor amplification of a fragment corresponding to the chromosome structure formed after the pop-out event was also detected with total DNA isolated from KuW1 cells after a few cultivations under nonselective conditions (absence of 5-FOA) (Fig. 5A, lane 2). Although we could not quantify the efficiency of the pop-out event, the results suggest that the molecular construct used here allows the pop-out recombination to occur at efficiencies that can be detected even under nonselective conditions. Furthermore, KUW1 was transformed with pUDHPOP, a vector designed for disruption of hisD and subsequent pop-out excision of pyrF using the same strategy. As a result of PCR analyses of the pyrF, trpE, and hisD loci (Fig. 5B, lanes 3, 6, and 9), we confirmed that the final isolate, KUWH1, was a triple mutant with the expected genotype (ΔpyrF ΔtrpE ΔhisD). These results demonstrated that by using type I pop-out vectors, we can repeatedly utilize the pyrF marker for multiple gene disruptions.
FIG. 5.
PCR analyses of pyrF-trpE double deletion mutants and pyrF-trpE-hisD triple deletion mutants of T. kodakaraensis constructed by repeated utilization of the pyrF marker using pop-out strategy. (A) Amplification of the trpE locus in strains KU216, KuW1, KUW1, KuWc1, and KUWc1 using CHDTRP-R/CHDTRP-F as a primer set. (B) Amplification of pyrF, trpE, and hisD loci in strains KU216, KUW1, and KUWH1 using CHDPYR-R/CHDPYR-F, CHDTRP-R/CHDTRP-F, and CHDHID-R/CHDHID-F as primer sets, respectively. (C) Amplification of pyrF, trpE, and hisD loci in strains KU216, KUWc1, and KUWcHc1 using CHDPYR-R/CHDPYR-F, CHDTRP-R/CHDTRP-F, and CHDHID-R/CHDHID-F as primer sets, respectively. Primer sets used for these analyses were displayed in Fig. 1 and 4. M represents the DNA size marker, HindIII-digested λ DNA.
In another strategy, using type II vectors, an exogenous DNA sequence was applied for the tandem repeats, as in a strategy described previously (3) (Fig. 4B). We adopted a 0.35-kbp sequence derived from the 2μ region in the yeast plasmid pYES2 as the exogenous sequence, designated as 2μ′, and constructed a new cassette consisting of the pyrF marker flanked on both sides by the 2μ′ regions. The 2μ′-pyrF-2μ′ fusion was then inserted between the 5′- and 3′-flanking regions of trpE for homologous recombination, and KU216 was transformed with the resulting vector, pUDTPOPC. As seen in the case of KuW1, PCR analysis of a pyrF+ intermediate strain, KuWc1, exhibited a major band corresponding to the intended replacement of trpE by the 2μ′-pyrF-2μ′ cassette, along with a faint band indicating subsequent pop-out recombination without 5-FOA (Fig. 5A, lane 4). By positive selection with 5-FOA, we could obtain KUWc1 (ΔpyrF ΔtrpE::2μ′) harboring one copy of the 2μ′ region in the place of trpE. In the case of this experiment, the frequency of the generation of 5-FOA-resistant colonies was 8 × 10−4. Further transformation with a similar type II vector, pUDHPOPC, for hisD disruption followed by the pop-out event gave the strain KUWcHc1 (ΔpyrF ΔtrpE::2μ′ ΔhisD::2μ′). Genotypes of these strains were confirmed by PCR analyses of the pyrF, trpE, and hisD loci (Fig. 5C, lanes 2 and 3, 5 and 6, and 8 and 9). In conclusion, the exogenous sequence derived from yeast plasmid could also be applied as tandem repeats for repeated utilization of the pyrF marker.
Utilization of two genetic markers, pyrF and trpE, in a double deletion mutant, KUW1.
As described above, we created the double deletion mutant KUW1, in which pyrF and trpE genes were almost entirely removed (ΔpyrF ΔtrpE), and confirmed that the pyrF marker was applicable for transformation of KUW1 in the course of constructing strain KuWH1 (ΔpyrF ΔtrpE ΔhisD::3′-hisD-pyrF). Using the trpE marker, we further constructed the plasmid pUDLysV for disruption of the lysV gene in a predicted lysine biosynthesis operon and performed transformation experiments on KUW1 and KuWH1. The lysV deletion mutants were successfully obtained from these host strains by using trpE as a selectable marker (data not shown), indicating the usefulness of KUW1 as a host strain in which both independent and sequential utilization of these two markers are possible.
DISCUSSION
This study reports the development of improved transformation systems for the hyperthermophilic archaeon T. kodakaraensis. Strains KU216 (ΔpyrF), KW128 (ΔpyrF ΔtrpE::pyrF), and KUW1 (ΔpyrF ΔtrpE) were constructed as hosts by directed gene deletion through homologous recombination. Therefore, there is no longer a need to consider unknown mutations caused by random mutagenesis. Furthermore, undesirable recombination between marker genes and the chromosome alleles will no longer be problematic. KU216 was used as a basic strain for the construction of further mutants of T. kodakaraensis. The trpE deletant KW128 and the trpE gene have already been applied as a host and a selectable marker for disruption of rgyTk (6), impTk, and fbpTk genes (22), demonstrating the usefulness of this system for various gene disruptions.
The use of the trpE marker made it possible not only to isolate transformants by a simple selection procedure based on the strict tryptophan auxotrophy of ΔtrpE strains but also to score the transformation efficiency. The disruption of the hisD gene in KW128 was performed using the trpE marker with an efficiency of approximately 102/μg DNA. In addition to our previous finding that transformation of T. kodakaraensis could occur without CaCl2 treatment of the recipient cells (21), it was further clarified here that the CaCl2 treatment did not have an apparent effect on the transformation efficiency. This natural competency of T. kodakaraensis was a property quite distinct from that of the closely related archaeon Pyrococcus abyssi, of which transformation with a shuttle vector required the use of a polyethyleneglycol (PEG)-mediated spheroplast method for uptake of extracellular DNA (102 to 103/μg DNA) (14). Gene disruption by double-crossover homologous recombination has recently been reported in S. solfataricus using electroporation; however, the efficiencies and the effects of transformation conditions were not documented (29). When compared to the efficiencies of natural transformation of mesophilic archaea through single-crossover homologous recombination, the efficiency for T. kodakaraensis was larger than the 101/μg DNA reported for Methanococcus voltae PS (17) and similar to the levels of 100∼103/μg DNA observed for Methanococcus maripaludis (20, 27). However, it was lower than those for M. voltae by an electroporation-mediated procedure (103/μg DNA) (17) and for M. maripaludis by a PEG-mediated procedure (105/μg DNA) (27). In most cases in the transformation of mesophilic archaea with autonomously replicating plasmids, much higher efficiencies (105∼108/μg DNA) have been reported by using PEG- or liposome-mediated methods (15, 23). In general, transformation through homologous recombination is supposed to be affected by DNA uptake efficiency, intracellular stability of the exogenous DNA, and recombination efficiency in host cells. Although it has not been clarified which factor is mainly responsible for the transformation efficiency in T. kodakaraensis, adoption of PEG, liposome, or electroporation methodology in the transformation procedure may further enhance the efficiency. Indeed, in the transformation of M. voltae and M. maripaludis with integration vectors, dramatic improvements of efficiencies over those of natural transformation were achieved by electroporation- and PEG-mediated transformation, respectively.
In T. kodakaraensis, efficient recombination at the target locus was possible with homologous regions of 1,000 bp. Unlike the case of M. voltae (17), the type of added DNA, circular or linear, did not seriously affect the transformation efficiency. This result may reflect the different DNA uptake and restriction machineries between these organisms. Reducing the length of the homologous regions by half (500 bp) still led to recombination but with lower efficiencies, whereas 100-bp homologous regions seem to be too short. Under our conditions reported here, homologous regions longer than at least 100 bp appear to be necessary for effective double-crossover recombination in T. kodakaraensis. This fact hampers our use of the far-easier PCR-based molecular construction reported in several yeast strains, where constructs with homologous regions of only about 50 bp are applicable (7, 11, 13). However, there is the possibility that higher intracellular concentration of DNAs effective for formation of recombination complexes, which can be achieved by enhancement of uptake efficiency and/or stability of exogenous DNAs, will allow recombination with extremely shortened homologous regions.
In addition to the new host-marker systems, we demonstrated the repeated utilization of the single pyrF marker through pop-out recombination between tandem repeats flanking the marker genes, followed by positive selection of the pyrF-excised strains with 5-FOA. Both an endogenous 3′ region of the target (type I vectors) and an exogenous 2μ region from a yeast plasmid (type II vectors) could be applied, and the pop-out recombination was found to occur in T. kodakaraensis even in the absence of 5-FOA. The type II vectors, using the 2μ′-pyrF-2μ′ fusion, can be applied as a universal cassette for any gene disruption and subsequent reuse of the marker, saving us several steps to tailor tandem repeat regions for each target gene. In this strategy, one copy of the exogenous sequence will consequently remain on the host chromosome with each gene disruption. Multiple copies of the sequence after repeated utilization may lead to instability of the chromosome caused by removal of regions between the exogenous sequences, especially when they are introduced at nearby position from each other in the same orientation on the chromosome. However, the facile occurrence of pop-out recombination in T. kodakaraensis might be applicable to promote artificial large-scale rearrangement of the genome, for example, for creation of a minimum genome for hyperthermophilic life. Using the pop-out strategy, we have constructed a double deletion mutant, KUW1 (ΔpyrF ΔtrpE), and a triple deletion mutant, KUWH1 (ΔpyrF ΔtrpE ΔhisD), using type I vectors. The strain KUW1 is, as demonstrated above, useful for further multiple genetic manipulations of T. kodakaraensis with two kinds of markers, trpE and the repeatedly utilizable pyrF. Along with the recent complete genome analysis of T. kodakaraensis (10), the multiple-transformation system developed in this study is expected to expand the versatility of using this archaeon as a model organism for research on hyperthermophilic archaea.
Acknowledgments
This study was supported by a grant-in-aid for scientific research to T.I. (no. 14103011) and was partly supported by that for JSPS fellows to T.S. (no. 15005649) from the Ministry of Education, Science, Culture, and Technology.
REFERENCES
- 1.Aagaard, C., I. Leviev, R. N. Aravalli, P. Forterre, D. Prieur, and R. A. Garrett. 1996. General vectors for archaeal hyperthermophiles: strategies based on a mobile intron and a plasmid. FEMS Microbiol. Rev. 18:93-104. [DOI] [PubMed] [Google Scholar]
- 2.Adams, M. W., and R. M. Kelly. 1998. Finding and using hyperthermophilic enzymes. Trends Biotechnol. 16:329-332. [DOI] [PubMed] [Google Scholar]
- 3.Alani, E., L. Cao, and N. Kleckner. 1987. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics 116:541-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aravalli, R. N., and R. A. Garrett. 1997. Shuttle vectors for hyperthermophilic archaea. Extremophiles 1:183-191. [DOI] [PubMed] [Google Scholar]
- 5.Atomi, H., T. Fukui, T. Kanai, M. Morikawa, and T. Imanaka. 2004. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atomi, H., R. Matsumi, and T. Imanaka. 2004. Reverse gyrase is not a prerequisite for hyperthermophilic life. J. Bacteriol. 186:4829-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baudin, A., O. Ozier-Kalogeropoulos, A. Denouel, F. Lacroute, and C. Cullin. 1993. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 21:3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeke, J. D., F. LaCroute, and G. R. Fink. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197:345-346. [DOI] [PubMed] [Google Scholar]
- 9.Cannio, R., P. Contursi, M. Rossi, and S. Bartolucci. 1998. An autonomously replicating transforming vector for Sulfolobus solfataricus. J. Bacteriol. 180:3237-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukui, T., H. Atomi, T. Kanai, R. Matsumi, S. Fujiwara, and T. Imanaka. 2004. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 15:352-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klinner, U., and B. Schäfer. 2004. Genetic aspects of targeted insertion mutagenesis in yeasts. FEMS Microbiol. Rev. 28:201-223. [DOI] [PubMed] [Google Scholar]
- 12.Lange, M., and B. K. Ahring. 2001. A comprehensive study into the molecular methodology and molecular biology of methanogenic Archaea. FEMS Microbiol. Rev. 25:553-571. [DOI] [PubMed] [Google Scholar]
- 13.Längle-Rouault, F., and E. Jacobs. 1995. A method for performing precise alterations in the yeast genome using a recyclable selectable marker. Nucleic Acids Res. 23:3079-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas, S., L. Toffin, Y. Zivanovic, D. Charlier, H. Moussard, P. Forterre, D. Prieur, and G. Erauso. 2002. Construction of a shuttle vector for, and spheroplast transformation of, the hyperthermophilic archaeon Pyrococcus abyssi. Appl. Environ. Microbiol. 68:5528-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metcalf, W. W., J. K. Zhang, E. Apolinario, K. R. Sowers, and R. S. Wolfe. 1997. A genetic system for archaea of the genus Methanosarcina: liposome-mediated transformation and construction of shuttle vectors. Proc. Natl. Acad. Sci. USA 94:2626-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morikawa, M., Y. Izawa, N. Rashid, T. Hoaki, and T. Imanaka. 1994. Purification and characterization of a thermostable thiol protease from a newly isolated hyperthermophilic Pyrococcus sp. Appl. Environ. Microbiol. 60:4559-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel, G. B., J. H. E. Nash, B. J. Agnew, and G. D. Sprott. 1994. Natural and electroporation-mediated transformation of Methanococcus voltae protoplasts. Appl. Environ. Microbiol. 60:903-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peck, R. F., S. DasJarma, and M. P. Krebs. 2000. Homologous gene knockout in the archaeon Halobacterium salinarum with ura3 as a counterselectable marker. Mol. Microbiol. 35:667-676. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Sandbeck, K. A., and J. A. Leigh. 1991. Recovery of an integration shuttle vector from tandem repeats in Methanococcus maripaludis. Appl. Environ. Microbiol. 57:2762-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato, T., T. Fukui, H. Atomi, and T. Imanaka. 2003. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 185:210-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato, T., H. Imanaka, N. Rashid, T. Fukui, H. Atomi, and T. Imanaka. 2004. Genetic evidence identifying the true gluconeogenic fructose-1,6-bisphosphatase in Thermococcus kodakaraensis and other hyperthermophiles. J. Bacteriol. 186:5799-5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sowers, K. R., and H. J. Schreier. 1999. Gene transfer systems for the archaea. Trends Microbiol. 7:212-219. [DOI] [PubMed] [Google Scholar]
- 24.Stedman, K. M., C. Schleper, E. Rumpf, and W. Zillig. 1999. Genetic requirements for the function of the archaeal virus SSV1 in Sulfolobus solfataricus: construction and testing of viral shuttle vectors. Genetics 152:1397-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stetter, K. O. 1996. Hyperthermophilic prokaryotes. FEMS Microbiol. Rev. 18:149-158. [Google Scholar]
- 26.Stetter, K. O. 1999. Extremophiles and their adaptation to hot environments. FEBS Lett. 452:22-25. [DOI] [PubMed] [Google Scholar]
- 27.Tumbula, D. L., R. A. Makula, and W. B. Whitman. 1994. Transformation of Methanococcus maripaludis and identification of a PstI-like restriction system. FEMS Microbiol. Lett. 121:309-314. [Google Scholar]
- 28.Vieille, C., and G. J. Zeikus. 2001. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 65:1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worthington, P., V. Hoang, F. Perez-Pomares, and P. Blum. 2003. Targeted disruption of the α-amylase gene in the hyperthermophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 185:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]