Abstract
We describe the case of a patient with improving invasive aspergillosis and paradoxically rising serum galactomannan levels in the presence of chronic renal failure and ongoing hemodialysis. Dialysate tested negative for galactomannan, demonstrating the inability of treatments such as hemodialysis to clear Aspergillus antigen from serum. In patients with renal failure and aspergillosis, rising serum galactomannan levels may not necessarily signify progressive infection.
CASE REPORT
Shortly after beginning chemotherapy in April 2002, a 16-year-old white male with acute myeloblastic leukemia subtype M4 developed bilateral pulmonary infection with Aspergillus flavus. The organism was recovered in culture of a specimen from computed tomography (CT)-guided needle biopsy, and a full diagnostic workup revealed no involvement of other organs. A. flavus was also recovered in culture of all surgical specimens from a lobectomy of the right lower lung lobe and wedge resection of the left upper lobe. The patient received combination antifungal therapy with voriconazole and amphotericin B deoxycholate for 4 months. He showed improvement both clinically and radiographically. Following combination antifungal therapy, the patient was maintained on voriconazole therapy (200 mg orally twice daily). After his leukemia failed to respond to conventional cancer chemotherapy, the patient received a matched unrelated donor hematopoietic stem cell transplant in December 2002. Following development of graft failure, he received a haploidentical stem cell transplant from his father in June 2003.
The day following the second transplant and prior to cellular engraftment, the patient developed a right mandibular deep soft tissue lesion, which rapidly progressed in size over the next few days. The lesion was biopsied, and the biopsy specimen subsequently grew Saccharomyces cerevisiae and Aspergillus terreus. The patient was still receiving voriconazole therapy at this time. Radiographic studies showed lesions suggestive of invasive aspergillosis (IA) in the lungs, kidneys, and brain (Fig. 1A). The patient continued to receive voriconazole, and antifungal coverage was broadened to include amphotericin B lipid complex (10 mg/kg of body weight/day) and caspofungin (70-mg loading dose followed by 50 mg daily). Although not a standard of care, this triple antifungal regimen is used at St. Jude Children's Research Hospital, Memphis, Tenn., for treatment of refractory cases of aspergillosis. After 2 weeks of treatment, no improvement was documented, either clinically or radiologically. Voriconazole and caspofungin treatments were discontinued, and treatment with oral posaconazole was initiated at 200 mg every 6 h.
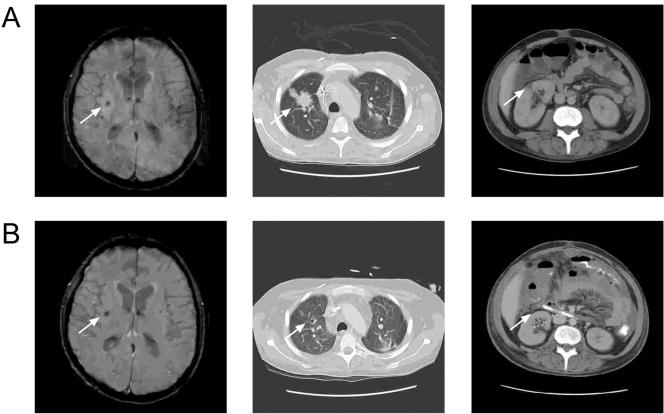
FIG. 1.
Evidence of improvement in fungal infection upon CT and magnetic resonance imaging. Panel A shows images from the MRI of the brain and CT scans of the chest and abdomen at the initial workup of the infection. Panel B shows repeat imaging demonstrating improvement: the two lesions identified in the basal ganglia bilaterally are stable in size, with an apparent decrease in the surrounding edema. The prominent invasive fungal lesion in the upper lobe of the right lung has markedly improved, and the solitary right renal lesion is shown to have resolved upon follow-up CT. Arrows indicate lesion sites.
The patient had developed renal insufficiency, and approximately 6 weeks after the second transplant, he developed respiratory failure following progressive fluid overload and resulting respiratory compromise. He required intubation, and initial endotracheal aspirates grew S. cerevisiae and A. terreus. No fungal organisms were recovered from a needle biopsy specimen from one of the lung lesions. One week following transfer of the patient to the intensive care unit, the patient's baseline renal insufficiency progressed into renal failure (creatinine level, 2.3) and continuous venovenous hemofiltration was initiated. Five days later, intermittent hemodialysis was initiated using the Baxter System 1000 series single-patient hemodialysis delivery system. The patient's respiratory status improved significantly, and he was extubated within a week of beginning hemodialysis. The patient continued to receive both amphotericin B lipid complex and posaconazole, with complete resolution of the mandibular lesion, significant improvement of the pulmonary and renal lesions, and reduction of edema surrounding stable cerebral foci noted upon repeat CT scan (Fig. 1B). The patient showed no evidence of active fungal infection and remained free from respiratory support but suffered a relapse of his primary disease and died approximately 4 months after the second transplant.
Twice weekly screening of the patient's blood for galactomannan (GM), using the Platelia Aspergillus enzyme immunoassay, was performed serially for 10 consecutive weeks, beginning 61 days after the patient's second stem cell transplant and continuing until his death. Urine and dialysate were tested for GM at single time points corresponding to the period of high serum antigenicity (serum antigen level of 7.4). At that time, the urine GM index (GMI) was 10.8, while the GMI of the dialysate (collected 4 days after the urine specimen) was 0.06. The test's positive cutoff for serum GMI was set at 0.5, in accordance with the U.S. package insert for this product (Bio-Rad). As illustrated in Fig. 2, there was near complete resolution of clinical and radiographic signs of fungal infection despite a progressive increase in serum GMI. The increasing GMI seemed to correspond to the patient's reduced renal function and implementation of renal replacement therapy.
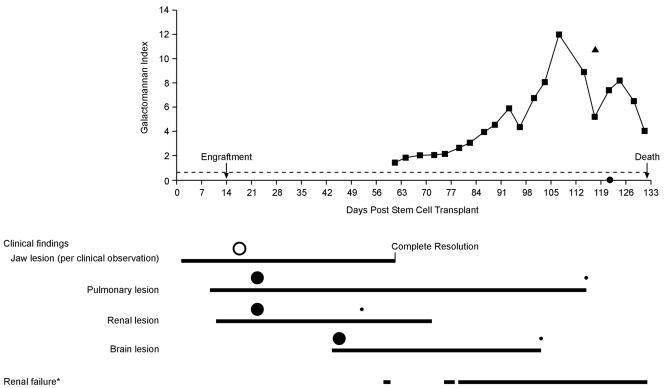
FIG. 2.
Progression of GMI over time in relation to clinical findings and renal failure after transplantation. The solid squares represent individual serum GMIs. The solid triangle indicates urine GMI (posttransplantation day 117). The solid circle on the x axis indicates dialysate GMI (posttransplantation day 121). The hollow and solid circles below the graph indicate relative lesion sizes determined by clinical observation (hollow circle) and by imaging (solid circles). Imaging corresponds to that shown in Fig. 1, demonstrating marked improvement in pulmonary, renal, and brain lesions. The dotted line indicates the threshold value of 0.5. *, hemodialysis was performed during this period of renal failure as indicated in the graph.
IA has emerged as a common infection in hematopoietic stem cell recipients and is associated with high rates of morbidity and mortality (5). This poor prognosis can partly be explained by the diagnostic challenges posed by invasive fungal disease in vulnerable patient populations. In such patients, the already limited utility of culture-based methods is further hampered by the difficulty of aggressive measures such as lung biopsy. Recently approved for use in the United States, the Platelia Aspergillus enzyme immunoassay is an antigen detection assay targeting GM, a cell wall constituent of Aspergillus spp. (4). Three reports have documented the utility of this test as a screening tool for the early diagnosis of IA (6, 7, 9). When used in conjunction with appropriate imaging techniques, detection of serial antigenemia has been demonstrated to be a sensitive and noninvasive indicator of IA in high-risk patients with cancer (7). As outcome is often dependent on early diagnosis, this test may help establish a diagnosis before fungal proliferation becomes overwhelming and therapeutic interventions are no longer effective.
Little is known regarding the kinetics of GM in body fluids. GM antigen has been detected in cerebrospinal fluid, bronchoalveolar lavage fluid, and urine (11). Serum GM levels are influenced by the tissue burden of infection, renal clearance, use of antifungal therapy, and the patient's disease status (2, 8). GM antigens of Aspergillus species appear to be cleared in large part by glomerular filtration in the kidneys (3). Renal clearance in turn depends on the molecular mass of the antigen, formation of immune complexes, and renal function (9). The estimated molecular masses of circulating antigens in patients with IA varies from 31 to 200 kDa (12), and in early animal studies, 35% of radiolabeled GM was concentrated in the liver 1 h after injection, with the same amount excreted in the urine over a period of 24 h (1). Although urine has been shown to be inferior to serum for GM testing (14), GM appears in urine promptly after infection and can be detected by commercially available antigen detection tests.
Recently, some authors have also advocated the use of the Platelia Aspergillus enzyme immunoassay for monitoring of therapeutic response and evaluation of prognosis. Decline of GM antigenemia has indeed been shown to correlate in experimental pulmonary aspergillosis with improved odds of survival, reduced residual fungal burden, decreased organism-mediated pulmonary injury, and resolution of pulmonary infiltrates in response to antifungal therapy (10). Consistent with observations in animals, some authors have suggested that the clinical course of IA and the effectiveness of antifungal therapy in patients with IA may be monitored by clearance of antigen from serum or rebound of antigen levels (2, 8). Boutboul et al. retrospectively evaluated the kinetics of the GMI and correlated serum GMI values to therapeutic outcomes for allogeneic stem cell recipients treated for IA. GMI values were stable in patients showing a clinical response to antifungal therapy and rose in patients who were without evidence of response to therapy. An increase in GMI of at least 1.0 above baseline during the first week of observation was predictive of treatment failure (2). Marr et al. further demonstrated a positive correlation between tissue fungal burden and the level of serum antigenemia (8). Other studies have suggested that the course of antigenemia might be affected by treatment with mold-active antifungal agents (7, 11), with clinical response to therapy corresponding to a reduction in circulating GM. Aspergillus antigenemia is closely related to angioinvasiveness (9), and it appears that persistently elevated antigen levels suggest disease progression. However, little is known about the kinetics of GM antigen in patients with IA and renal failure or those undergoing hemodialysis.
The clinical case described above demonstrates an unusual situation, with rising and persistently elevated serum GM levels in the presence of objective clinical improvement as evidenced by resolution of the mandibular mass and improvement in radiographic appearance of the lung, brain, and kidney lesions. The serum GMI values remained high despite clinical improvement and hemodialysis, and the dialysate was negative for GM, indicating that the GM could not be cleared. Although the possibility of subclinical residual infection as the reason for persistent antigenemia cannot be ruled out, it is highly unlikely that this would explain these high GMIs, which are usually seen in invasive disease and/or treatment failure. Some conditions have been associated with false positive results, with the administration of piperacillin/tazobactam being the most recently recognized factor (13, 14). In our case, the patient did not receive this antibiotic.
The ability to eliminate molecules by hemodialysis depends to a large extent on the pore size of the dialysis membrane. The molecular mass cutoff for large-pore (or high-flux) membranes, including the one used here, is generally around 5 to 10 kDa. Therefore, hemodialysis systems are unable to clear larger molecules such as Aspergillus GM from the circulation. To our knowledge, the kinetics of GM antigen in patients undergoing hemodialysis has not been systematically documented.
This report documents a case of persistence of Aspergillus antigenemia secondary to renal failure in a patient with clinically improving IA. GM continued to accumulate in the serum, despite multiple hemodialysis treatments. This previously undescribed phenomenon may have significant implications, as renal impairment is relatively common in IA patients. In such patients, a rise in or persistent elevation of serum GM levels should be interpreted with caution. In this setting, while the GM assay may be of continued value in the initial diagnosis of IA, it may have diminished utility in the assessment of therapeutic responsiveness. Any results must be correlated with clinical and radiographic findings, thereby sparing these vulnerable patients the added risks of unwarranted, frequently invasive tests and changes of therapy.
Acknowledgments
We thank the staff of the bone marrow transplantation team at St. Jude Children's Research Hospital.
This work was supported in part by National Cancer Institute Cancer Center Support CORE grant P30 CA 21765 and by the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.Bennett, J. E., M. M. Friedman, and B. Dupont. 1987. Receptor-mediated clearance of Aspergillus galactomannan. J. Infect. Dis. 155:1005-1010. [DOI] [PubMed] [Google Scholar]
- 2.Boutboul, F., C. Alberti, T. Leblanc, A. Sulahian, E. Gluckman, F. Derouin, and P. Ribaud. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: increasing antigenemia is associated with progressive disease. Clin. Infect. Dis. 34:939-943. [DOI] [PubMed] [Google Scholar]
- 3.Dupont, B., M. Huber, S. J. Kim, and J. E. Bennett. 1987. Galactomannan antigenemia and antigenuria in aspergillosis: studies in patients and experimentally infected rabbits. J. Infect. Dis. 155:1-11. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. 16 May 2003, posting date. FDA clears rapid test for aspergillus infection. FDA News. [Online.] http://www.fda.gov/bbs/topics/NEWS/2003/NEW00907.html.
- 5.Fukuda, T., M. Boeckh, R. A. Carter, B. M. Sandmaier, M. B. Maris, D. G. Maloney, P. J. Martin, R. F. Storb, and K. A. Marr. 2003. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood 102:827-833. [DOI] [PubMed] [Google Scholar]
- 6.Maertens, J., J. Van Eldere, J. Verhaegen, E. Verbeken, J. Verschakelen, and M. Boogaerts. 2002. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J. Infect. Dis. 186:1297-1306. [DOI] [PubMed] [Google Scholar]
- 7.Maertens, J., J. Verhaegen, K. Lagrou, J. Van Eldere, and M. Boogaerts. 2001. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood 97:1604-1610. [DOI] [PubMed] [Google Scholar]
- 8.Marr, K. A., S. A. Balajee, L. McLaughlin, M. Tabouret, C. Bentsen, and T. J. Walsh. 2004. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J. Infect. Dis. 190:641-649. [DOI] [PubMed] [Google Scholar]
- 9.Mennink-Kersten, M. A., J. P. Donnelly, and P. E. Verweij. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4:349-357. [DOI] [PubMed] [Google Scholar]
- 10.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, S. Piscitelli, M. Candelario, A. Field-Ridley, N. Avila, J. Bacher, and T. J. Walsh. 2001. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob. Agents Chemother. 45:857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salonen, J., O. P. Lehtonen, M. R. Terasjarvi, and J. Nikoskelainen. 2000. Aspergillus antigen in serum, urine and bronchoalveolar lavage specimens of neutropenic patients in relation to clinical outcome. Scand. J. Infect. Dis. 32:485-490. [DOI] [PubMed] [Google Scholar]
- 12.Sarfati, J., D. G. Boucias, and J. P. Latge. 1995. Antigens of Aspergillus fumigatus produced in vivo. J. Med. Vet. Mycol. 33:9-14. [PubMed] [Google Scholar]
- 13.Walsh, T. J., S. Shoham, R. Petraitiene, T. Sein, R. Schaufele, A. Kelaher, H. Murray, C. Mya-San, J. Bacher, and V. Petraitis. 2004. Detection of galactomannan antigenemia in patients receiving piperacillin-tazobactam: in vitro, in vivo, and clinical correlations. J. Clin. Microbiol. 42:4744-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheat, L. J. 2003. Rapid diagnosis of invasive aspergillosis by antigen detection. Transplant Infect. Dis. 5:158-166. [DOI] [PubMed] [Google Scholar]