Abstract
Vibrio cholerae is a halophilic facultative human pathogen found in marine and estuarine environments. Accumulation of compatible solutes is important for growth of V. cholerae at NaCl concentrations greater than 250 mM. We have identified and characterized two compatible solute transporters, OpuD and PutP, that are involved in uptake of glycine betaine and proline by V. cholerae. V. cholerae does not, however, possess the bet genes, suggesting that it is unable to synthesize glycine betaine. In contrast, many Vibrio species are able to synthesize glycine betaine from choline. It has been shown that many bacteria not only synthesize but also secrete glycine betaine. We hypothesized that sharing of compatible solutes might be a mechanism for cooperativity in microbial communities. In fact, we have demonstrated that, in high-osmolarity medium, V. cholerae growth and biofilm development are enhanced by supplementation with either glycine betaine or spent media from other bacterial species. Thus, we propose that compatible solutes provided by other microorganisms may contribute to survival of V. cholerae in the marine environment through facilitation of osmoadaptation and biofilm development.
Estuarine ecosystems are positioned at the interface between freshwater systems and the ocean. Consequently, their inhabitants must continually adapt to temporal and spatial fluctuations in salt concentration. Bacteria living in these ecosystems have developed a variety of strategies to cope with osmotic stress. Decreases in external osmotic pressure are overcome by rapid expulsion of a nonrandom assortment of osmotically active molecules through mechanosensitive channels (1, 21). Bacteria adapt to increases in external osmolarity both by importing charged ions from the environment and by importing or synthesizing cytoplasmic solutes. Although charged ions are readily available in seawater, osmoadaptation by import of charged ions alone may increase the ionic strength of the cytoplasm to a point at which optimal functioning of bacterial enzymes is not possible. An alternative strategy is to accumulate compatible solutes, which are small, zwitterionic, highly soluble organic molecules that are thought to stabilize proteins and lead to hydration of the cell (25, 26). However, compatible solutes are not as readily available in seawater as charged ions are, and synthesis of compatible solutes in the face of osmotic stress is costly. The cytoplasm of halophilic or salt-loving bacteria contains enzymes that function best at elevated ionic strength. Thus, they have an absolute requirement for charged ions in their cytoplasm, and the optimum habitats of halophiles are determined by their salt requirements. Halophiles use compatible solutes rather than charged ions to compensate for the high environmental osmolarity only if the optimum environmental salt concentration is exceeded.
Compatible solutes are diverse, including amino acids such as proline and glutamate, amino acid derivatives such as glycine betaine and ectoine, and sugars such as trehalose and sucrose. Uptake of released compatible solutes from the environment is the preferred mechanism for osmoadaptation, and most bacteria are able to import at least one compatible solute. Five uptake systems are known in Bacillus subtilis. OpuA, OpuB, and OpuC are ABC-type transporters, whereas OpuD and OpuE are secondary transporters of the BCCT family (betaine carnitine choline transporter) and the sodium/solute transporter family, respectively (1). OpuD is involved in glycine betaine uptake and is regulated by osmolarity both at the transcriptional level and at the level of transporter activity (5). OpuE is a proline transporter whose transcription is activated by high osmolarity, suggesting a role in osmoadaptation (27, 30). The PutP transporter of Escherichia coli is homologous to OpuE. Unlike OpuE, however, uptake of proline via PutP serves only metabolic purposes, while the additional transporters, ProP and ProU, are utilized for proline uptake during osmotic stress (18). A Vibrio vulnificus PutP homolog has been identified as well. Transcription of the gene encoding this transporter is activated by NaCl, proline, and the cyclic AMP receptor protein (14).
If compatible solutes are not available in the environment, they may be synthesized either de novo or from precursor molecules. For example, many bacteria possess the betAB and ectABC genes, which encode enzymes responsible for the oxidation of choline to glycine betaine and the conversion of l-aspartate-β-semialdehyde to ectoine, respectively (6, 11, 12, 17, 24).
V. cholerae is not only the infectious agent of the diarrheal disease cholera but also a halophilic bacterium found in estuarine and coastal waters (15). In fact, increases in estuarine salinity have been associated with peaks in cholera incidence (19). We have previously demonstrated that V. cholerae grows best in medium containing 200 mM NaCl when K+ is present, suggesting that V. cholerae accumulates K+ in its cytoplasm. When V. cholerae is grown in medium containing NaCl concentrations higher than 200 mM, compatible solutes accumulate within its cytoplasm (24). These compatible solutes may be derived from synthesis of ectoine or transport of glycine betaine. We have previously shown that a V. cholerae ΔectA mutant lacking one of the genes in the ectoine synthesis gene cluster grows quite poorly in medium containing 500 mM NaCl (24).
In the marine environment, V. cholerae has been found in association with the surfaces of plants, algae, phytoplankton, and zooplankton, and plankton blooms in the Bay of Bengal have been associated with cholera outbreaks in Bangladesh, an area endemic for the pathogen (2, 15, 16, 28). Previous studies have shown that V. cholerae O139 uses at least two strategies for surface attachment or biofilm formation in the presence of specific environmental activators (7). Monosaccharides induce synthesis of the VPS exopolysaccharide by enzymes that are encoded by the vps genes. This leads to vps-dependent biofilm development. A different type of biofilm is formed in seawater. Intercellular interactions in this biofilm depend on Ca2+ ions, which are thought to bridge the negative charges of the capsule and/or O-antigen (8).
Compatible solutes are released into the environment from the roots of living plants, decaying plants and animals, and osmotically downshocked cells (1, 3). Because compatible solutes would rapidly disperse after release into the aquatic environment, we hypothesize that, within diffusion-limited microbial communities such as biofilms, concentrations of these released compatible solutes would be sufficient to enhance bacterial growth in high-osmolarity environments. Glycine betaine, one of the most widespread compatible solutes, is found in plants, mammals, archea, and bacteria (1, 25, 26). To address our hypothesis, we have identified and characterized two transporters of V. cholerae that are involved in the uptake of glycine betaine. We have then shown that the compatible solute glycine betaine is present in the spent media of other Vibrio species in concentrations high enough to promote growth of V. cholerae in a high-osmolarity minimal medium. In addition to enhancing growth in this medium, glycine betaine also enhances biofilm formation, an effect that can be reproduced by supplementing high-osmolarity media with the spent supernatants of other Vibrio species. Finally, we have demonstrated that enhancement of biofilm formation by glycine betaine occurs at the transcriptional level by activation of vps gene transcription. We, therefore, hypothesize that glycine betaine may play an important role in establishment and survival of surface-attached microbial communities in estuarine and marine environments.
MATERIALS AND METHODS
Bacterial strains and media.
The V. cholerae strains used in this study are shown in Table 1. The minimal medium (MM) used for osmoadaptation experiments has been described elsewhere (24). In this medium, a mixture of amino acids provides the sole carbon and nitrogen source. V. cholerae vps-dependent biofilms were formed in modified MM (biofilm MM) designed as follows to maximize exopolysaccharide synthesis: (i) half the amino acid concentration was used, (ii) 0.5% glucose was added, and (iii) the medium was supplemented with 17 μM FeSO4 and 3.3 mM potassium phosphate (pH 7). The various media were supplemented with NaCl, glycine betaine, proline, and choline as specified in the text. All chemicals were purchased from Sigma unless otherwise noted.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| V. cholerae strains | ||
| MO10 | Clinical O139 isolate, India 1992; Smr | 31 |
| PW411 | MO10ΔectA | 24 |
| PW477 | MO10ΔopuD | This study |
| PW478 | MO10ΔputP | This study |
| PW653 | MO10ΔputPΔopuD | This study |
| PW654 | MO10ΔectAΔputPΔopuD lacZ::gfp | This study |
| PW357 | MO10 lacZ::pAJH2 | 4 |
| PW647 | MO10ΔopuD lacZ::pAJH2 | This study |
| V. parahaemolyticus | Clinical isolate | S. Calderwood |
| V. fluvialis | Clinical isolate | S. Calderwood |
| V. vulnificus | Clinical isolate | S. Calderwood |
| Plasmids | ||
| pAJH2 | vpsL (VC0934) promoter-lacZ gene fusion | 4 |
| pJZ111 | pCVD442::Plac::gfp::lacZ | J. Zhu |
| pDK1 | pWM91::ΔopuD | This study |
| pDK2 | pWM91::ΔputP | This study |
Construction of in-frame deletion mutants.
Strains harboring 1,051-bp and 1,132-bp in-frame deletions in the chromosomal loci VC1279 (opuD) and VCA1071 (putP), respectively, were constructed by double homologous recombination as previously described (4). The primer pairs used to amplify regions flanking the deletions were as follows: (i) 5′ end of opuD, PopuD1 (GTT TGG GAT CAG TGC AGG TT) and PopuD2 (TTA CGA GCG GCC GCA TCC ACC TAA CCG AAT TTT GC); (ii) 3′ end of opuD, PopuD3 (TGC GGC CGC TCG TAA AGT ATT ACC TCA GGC GGC AAA) and PopuD4 (CCA TGA ACT CAG GTT CGG TT); (iii) 5′ end of putP, PputP1 (AAC GGA TCT GCT TAC CTT GC) and PputP2 (TTA CGA GCG GCC GCA CCA GCC ACT CAT ATC GGA TG); and (iv) 3′ end of putP, PputP3 (TGC GGC CGC TCG TAA GTG TGA CGA TTG TGG TTT GG) and PputP4 (CTT TTT GAA GGT GGC GTG TT).
Planktonic and biofilm growth assays.
For planktonic growth experiments, V. cholerae strains were first grown overnight at 27°C in 5 mM NaCl-MM and then diluted 100-fold into wells containing the medium described in the text. Growth experiments were conducted in 96-well plates at 27°C, and cell densities were measured as the optical density at 655 nm (OD655) in a microplate reader (model 680; Bio-Rad). Each growth experiment was conducted in triplicate.
For biofilm growth experiments, cells were grown overnight on LB-agar plates and resuspended in biofilm MM. The cell suspension was used to inoculate borosilicate tubes filled with 300 μl of the indicated growth medium to yield an initial OD655 of 0.05. After incubation of the biofilms for 24 h at 27°C, planktonic cells were removed, 300 μl of fresh medium was added to the tubes, and biofilm cells were dislodged by vortexing in the presence of 1-mm borosilicate beads (Biospec). The density of planktonic and biofilm cells was measured in a microplate reader as described above.
Preparation of supernatants.
Fifty milliliters of 500 mM NaCl-MM with or without 1 mM choline was introduced into a 250-ml flask and inoculated with a small amount of V. cholerae, Vibrio parahaemolyticus, V. vulnificus, or Vibrio fluvialis grown overnight on an LB-agar plate. This preparation was incubated with shaking for 48 h at 27°C. Cells were pelleted by gentle centrifugation, and the resultant supernatants were filter sterilized with 0.22-μm syringe filters (Fisher Scientific). The sterile supernatants were diluted fourfold in MM or biofilm MM as described in the text.
β-Galactosidase activity.
To measure transcription of vpsL, the relevant V. cholerae strain harboring a vpsL-lacZ fusion was inoculated into a 50-ml polypropylene tube containing 5 ml of the relevant growth medium to obtain a final OD655 of 0.05. Planktonic cells were removed after 24 h of growth at 27°C under static conditions. One milliliter of Z-buffer and borosilicate beads were added to the remaining adherent cells and then cells were dislodged by vortexing (20). The optical densities of both planktonic and biofilm cell suspensions were measured. Planktonic cells were then pelleted by gentle centrifugation and resuspended in 700 μl of Z-buffer. For lysis, cells were subjected to three freeze-thaw cycles (−80°C to 42°C). Cell debris was pelleted at 13,000 rpm. The protein concentration of each lysate was measured in triplicate using the Coomassie Plus protein assay (Pierce). A 100-μl volume of 2-nitrophenyl β-d-galactopyranoside was added to the remaining lysate, and this mixture was incubated overnight at 37°C. Before quantification of β-galactosidase activity by measurement of the OD405, the mixture was spun at high speed to remove particulates. Relative β-galactosidase activity was calculated by division of the measured OD405 by the protein concentration and multiplication by 1,000.
Preparation of cell extract and NMR analysis.
V. vulnificus was incubated in a 2-liter Erlenmeyer flask with shaking at 37°C in 500 ml of MM supplemented with choline. When stationary phase was reached, cells were pelleted gently, and the spent growth medium was removed. Cell pellets were subjected to three freeze-thaw cycles to enhance lysis. Pellets were then resuspended in 750 μl of ethanol. Debris was removed by centrifugation, and the ethanol extract was transferred to a clean tube. The ethanol was subsequently removed from the extracted material by evaporation under vacuum (Speedvac DNA A-110; Savant). The resulting pellet was resuspended in 800 μl of D2O (Aldrich). After removal of insoluble material by centrifugation, the solution was transferred to a 5-mm nuclear magnetic resonance (NMR) tube for analysis. One-dimensional, double-filtered quantum coherence and heteronuclear multiple-quantum coherence experiments were performed on an AMX500 spectrometer (Bruker).
RESULTS
Compatible solute transporters of V. cholerae.
In order to identify the glycine betaine uptake system of V. cholerae, we searched the V. cholerae genome for homologs of known glycine betaine transporters. This revealed the presence of a gene at locus VC1279 encoding a protein of 540 amino acids in the BCCT family of secondary transporters, which includes well-characterized members such as OpuD of Bacillus subtilis (5) and BetP of Corynebacterium glutamicum (10). Based on homology and the studies presented here, we have named this protein OpuD. V. cholerae OpuD is most similar to the putative transporters of the related halophiles V. parahaemolyticus (locus VP1905; 76% identical, 85% similar), Photobacterium profundum SS9 (locus PBPRA2516; 74% identical, 85% similar), and V. vulnificus strains CMCP6 and YJ016 (locus VV12243/VV2103; 74% identical, 84% similar).
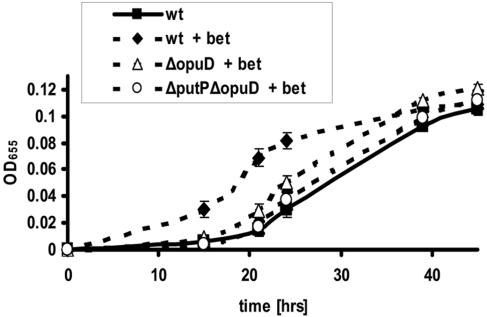
To determine the role of V. cholerae OpuD in osmoadaptation, a mutant harboring an in-frame deletion within the opuD coding sequence was constructed. When grown in 500 mM NaCl-MM alone, growth of the ΔopuD mutant was similar to that of wild-type V. cholerae (data not shown). However, in the presence of 0.25 mM glycine betaine, the ΔopuD mutant exhibited a longer growth delay than wild-type V. cholerae (Fig. 1). Whereas wild-type V. cholerae displayed no delay in growth upon transfer to 500 mM NaCl-MM supplemented with glycine betaine, growth of the ΔopuD mutant began only after a delay of approximately 15 h. We reasoned that if glycine betaine transport were absent in the ΔopuD mutant, the growth of the ΔopuD mutant in 500 mM NaCl-MM supplemented with glycine betaine should parallel growth of wild-type V. cholerae in unsupplemented 500 mm NaCl-MM. In fact, the ΔopuD mutant grew slightly more rapidly in the presence of glycine betaine than wild-type V. cholerae in unsupplemented 500 mM NaCl-MM (Fig. 1). Thus, we suspected the presence of an additional, minor glycine betaine uptake system.
FIG. 1.
Growth phenotype of wild-type V. cholerae (MO10; wt) and the glycine betaine transporter mutants ΔopuD and ΔputP ΔopuD. Cultures were grown in 500 mM NaCl-MM with or without supplementation with 0.25 mM betaine (bet).
Many transporters involved in bacterial osmoadaptation are able to import both proline and glycine betaine into bacterial cells (1). Therefore, we searched the V. cholerae genome for homologs of proline transporters that might also carry out glycine betaine transport in the absence of opuD. A putative protein encoded by locus VCA1071 with 42% identity and 60% similarity to OpuE of B. subtilis was identified in a BLAST search. Because this protein is most similar to PutP, a recently characterized proline permease of Vibrio vulnificus, we have named the V. cholerae protein PutP as well. To assess the role of PutP in glycine betaine uptake, a mutant harboring mutations in both opuD and putP was constructed, and its growth in 500 mM NaCl-MM supplemented with glycine betaine was assessed. As shown in Fig. 1, growth of the V. cholerae ΔopuD ΔputP mutant in 500 mM NaCl-MM supplemented with glycine betaine was similar to that of wild-type V. cholerae in unsupplemented 500 mM NaCl-MM. These results suggest that, under these growth conditions, transport of glycine betaine into V. cholerae is primarily carried out by OpuD and PutP.
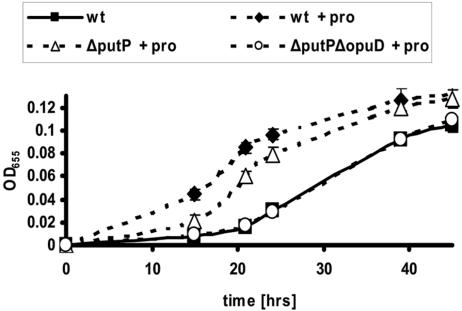
Because PutP is most homologous to a proline permease, we hypothesized that PutP could transport proline as well as glycine betaine into V. cholerae. To assess the role of the PutP permease in proline uptake, we constructed a mutant harboring an in-frame deletion within the putP gene and compared its growth in high-osmolarity medium to that of wild-type V. cholerae. In 500 mM NaCl-MM, no difference in growth was observed between wild-type V. cholerae and the ΔputP mutant (data not shown). In the presence of 0.1 mM proline, growth of the ΔputP mutant was only modestly slower than that of wild-type V. cholerae grown in similar medium and still much greater than wild-type V. cholerae grown in 500 mM NaCl-MM alone (Fig. 2). Furthermore, growth of the ΔputP mutant was not significantly different from that of wild-type V. cholerae at higher proline concentrations (data not shown). This suggested to us that an additional proline transporter was encoded in the V. cholerae genome. We hypothesized that this transporter of proline might be OpuD. As shown in Fig. 2, growth of a mutant harboring deletions in opuD and putP was indistinguishable from that of wild-type V. cholerae in 500 mM NaCl-MM. This suggests that in 500 mM NaCl-MM, OpuD and PutP are responsible for most of the proline uptake by V. cholerae.
FIG. 2.
Growth phenotype of wild-type V. cholerae (MO10; wt) and the proline transporter mutants ΔputP and ΔputP ΔopuD. Cultures were grown in 500 mM NaCl-MM with or without 0.1 mM proline (pro).
Other species serve as a source for compatible solutes.
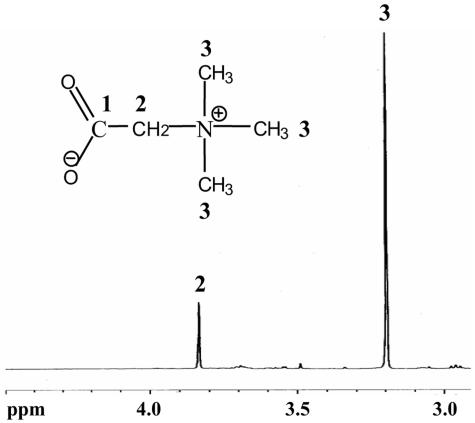
We have shown that V. cholerae is able to transport glycine betaine. However, the genome of V. cholerae does not contain genes homologous to the known glycine betaine synthesis genes, bet and gbs, suggesting that it lacks the ability to synthesize glycine betaine from choline. Similarly, V. cholerae can only synthesize ectoine, while transporters of ectoine have been identified in other halophiles (24, 29). This suggested to us that a microbe might expand its repertoire of osmoadaptive mechanisms by living within a microbial community collectively possessing the ability to synthesize many types of compatible solutes. We first examined the genomes of other Vibrio species for homologs of the bet genes, which are responsible for glycine betaine synthesis from choline. A BLAST search revealed that the chromosomes of V. parahaemolyticus and V. vulnificus both encode such homologs. To show that V. vulnificus is indeed able to synthesize glycine betaine from choline, a cell extract of V. vulnificus grown in 500 mM NaCl-MM supplemented with choline was analyzed by NMR. The NMR spectrum documents the presence of glycine betaine in the cytoplasm of V. vulnificus grown in high-osmolarity medium (Fig. 3).
FIG. 3.
One-dimensional 1H-NMR spectrum of V. vulnificus cell extracts grown in 500 mM NaCl-MM supplemented with 1 mM choline. V. vulnificus accumulates the compatible solute glycine betaine within its cytoplasm, which is synthesized from choline. The numbers above the peaks correspond to the carbon atoms to which the hydrogen atoms are attached.
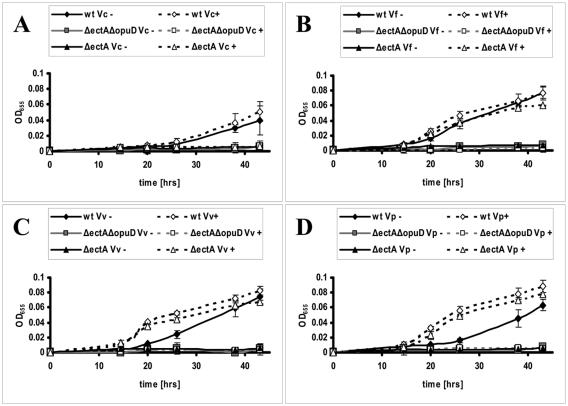
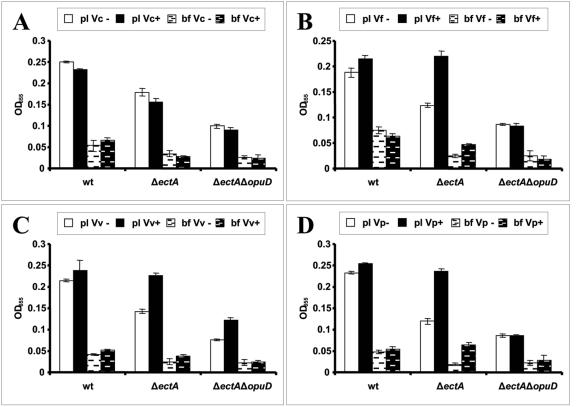
We hypothesized that V. cholerae OpuD might import glycine betaine released into the environment by osmoadapted species capable of synthesizing and secreting glycine betaine. We used the following bioassay to determine whether glycine betaine was released into the environment by osmoadapted vibrios grown in the presence of choline. To allow for osmoadaptation, V. cholerae, V. vulnificus, V. parahaemolyticus, and V. fluvialis were grown for 48 h in either 500 mM NaCl-MM alone or 500 mM NaCl-MM supplemented with 1 mM choline. The spent media from these cultures were collected, filter sterilized, and mixed in a ratio of 1:3 with fresh 500 mM NaCl-MM. We tested the ability of the resulting media to enhance growth of wild-type V. cholerae, a ΔectA mutant, and a ΔectA ΔopuD mutant (Fig. 4). Spent media from cultures of V. cholerae grown in 500 mM NaCl-MM both alone and supplemented with choline had no effect on growth of wild-type V. cholerae following osmotic shock. This supports our hypothesis that V. cholerae is not able to synthesize glycine betaine from choline. Furthermore, spent media from cultures of other Vibrio species grown in 500 mM NaCl-MM alone also did not have an effect on growth of wild-type V. cholerae. This suggests that these vibrios do not secrete significant amounts of glycine betaine in the absence of choline. In contrast, spent media from cultures of V. parahaemolyticus, V. fluvialis, and V. vulnificus grown in the presence of choline enhanced growth of wild-type V. cholerae and the ΔectA mutant but did not enhance growth of the ΔectA ΔopuD mutant, which is defective in glycine betaine uptake. These results suggest that, when grown in high-osmolarity medium supplemented with choline, some Vibrio species synthesize glycine betaine from choline and release a portion of it into the environment. This glycine betaine may, in turn, be transported by other bacteria for use in osmoadaptation.
FIG. 4.
Growth of wild-type V. cholerae (wt) and mutant strains in MM supplemented with supernatants from a number of Vibrio species. The supernatants were prepared by growing cultures of (A) V. cholerae (Vc), (B) V. fluvialis (Vf), (C) V. vulnificus (Vv), and (D) V. parahaemolyticus (Vp) in 500 mM NaCl-MM with (+) or without (-) 1 mM choline.
Supplementation with glycine betaine increases V. cholerae surface attachment in 500 mM NaCl-MM.
Our experiments with spent media suggested that compatible solutes released by microorganisms within a microbial community would be available for uptake by other members. We hypothesized that the diffusion-limited environment of the biofilm would provide the ideal milieu for such a process. Thus, we chose to study the influence of osmoadaptation on biofilm formation. In MM, V. cholerae biofilm formation occurs only in the presence of monosaccharides and is dependent on the synthesis of the VPS exopolysaccharide (7). To maximize vps-dependent surface attachment, we used biofilm MM consisting of 500 mM NaCl-MM supplemented with glucose, iron, and phosphate as described above. For these experiments, wild-type V. cholerae or mutants were harvested from LB-agar plates and incubated in either 5 mM NaCl or 500 mM NaCl-biofilm MM. Growth of planktonic and biofilm cells was measured. Under these conditions, growth of planktonic wild-type V. cholerae and ΔectA and ΔectA ΔopuD ΔputP mutants in 5 mM NaCl-MM and 500 mM NaCl-MM did not differ by more than approximately 10% (Fig. 5). In contrast, when wild-type V. cholerae was cultured in 500 mM NaCl-biofilm MM, the OD655 of surface-attached cells was 40% less than that measured for cells cultured in 5 mM NaCl-biofilm MM. In 500 mM NaCl-biofilm MM, growth of ΔectA and ΔectA ΔopuD ΔputP mutant biofilms was even more profoundly affected. However, growth of wild-type V. cholerae as well as the ΔectA mutant biofilms in 500 mM NaCl-biofilm MM increased to levels observed for cells grown in 5 mM NaCl-biofilm MM when the medium was supplemented with glycine betaine. Biofilm formation in 500 mM NaCl-biofilm MM by the ΔectA ΔopuD ΔputP mutant, which neither synthesizes ectoine nor transports glycine betaine, was unaffected by the presence of glycine betaine. Glycine betaine had no effect on surface accumulation of wild-type V. cholerae or the ΔectA and ΔectA ΔopuD ΔputP mutants in 5 mM NaCl-biofilm MM.
FIG. 5.
OD655 of biofilm cells of V. cholerae MO10 wild-type (wt) and mutant strains after 24 h in the presence or absence of 0.25 mM betaine (bet) in 5 mM or 500 mM NaCl-biofilm MM.
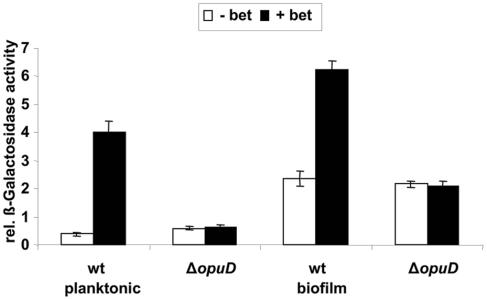
In 500 mM NaCl-biofilm MM, biofilm formation by wild-type V. cholerae as well as a ΔectA mutant increased in the presence of glycine betaine. We have previously identified nucleosides and monosaccharides as environmental signals that increase V. cholerae biofilm formation by activating transcription of the vps genes that are located in an operon involved in VPS exopolysaccharide synthesis (4, 7, 32). We questioned whether glycine betaine might also enhance biofilm formation by increasing vps gene transcription. We, therefore, measured vpsL transcription using a previously constructed strain harboring a chromosome-based reporter fusion of the vpsL promoter to the lacZ gene (4). Biofilms were formed in 500 mM NaCl-biofilm MM alone or supplemented with 0.25 mM glycine betaine. Supplementation with glycine betaine did, in fact, lead to a large increase in vpsL transcription in both planktonic and biofilm-associated wild-type V. cholerae (Fig. 6). In contrast, supplementation of 500 mM NaCl-biofilm MM with glycine betaine did not affect vpsL transcription in a ΔopuD mutant. These results suggest that glycine betaine transport into the cell increases surface association by increasing vps gene transcription and, hence, VPS synthesis.
FIG. 6.
Relative β-galactosidase activity of a chromosomal vpsL promoter-lacZ construct in V. cholerae planktonic and biofilm cells. Biofilms were formed over 24 h in 500 mM NaCl-biofilm MM with or without 0.25 mM betaine (bet). Strains MO10 lacZ::pAJH2 and glycine betaine transporter mutant MO10ΔopuD lacZ::pAJH2 were used to show the effect of betaine on vpsL transcription.
Other bacterial species may enhance V. cholerae biofilm formation in high salt.
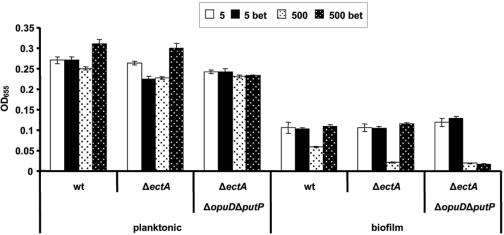
We have shown above that glycine betaine is synthesized from choline by selected Vibrio species and can accelerate growth of V. cholerae in high-osmolarity medium. Since glycine betaine enhances biofilm formation in 500 mM NaCl-biofilm MM, we hypothesized that spent supernatants of these Vibrio species would also increase biofilm formation by V. cholerae at high osmolarity. As described above, we mixed spent supernatants with fresh 500 mM NaCl-biofilm MM in a ratio of 1:3 and then allowed wild-type V. cholerae and mutants to form biofilms in this medium. As can be seen in Fig. 7, addition of wild-type V. cholerae supernatants to 500 mM NaCl-biofilm MM did not affect growth or biofilm formation by the ΔectA or ΔectA ΔopuD V. cholerae mutants. Addition of supernatants from the other three Vibrio species grown in the absence of choline also showed no effect. In contrast, addition of spent supernatants from other Vibrio species grown in 500 mM NaCl-MM supplemented with choline increased both planktonic growth and biofilm formation by the V. cholerae ΔectA mutant but had no effect on biofilm formation by the ΔectA ΔopuD mutant. These results suggest that glycine betaine in the spent supernatants is responsible for increased planktonic growth and biofilm formation by V. cholerae.
FIG. 7.
OD655 of planktonic and biofilm cells of V. cholerae wild-type (wt) and mutant strains after 24 h in 500 mM NaCl-biofilm MM supplemented with supernatants of different Vibrio species. The supernatants were prepared by growing cultures of (A) V. cholerae (Vc), (B) V. fluvialis (Vf), (C) V. vulnificus (Vv), and (D) V. parahaemolyticus (Vp) in 500 mM NaCl-MM with (+) or without (-) 1 mM choline. Sterile supernatants of these cultures were mixed 1:3 with 500 mM NaCl-biofilm MM for this experiment.
DISCUSSION
ABC transporters utilize ATP to power transport, whereas secondary transporters rely on ion gradients across the cell membrane. Because Na+ gradients across cell membranes are a prerequisite of life in the sea, many secondary transporters of halophilic bacteria utilize Na+ symport. The search for compatible solute transporters of V. cholerae yielded two secondary transporters but no ABC-type transporters. Since V. cholerae is a natural inhabitant of estuarine ecosystems, which are subject to constant fluctuations in salt concentrations, secondary transporters might be the more economical solution to the recurrent need for efflux and uptake of compatible solutes.
Numerous studies have demonstrated the ability of V. cholerae to attach to biotic surfaces, and an association with plankton blooms has been established (15). In addition, seawater isolated during phytoplankton blooms supports growth of V. cholerae (22). Based on these observations, plankton are hypothesized to provide nutrients to adherent V. cholerae. Synthesis of compatible solutes is costly to bacterial cells (23). Thus, not only nutrients but also compatible solutes released from plankton may play an important role in the survival and growth of V. cholerae in seawater. Because glycine betaine is one of the most widespread compatible solutes, a high-affinity transporter of glycine betaine is likely to be of great advantage to a bacterial cell in the marine environment. The experiments we have performed with spent bacterial supernatants clearly show that compatible solutes synthesized by other bacteria are released in concentrations high enough to enhance growth of V. cholerae in high-osmolarity medium. Although the glycine betaine in our spent supernatants may have been released only from leaky or dead cells, there is evidence that bacteria release glycine betaine into the environment. Using E. coli bet mutants, Lamark et al. showed that E. coli excretes glycine betaine when grown in high-osmolarity medium supplemented with choline. This glycine betaine is then imported into cells by the ProU and ProP transporters (13). Efflux of glycine betaine has also been observed in Salmonella enterica serovar Typhimurium and was suggested to be a means of regulating the intracellular glycine betaine pool (9). In a microbial community such as a biofilm, it is likely that any bacterium possessing a high-affinity glycine betaine uptake system would benefit from this type of glycine betaine efflux.
We have previously identified nucleosides and monosaccharides as environmental signals that induce V. cholerae biofilm development (4, 7). We have now shown that glycine betaine not only increases growth of V. cholerae at high salt concentrations, but also enhances surface attachment through induction of vps gene transcription. Thus, glycine betaine is an additional environmental signal that induces biofilm development.
In most aquatic environments, microbes are found in association with surfaces. Because microbes synthesize and excrete glycine betaine, we hypothesize that concentrations of compatible solutes are increased in and around the biofilms formed on biotic surfaces. Furthermore, the presence of glycine betaine increases V. cholerae surface attachment at high salt concentrations, suggesting that V. cholerae favors the surface-attached state during times of osmotic stress. Because a microbial community such as a biofilm is composed of microbes with diverse synthetic capabilities, we propose that microbes expand their repertoire of responses to osmotic stress by joining the biofilm community. Thus, surface attachment in response to glycine betaine may be a beneficial adaptive response.
Acknowledgments
NMR experiments were performed at the Tufts University Biological NMR Center with the expert help of James Sudmeier. We acknowledge Stephen Calderwood for generously providing various non-cholerae Vibrio strains used in this work. We also thank Anne Kane of the GRASP Center and her staff for their patience with the development of the various growth media and their expert preparation of many reagents, which greatly accelerated the course of these experiments.
This work was supported by NIH R01 AI50032 and New England Medical Center GRASP Center NIH/NIDDK grant P30 DK34928.
REFERENCES
- 1.Bremer, R., and R. Kraemer. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria, p. 79-97. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 2.Chiavelli, D. A., J. W. Marsh, and R. K. Taylor. 2001. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl. Environ. Microbiol. 67:3220-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaasker, E., W. N. Konings, and B. Poolman. 1996. Glycine betaine fluxes in Lactobacillus plantarum during osmostasis and hyper- and hypo-osmotic shock. J. Biol. Chem. 271:10060-10065. [DOI] [PubMed] [Google Scholar]
- 4.Haugo, A. J., and P. I. Watnick. 2002. Vibrio cholerae CytR is a repressor of biofilm development. Mol. Microbiol. 45:471-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 178:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiene, R. P. 1998. Uptake of choline and its conversion to glycine betaine by bacteria in estuarine waters. Appl. Environ. Microbiol. 64:1045-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kierek, K., and P. I. Watnick. 2003. Environmental determinants of Vibrio cholerae biofilm development. Appl. Environ. Microbiol. 69:5079-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kierek, K., and P. I. Watnick. 2003. The Vibrio cholerae O139 O-antigen polysaccharide is essential for Ca2+-dependent biofilm development in sea water. Proc. Natl. Acad. Sci. USA 100:14357-14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo, S. P., C. F. Higgins, and I. R. Booth. 1991. Regulation of compatible solute accumulation in Salmonella typhimurium: evidence for a glycine betaine efflux system. J. Gen. Microbiol. 137:2617-2625. [DOI] [PubMed] [Google Scholar]
- 10.Kramer, R., and S. Morbach. 2004. BetP of Corynebacterium glutamicum, a transporter with three different functions: betaine transport, osmosensing, and osmoregulation. Biochim. Biophys. Acta 1658:31-36. [DOI] [PubMed] [Google Scholar]
- 11.Kuhlmann, A. U., and E. Bremer. 2002. Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Appl. Environ. Microbiol. 68:772-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamark, T., I. Kaasen, M. W. Eshoo, P. Falkenberg, J. McDougall, and A. R. Strom. 1991. DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol. Microbiol. 5:1049-1064. [DOI] [PubMed] [Google Scholar]
- 13.Lamark, T., O. B. Styrvold, and A. R. Strom. 1992. Efflux of choline and glycine betaine from osmoregulating cells of Escherichia coli. FEMS Microbiol. Lett. 96:149-154. [DOI] [PubMed] [Google Scholar]
- 14.Lee, J. H., N. Y. Park, M. H. Lee, and S. H. Choi. 2003. Characterization of the Vibrio vulnificus putAP operon, encoding proline dehydrogenase and proline permease, and its differential expression in response to osmotic stress. J. Bacteriol. 185:3842-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipp, E. K., A. Huq, and R. R. Colwell. 2002. Effects of global climate on infectious disease: the cholera model. Clin. Microbiol. Rev. 15:757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobitz, B., L. Beck, A. Huq, B. Wood, G. Fuchs, A. S. G. Faruque, and R. Colwell. 2000. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. USA 97:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis, P., and E. A. Galinski. 1997. Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 143:1141-1149. [DOI] [PubMed] [Google Scholar]
- 18.McFall, E., and E. B. Newman. 1996. Amino acids as carbon sources, p. 358-379. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 19.Miller, C. J., B. S. Drasar, and R. G. Feachem. 1982. Cholera and estuarine salinity in Calcutta and London. Lancet i:1216-1218. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 21.Morbach, S., and R. Kramer. 2002. Body shaping under water stress: osmosensing and osmoregulation of solute transport in bacteria. ChemBioChem 3:384-397. [DOI] [PubMed] [Google Scholar]
- 22.Mourino-Perez, R. R., A. Z. Worden, and F. Azam. 2003. Growth of Vibrio cholerae O1 in red tide waters off California. Appl. Environ. Microbiol. 69:6923-6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oren, A. 1999. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 63:334-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pflughoeft, K. J., K. Kierek, and P. I. Watnick. 2003. Role of ectoine in Vibrio cholerae osmoadaptation. Appl. Environ. Microbiol. 69:5919-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roesser, M., and V. Muller. 2001. Osmoadaptation in bacteria and archaea: common principles and differences. Environ. Microbiol. 3:743-754. [DOI] [PubMed] [Google Scholar]
- 26.Sleator, R. D., and C. Hill. 2002. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26:49-71. [DOI] [PubMed] [Google Scholar]
- 27.Spiegelhalter, F., and E. Bremer. 1998. Osmoregulation of the opuE proline transport gene from Bacillus subtilis: contributions of the sigma A- and sigma B-dependent stress-responsive promoters. Mol. Microbiol. 29:285-296. [DOI] [PubMed] [Google Scholar]
- 28.Tamplin, M. L., A. L. Gauzens, A. Huq, D. A. Sack, and R. R. Colwell. 1990. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 56:1977-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tetsch, L., and H. J. Kunte. 2002. The substrate-binding protein TeaA of the osmoregulated ectoine transporter TeaABC from Halomonas elongata: purification and characterization of recombinant TeaA. FEMS Microbiol. Lett. 211:213-218. [DOI] [PubMed] [Google Scholar]
- 30.von Blohn, C., B. Kempf, R. M. Kappes, and E. Bremer. 1997. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol. Microbiol. 25:175-187. [DOI] [PubMed] [Google Scholar]
- 31.Waldor, M. K., and J. J. Mekalanos. 1994. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect. Immun. 62:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yildiz, F. H., N. A. Dolganov, and G. K. Schoolnik. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPSETr-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 183:1716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]