Abstract
The performances of the gelatin particle agglutination test (GPAT) and enzyme-linked immunosorbent assay (ELISA) for the diagnosis of strongyloidiasis with reference to the results of the agar plate culture technique (APCT) were evaluated with samples from 459 individuals from communities in northeast Thailand where strongyloidiasis is endemic. The prevalence of strongyloidiasis in five sample groups determined by GPAT varied between 29.3 and 61.5% (mean, 38.8%). ELISA and APCT, employed concurrently, gave lower prevalence rates of 27.5% (range, 21.6 to 42.1%) and 22.7% (range, 12.7 to 53.8%), respectively. By using APCT as the standard method, the sensitivity of GPAT was generally higher than that of ELISA (81 versus 73%). The specificity of GPAT was slightly lower than that of ELISA (74 versus 86%). The resulting GPAT titers exhibited positive linear relationships with the ELISA values (optical density at 490 nm) (P < 0.05), which suggests that the GPAT titer also reflects the levels of specific antibody comparable to those reflected by the ELISA values. Based on the relative ease and simplicity of use of the technique as well as the acceptable rates of sensitivity and specificity of the test, GPAT is more practical for screening for strongyloidiasis than the conventional ELISA.
Strongyloides stercoralis is an intestinal nematode with a worldwide distribution, especially in tropical and subtropical areas (9). It has the unique ability to induce hyperinfection and disseminated infection, particularly in individuals immunosuppressed because of long-term or high-dose corticosteroid chemotherapeutic treatments. To avoid such a condition, employment of a sensitive and specific means of diagnosis and timely curative anthelmintic treatment is necessary. Conventional fecal examination methods are often unreliable for demonstration of the characteristic S. stercoralis larvae due to the low intensity of the infection and the low fecundity of the parasite (20). Improved parasitological methods, particularly the agar plate culture technique (APCT), considerably increased the detection rate, and it is currently regarded as the most sensitive fecal examination technique (1, 15). However, coprodiagnosis has drawbacks, and repeat fecal examinations three or more times is required to achieve a reliable diagnosis of the infection (23). In this regard, serological diagnosis, particularly the enzyme-linked immunosorbent assay (ELISA), has considerable advantages over parasitological methods, since only a single serum sample is required; and hence, it has been widely used as the test of choice for the diagnosis of strongyloidiasis (10, 21). However, ELISA must be performed in a well-equipped laboratory with specific equipment and trained technicians. Alternatively, the gelatin particle agglutination test (GPAT) is simpler to perform, as it requires no special equipment and it is less labor-intensive and much cheaper than ELISA. It has been widely used as a tool for the screening of several infectious diseases, such as leprosy (12), Mycobacterum leprae infection (7), human immunodeficiency virus infection (30), as well as strongyloidiasis (25).
In our recent study (28), we demonstrated by APCT that the prevalence of strongyloidiasis in rural communities in northeast Thailand is high and surpasses that of Opisthorchis viverrini infection, which has predominated in this area for decades. In that study, application of ELISA as a supplementary method of serodiagnosis revealed as many as 46% of additional positive cases. Therefore, serodiagnosis has become an important method of choice for the diagnosis of strongyloidiasis in areas of endemicity.
The aim of the present work was to assess the performance and effectiveness of GPAT and ELISA for the diagnosis of human strongyloidiasis in northeast Thailand by using APCT as the reference method.
MATERIALS AND METHODS
Sample population.
The sample population included villagers from three rural communities from the previous study (28), namely, Haui Ma Tho (HMT) in Kalasin Province and Nong Kae (NEK) and Chonnabot (KT) in Khon Kaen Province, and two additional communities, namely, Ban Moung (BM) and Nam Pong (NP) in Khon Kaen Province; all are in northeast Thailand. The total sample of 459 individuals, from whom paired fecal and serum specimens were available, were recruited for this study. The clinical samples were collected in the field and kept in an insulated icebox for transport to the laboratory.
Serum samples.
Three groups of serum samples were analyzed in this study. Group I consisted of 459 serum samples from the populations in the communities in northeast Thailand described above. Group II consisted of serum samples from people with one other parasite infection but in whom S. stercoralis was not demonstrated by APCT. This group included 14 individuals infected with O. viverrini, 28 individuals infected with Clonorchis sinensis, 23 individuals infected with echinostomes, 16 individuals infected with minute intestinal flukes (Phaneropsolus and Prostodendrium spp.), 12 individuals infected with Taenia, 34 individuals with hookworms, 1 individual infected with Angiostrongylus cantonensis, and 10 individuals infected with Giardia intestinalis. These serum samples were used to identify the cross-reactivity of GPAT and ELISA between S. stercoralis and other parasites, and no subjects with multiple infections were recruited into this group. Group III consisted of 50 serum samples from noninfected, healthy controls from northeast Thailand used to establish the cutoff values for serological diagnosis by both GPAT and ELISA.
Fecal examination methods.
The presence of S. stercoralis in the fecal samples was determined by the agar plate culture technique with approximately 4 g of feces, as described by Koga et al. (15). The other 2 g of fecal specimen was simultaneously processed for the modified formalin-ethyl acetate concentration technique (6) to search for concurrent parasite infections. Positive results by either method were taken as positive for strongyloidiasis and were taken as a “gold standard” diagnosis.
Antigen preparation.
The method for antigen preparation of the crude somatic extract of the third-stage filariform larvae (L3) of S. stercoralis was performed as described previously (28). In brief, the larvae were originally obtained from cultures of feces from an infected patient by the filter paper culture technique of Harada and Mori (11). After 4 to 6 days of culture of S. stercoralis-positive feces at room temperature, the larvae were harvested and washed several times in normal saline. When the amount of larvae was adequate, usually 2 to 3 ml, the larval pellet was suspended in phosphate-buffered saline (PBS) containing protease inhibitors and was disrupted by sonication (4). The homogenate was left at 4°C overnight and centrifuged at 17,266 × g for 30 min at 4°C. The supernatant was separated and used as the antigen, and the protein concentration was measured by the method of Lowry et al. (18) and kept at −20°C. The antigen was used for serodiagnosis by GPAT and ELISA.
ELISA.
The indirect (optical density [OD]-based) ELISA was performed as described previously (28). The protocol was similar to those described by other investigators (4, 29). The microplates (Maxisorb; Nunc, Roskilde, Denmark) were coated with 100 μl of 2 μg/ml of the antigen in carbonate buffer (pH 9.6) at 4°C overnight. After removal of unbound antigen by three 3-min washes with PBS containing 0.05% Tween (PBST), 200 μl of 5% skim milk in PBS was added to each well and the plate was kept at room temperature for 1 h. The wells were washed three times for 3 min each time with PBS, 100 μl of the serum samples (dilution, 1:1,500 in 2% skim milk in PBST, in duplicate) was added to the plate, and the plate was incubated for 1 h at 37°C. After the plate was washed three times, 100 μl of horseradish peroxidase anti-human immunoglobulin G (IgG; Zymed) in 2% skim milk in PBST (dilution, 1:5,000) was added to each well, and the plate was incubated at 37°C for 1 h. After the plate was washed, 100 μl of substrate solution (0.4 mg/ml of o-phenylenediamine, 0.001% H2O2 in citrate buffer, pH 5.0) was added to each well. After 15 min at 37°C, the reaction was stopped with 100 μl of 4 N H2SO4. The optical density of the reaction was measured at 490 nm with a microplate reader (Dynatech MR 5000). The ELISA result was judged to be positive when the OD was greater than the cutoff OD of 0.26. The cutoff OD was calculated from the mean OD for 50 negative control cases plus 3 standard deviations.
Gelatin particle agglutination assay.
GPAT was performed as described by Sato et al. (25). The unsensitized gelatin particles (Fujirebio Inc., Japan) were suspended in PBS (pH 6.4), washed four times in PBS, and adjusted to a concentration of 3%. Equal volumes of 0.01 mg/ml of tannic acid in PBS were added to the gelatin suspension and kept in ice for 15 min and mixed every 5 min. Then, the gelatins were washed three times with cold PBS and readjusted to the original concentration (3%). Based on the preliminary experiment, an equal volume of 60 μg/ml S. stercoralis antigen was then mixed with the gelatin suspension and the antigen absorption reaction was allowed to take place for 15 min in a 37°C water bath with continuous mixing. Any excess antigen was removed by washing four times with 0.2% bovine serum albumin (BSA) in PBS, and the final concentration of the gelatin suspension was adjusted to 1% and kept at 4°C until required for testing. The unsensitized gelatin particles for the control experiments were also prepared in the same manner, except that normal saline was used instead of the antigen. GPAT was performed in a U-bottom plate with a twofold dilution of 25 μl of the serum samples with 0.2% BSA in PBS and 25 μl of the 1% gelatin particle suspension. After gentle mixing of the contents of the plate, the plate was left at room temperature for 2 h prior to interpretation of the test result. The agglutination pattern was considered positive when the smooth mat of particles spread uniformly and covered the entire bottom of the well, and the edge of the mat was sometimes folded. If the gelatin particles were concentrated at the center of the well, it was interpreted as a negative reaction. If any equivocal pattern appeared, the samples were retested and the results were read by two individuals for final interpretation of the results. The highest antibody dilution that gave a positive agglutination reaction was taken as the antibody titer. The cutoff value for a positive titer for GPAT was 32, and this was obtained from the tests done with 50 negative control serum samples. For quality control, in each run of the GPAT, the titer of the positive control used for the entire experiment should be 1:1,024 ±1 doubling dilution. In addition, wells with unsensitized particles in the presence of the test serum and the sensitized particle in the presence of PBS should give negative reactions.
This study was reviewed and approved by the Ethics Committee on Human Research, Faculty of Medicine, Khon Kaen University.
Statistical analyses.
The OD and antibody titers were not normally distributed, and thus, the data were log transformed. Kendall's tau correlation coefficient was used to assess the relationship between the OD values and the antibody titers. Statistical tests were performed with the SPSS v.10 statistical package.
RESULTS
The prevalences of strongyloidiasis in rural northeast Thai communities measured by APCT, ELISA, and GPAT are presented in Table 1. GPAT gave a consistently higher rate of positivity in each community than the other two techniques. The prevalence in each community as determined by GPAT ranged from 4 to 27% and from 8 to 28% higher than those determined by ELISA and APCT, respectively. The overall prevalence by GPAT was 38.7%, while that by ELISA was 27.5%; and the lowest was 22.7%, as determined by APCT. In general, ELISA yielded higher prevalence rates than APCT in most groups of subjects except in one community (KT), where the rate of positivity by APCT was higher than that by ELISA.
TABLE 1.
Comparison of rates of positivity for S. stercoralis among populations sampled in northeast Thailand as determined by APCT, ELISA, GPAT
| Village | Total no. of patients | No. (%) positive by:
|
||
|---|---|---|---|---|
| APCT | ELISA | GPAT | ||
| HMT | 57 | 16 (28.1) | 24 (42.1) | 32 (56.1) |
| BM | 182 | 44 (24.1) | 45 (24.7) | 69 (37.9) |
| KT | 26 | 14 (53.8) | 9 (34.6) | 16 (61.5) |
| NP | 157 | 20 (12.7) | 40 (25.4) | 46 (29.3) |
| NEK | 37 | 10 (27.0) | 8 (21.6) | 15 (40.5) |
| Total | 459 | 104 (22.7) | 126 (27.5) | 178 (38.8) |
The sensitivity and specificity of GPAT and ELISA with reference to the results of APCT as the gold standard among subjects from the different communities are listed in Table 2. The sensitivities of GPAT were greater than those of ELISA in all the communities studied; and the overall sensitivity was 81% by GPAT, while that by ELISA was 73%. In contrast, the overall specificity of GPAT (74%) was slightly lower than that of ELISA (86%). The positive predictive value of ELISA was greater than that of GPAT, while the negative predictive values were similar between the two methods of serodiagnosis.
TABLE 2.
Sensitivities and specificities of ELISA and GPAT for diagnosis of S. stercoralis infection in the population sampled in northeast Thailanda
| Diagnostic method | Village | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| ELISA | HMT | 75 | 71 | 50 | 88 |
| BM | 75 | 91 | 73 | 92 | |
| KT | 43 | 75 | 67 | 53 | |
| NP | 85 | 83 | 43 | 97 | |
| NEK | 80 | 100 | 100 | 93 | |
| Overall | 73 | 86 | 60 | 92 | |
| GPAT | HMT | 81 | 81 | 41 | 88 |
| BM | 75 | 75 | 41 | 88 | |
| KT | 79 | 79 | 69 | 70 | |
| NP | 90 | 90 | 39 | 98 | |
| NEK | 90 | 90 | 60 | 95 | |
| Overall | 81 | 81 | 47 | 93 |
The results of APCT, performed with samples from the same individuals, were used as the reference. The analysis was based on a total sample size of 459 individuals.
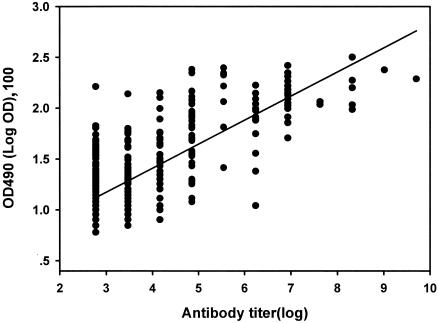
Statistical analyses of the relationship between the antibody titer by GPAT and the ELISA values (OD at 490 nm [OD490]) for each community revealed a significant positive correlation (Kendall's tau correlation coefficient = 0.44 to 0.63; P < 0.01; R = 0.78 to 0.88). The relationship of the overall data for the antibody titers by GPAT and the ELISA values was also significant (P < 0.01) (Fig. 1). A highly variable relationship was observed at an antibody titer <256, but a more proportionate correlation occurred at titers >256.
FIG. 1.
Relationship between antibody titer by GPAT and ELISA value (OD490) for samples from 459 individuals in five localities in northeast Thailand. The data shown are the observed values, and the solid line is the linear regression line with a slope of 0.241 and a y-axis intercept of 0.45 (t = 25.7; P < 0.001; R = 0.77).
To determine the specificity of the test, sera from subjects infected by other parasites common in the area and the available sera were analyzed for cross-reactivity in the GPAT and ELISA. Cross-reactions identified by GPAT and ELISA were found with the sera of individuals harboring O. viverrini and hookworms (Table 3). No cross-reaction was observed for sera from individuals infected with Taenia spp., echinostomes, minute intestinal fluke, Clonorchis sinensis, Angiostrongylus cantonensis, and Giardia intestinalis.
TABLE 3.
Comparison of cross-reactions of S. stercoralis antigen with sera from patients with other parasitic infections determined by GPAT and ELISAa
| Parasite | Total no. of patients | No. (%) positive
|
|
|---|---|---|---|
| GPAT | ELISA | ||
| Opisthorchis viverrini | 14 | 1 (7.1) | 1 (7.1) |
| Clonorchis sinensis | 28 | 0 (0) | 0 (0) |
| Echinostomes | 23 | 0 (0) | 0 (0) |
| Minute intestinal flukes | 16 | 0 (0) | 0 (0) |
| Taenia spp. | 12 | 0 (0) | 0 (0) |
| Hookworms | 34 | 2 (5.7) | 1 (2.8) |
| Angiostrongylus cantonensis | 1 | 0 (0) | 0 (0) |
| Giardia intestinalis | 10 | 0 (0) | 0 (0) |
| Total | 138 | 3 (2.2) | 2 (1.4) |
The GPAT result was positive when the titer was ≥32. The ELISA result was positive when the OD490 was ≥0.26.
Within the group of individuals APCT negative for S. stercoralis (n = 355), 94 of them (26.5%) were GPAT positive. A comparison of the rates of concurrent parasitic infections among the GPAT-negative and GPAT-positive groups is shown in Table 4. The rates of infection with the two parasites shown to result in cross-reactions between GPAT and ELISA (Table 3), namely, O. viverrini and hookworm, were comparable. The other parasites, i.e., minute intestinal fluke, echinostomes, Taenia, and G. intestinalis, occurred more frequently in the GPAT-positive individuals than in their GPAT-negative counterparts. The total rate of infection in GPAT-positive individuals was also higher than that in the GPAT-negative group.
TABLE 4.
Rates of parasitic infections among subjects who were negative for strongyloidiasis by APCTa
| Parasite | No. (%) of subjects with the following GPAT result:
|
|
|---|---|---|
| Negative (n = 261) | Positive (n = 94) | |
| O. viverrini | 22 (8.42) | 8 (8.51) |
| Hookworms | 48 (18.39) | 16 (17.02) |
| Minute intestinal fluke | 13 (4.98) | 14 (14.89) |
| Echinostomes | 1 (0.38) | 4 (4.26) |
| Taenia | 1 (0.38) | 2 (2.12) |
| Hymenolepis diminuta | 1 (0.38) | 0 (0) |
| Giardia intestinalis | 16 (6.13) | 10 (10.63) |
| Total | 102 (39.08) | 54 (57.4) |
Samples from 355 subjects were tested. These subjects were classified as serologically negative and positive by GPAT. Coprodiagnosis for the concurrent parasitic infections was determined by formalin-ethyl acetate concentration technique (FECT), except for hookworm, for which the results of both APCT and FECT were combined.
DISCUSSION
In our study, GPAT for the diagnosis of strongyloidiasis in communities in northeast Thailand where the disease is endemic was established, and its efficacy was compared with that of ELISA by using coprodiagnosis as the reference method. In general, GPAT gave higher rates of positivity than ELISA and the parasitological method, APCT. It is interesting that all of the proven cases of strongyloidiasis based on the results of APCT were positive by GPAT, and the sensitivity of GPAT was comparable to or better than that of conventional ELISA in terms of both the rate of positivity and the sensitivity. However, a slightly lower specificity was obtained compared with that of ELISA, and the rates of cross-reactions with hookworms by GPAT were also higher than those by ELISA. The rates of cross-reactivity with O. viverrini and S. stercoralis were comparable between ELISA and GPAT. In this study, the activities of the sensitized gelatin particles were relatively stable when they were kept at 4°C for at least 3 months. With further study on the method of long-term preservation and the shelf life of the sensitized gelatin particles, GPAT could be a promising candidate for mass screening for strongyloidiasis in areas of endemicity. Such screening would help detect patients with unidentified chronic infections and high-risk individuals for a more definite diagnosis, thus reducing costs and the time needed for repeat stool examinations for the diagnosis of strongyloidiasis.
The high variability in the prevalence of S. stercoralis encountered among different communities in this study was not unexpected, because a recent study in northeast Thailand (n = 1,233) also found a similar pattern of infection (13), with the prevalences in different provinces ranging from 13.3 to 61%. From that study (13), the prevalence of S. stercoralis infection in Khon Kaen was 16.5%, whereas it ranged from 12.7 to 53.8% in four communities (BM, KT, NP, and NEK) in the same province. Likewise, the prevalence in Kalasin Province was 61%, whereas it was 28.1% in HMT, which is a community in Kalasin Province. Therefore, the background prevalence of S. stercoralis in each province may not be the sole factor contributing to the differences in prevalence observed in the communities evaluated in this study.
The observed cross-reaction of GPAT with the sera from patients infected with O. viverrini and hookworms was not entirely unexpected, since several species of parasites coexist in the area and it is uncommon to find individuals infected with a single species of parasite. The observed cross-reactivity was therefore probably associated with multiple infections or previous exposure to the helminths and was partially due to the nature of the crude somatic extract of the antigen of S. stercoralis used in GPAT and ELISA. A similar cross-reactivity of GPAT to hookworms and the agents responsible for angiostrongyliasis, sparganosis, and cysticercosis was reported previously (25). The sera of individuals with occult filariasis and schistosomiasis also showed consistent cross-reactions by the indirect hemagglutination test (8). The use of the partially purified third-stage larval antigen for ELISA has slightly improved the efficiency of the ELISA over that obtained with crude or unpurified antigen fractions (19). Recently, in the immediate hypersensitivity skin test for strongyloidiasis with crude and excretory-secretory antigen, cross-reaction with filaria-positive sera was also encountered (22). These cross-reactions may be minimized by using a more complicated Western blot analysis, whose sensitivity and specificity are greatly increased (5, 17). It appears, therefore, that the identification of a specific antigen(s) is the precondition for elimination of these cross-reactions with other helminths (27).
Additionally, in the case of infection with multiple parasites, preincubation of the sera with certain parasite antigens to reduce the cross-reacting antibodies improved the specificity of the serological test by ELISA (4). Those authors found that for patients with hookworm infections, when the sera were preincubated with an extract of Ascaris lumbricoides, the cross-reacting IgG could be effectively reduced. It remains to be determined whether this approach will similarly improve the specificity of GPAT.
In our study, a single fecal sample from each subject was collected for examination, and thus, GPAT gave the correct test results not only for all of the fecal sample-positive individuals but also for the fecal-negative individuals. Although triple stool examinations are recommended (23), it would be interesting to investigate how many more fecal sample-positive cases could be retrieved by repeated fecal sampling for analysis by APCT (among the fecal sample-negative individuals). Although concurrent parasitic infections were commonly observed in this study, two major cross-reactive parasites, O. viverini and hookworm, occurred in similar percentages of GAPT-positive and -negative individuals. It is unlikely that they may cause false-positive test results. However, the influence of higher rates of infection with other parasites for the GPAT-positive group than the GPAT-negative group upon the false-positive test result is difficult to rule out. More study is needed to resolve this issue. By contrast, in the case of ELISA, the rates of false-negative test results for patients with proven strongyloidiasis ranged from 16 to 20% (2, 3, 24). Such findings indicate that the wide spectrum of host immune responses against S. stercoralis infection influences the outcome of the serodiagnostic assays. This difference in the individual response to infection may affect not only serodiagnosis but also the effectiveness of drug treatment. For example, elevated S. stercoralis-specific IgG4 has been found to be associated with albendazole resistance (26) or ivermectin resistance (16).
The finding that the antibody titer by GPAT positively correlated with the OD values obtained by ELISA suggested that GPAT provided not only a qualitative measurement but also a semiquantitative measurement. Whether the rate of change in these values after chemotherapy will be proportional and which measurement is a better marker of curative treatment is not known. ELISA was employed to evaluate the outcome of treatment (14), but no investigation with GPAT has been done.
In conclusion, the results of our study demonstrated that GPAT is more sensitive than ELISA for the serodiagnosis of strongyloidiasis. The results also demonstrated that GPAT is superior to ELISA. In particular, GPAT is easy to perform and there is no need for specialized equipment. In addition, the gelatin particles have many advantages as an antigen carrier, e.g., in handling and reading of the resulting pattern. The test was considered to be more convenient than the conventional ELISA for mass screening for strongyloidiasis in an area of endemicity and may be applied as a routine laboratory test to rule out strongyloidiasis in immunosuppressed patients and patients with malignancies.
Acknowledgments
This study was supported by the Faculty of Medicine and the National Research Council of Thailand.
We thank Fujirebio Inc., Japan, for providing the gelatin particles for this study. We also thank Trevor Petney and Bryan Roderick Hamman for assisting with the English-language presentation of the manuscript. The kind donation of sera from patients with clonorchiasis by Sung-Tae Hong is greatly appreciated.
REFERENCES
- 1.Arakaki, T., M. Iwanaga, F. Kinjo, A. Saito, R. Asato, and T. Ikeshiro. 1990. Efficacy of agar-plate culture in detection of Strongyloides stercoralis infection. J. Parasitol. 76:425-428. [PubMed] [Google Scholar]
- 2.Badaro, R., E. M. Carvalho, R. B. Santos, A. A. Gam, and R. M. Genta. 1987. Parasite-specific humoral responses in different clinical forms of strongyloidiasis. Trans. R. Soc. Trop. Med. Hyg. 81:149-150. [DOI] [PubMed] [Google Scholar]
- 3.Carroll, S. M., K. T. Karthigasu, and D. I. Grove. 1981. Serodiagnosis of human strongyloidiasis by an enzyme-linked immunosorbent assay. Trans. R. Soc. Trop. Med. Hyg. 75:706-709. [DOI] [PubMed] [Google Scholar]
- 4.Conway, D. J., N. S. Atkins, J. E. Lillywhite, J. W. Bailey, R. D. Robinson, J. F. Lindo, D. A. Bundy, and A. E. Bianco. 1993. Immunodiagnosis of Strongyloides stercoralis infection: a method for increasing the specificity of the indirect ELISA. Trans. R. Soc. Trop. Med. Hyg. 87:173-176. [DOI] [PubMed] [Google Scholar]
- 5.Conway, D. J., J. W. Bailey, J. F. Lindo, R. D. Robinson, D. A. Bundy, and A. E. Bianco. 1993. Serum IgG reactivity with 41-, 31-, and 28-kDa larval proteins of Strongyloides stercoralis in individuals with strongyloidiasis. J. Infect. Dis. 168:784-787. [DOI] [PubMed] [Google Scholar]
- 6.Elkins, D. B., M. R. Haswell-Elkins, E. Mairiang, P. Mairiang, P. Sithithaworn, S. Kaewkes, V. Bhudhisawasdi, and T. Uttaravichien. 1990. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in north-east Thailand. Trans. R. Soc. Trop. Med. Hyg. 84:715-719. [DOI] [PubMed] [Google Scholar]
- 7.Escobar-Gutierrez, A., M. E. Amezcua, S. Pasten, F. Pallares, J. V. Cazares, R. M. Pulido, O. Flores, E. Castro, and O. Rodriguez. 1993. Comparative assessment of the leprosy antibody absorption test, Mycobacterium leprae extract enzyme-linked immunosorbent assay, and gelatin particle agglutination test for serodiagnosis of lepromatous leprosy. J. Clin. Microbiol. 31:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gam, A. A., F. A. Neva, and W. A. Krotoski. 1987. Comparative sensitivity and specificity of ELISA and IHA for serodiagnosis of strongyloidiasis with larval antigens. Am. J. Trop. Med. Hyg. 37:157-161. [DOI] [PubMed] [Google Scholar]
- 9.Genta, R. M. 1989. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev. Infect. Dis. 11:755-767. [DOI] [PubMed] [Google Scholar]
- 10.Genta, R. M. 1988. Predictive value of an enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of strongyloidiasis. Am. J. Clin. Pathol. 89:391-394. [DOI] [PubMed] [Google Scholar]
- 11.Harada, Y., and O. Mori. 1955. A new method for culturing hookworm. Yagano Acta Med. 1:177-179. [Google Scholar]
- 12.Izumi, S., T. Fujiwara, M. Ikeda, Y. Nishimura, K. Sugiyama, and K. Kawatsu. 1990. Novel gelatin particle agglutination test for serodiagnosis of leprosy in the field. J. Clin. Microbiol. 28:525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jongsuksuntigul, P., P. M. Intapan, T. Wongsaroj, S. Nilpan, S. Singthong, S. Veerakul, and W. Maleewong. 2003. Prevalence of Strongyloides stercoralis infection in northeastern Thailand (agar plate culture detection). J. Med. Assoc. Thai. 86:737-741. [PubMed] [Google Scholar]
- 14.Kobayashi, J., Y. Sato, H. Toma, M. Takara, and Y. Shiroma. 1994. Application of enzyme immunoassay for postchemotherapy evaluation of human strongyloidiasis. Diagn. Microbiol. Infect. Dis. 18:19-23. [DOI] [PubMed] [Google Scholar]
- 15.Koga, K., S. Kasuya, C. Khamboonruang, K. Sukhavat, M. Ieda, N. Takatsuka, K. Kita, and H. Ohtomo. 1991. A modified agar plate method for detection of Strongyloides stercoralis. Am. J. Trop. Med. Hyg. 45:518-521. [DOI] [PubMed] [Google Scholar]
- 16.Lindo, J. F., N. S. Atkins, M. G. Lee, R. D. Robinson, and D. A. Bundy. 1996. Parasite-specific serum IgG following successful treatment of endemic strongyloidiasis using ivermectin. Trans. R. Soc. Trop. Med. Hyg. 90:702-703. [DOI] [PubMed] [Google Scholar]
- 17.Lindo, J. F., D. J. Conway, N. S. Atkins, A. E. Bianco, R. D. Robinson, and D. A. Bundy. 1994. Prospective evaluation of enzyme-linked immunosorbent assay and immunoblot methods for the diagnosis of endemic Strongyloides stercoralis infection. Am. J. Trop. Med. Hyg. 51:175-179. [DOI] [PubMed] [Google Scholar]
- 18.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 19.Mangali, A., W. Chaicumpa, P. Nontasut, P. Chantavanij, P. Tapchaisri, and C. Viravan. 1991. Enzyme-linked immunosorbent assay for diagnosis of human strongyloidiasis. Southeast Asian J. Trop. Med. Public Health 22:88-92. [PubMed] [Google Scholar]
- 20.Neva, F. A. 1986. Biology and immunology of human strongyloidiasis. J. Infect. Dis. 153:397-406. [DOI] [PubMed] [Google Scholar]
- 21.Neva, F. A., A. A. Gam, and J. Burke. 1981. Comparison of larval antigens in an enzyme-linked immunosorbent assay for strongyloidiasis in humans. J. Infect. Dis. 144:427-432. [DOI] [PubMed] [Google Scholar]
- 22.Neva, F. A., A. A. Gam, C. Maxwell, and L. L. Pelletier. 2001. Skin test antigens for immediate hypersensitivity prepared from infective larvae of Strongyloides stercoralis. Am. J. Trop. Med. Hyg. 65:567-572. [DOI] [PubMed] [Google Scholar]
- 23.Sato, Y., J. Kobayashi, H. Toma, and Y. Shiroma. 1995. Efficacy of stool examination for detection of Strongyloides infection. Am. J. Trop. Med. Hyg. 53:248-250. [DOI] [PubMed] [Google Scholar]
- 24.Sato, Y., M. Takara, and M. Otsuru. 1985. Detection of antibodies in strongyloidiasis by enzyme-linked immunosorbent assay (ELISA). Trans. R. Soc. Trop. Med. Hyg. 79:51-55. [DOI] [PubMed] [Google Scholar]
- 25.Sato, Y., H. Toma, S. Kiyuna, and Y. Shiroma. 1991. Gelatin particle indirect agglutination test for mass examination for strongyloidiasis. Trans. R. Soc. Trop. Med. Hyg. 85:515-518. [DOI] [PubMed] [Google Scholar]
- 26.Satoh, M., H. Toma, Y. Sato, M. Kikuchi, M. Takara, Y. Shiroma, S. Kiyuna, and K. Hirayama. 1999. Production of a high level of specific IgG4 antibody associated with resistance to albendazole treatment in HLA-DRB1*0901-positive patients with strongyloidiasis. Am. J. Trop. Med. Hyg. 61:668-671. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui, A. A., C. S. Stanley, and S. L. Berk. 2000. Cloning and expression of isocitrate lyase from human round worm Strongyloides stercoralis. Parasite 7:233-236. [DOI] [PubMed] [Google Scholar]
- 28.Sithithaworn, P., T. Srisawangwong, S. Tesana, W. Daenseekaew, J. Sithithaworn, Y. Fujimaki, and K. Ando. 2003. Epidemiology of Strongyloides stercoralis in north-east Thailand: application of the agar plate culture technique compared with the enzyme-linked immunosorbent assay. Trans. R. Soc. Trop. Med. Hyg. 97:398-402. [DOI] [PubMed] [Google Scholar]
- 29.Uparanukraw, P., S. Phongsri, and N. Morakote. 1999. Fluctuations of larval excretion in Strongyloides stercoralis infection. Am. J. Trop. Med. Hyg. 60:967-973. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida, T., T. Matsui, S. Kobayashi, and N. Yamamoto. 1987. Evaluation of passive particle agglutination test for antibody to human immunodeficiency virus. J. Clin. Microbiol. 25:1433-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]