Abstract
Routine bacterial cultures of corneal scrapings from seven cats with either ulcerative feline keratitis, keratomalacia, or both yielded colonies which were identified by 16S rRNA gene sequencing as Mycoplasma felis (six cases) and Mycoplasma gateae (one case). Identification of the pathogens allowed the use of less empirical and more organism-specific therapy.
Mycoplasma species are part of the normal flora of the conjunctiva and upper respiratory tract of cats (1, 5, 16, 21, 25). However, Mycoplasma species have been implicated as etiological agents of feline conjunctivitis, lower respiratory tract infections, and polyarthritis (7, 9, 11, 12, 13, 19, 20). Historically, the detection of Mycoplasma in and diagnosis of cases of feline conjunctivitis and ulcerative keratitis have been based on clinical presentation and judgment, the observation of small basophilic inclusion bodies in stained cytology preparations of epithelial cells, and culture of clinical specimens. However, cytology is not specific for Mycoplasma, and culture for Mycoplasma is not a particularly sensitive test. The routine antimicrobial agents (usually triple-antibiotic ointments and gentamicin) used by veterinarians to treat feline ulcerative keratitis empirically are not effective against Mycoplasma. In addition, other antimicrobial agents (e.g., tetracycline and chloramphenicol), if given topically, can cause cats so much irritation that treatment must be discontinued. Therefore, veterinarians and their feline patients could benefit from rapid and accurate identification of Mycoplasma involved in feline ulcerative keratitis so that Mycoplasma-specific antimicrobial agents can be administered as soon as possible.
Clinical presentations.
From March 2000 to March 2004, seven cats with severe stromal ulcerative keratitis, keratomalacia, or both were examined, cultured for bacteria routinely encountered in a clinical microbiology laboratory, and treated by a veterinary ophthalmologist. These cases were unusual because (i) each case presented as severe stromal ulcerative keratitis, keratomalacia, or both and (ii) corneal specimens from each of the cases were culture positive for growth which was suggestive of Mycoplasma. Four cats had a history of recent feline herpetic keratitis or concurrent corneal ulcerations which were typical of feline herpetic keratitis. All of the cats had recently received systemic or topical corticosteroids prior to presentation, or had received either topical or systemic antibiotics which were not effective against Mycoplasma. Three representative cases are presented here.
Case 1.
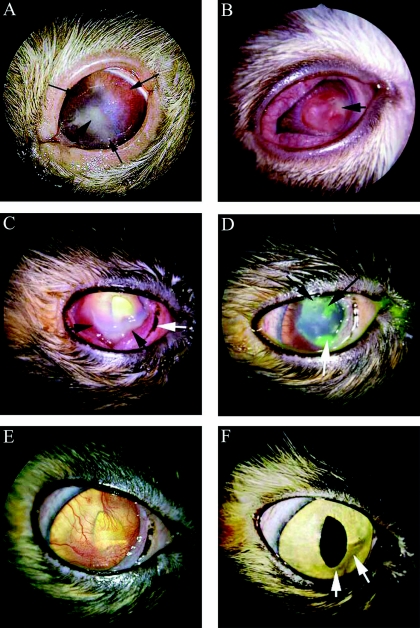
A 15-year-old Birman cat presented with a classical dendritic herpetic ulcer in the right eye and a large corneal ulcer with central necrosis and diffuse corneal vascularization in the left eye (Fig. 1A). Prior to presentation, both eyes had been treated with topical prednisolone for an unknown length of time. The treatment with prednisolone likely had activated a latent herpesvirus infection. Corneal scrapings from the left eye were submitted for routine bacterial culture. The cat was treated with erythromycin, oral interferon, and trifluridine, and showed significant improvement until the cat was euthanized because of an advancing nasal carcinoma.
FIG. 1.
(A) Case 1. Corneal herpetic ulcer and stromal ulceration in the left eye of a 15-year-old Birman cat at presentation. The thin long black arrows delineate the edge of the large ulceration, which is surrounded by corneal vessels. The short black arrow points to the center of the stromal area of corneal necrosis. (B) Case 2. Severe corneal necrosis of a 7-week-old domestic shorthair cat at presentation. The short black arrow indicates the cream-colored infiltrate and corneal necrosis. The entire cornea was vascularized. Raised corneal vascularization is present to the left of the necrotic lesion. (C) Case 3. Severe corneal ulceration in the right eye of a 1.5-year-old domestic shorthair cat at presentation. The conjunctiva is moderately hyperemic, and the nictitating membrane (white arrow) is prolapsed. The cornea is vascularized from the limbus to the stromal ulcer (black arrows). The cream-colored necrotic margins of the ulcer can be seen at the black arrows. (D) Case 3. Improved condition of corneal ulceration 4 days posttreatment The cornea has been stained with fluorescein dye. The white arrow indicates the site of the original ulcer. This ulcer extended under the nictitating membrane. New ulcers, typical of herpetic keratitis, can be seen at the black arrows. (E) Case 3. Improved condition of corneal ulceration 3 weeks posttreatment. The cornea has diffuse superficial vessels. The cornea is now smooth, and no corneal ulcers are present. (F) Case 3. Improved condition of corneal ulceration 84 days posttreatment. Corneal ghost vessels could be seen only with biomicroscopy. A very faint corneal scar is barely discernible (white arrows) at the site of the original stromal ulcer.
Case 2.
Prior to presentation, a 7-week-old domestic shorthair kitten had been treated with a topical triple-antibiotic ointment (neomycin-polymyxin B-bacitracin) for severe conjunctivitis and ulcerative keratitis in both eyes. At presentation, the lesions in both eyes had advanced to severe corneal necrosis, ulceration, chemosis, vascularization, and hyperemia of the conjunctiva (Fig. 1B). Corneal scrapings from both eyes were submitted for routine bacterial culture. The cat was treated with topical ofloxacin, l-lysine, and idoxuridine, and showed significant improvement until the cat was lost to follow-up.
Case 3.
Prior to presentation, a 1.5-year-old domestic shorthair cat had been treated in the right eye with a topical triple-antibiotic ointment, trifluridine, and atropine sulfate for presumed herpetic keratitis. The cat presented with a corneal ulcer, cream-colored corneal stroma, and severe corneal vascularization which extended to a large area of keratomalacia of the right eye (Fig. 1C). A corneal scraping was collected from the necrotic area and submitted for routine bacterial culture. The cat was treated with ofloxacin, azithromycin, atropine sulfate ointment, and l-lysine. Four days after the initial examination and treatment, the condition of the eye had improved (Fig. 1D). However, the cat developed superficial axial corneal herpetic ulcers, the conjunctiva continued to be hyperemic, and the cat was still experiencing severe pain manifested as blepharospasms. The aforementioned medications were continued, and trifluridine solution for herpetic keratitis was added. Twenty-five days after the initial examination, the cornea was smooth and there was no corneal ulceration (Fig. 1E). Sixty-seven days after the initial examination, the cornea was clear, and all medications except l-lysine were discontinued. Forty-two days after discontinuation of all topical medications and 84 days after the initial presentation, the cornea remained clear (Fig. 1F).
Microbiological cultures.
Corneal scrapings from all cats were ground and cultured onto solid tryptic soy medium with 5% sheep blood, onto solid chocolate II medium, and into tryptic soy broth. Cultures were incubated at 35 to 37°C under an atmosphere of 5 to 10% CO2 for 3 days. The presence of Mycoplasma was suggested by the observation of numerous, clear, pinpoint, wet colonies which (i) were growing on the sheep blood medium after 2 or 3 days of incubation, (ii) did not stain with Gram stain, and (iii) were able to be subcultured on the sheep blood medium. The colonies were removed with sterile swabs, placed in sterile 0.85% NaCl, and frozen at −70°C until they were shipped for identification.
16S rRNA gene amplification and sequencing.
A loopful of each purified bacterial isolate was put into 1 ml of distilled water and heated at 95°C for 7 min, the suspension was centrifuged, and 1 μl of supernatant was used for PCR amplification (22). PCR amplification of the first 500 base pairs of the 16S rRNA gene was performed using by using the MicroSeq 500 16S bacterial sequencing kit according to the manufacturer's instructions and as previously described (23). The OpenGene DNA sequencing system (Bayer Corporation, Diagnostics Division, Tarrytown, NY) and two additional primers (0008F [5′-AGA GTT TGA TCC TGG CTC AG-3′] and 0532R [5′-TAC CGC GGC TGC TGG CAC-3′)]; Applied Biosystems, Foster City, CA) were used to determine both directional sequences of the first 527 base pairs of the 16S rRNA gene. Neighbor-joining phylogenetic analysis was performed by online analysis at the Ribosomal Database Pro-ject II site (http://rdp8.cme.msu.edu/html/index.html) and the MicroSeq Database Library (Applied Biosystems, Foster City, CA) as previously described (15, 23). The process of amplifying and sequencing the 16S rRNA gene of most isolates required 1 to 2 days (23).
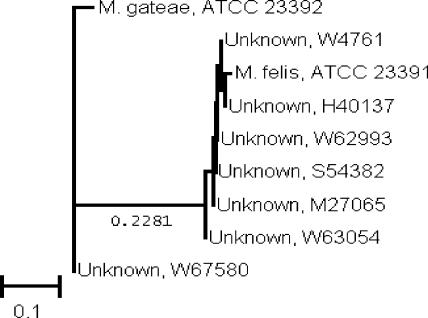
The sequences of the first 500 base pairs of the 16S rRNA gene indicated that six of the isolates were most closely related to Mycoplasma felis and one isolate was most closely related to Mycoplasma gateae (2). A neighbor-joining phylogenetic analysis indicated that the seven isolates had similarities of 98.7 to 99.8% to the ATCC prototype sequences (Fig. 2).
FIG. 2.
Neighbor-joining analysis of DNA sequences from seven Mycoplasma species with homology to previously published rRNA gene sequences of M. felis and M. gateae. Phylogenetic analysis was based on the first 500 base pairs of the 16S rRNA gene sequences. The scale indicates relative phylogenetic distance.
The use of 16S rRNA gene sequencing to identify the seven isolates as M. felis and M. gateae allowed the veterinary ophthalmologist to (i) detect and identify a possible pathogen capable of permanently altering the vision of the cats and (ii) treat the cats with more organism specific therapy.
Identification of Mycoplasma species grown from clinical specimens traditionally has been performed only by reference laboratories, employing methods which use biochemical reactions and/or species-specific specific antisera in procedures such as growth inhibition, immunofluorescence, immunoperoxidase staining, and immunoblotting (3, 6, 8, 18, 24, 25). However, for most of these methods, species-specific antisera might not be commercially available. In addition, biochemical reactions usually are not sufficient for definitive identification of Mycoplasma species, provide only a presumptive identification, or limit identification to only a few Mycoplasma species pathogenic for humans (3). Recently, molecular methods, e.g., PCR and 16S rRNA gene sequencing techniques, have been used to identify Mycoplasma species isolated from humans and animals (2, 4, 10, 14, 17, 24).
Clinical utility of 16S rRNA gene sequencing.
In the seven cases presented here, and potentially in other, similar cases, 16S rRNA gene sequencing appears to be particularly well suited as a clinical laboratory diagnostic tool because use of viable organisms is not necessary, only minute amounts of Mycoplasma growth are required, primers that are specific for well-characterized, widely known conserved bacterial 16S rRNA genes are available, and gene banks with enough information to identify species-specific sequences within 16S rRNA genes are available. In addition, the turnaround time from receipt of the specimen to identification is clinically relevant (2 days or less). Once the identification is available, a veterinarian can decide either to continue empirical therapy or to initiate Mycoplasma-specific therapy.
16S rRNA gene sequencing is neither widely available nor routinely performed in clinical laboratories. However, as the technique becomes increasingly more available and as veterinarians become increasingly more aware of the technique, 16S rRNA gene sequencing could become a valuable method for use in the timely and accurate laboratory detection and identification of important isolates thought to be Mycoplasma in cases of feline keratitis.
Mycoplasma feline keratitis.
This is the first report of M. felis and M. gateae being detected in and associated with cases of feline ulcerative keratitis. Although M. felis and M. gateae likely were not the primary etiological agents in these cases, M. felis and M. gateae were believed to be clinically relevant and to be significant threats to the cats' vision. Two observations suggest that M. felis and M. gateae could be clinically relevant pathogens in feline ulcerative keratitis: (i) all of the cats' ocular conditions improved only when the cats were treated with Mycoplasma-specific antimicrobial agents, and (ii) all of the cats from whom Mycoplasma was cultured had a unique presentation: severe stromal ulcerative keratitis, keratomalacia, or both.
Additional research is needed to prove that M. felis and M. gateae are etiological agents of feline ulcerative keratitis and to determine if this infection always manifests as atypical herpes keratitis.
Nucleotide sequence accession numbers.
The partial 16S rRNA gene sequences of the seven strains have been deposited in the GenBank sequence database under accession numbers AY769698 to AY769703 (M. felis) and AY769704 (M. gateae).
REFERENCES
- 1.Blackmore, D. K., A. Hill, and O. F. Jackson. 1971. The incidence of mycoplasmas in pet colony maintained cats. J. Small Anim. Prac. 12:207-216. [DOI] [PubMed] [Google Scholar]
- 2.Brown, D. R., G. S. McLaughlin, and M. B. Brown. 1995. Taxonomy of the feline mycoplasmas Mycoplasma felifaucium, Mycoplasma feliminutum, Mycoplasma felis, Mycoplasma gateae, Mycoplasma leocaptivus, Mycoplasma leopharyngis, and Mycoplasma simbae by 16S rRNA gene sequence comparisons. Int. J. Syst. Bacteriol. 45:560-564. [DOI] [PubMed] [Google Scholar]
- 3.Brown, M. B., P. Gionet, and D. F. Senior. 1990. Identification of Mycoplasma felis and Mycoplasma gateae by an immunobinding assay. J. Clin. Microbiol. 28:1870-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalker, V. J., W. M. A. Owen, C. J. I. Paterson, and J. Brownlie. 2004. Development of a polymerase chain reaction for the detection of Mycoplasma felis in domestic cats. Vet. Microbiol. 100:77-82. [DOI] [PubMed] [Google Scholar]
- 5.Chandler, J. C., and M. R. Lappin. 2002. Mycoplasmal respiratory infections in small aminals: 17 cases (1988-1999). J. Am. Anim. Hosp. Assoc. 38:111-119. [DOI] [PubMed] [Google Scholar]
- 6.Clyde, W. A. 1964. Mycoplasma species identification based on growth inhibition by specific antisera. J. Immunol. 94:1451-1458. [PubMed] [Google Scholar]
- 7.Crisp, M. S., S. J. Birchard, A. E. Lawrence, and J. Fingeroth. 1987. Pulmonary abscess caused by a Mycoplasma species in a cat. J. Am. Vet. Med. Assoc. 191:340-342. [PubMed] [Google Scholar]
- 8.DelGuidice, R. A., and H. E. Hopps. 1977. Microbiological methods and fluorescent microscopy for the direct demonstration of Mycoplasma infection of cell cultures, p. 57-59. In G. J. McGarrity, D. G. Murphy, and W. W. Nichols (ed.), Mycoplasma infection of cell cultures. Plenum Publishing Corp., New York, N.Y.
- 9.Foster, S. F., P. Martin, J. A. Braddock, and R. Malik. 2004. A retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995-2000). J. Feline Med. Surg. 6:189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilroy, C. G., and D. Taylor-Robinson. 1999. Detection of genital mycoplasmas. Methods Mol. Med. 20:81-102. [DOI] [PubMed] [Google Scholar]
- 11.Haesebrouck, F., L. A. Devriese, B. van Rijssen, and E. Cox. 1991. Incidence and significance of isolation of Mycoplasma felis from conjunctival swabs of cats. Vet. Microbiol. 26:95-101. [DOI] [PubMed] [Google Scholar]
- 12.Hooper, P. T., L. A. Ireland, and A. Carter. 1985. Mycoplasma polyarthritis in a cat with probable severe immune deficiency. Aust. Vet. J. 62:352. [DOI] [PubMed] [Google Scholar]
- 13.Hoskins, J. D., and J. Taboata. 1994. Specific treatment of infectious causes of respiratory disease in dogs and cats. Vet. Med. 89:443-452. [Google Scholar]
- 14.Johnson, L. R., N. L. Drazenovich, and J. E. Foley. 2004. A comparison of routine culture with polymerase chain reaction technology for the detection of Mycoplasma species in feline nasal samples. J. Vet. Diagn. Investig. 16:347-351. [DOI] [PubMed] [Google Scholar]
- 15.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randolph, J. F., N. S. Moise, J. M. Scarlett, S. J. Shin, J. T. Blue, and J. R. Corbett. 1993. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and of mycoplasmal recovery from pharyngeal swab specimens in cats with or without pulmonary disease. Am. J. Vet. Res. 54:897-900. [PubMed] [Google Scholar]
- 17.Razin, S. 1994. DNA probes and PCR in diagnosis of mycoplasmal infections. Mol. Cell. Probes 8:497-511. [DOI] [PubMed] [Google Scholar]
- 18.Rosendal, S. 1975. Canine mycoplasmas: serological studies of type and reference strains with a proposal for the new species, Mycoplasma opalescens. Acta Pathol. Microbiol. Scand. Sect. B 83:463-470. [DOI] [PubMed] [Google Scholar]
- 19.Rosendal, S. 1979. Canine and feline mycoplasmas, p. 217-234. In J. G. Tully and R. F. Whitcomb (ed.), The mycoplasmas, vol. 2. Academic Press, New York, N.Y. [Google Scholar]
- 20.Tan, R. J. S. 1974. Susceptibility of kittens to Mycoplasma felis infection. Jpn. J. Exp. Med. 44:235-240. [PubMed] [Google Scholar]
- 21.Tan, R. J. S., E. W. Lim, and B. Ishak. 1977. Ecology of mycoplasmas in clinically healthy cats. Aust. Vet. J. 53:515-518. [DOI] [PubMed] [Google Scholar]
- 22.Tang, Y. W., M. K. Hopkins, C. P. Kolbert, P. A. Hartley, P. J. Severance, and D. H. Persing. 1998. Bordetella holmesii-like organisms associated with septicemia, endocarditis, and respiratory failure. Clin. Infect. Dis. 26:389-392. [DOI] [PubMed] [Google Scholar]
- 23.Tang, Y. W., A. Von Graevenitz, W. G. Waddington, M. K. Hopkins, D. H. Smith, H. J. Li, C. P. Kolbert, S. O. Montgomery, and D. H. Persing. 2000. Identification of coryneform bacterial isolates by ribosomal DNA sequence analysis. J. Clin. Microbiol. 38:1676-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waites, K. B., C. M. Bebear, J. A. Robertson, D. F. Talkington, and G. E. Kenny. 2001. Cumitech 34, Laboratory diagnosis of mycoplasmal infections. Coordinating ed., F. S. Nolte. American Society for Microbiology, Washington, D.C.
- 25.Waites, K. B., Y. Rikihisa, and D. Taylor-Robinson. 2003. Mycoplasma and Ureaplasma, p. 972-990. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.