Abstract
We describe the first transmission of Mycobacterium tuberculosis from human to cattle confirmed by molecular typing of isolates involved in the transmission. IS6110-based restriction fragment length polymorphism analysis showed that the isolates from the cattle and farm worker who suffered from pulmonary tuberculosis 1 year prior to this case were the same strains.
CASE REPORT
In May 2000, four cows on a small cattle farm (78 animals) near the Slovenian capital, Ljubljana, reacted inconclusively to bovine tuberculin (Bioveta, Ivanovice na Hane, Czech Republic) in a monotest during compulsory skin testing for tuberculosis (TB). All animals on the farm tested negative to bovine tuberculin during previous routine TB testing in May 1999. In July 2000, all animals on the farm were retested using a comparative skin test with bovine and avian (Bioveta) tuberculin. All skin tests were performed according to the European Council Directive 64/432/EEC (3). According to the Directive criteria (3), 16 animals were considered to have positive reactions to bovine tuberculin, and 3 animals with the strongest reactivity to tuberculin (2- and 2.5-year-old cows and a 6-month-old calf) were slaughtered. The mandibular, mediastinal, and portal lymph nodes were collected and sent for bacteriological examination. All 16 tuberculin-reactive animals, including 3 slaughtered animals, were clinically examined in detail and considered completely healthy.
Except for slight macroscopic enlargement, no gross pathological changes were visible on any lymph node specimen collected from the three slaughtered animals. Histopathological examination of hematoxylin-eosin-stained tissues revealed nonspecific findings. No acid-fast bacilli were detected on Ziehl-Neelsen-stained impression smears. However, after a 28-day incubation at 37°C, a few colonies appeared on a Löwenstein-Jensen slant inoculated with the mixed specimen of mediastinal and portal lymph nodes collected from the 2-year-old cow. No growth was detected on Stonebrink medium, Middlebrook 7H10 medium, and the Mycobacterium Growth Indicator Tube (Becton Dickinson, Franklin Lakes, N.J.) in spite of a prolonged, 8-week incubation. The colonies that appeared on the Löwenstein-Jensen slant were Ziehl-Neelsen stained, and acid-fast culture isolates were subcultured on Middlebrook 7H10 medium and identified by growth characteristics and standard biochemical tests (9). Biochemical testing, including niacin accumulation (positive), nitrate reduction (positive), thiophene-2-carboxylic acid hydrazide susceptibility (negative), pyrazinamide susceptibility (positive), and Lebek test for oxygen preference (aerobic), identified the isolate from the 2-year-old cow as Mycobacterium tuberculosis. Additionally, both the AccuProbe Mycobacterium tuberculosis Complex Assay (GenProbe, San Diego, Calif.) and an in-house PCR, targeting the 123-bp portion of IS6110 (17), identified the isolate as a member of M. tuberculosis complex. A banding pattern specific for M. tuberculosis/Mycobacterium africanum II/Mycobacterium canettii was obtained using the GenoType MTBC Assay (Hain Lifescience, Nehren, Germany).
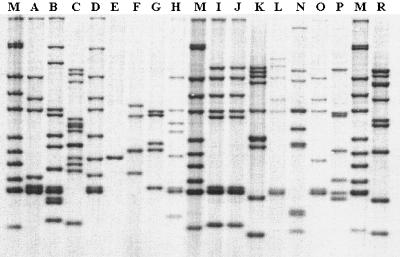
A detailed epidemiological investigation was conducted on the cattle farm in November 2000. It was found out that one of the farm workers, a 59-year-old man, suffered from bilateral cavitary pulmonary tuberculosis in 1999. After isolation of M. tuberculosis from his sputa in April 1999, he successfully completed the standard 6-month treatment in December 1999. Therefore, M. tuberculosis isolates from the cow and farm worker, together with a number of epidemiologically unrelated strains, were analyzed by IS6110-based restriction fragment length polymorphism (RFLP) typing, following the standardized protocol (32). As presented in Fig. 1, the M. tuberculosis isolates from the cow and farm worker show an identical RFLP pattern. A 100% homology of the two isolates was also confirmed by computer-assisted analysis of RFLP patterns.
FIG. 1.
IS6110 restriction fragment length polymorphism results. Lanes M, reference strain M. tuberculosis MT 14323; lane I, isolate from farm worker; lane J, isolate from cattle; lanes A to H and lanes K to R, epidemiologically unrelated Slovenian M. tuberculosis isolates.
All animals on the cattle farm (including 13 previously positive animals that were not slaughtered) tested completely negative to bovine tuberculin (Bioveta) during the consecutive compulsory skin TB testing performed in May 2001.
Bovine tuberculosis caused by Mycobacterium bovis is a significant veterinary disease that can occasionally spread to humans (22, 23, 27, 31). In contrast, M. tuberculosis is considered primarily a human pathogen. Apart from humans, M. tuberculosis infection has been reported in a wide range of domestic or wildlife animal species, most frequently living in close, prolonged contact with humans: e.g., in captive settings (2, 4, 18, 19, 28). Among domestic animals, infection with M. tuberculosis has been most frequently identified in cattle (6, 27, 30). According to published data, the prevalence of M. tuberculosis infection in cattle herds did not exceed 1% in the majority of studies (13, 21, 24-26, 30). However, a few exceptions, like Algeria and Sudan with 6.2% and 7.4% prevalence, respectively, were described (5, 29) as a most probable consequence of the high prevalence of human TB in these two African countries.
According to the present knowledge, M. tuberculosis does not appear to have an indigenous animal host or reservoir and the animals that become infected represent most probably accidental hosts (27, 31). Thus, although never directly proved, humans suffering from active TB are strongly believed to represent the main source of M. tuberculosis in animals, including cattle (27, 30, 31). To the best of our knowledge, we describe in this report the first transmission of M. tuberculosis from human to cattle unequivocally confirmed by molecular typing of appropriate isolates involved in the transmission. For molecular typing, we used IS6110 RFLP analysis, which has recently become the worldwide standard fingerprinting technique for comparison of M. tuberculosis isolates on the strain level. Apart from molecular confirmation of numerous cases of human-to-human and some cases of animal-to-animal transmission of M. tuberculosis (1, 16, 32, 33), IS6110 RFLP has, to the best of our knowledge, been used only three times for molecular confirmation of transmission of mycobacteria between humans and animals. Thus, IS6110 RFLP has recently been used to confirm the transmission of M. tuberculosis between a marmoset (Callithrix jacchus) and the monkey's owner (15), between four elephants and the elephant handler (14), and between a TB patient and her dog (8).
Numerous M. tuberculosis infections in cattle, including the one presented in this paper, have been identified on the basis of routine tuberculin testing of cattle herds (11, 26, 27). Although M. tuberculosis in cattle most frequently produces a quickly vanishing infection rather than a progressive disease, the infected animals do react positively when challenged with tuberculin (7, 10). The duration of sensitization to tuberculin is usually short, and the reactivity disappears when the infection source is removed (11). Thus, when a tuberculin-positive animal is recognized during a routine tuberculin testing for the first time in the previously tuberculin-negative herd, and particularly when the tuberculin-positive animal is young, the possibility of human TB infection among farm workers or animal attendants should be considered (11). As reported by several authors (11, 12, 20, 26), animal attendants with active pulmonary TB represent an important source of M. tuberculosis for animals, spreading the mycobacterium via sputum, urine, or feces (31). As a result of such spreading, the classical form of TB may occasionally develop in animals living in close contact with humans with active TB (10).
In conclusion, although indirect data suggesting that humans suffering from active TB are the most probable source of M. tuberculosis in cattle have been described before, we consider this report as the first unequivocal evidence of human-to-cattle transmission of M. tuberculosis. Namely, we were able to confirm molecularly the epidemiological relatedness of the strains infecting the human and cattle involved in the transmission. According to our experience, in the areas where bovine TB and human TB coexist, a detailed microbiological investigation of the specimens of slaughtered tuberculin-positive animals should always be performed in order to discriminate between M. tuberculosis and M. bovis infections.
REFERENCES
- 1.Alexander, K. A., E. Pleydell, M. C. Williams, E. P. Lane, J. F. Nyange, and A. L. Michel. 2002. Mycobacterium tuberculosis: an emerging disease of free-ranging wildlife. Emerg. Infect. Dis. 8:598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfonso, R., R. E. Romero, A. Diaz, M. N. Calderon, G. Urdaneta, J. Arce, M. E. Patarroyo, and M. A. Patarroyo. 2004. Isolation and identification of mycobacteria in New World primates maintained in captivity. Vet. Microbiol. 98:285-295. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1964. Council Directive 64/432/EEC of 26 June 1964 on animal health problems affecting intra-Community trade in bovine animals and swine. Official Journal of the European Communities L 121. Office for Official Publication of the European Communities, Luxembourg.
- 4.Aranaz, A., E. Liébana, E. Gómez-Mampaso, J. C. Galán, D. Cousins, A. Ortega, J. Blázquez, F. Baquero, A. Mateos, G. Súarez, and L. Domínguez. 1999. Mycobacterium tuberculosis subsp. caprae subsp. nov.: a taxonomic study of a new member of the Mycobacterium tuberculosis complex isolated from goats in Spain. Int. J. Syst. Bacteriol. 49:1263-1273. [DOI] [PubMed] [Google Scholar]
- 5.Boulahbal, F., A. Benelmouffok, and K. Brahimi. 1978. Role de Mycobacterium tuberculosis dans la tuberculose bovine. Arch. Inst. Pasteur Alger. 53:155-164. [PubMed] [Google Scholar]
- 6.Chandrasekharan, K. P., and R. Ramakrishnan. 1969. Bovine tuberculosis due to the human strain of Mycobacterium tuberculosis. Indian J. Tuberc. 16:103-105. [Google Scholar]
- 7.Erler, W., G. Martin, K. Sachse, L. Naumann, D. Kahlau, J. Beer, M. Bartos, G. Nagy, Z. Cvetnic, M. Zolnir-Dovc, and I. Pavlik. 2004. Molecular fingerprinting of Mycobacterium bovis subsp. caprae isolates from Central Europe. J. Clin. Microbiol. 42:2234-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erwin, P. C., D. A. Bemis, D. I. Mawby, S. B. McCombs, L. L. Sheeler, I. M. Himelright, S. K. Halford, L. Diem, B. Metchock, T. F. Jones, M. G. Schilling, and B. V. Thomsen. 2004. Mycobacterium tuberculosis transmission from human to canine. Emerg. Infect. Dis. 10:2258-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology: a guide for level III laboratory. Centers for Disease Control and Prevention, Atlanta, Ga.
- 10.Krishnaswami, K. V., and K. R. Mani. 1983. Mycobacterium tuberculosis humanis causing zoonotic tuberculosis among cattle. Indian J. Public Health 27:60-63. [PubMed] [Google Scholar]
- 11.Lesslie, I. W. 1960. Tuberculosis in attested herds caused by the human type tubercle bacillus. Vet. Rec. 72:218-224. [Google Scholar]
- 12.Lesslie, I. W. 1968. Cross infections with Mycobacterium between animals and man. Bull. Int. Union Tuberc. 41:285-288. [PubMed] [Google Scholar]
- 13.Lesslie, I. W., and K. J. Birn. 1970. Mycobacterium avium infections in cattle and pigs in Great Britain. Tubercle 51:446-451. [DOI] [PubMed] [Google Scholar]
- 14.Michalak, K., C. Austin, S. Diesel, M. J. Bacon, P. Zimmerman, and J. N. Maslow. 1998. Mycobacterium tuberculosis infection as a zoonotic disease: transmission between humans and elephants. Emerg. Infect. Dis. 4:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel, A. L., and H. F. Huchzermeyer. 1998. The zoonotic importance of Mycobacterium tuberculosis: transmission from human to monkey. J. S. Afr. Vet. Assoc. 69:64-65. [DOI] [PubMed] [Google Scholar]
- 16.Michel, A. L., L. Venter, I. W. Espie, and M. L. Coetzee. 2003. Mycobacterium tuberculosis infections in eight species at the National Zoological Gardens of South Africa, 1991-2001. J. Zoo Wildl. Med. 34:364-370. [DOI] [PubMed] [Google Scholar]
- 17.Miller, J., A. Jenny, J. Rhyan, D. Saari, and D. Suarez. 1997. Detection of Mycobacterium bovis in formalin-fixed, paraffin-embedded tissues of cattle and elk by PCR amplification of an IS6110 sequence specific for Mycobacterium tuberculosis complex organisms. J. Vet. Diagn. Investig. 9:244-249. [DOI] [PubMed] [Google Scholar]
- 18.Montali, R. J., S. K. Mikota, and L. I. Cheng. 2001. Mycobacterium tuberculosis in zoo and wildlife species. Rev. Sci. Tech. Off. Int. Epizoot. 20:291-303. [DOI] [PubMed] [Google Scholar]
- 19.Oh, P., R. Granich, J. Scott, B. Sun, M. Joseph, C. Stringfield, S. Thisdell, J. Staley, D. Workman-Malcolm, L. Borenstein, E. Lehnkering, P. Ryan, J. Soukup, A. Nitta, and J. Flood. 2002. Human exposure following Mycobacterium tuberculosis infection of multiple animal species in a metropolitan zoo. Emerg. Infect. Dis. 8:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlas, M., and L. Mezensky. 1982. The epizootiological significance of positive bacteriological findings on Mycobacterium tuberculosis and Mycobacterium bovis in humans. Vet. Med. (Prague) 27:641-649. (In Czech.) [PubMed] [Google Scholar]
- 21.Pavlik, I., W. Yayo Ayele, I. Parmova, I. Melicharek, M. Hanzlikova, B. Körmendy, G. Nagy, Z. Cvetnic, V. Katalinic-Jankovic, M. Ocepek, M. Zolnir-Dovc, and M. Lipiec. 2003. Mycobacterium tuberculosis in animal and human populations in six Central European countries during 1990-1999. Vet. Med. Czech. 48:83-89. [Google Scholar]
- 22.Pavlik, I., W. Yayo Ayele, I. Parmova, I. Melicharek, M. Hanzlikova, B. Körmendy, G. Nagy, Z. Cvetnic, M. Ocepek, N. Fejzic, and M. Lipiec. 2002. Incidence of bovine tuberculosis in cattle in seven Central European countries during the years 1990-1999. Vet. Med. Czech. 47:45-51. [Google Scholar]
- 23.Pavlik, I., W. Yayo Ayele, M. Havelkova, M. Svejnochova, V. Katalinic-Jankovic, and M. Zolnir-Dovc. 2003. Mycobacterium bovis in human population in four Central European countries during 1990-1999. Vet. Med. Czech. 48:90-98. [Google Scholar]
- 24.Popluhar, L., S. Haladej, B. Havelka, and J. Zubaj. 1974. Occurrence of tuberculosis in husbandry animals in Slovakia in 1972. Veterinarstvi. 24:33-34. (In Slovak.) [Google Scholar]
- 25.Schliesser, T. 1976. Vorkommen und Bedeutung von Mykobakterie bei Tieren. Zentbl. Bakteriol. Hyg. I Abt. Orig. A 235:184-194. [PubMed] [Google Scholar]
- 26.Smith, I. G. N. 1984. A herd breakdown due to Mycobacterium tuberculosis. State Vet. J. 38:40-44. [Google Scholar]
- 27.Steele, J. H. 1980. Human tuberculosis in animals, p. 141-159. In J. H. Steele (ed.), CRC handbook series in zoonoses. Section A. Bacterial, rickettsial and mycotic diseases, vol. 2. CRC Press, Inc., Boca Raton, Fla.
- 28.Sternberg, S., K. Bernodt, A. Holmstrom, and B. Roken. 2002. Survey of tuberculin testing in Swedish zoos. J. Zoo Wildl. Med. 33:378-380. [DOI] [PubMed] [Google Scholar]
- 29.Sulieman, M. S., and M. E. Hamid. 2002. Identification of acid fast bacteria from caseous lesions in cattle in Sudan. J. Vet. Med. B 49:415-418. [DOI] [PubMed] [Google Scholar]
- 30.Thoen, C. O., A. G. Karlson, and E. M. Himes. 1981. Mycobacterial infections in animals. Rev. Infect. Dis. 3:960-972. [DOI] [PubMed] [Google Scholar]
- 31.Thoen, C. O., and J. H. Steele. 1995. Mycobacterium bovis infections in animals and humans. Iowa State University Press, Ames.
- 32.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Žolnir-Dovč, M., M. Poljak, D. Eržen, and J. Šorli. 2003. Molecular epidemiology of tuberculosis in Slovenia: results of one-year (2001) nation-wide study. Scand. J. Infect. Dis. 35:863-868. [DOI] [PubMed] [Google Scholar]