Abstract
The cGAS–STING pathway plays an important role in ischemia-reperfusion injury in the heart, liver, brain, and kidney, but its role and mechanisms in cerebral ischemia-reperfusion injury have not been systematically reviewed. Here, we outline the components of the cGAS–STING pathway and then analyze its role in autophagy, ferroptosis, cellular pyroptosis, disequilibrium of calcium homeostasis, inflammatory responses, disruption of the blood–brain barrier, microglia transformation, and complement system activation following cerebral ischemia-reperfusion injury. We further analyze the value of cGAS–STING pathway inhibitors in the treatment of cerebral ischemia-reperfusion injury and conclude that the pathway can regulate cerebral ischemia-reperfusion injury through multiple mechanisms. Inhibition of the cGAS–STING pathway may be helpful in the treatment of cerebral ischemia-reperfusion injury.
Keywords: calcium homeostasis, cellular autophagy, cerebral ischemia-reperfusion injury, cGAS–STING pathway, ferroptosis, gut–brain–microbiota axis, inflammatory, light chain 3, microglial cells, Syntaxin-17 protein
Introduction
Stroke is a major cause of death and chronic disability globally (Venketasubramanian et al., 2017; GBD 2017 Causes of Death Collaborators, 2018; Pandian et al., 2018; Saposnik et al., 2022). Vascular recanalization may be able to save the ischemic penumbra and the neurological deficits, but there are not many other options for therapy (Belayev et al., 2018). Unfortunately, owing to their limitations, including the small time window for intervention and concomitant issues such as bleeding and allergies, the presently available procedures thrombolysis and mechanical thrombectomy cannot adequately treat many strokes (Berge et al., 2016; Demaerschalk et al., 2016). Just 11% of stroke patients are appropriate candidates for recombinant tissue plasminogen activator in routine clinical practice because of the limited temporal window after a stroke, weak recanalization rates, and other clinical contraindications (Tuo et al., 2022b). Furthermore, only 50% of patients see improvements in their health with recombinant tissue plasminogen activator (Yeo et al., 2013). Mechanical thrombectomy, while offering a larger temporal window, is not always effective in treating microvessel- and occlusion-related strokes. Moreover, the blood flow restoration that takes place outside of the reperfusion window may result in cerebral ischemia-reperfusion (IR) injury (CIRI). The series of events that define this syndrome start with mitochondrial damage and continue through energy depletion, excess calcium, inflammatory responses, secondary damage or injury, and function loss (Hu et al., 2015; Anrather and Iadecola, 2016; Chen et al., 2020b; Liao et al., 2020a; Qiu et al., 2023; Dai et al., 2024). When many impairments combine, it creates a “waterfall” effect that injures neighboring healthy cells, intensifying the initial damage and resulting in inflammation and neuronal death, which impairs function (Lipinski et al., 2015). Research has shown that maintaining mitochondrial integrity protects neural cells and shortens the pathogenic course of CIRI (Liu et al., 2020). The mitochondria-associated endoplasmic reticulum (ER) membrane (MAM), a dynamic cellular structure, strengthens the connection between the mitochondria and ER without merging the two. The MAM affects a wide range of cellular processes, including autophagy, lipid metabolism, apoptosis, inflammation, mitochondrial kinetics, calcium ion (Ca2+) homeostasis, and oxidative stress (Zhou et al., 2018). Synaptic fusion protein 17, known also as syntaxin 17 (STX17), mediates many functions of MAM. STX17 decreases ER stress and restores autophagic flow by inhibiting ischemic neuronal injury (Chen et al., 2020a). Intracellular homeostasis disturbances may lead to DNA damage, the disruption of mitochondria and exosomes, and the activation of cyclic guanosine monophosphate-adenosine synthase (cGAS) (Gentili et al., 2019; Li et al., 2020). When cGAS attaches to double-stranded DNA (dsDNA) and activates its enzymatic activity, 2′3′ cyclic guanosine monophosphate-adenosine monophosphate (cGAMP), a potent stimulator of interferon genes (STING) agonist and second messenger molecule, is created (Ishikawa et al., 2009; Ablasser et al., 2013; Diner et al., 2013; Gao et al., 2013; Sun et al., 2013; Wu et al., 2013; Zhang et al., 2013). Studies on STING-deficient mice have shown the critical role STING plays in initiating the body’s defense mechanism against interferon (IFN)-β (Sun et al., 2009). The STING targets interferon regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB), which may provide STING-activating signals that, in turn, may cause IFN production, essential for neuroinflammation and cell death (Ding et al., 2016; Guo et al., 2019; Gamdzyk et al., 2020).
A recent study summarized the evidence that cGAS–STING plays a pivotal role in IR injury of the heart, liver, brain, kidney, and other organs (Lv et al., 2023). However, a more systematic review of cGAS-STING’s role in CIRI is lacking. In this paper, we mainly review the functions of the cGAS–STING pathway in CIRI, such as microglia transformation, disruption of the blood–brain barrier (BBB) by the inflammatory response, dysregulation of calcium homeostasis, autophagy, ferroptosis, pyroptosis, etc., and summarize the inhibitors related to cGAS–STING with a view to assisting research into the treatment of CIRI.
Search Strategy
There have been a lot of similar investigations on cGAS–STING in CIRI, but they have often focused on just one or a few of the pathogenic pathways. In this narrative review, we summarize the influence of the cGAS–STING signaling pathway on the pathological mechanisms of CIRI in a more comprehensive manner, with a focus on a single disease, CIRI. The pathological mechanisms are also elaborated in greater detail. Furthermore, unlike earlier investigations, this article focuses on the idea of the amount of time following a cerebral infarction; after all, “time is brain.” From February to July 2023, we searched for relevant literature online using the PubMed database. The search strategy and screening criteria included the following keywords: cerebral IR, cGAS, STING, STX17, syntaxin 17, cellular autophagy, inflammation, pharmacological intervention, pharmacological treatment, neuroprotection, mode of cell death, time after cerebral ischemia, mitochondria, and calcium homeostasis. To conduct a thorough search of the literature, we employed numerous combinations of the aforementioned phrases. Initially, we assessed the relevance of the titles and abstracts to the pathogenic processes of CIRI using the most relevant search phrase. If they were found to be significant, the full publication was reviewed to verify that it contained an adequate explanation of the link between CIRI and signal pathways. Articles published in English that discussed the importance of characterizing the cGAS–STING signaling pathway and the pathogenic processes of CIRI were included. Articles that did not concentrate on CIRI or had pathogenic processes unrelated to CIRI were removed, as were those that were not published in English. The majority of the chosen literature (80% of total references) was published between 2017 and 2023.
Overall Mechanism and Treatment Strategies of Cerebral Ischemia-Reperfusion Injury
Because research is revealing more of the multiple mechanisms of CIRI, treating it is becoming more complicated, which presents a problem in clinical work. Numerous free radicals are produced by oxidative stress during cerebral ischemia or reperfusion injury. These radicals act on polyvalent unsaturated fatty acids, causing lipid peroxidation, and they can also induce the cross-linking of DNA, RNA, polysaccharides, and amino acids, among other substances, decreasing their function or activity. Finally, they can encourage the polymerization and breakdown of polysaccharide molecules (Sun et al., 2018). CIRI damage occurs when there is excess Ca2+ (Lipton, 1999). In addition, to block ATP synthesis, which hinders the synthesis of energy, calcium-dependent proteases are activated by Ca2+. These proteases change the intracellular xanthine dehydrogenase enzyme from its harmless form to xanthine oxidase, which produces a lot of oxygen free-radicals. Excitatory amino acid toxicity, which defines neuronal death, results from the activation of excitatory amino acid receptors and serves as a major catalyst and mediator of cerebral ischemia injury (Haskew-Layton et al., 2005), which is often accompanied inflammation and immune responses. Immune responses and inflammation frequently happen at the same time when CIRI occurs.
In addition to the increased neutrophil count that causes the “no-reflow” phenomenon (El Amki et al., 2020), other events that are involved in the inflammatory reaction include the development of neutrophil extracellular traps (NETs) (Wang et al., 2019b; Li et al., 2024; Lou et al., 2024); the activation of the NF-κB pathway, which mediates apoptosis (Li et al., 2022b); and disruption of the BBB (Hammond et al., 2014; Giraud et al., 2015; Neumann et al., 2015). However, the treatment of inflammatory storms should not be limited to agents that induce the excessive suppression of inflammation. The body needs adequate inflammation, as it has a protective function (Medzhitov, 2021). Preventing the over-inhibition of inflammation requires further research to enhance our understanding of the process. Additionally, the cell death processes apoptosis, necroptosis, ferroptosis, and cuproptosis are linked to CIRI (Tuo et al., 2022a; Guo et al., 2023). Scientists are discovering a multitude of other mechanisms related to CIRI as the research progresses. These mechanisms include heat shock protein activity, exosomes, microRNA regulation, and the either augmentation or repression of gene expression (Xu et al., 2021b; Peng et al., 2022; Wei et al., 2023).
Treatment approaches for CIRI usually focus on influencing several targets within these pathways due to their intricate nature. In order to prevent the oversuppression of processes that could be advantageous at specific periods of injury and repair, interventions must be balanced. A few popular therapy modalities include the following: (1) Anti-inflammatory treatments, including the crucial cytokine inhibitor drugs that restrict peripheral immune cell infiltration and microglial cell activation (Yu et al., 2022a). It is important therapy is directed toward suppressing the specific damaging components of the inflammatory response rather than the entire reaction, which could impede the healing process. (2) Antioxidants, which lessen oxidative stress by mitigating reactive oxygen species (ROS) (Tang et al., 2024). The timing and dosage (Li et al., 2022c) of antioxidants, however, need to be carefully managed to prevent interference with ROS’s normal cell signaling function. (3) Neuroprotective drugs, such as N-methyl-D-aspartate receptor antagonists, inhibit glutamate receptors and can lessen excitotoxicity (Jin et al., 2019). In a similar vein, extreme caution must be exercised when using these drugs to prevent disruptions to normal glutamatergic signaling, which is essential for memory and learning. (4) Apoptosis inhibitors can avoid neuronal death by targeting important stages of the apoptosis process (Xu et al., 2021a). Furthermore, the too broad-scope suppression of apoptosis could result in compromised cell function or possibly cancer-causing cell survival. (5) Lowering the body temperature via therapeutic hypothermia may lessen the effects of ischemic injury and delay the emergence of secondary injury mechanisms (Liu et al., 2018). This is achieved by lowering the metabolic rate. (6) Stem cell therapy and regenerative medicine approaches can help with brain healing by attempting to restore missing neurons (Tang et al., 2023). Although these approaches seem promising, they need to be finely adjusted to prevent negative consequences such as tumor development. (7) Targeted therapy is a popular strategy that involves customizing the treatment course according to certain aspects of the patient and their illness, including their genetic predisposition, degree of damage, target proteins, and pathways (Bai et al., 2023). Optimizing the timing of therapy interventions, modifying drug combinations, and tweaking dosages, may enhance the outcomes.
Research on more appropriate and successful treatments for CIRI is still ongoing and necessitates a sophisticated comprehension of the disease’s pathogenesis. The ultimate objective is to improve outcomes for people with CIRI by promoting intrinsic brain healing processes and minimizing damage.
Overview of the cGAS–STING Signaling Pathway
The initiation of cGAS-mediated antiviral activities across different species relies on the production of cGAMP (Ablasser et al., 2013; Diner et al., 2013; Gao et al., 2013; Sun et al., 2013; Wu et al., 2013; Zhang et al., 2013). Researchers have found positively charged DNA-binding sites on cGAMP, with site A serving as the principal point of contact for dsDNA and site B serving as a complementary binding site for additional dsDNA molecule sugar-phosphate backbones (Wan et al., 2020; Decout et al., 2021). When DNA attaches to the A site, it undergoes a conformational shift that facilitates the most efficient interaction between the substrates ATP and GTP (Ishikawa et al., 2009; Diner et al., 2013). In the core 2:2 cGAS DNA complex, where binding is based on DNA length rather than sequence, the B site contributes significantly to the creation of a compact, functional enzyme unit (Andreeva et al., 2017; Wan et al., 2020; Decout et al., 2021). Recognition of b-type dsDNA by cGAS is often the only trigger for cGAS activation, as opposed to a-type dsDNA or RNA (Hu et al., 2015; Anrather and Iadecola, 2016). In order to induce biological effects, cGAS dimers must organize on long dsDNA in ladder-like networks with phase-separated organelles (Andreeva et al., 2017; Luecke et al., 2017; Du and Chen, 2018). There is a brief N-terminal segment in the cytoplasm of STING, followed by a four-span transmembrane structural domain, a linker region, and finally, a C-terminal tail linked to a ligand-binding domain in the cytoplasm (Decout et al., 2021). STING is redirected from the ER to the Golgi or endosomal and autophagy-associated compartments via the ER-Golgi intermediate compartment when it binds to cGAMP, which separates the binding of STING and STIM and allows STING to bind to selenocysteine 24C (SEC24C) protein (Saitoh et al., 2009; Dobbs et al., 2015). When STING reaches these regions, it enhances the autophosphorylation of TANK-binding kinase 1 (TBK1) at its C-terminal tail, which in turn activates the phosphorylation of IRF3 (Berke et al., 2013; Dobbs et al., 2015; Chen et al., 2016). The production of microtubule-associated protein I light chain 3 (LC3)-positive autophagic vesicles is also aided by STING when it exits the ER (Gui et al., 2019). As a mechanism for self-degradation, the bridging protein surfeit locus protein 4 (SURF4) interacts with STING at the Golgi apparatus to enable the retrograde trafficking of coat protein complex 1 vesicles encapsulating STING (Deng et al., 2020; Mukai et al., 2021; Figure 1).
Figure 1.
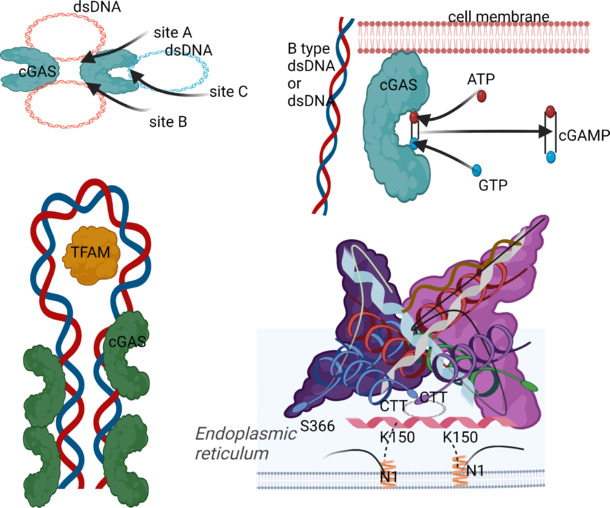
Structural characteristics of the cGAS–STING pathway.
The DNA binds to the master site A, inducing a conformational change (i.e., the interaction of the loose dsDNA with cGAS at the site of these two DNA interface residues causes the dsDNA to bend and allows it to bind more easily to the next adjacent cGAS, thereby rearranging the enzyme’s catalytic bag). Site B is complementary and binds to another dsDNA. The binding of DNA to the juxtaposition of B sites is essential for the formation of the core 2:2 cGAS DNA complex, which is the smallest active enzyme unit. Created with BioRender.com. cGAMP: Cyclic GMP-AMP; cGAS: cyclic GMP-AMP synthase; CTT: cytoplasmic tail; dsDNA: double-stranded DNA; TFAM: mitochondrial transcription factor A.
cGAS not only activates STING as a second messenger via its catalytic activity but also plays a non-catalytic role by inhibiting the homology of DNA breaks in the cell nucleus (Hopfner and Hornung, 2020). After being activated, STING displays a wide array of cellular roles. Activation of STING not only causes IRF3 phosphorylation, which in turn drives the expression of several antiviral genes, but also activates NF-κB and MAPK, regulates autophagy, and produces lysosome-dependent cell death by mediating lysosomal transport (de Oliveira Mann et al., 2019; Hopfner and Hornung, 2020).
Cerebral Ischemia-Reperfusion–Induced Brain Damage and cGAS–STING Pathway
In CIRI, the cGAS–STING pathway is pivotal. Its interaction with proteins in the caspase family causes apoptosis and stimulates the NF-κB pathway, which is involved in the inflammatory response. Furthermore, it is necessary for ferroptosis to take place, regulating calcium homeostasis, and encouraging microglia to convert to the M1 type. The CIRI cGAS–STING pathway’s particular roles will be detailed in the sections that follow.
Correlation of cGAS–STING with inflammatory reactions in cerebral ischemia-reperfusion injury
Importantly, neutrophils are among the cells that are susceptible to inflammatory reactions. Depleting neutrophils might decrease BBB damage, according to research that used a mouse model of neutrophil-associated middle cerebral artery occlusion (MCAO). It was shown that STING-mediated vascular remodeling is caused by NETs, and this reduction improved neovascularization in 14 days. Neutrophil inhibition promoted vascular plasticity and inhibited STING, which in turn reduced damage to the BBB (Kang et al., 2020).
Thromboinflammation, enhanced excitotoxicity, cell lysis, oxidative stress, and BBB disruption are among the ways in which inflammation worsens brain injury during the acute phase (Shi et al., 2019).
Crucial to the maintenance of the CNS microenvironment, the BBB controls the entrance of substances into the brain and is made up of brain endothelial cells, pericytes, extracellular matrix, and astrocyte end-feet (Xu et al., 2021c; Jia et al., 2024). Acute ischemic stroke patients may have a worse chance of survival as a result of the BBB instability and permeability alterations that occur during cerebral ischemia (Horsch et al., 2016). According to many studies, after cerebral ischemia, the BBB is most often disrupted by the inflammatory response (Huang et al., 2020; Qiu et al., 2021; Xu et al., 2021c; Cui et al., 2023; Chen et al., 2024). Neutrophils are the most delicate cells in the body’s inflammatory system. Researchers found that neutrophil depletion for 14 days reduced BBB disruption and enhanced neovascularization in a mouse model of neutrophil-associated MCAO. Moreover, vascular remodeling mediated by STING is carried out by NETs. Suppressing STING reduction, decreasing BBB damage, and increasing vascular plasticity are the results of inhibiting neutrophils (Kang et al., 2020).
Sterile inflammation is initiated by cellular stress in CIRI. Pattern recognition receptors are cellular sensors that can detect various signals, such as apoptosis, exosomes, DNA damage, mitochondrial damage, pathogen-associated molecular patterns, and the danger-associated molecular patterns produced by DNA viruses, retroviruses, and microbes (Wan et al., 2020; Hu et al., 2022). The genetic material of most eukaryotic organisms is typically contained inside the nucleus and mitochondria. But in CIRI, DNA from either within or outside the cell may activate DNA sensors such as cGAS to trigger innate immune responses (Hartmann, 2017; Benmerzoug et al., 2019; Zahid et al., 2020). In order to initiate IFN production, STING activation causes the phosphorylation of IRF3, but this is only the beginning of the immunostimulatory response induced by type I IFN signaling. Type I IFN-stimulated genes are transcribed when the Janus kinase signaling sensor and activator of transcription pathway are activated (González-Navajas et al., 2012; Schneider et al., 2014). Through cGAS–STING pathway activity, the expression of various IFN-stimulated genes is induced, including IFN-induced proteins with tetratricopeptide repeats and pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-6, C–X–C motif chemokine ligand 10 (CXCL10), and chemokine (C–C motif) ligand 5 (CCL5) (Prabakaran et al., 2018). A positive-feedback loop in the propagation of IFN signaling results from the increased expression of cGAS and STING (Ma et al., 2015a, b). As this response continues to be maintained by positive-feedback control, the inflammatory reaction will continue indefinitely, and consequently, STING will continue to strengthen CIRI (Figure 2).
Figure 2.
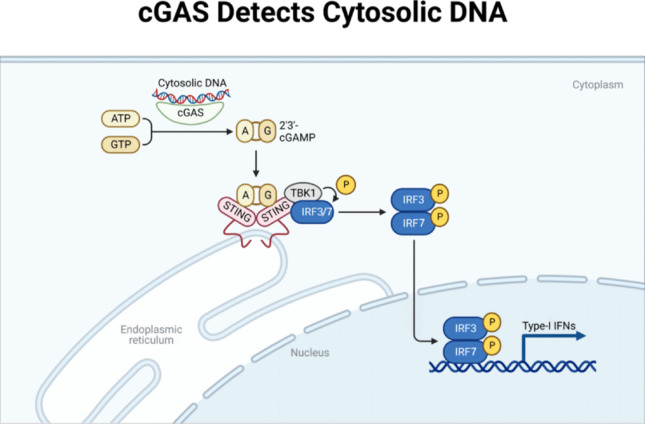
cGAS detects cytosolic DNA.
After recognizing double-stranded DNA, cGAS promoted STING activation. Meanwhile, TANK-binding kinases 1 (TBK1) were recruited to STING’s highly conserved CTT region (C-terminal tail domain), thereby phosphorylating and activating TBK1. Activated TBK1 phosphorylates the interferon regulatory factors IRF3 and IRF7 (IRF3/7), causing the dimerization and transfer of IRF3 and IRF7 to the nucleus and ultimately activating the transcription of type I IFN and inflammatory cytokine genes. Created with BioRender.com. A: ATP; cGAS: cyclic GMP-AMP synthase; G: GTP; IRF3: interferon regulatory factor 3; IRF7: interferon regulatory factor 7; IFN: interferon; P: phosphorylation; STING: stimulator of interferon genes.
When STING activity is turned on, it may activate the inhibitor of the NF-κB (IκB) kinase complex and NF-κB transcription by promoting the synthesis of NF-κB via TBK1 and its homolog IκB kinase ε (Balka et al., 2020; Yu et al., 2022b). Despite CIRI’s relevance and the high degree of conservation in the downstream activity of STING-mediated NF-κB transcription, the molecular connection between STING and components of the NF-κB pathway remains poorly known (Decout et al., 2021). The involvement of NF-κB is critical in several CIRI processes, such as inflammatory promotion, apoptotic induction, and mediating free-radical damage. It is possible that NF-κB controls the synthesis of IL-6, IL-1β, and TNF-α, three pro-inflammatory factors expressed in astrocytes (Hwang et al., 2015; Phuagkhaopong et al., 2017). According to prior research, reducing macrophage and T cell infiltration may be achieved by selectively decreasing NF-κB (Gabel et al., 2016; Yuan et al., 2017; Xu et al., 2018). This is because this pathway downregulates chemokines generated by astrocytes, which in turn inhibits astrocyte activation. As a result, secondary system illnesses caused by inflammatory damage are reduced (Gabel et al., 2016; Yuan et al., 2017; Xu et al., 2018). Furthermore, non-canonical NF-κB responses may be induced by stimulating the cGAS-STING pathway, which in turn promotes the nuclear translocation of p52-RELB (p52-REL proto-oncogene, NF-κB subunit) (Bakhoum et al., 2018; Hou et al., 2018). Hence, to combat CIRI, it may be necessary to suppress the cGAS-STING pathway in CIRI. This might impact the production of IFN, NF-κB, and other proteins, which in turn could lessen the detrimental effects of sterile inflammation on the BBB.
cGAS-STING and gut–brain–microbiota axis
The gut–brain–microbiota axis has been the subject of numerous investigations in the last few years. The gut microbiota is involved in a cGAS-STING-dependent route that leads to the generation of type I IFN, and it has been shown that the dsDNA generated by numerous gastrointestinal bacteria may activate the STING pathway (Erttmann et al., 2022). Dendritic cells activate a cascade response involving cGAS-STING-TBK1-IRF7-IFNβ when exposed to the common probiotic Lactobacillus rhamnosus GG in the intestines.
The integrity of STING signal transduction is necessary for the maintenance of intestinal homeostasis. When it comes to the intestinal environment, type I IFN is the most important element that regulates CD4+Foxp3+ regulatory T cells (Tregs) (Dooyema et al., 2022). It is worth mentioning that animals lacking the STING gene show a decrease in the functioning and numbers of Tregs (Dooyema et al., 2022). The intricate nature of the STING pathway’s functional involvement in regulating intestinal permeability and homeostasis is highlighted by this.
The cGAS–STING signaling system modulates immunological regulatory mechanisms, mucosal protection, and intestinal permeability. By acting through the gut-brain-microbiota axis, this has additional effects on CIRI.
The cGAS–STING pathway and cellular autophagy in cerebral ischemia-reperfusion injury
Reducing cGAS–STING-mediated excessive autophagy can mitigate ischemic injury to neurons in CIRI animals, according to in vivo research using C57BL6 mice and an in vitro study using the OGD/R primitive neuronal cell model (Liu et al., 2023). This indicates that cGAS–STING is a potential target for therapies aimed at controlling autophagy in CIRI.
In CIRI, dysfunctional lysosomal storage and consequent neuronal cell death may result from upregulating neuronal autophagy (Zhang et al., 2021). Metabolic stress, component breakdown, and cell death may result from hyperactive autophagy in the latter phases of reperfusion (Haag et al., 2018). Although, there is as yet no evidence that autophagy, CIRI, and the beginning of plasmatic alterations are related. Initial autophagic alterations are amplified, and lysosome number and size are increased during acute ischemia, at which stage dysfunctional lysosomes start storing chemicals. Because initial mammalian target of rapamycin (mTOR)-dependent lysosomal biogenesis during reperfusion is inadequate to remove the temporarily elevated autophagic cargo, faulty autophagy follows defective lysosomal storage (Zhang et al., 2021).
LC3 and chelator 1 (SQSTM1/p62) are two of the more prevalent indicators of autophagy (Mizushima and Murphy, 2020). Increased p62 levels are often thought of as a sign of suppressed autophagic activity (Kim et al., 2021). The autophagy receptor p62/SQSTM1 is crucial for selective autophagy in cells. Cellular autophagy can be prompted by cGAS–STING activation, and once autophagy has begun, cGAS–STING ubiquitinates and binds to p62. Once bound STINGs are integrated into the cellular autophagosome and bind to lysosomes, they form autophagic lysosomes, which finally finish the entire process of autophagy (Hu et al., 2022). Autophagic lysosomes will promptly break down cGAS, also known as STING, after the brief activation of downstream signaling (Gui et al., 2019). Simultaneously, the cGAS-STING pathway is triggered by cytoplasmic dsDNA, and STING phosphorylates p62 to induce STING breakdown and attenuation (Prabakaran et al., 2018). p62 proteins and autophagy are involved in negative-feedback loops that prevent the prolonged overactivation of the system and guarantee transitory cGAS-STING signaling (Gui et al., 2019). A pathophysiological vicious circle was discovered in an experiment using the MCAO model in C57 mice, where it was discovered that certain pathological mechanisms of CIRI itself block autophagosome degradation with p62-mediated autophagosome formation. Undegraded autophagosomes are secreted extracellularly via exocytosis, which causes an inflammatory cascade that further damages mitochondria, increasing ROS accumulation and impeding autophagosome degradation (Zeng et al., 2022). Evidently, the STING pathway is overactivated when autophagosome degradation is interrupted. This p62 exacerbates the vicious cycle of CIRI by preventing the breakdown of further autophagic vesicles.
One of the most important steps in the process of cellular autophagy is the lipidation of LC3. Only lipidated LC3 protein can attach to the autophagosome membrane and take part in the cellular autophagy process that follows. It has been shown that cGAMP is independent of the C-terminal signaling domains of TBK1 and STING, which are required for type I IFN production, and it may directly promote LC3 lipidation (Tanaka and Chen, 2012; Liu et al., 2015). It is also true that STING-induced cytosolic autophagy and IFN-induced activity can be distinguished from one another, as evidenced by experiments showing that autophagy-associated protein-5-deficient cells with impaired LC3 lipidation and autophagosome formation still exhibited intact TBK1 and IRF3 activity (Schmeisser et al., 2013). Moreover, autophagy and the STING-mediated inflammatory response are still impacted by one another even after their separation. One possible theory is that p62 deficiency leads to decreased STING degradation, increased STING-mediated IFN synthesis, and an increased inflammatory response. It was discovered that TBK1 in normal cells not only triggers IFN but also indirectly activates p62, resulting in p62 phosphorylation and LC3 lipidation. After the occurrence of autophagy, STING is broken down (Prabakaran et al., 2018). Autophagy maintains a dynamic equilibrium and prevents the persistent overactivation of inflammation by regulating the protein quantities of the cGAS-STING signaling pathway in a normal state. Depending on how autophagy develops during cerebral ischemia or reperfusion, the activation of cGAS-STING will have distinct effects. Autophagosomes are more difficult for lysosomes to attach to, making them more difficult to break down, and it becomes more likely the STING pathway will be overactivated as more time elapses after reperfusion. When the pathway is out of balance, autophagy and the inflammatory response will be detrimental.
There are two primary forms of the mitochondrial autophagy process: the receptor-mediated pathway and the ubiquitin-mediated system (Onishi et al., 2021). Mitochondrial autophagy plays a critical role in the pathological processes linked to the existence of mitochondria in the presence of CIRI, which include oxidative stress, inflammatory response, calcium excess, and mitochondria-dependent apoptosis (Shen et al., 2021). Another name for mitochondrial DNA is dsDNA. When mitochondria are damaged, dsDNA activates cGAS receptors, which in turn triggers the cGAS-STING pathway. In tumor models xenografted from naked mice, the cGAS-STING pathway was demonstrated to correlate with mitochondrial autophagy (Li et al., 2022d). Furthermore, Liu et al.’s experiments with sepsis acute lung injury models revealed the occurrence of p62 accumulation and LC3-II conversion in Wistar rats but not STING−/− C57BL/6 mice (Liu et al., 2021). An important factor in sepsis acute lung injury was the increased level of cyclic mitochondrial DNA, which disrupted autophagy and activated the STING pathway. Based on the aforementioned research, we can determine that STING may affect the process of mitochondrial autophagy and that it temporally activates to impact LC3-II and p62. Because of its limitations, the CIRI model has not been used to validate this research. The research is still somewhat significant, however, as mitochondrial disruption is a major contributor to the pathogenic mechanisms of CIRI, and cGAS is a dsDNA sensor that is present in many cells (Figures 3 and 4).
Figure 3.
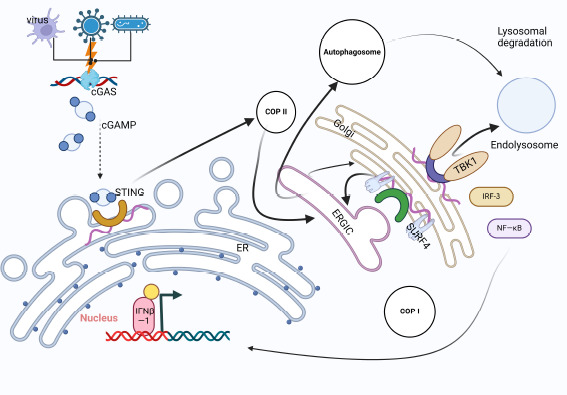
The process of STING-mediated cellular autophagy.
Bacteria and viruses lead to the emergence of double-stranded DNA, and STING is activated after cGAS recognizes double-stranded DNA and STING is transferred to the endoplasmic reticulum. Activated STING followed COP-II to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) and fused with it to become a new ERGIC. Subsequently, STING-induced LC3 lipidization and autophagosome formation. In the interior of the intermediate region, the SURF4 protein, which acts as a freight receptor, is also involved in the transport of various proteins between the ER and the Golgi apparatus. Autophagosomes need to bind to lysosomes to become autophagolysosomes for degradation, and this process requires the participation of TBK1. Created with BioRender.com. cGAMP: Cyclic GMP-AMPP; cGAS: cyclic GMP-AMP synthase; COPI: coat protein I; COPII: coat protein II; ERGIC: endoplasmic reticulum-Golgi intermediate compartment; IRF3: interferon regulatory factor 3; IFNβ-1: interferon-β-1; NF-κB: nuclear factor kappa-B; STING: stimulator of interferon genes; SURF4: surfeit locus protein 4; TBK1: TANK-binding kinase 1.
Figure 4.
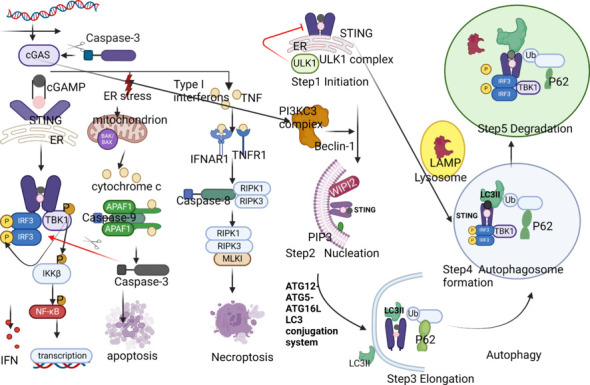
Illustration of the three main tasks of STING throughout the autophagic process.
First, STING is essential in the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) formation associated with autophagy. Upon binding to cGAMP, STING interacts with the capsid protein SEC24C on COPII vesicles, further undergoing highly targeted membrane vesicle transport with COPII to reach the ERGIC and fuse with it, completing the co-localization of STING and ERGIC. Secondly, STING carries cGAMP and induces LC3 lipidation in ERGIC, providing a membrane source for the recruitment of WIPI2 protein. During the co-localization of STING and LC3, STING undergoes translocation mediated by the WIPI2 protein. Thirdly, cGAMP-bound STING is transported to the lysosome via the endosome or trans-Golgi network (TGN) for degradation via the multivesicular body (MVB) pathway, accompanied by TBK1 phosphorylation and RAB7+ protein-dependent binding of STING to AP-1 protein. Created with BioRender.com. APAF1: Apoptotic protease activating factor-1; Caspase-3: cysteinyl aspartate specific proteinase 3; Caspase-8: cysteinyl aspartate specific proteinase 8; cGAMP: cyclic GMP-AMP; cGAS: cyclic GMP-AMP synthase; COPI: coat protein I; COPII: coat protein II; ERGIC: Endoplasmic Reticulum-Golgi Intermediate Compartment; IFN: Interferon; IFNAR1: interferon alpha and beta receptor subunit 1; IFNβ-1: Interferon-β-1; IKKβ: inhibitory kappa-B kinase beta; IRF3: interferon regulatory factor 3; LC3II: light chain 3; MLKL: mixed lineage kinase domain-like protein 1; NF-κB: nuclear factor kappa-B; P: phosphorylation; PIP3: phosphatidylinositide 3-kinases; RIPK1: receptor-interacting protein kinase 1; RIPK3: receptor-interacting protein kinase 3; STING: stimulator of interferon genes; SURF4: Surfeit locus protein 4; TBK1: TANK-binding kinase 1; TNF: tumor necrosis factor; TNFR1: tumor necrosis factor receptor 1; Ub: ubiquitin; ULK1: UNC-51-like kinase 1; WIPI2: WD repeat domain, phosphoinositide interacting 2.
By increasing autophagic flow and decreasing ER-stress-dependent neuronal apoptosis, STX17 may improve CIRI-induced neuronal damage, according to research by Chen et al. (2020a). It was found that, after overexpression of exogenous STX17, neuronal damage was alleviated and lysosome function was restored to a certain extent. STX17 plays a pivotal role during autophagic vesicle production, namely during the initiation and fusion phases (Baskaran et al., 2014; Kumar et al., 2021). When nutrients are lacking, acetyltransferase becomes inactive, and STX17’s acetylation level drops. This allows STX17 to more easily assemble with soluble N-ethylmaleimide-sensitive factor attachment protein receptor molecules, vesicle-associated membrane protein 8 (VAMP8), and synaptosome-associated protein 29 (SNAP29), forming a complex that enables autophagosomal-lysosomal fusion (Wollert, 2019). Alternatively, in a study examining energy stress-induced autophagy in Drosophila, a C57BL/6 mouse model, and STING-deficient fruit fly fat body cells, it was discovered that, in a well-nourished and inactivated innate immune system setting, the STING molecule can down-regulate autophagic flux by binding to STX17, a crucial protein for autophagic membrane fusion, causing it to reside in the ER (Rong et al., 2022). The stress-dependent sequestration of STX17 at the ER by STING was discovered; the translocation of STX17 was shown to be reliant on STING, and the dissociation of STING-STX17 was found to be energy-crisis-dependent. Autophagy does not occur under typical circumstances of sufficient nutrition, and this discovery provides strong proof of that. CIRI disease is basically a condition of starvation and subsequent nutritional recovery. In the pre-STX17 stage, autophagy is enhanced, and lysosomal binding is accelerated. However, in the late stage of CIRI, when blood and energy supplies are restored and lysosomal dysfunction occurs, autophagic vesicles are again generated continuously, and STX17 levels decrease. Additionally, lysosome and autophagosome binding are restricted when STX17 is depleted. The destination of the STX17 protein, as controlled by STING, is affected by the duration of, and energy recovery after, reperfusion, which in turn impacts the autophagy process in neuronal cells.
An excess amount of ROS is produced in CIRI, resulting in cellular oxidative stress that eventually leads to cell death (Jangholi et al., 2020). The endogenous ROS produced mainly comes from mitochondria. In an experiment of STX17-knockout cells, it was found that the complete loss of STX17 negatively regulated mitochondrial elongation and mitochondrial ROS production (Wang et al., 2019a). STX17-knockout cells showed strong resistance to oxidative stress, mainly due to the function of STX17 in mitochondrial division rather than autophagy (Wang et al., 2019a). It has also been found that the elongation of mitochondria in STX17-knockout cells prevents autophagy, which may provide protection against apoptosis (Gomes et al., 2011; Rambold et al., 2011). In conclusion, the absence of STX17 is beneficial to the protection of cells from apoptosis. Therefore, STX17 has opposing effects on autophagy and mitochondrial function during CIRI. However, analysis found that the STX17-gene knockout represented the congenital loss of STX17 protein, and mitochondrial function had been formed, which had antioxidant effects on cells. In normal cells, the depletion of STX17 protein is not instantaneous; there is no rapid change in mitochondrial function, and the cell does not suddenly increase its antioxidant capacity naturally.
In an experimental designed to simulate IR-injured neurons, researchers found that reactivated mechanistic target of rapamycin complex 1 repressed the transcriptional levels of relevant mRNAs (e.g., LC3, Stx17, Vamp8, and Snap29) (Hua et al., 2019); this surely explains the inhibitory effects of mTOR on transcriptional and translational levels of autophagy-associated proteins in neurons injured by IR. In addition, the above findings were reconfirmed in a study on the specific mechanism by which the Akt-mTOR pathway regulates neuron-specific autophagy to improve the prognosis of neurological function in mice after IR injury, and the role of STX17 in the lysosomal degradation of autophagy was once again affirmed (Hua et al., 2018). Rizwan et al. (2020) found that the escape of damaged mtDNA into cytoplasmic lysosomes was involved in the activation of mTOR via cGAS-STING with a high-glucose-environment-induced elevation of ROS levels. From this experiment, we can infer that STING may be able to inhibit the transcriptional regulation of the STX17 protein by activating mTOR. In addition to the above model of energy regulation, it was also found that STING interacts with STX17 through its dimerization domain, and STING regulates the STING-STX17 interaction through its own polymerization, phosphorylation, and degradation (Song et al., 2024). Song et al.’s study also explored the relationship between STING and STX17 in terms of temporal dynamics, which has not yet been validated in CIRI. Therefore, there are future opportunities to continue exploring the temporal changes of STING-STX17 after CIRI.
The cGAS–STING pathway affects microglial cells in cerebral ischemia-reperfusion injury
After brain traumas, such as IR damage, microglia are essential players in starting the inflammatory response (Li et al., 2017; Neher and Cunningham, 2019). The M1 and M2 phenotypes of microglia, which are involved in tissue damage and healing, respectively, may be quickly induced by various stresses (Liu et al., 2016; Wang et al., 2018a; Feng et al., 2019). Therapeutic strategies for cerebral ischemia that aim to regulate the balance between these characteristics have shown promise (He et al., 2020; Wang et al., 2020a; Li et al., 2021b). Metabolic reprogramming and autophagy play crucial roles in the transition between M1 and M2 states (Jin et al., 2018). An M1-type polarization marker protein, inducible nitric oxide synthase, and an M2-type polarization marker protein, recombinant human arginase-1, are expressed as polarization marker proteins by macrophages (Lisi et al., 2017). The concentration of microglia near injury sites is caused by CIRI’s activation of cellular stress, which in turn produces a cascade of inflammatory chemicals and cytotoxic compounds that worsen tissue damage. According to the hypothesis mentioned earlier, a higher percentage of M2-type microglia after CIRI may mitigate these effects (Figure 5).
Figure 5.
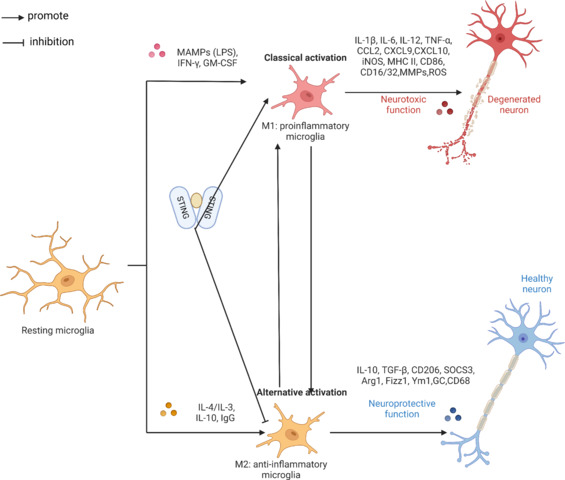
STING promotes M1 microglial cell activation and inhibits M2 microglial cell activation.
STING could promote the polarization of M1 microglia and act as an obstacle to M2 microglia. Created with BioRender.com. ArG1: Arginase-1; CCL2: C–C motif chemokine ligand 2; CXCL: C–X–C motif chemokine ligand; GM-CSF: granulocyte-macrophage colony stimulating factor; IFN: interferon; IL: interleukin; iNOS: Inducible nitric oxide synthase; MAMPs: metabolism-associated molecular patterns; MHCII: major histocompatibility complex; MMPs: matrix metalloproteinases; OS: reactive oxygen species; STING: stimulator of interferon genes; TGF: transforming growth factor; TNF: tumor necrosis factor.
Findings from in vivo and in vitro investigations show that STING is essential for microglia to adopt an M1 phenotype and may impede M2 microglia polarization by activating downstream pathways such as IRF3 and NF-κB (Kong et al., 2022). Work that focused on microglial cells and neuroinflammation using transient MCAO models in C57BL/6 mice later confirmed this conclusion. Results from in vivo experiments showed that microglial cells lacking cGAS reduced neuroinflammation and IR-induced brain damage (Liao et al., 2020b). Thus, STING in CIRI promotes the presence of M1-type microglia, and blocking STING might be a way to treat neuroinflammation after an ischemic stroke.
The inflammatory responses mediated by STING have been discussed in our earlier paper. By modulating microglial inflammation and decreasing M1 polarization, blocking this pathway does more than decrease cGAS; it also inhibits the pro-inflammatory cytokines IL-6 and TNF. Findings from an investigation using a C57BL/6 mouse MCAO model verified that inflammatory mediators influence changes in microglia polarization. A clinical study has demonstrated that cGAS inhibition alters polarization changes in microglia (Jiang et al., 2021). By activating transcription, pro-inflammatory cytokines, including IFN-γ, TNF-α, and IL-1β, regulate inducible nitric oxide synthase levels (Bogdan, 2015). A notable improvement in the reparative microglia phenotype (M2 microglia) was seen after cGAS-knockdown-induced suppression of the cGAS-STING pathway. This discovery raises the possibility that cGAS might be a therapeutic target for IS.
We learned more about the regulatory mechanisms and signaling pathways linked to microglial cell M1/M2 polarization in a thorough analysis of microglial cells in ischemic stroke (Jiang et al., 2020). The investigation showed that autophagy is associated with the polarization of microglia. In this cascade, STING modulates autophagy to affect microglial cell polarization and has a unique effect on microglial cell regulation.
Researchers have discovered a new cell type that may offer protection against acute ischemic stroke by utilizing human blood reperfusion in CIRI and the mouse middle cerebral artery embolism model. This cell type is different from the conventional M1/M2 phase of polarization due to the presence of FOXP3+ macrophages (Cai et al., 2023). After a thorough investigation, we found that FOXP3+ is related to inflammation, macrophages, and autophagy. We confidently claim that STING controls FOXP3+ macrophages by using this information. Research that focused on T cells in cervical cancer using C57BL/6 mice, blood samples, and tumors from cancer patients lends strong support to this idea (Ni et al., 2022).
It is evident from the data acquired that STING and cGAS are chemicals that influence the polarisation of microglia. Additionally, both impede M2 type microglia’s polarisation shift. Additionally, a possible association between STING and FOXP3+ macrophages has been identified. Though further research is required, as we still do not know how STING regulates CIRI damage or what variables impact this control.
The different microglia phenotypes that emerge throughout the progression of illnesses have been revealed in recent years with the emergence of novel single-cell/nucleus sequencing tools (Dumas et al., 2021; Li et al., 2022a). These phenotypes vary greatly from the M1/M2 state. The human CNS’s region-specific microglia, such as white matter and gray matter microglia, are heterogeneous, as shown by single-cell sequencing (Sankowski et al., 2019). Furthermore, fresh subtypes of midbrain microglia have been discovered (Uriarte Huarte et al., 2021). Depending on the disease, other subtypes have emerged. For example, in Alzheimer’s disease, the increased numbers of microglia surrounding Aβ plaques are linked to a significant transcriptional activation feature known as disease-associated microglia, which is different from homeostatic microglia (Keren-Shaul et al., 2017). These findings are among some of the seminal discoveries in microglia phenotypes made possible by single-cell sequencing. While white-matter-associated microglia have been discovered in senescent white matter, microglia associated with proliferative areas have also been found in developing white matter (Li et al., 2019; Safaiyan et al., 2021; Figure 5).
Recent research examined changes in gene expression in microglia in the brain of a mouse model of ischemic stroke by combining spatial transcriptomics with single-cell RNA sequencing. The researchers identified microglial subclusters associated with CIRI in the brains of mice undergoing MCAO (Li et al., 2023a). Cluster 2 cells made up 94% of the cells in normal mice, whereas cluster 1 surrounded the region of tissue damage, and cluster 3 was found in the center of the area affected by a lack of blood supply (Li et al., 2023a). Based termed cluster 1 cells as ischemic penumbra-associated microglia and cluster 3 as ischemic core-associated microglia based on the unique marker genes and features of the microglia subpopulations (Li et al., 2023a). Ischemic penumbra-associated microglia may possess neuroprotective qualities that are critical for the survival of ischemic penumbra cells, whereas ischemic-core-associated microglia may contribute to hyper-neuroinflammation and worsen brain injury (Li et al., 2023a). Research on cardiovascular illness has shown that STING has a role in increasing ischemic-core-associated microglia in vascular endothelial cells (Oduro et al., 2022). Although the research was carried out in cardiovascular disease models, these findings pertain to cerebral blood vessels and are specific to the vascular endothelium.
The cGAS–STING pathway and regulation of calcium homeostasis
The ER is an essential cellular organelle that plays a role in protein synthesis, maturation, and the regulation of intracellular Ca2+ levels (Fu et al., 2021). Intracellular Ca2+ signals regulate a wide variety of cellular processes, including secretion, contraction, gene transcription, cell proliferation, and cell death. The ER collaborates and coordinates with mitochondria, lysosomes, and plasma membranes to regulate cellular calcium homeostasis (Marchi et al., 2018), which is necessary for important cellular processes, including the transmission of intracellular Ca2+ (Figure 6).
Figure 6.
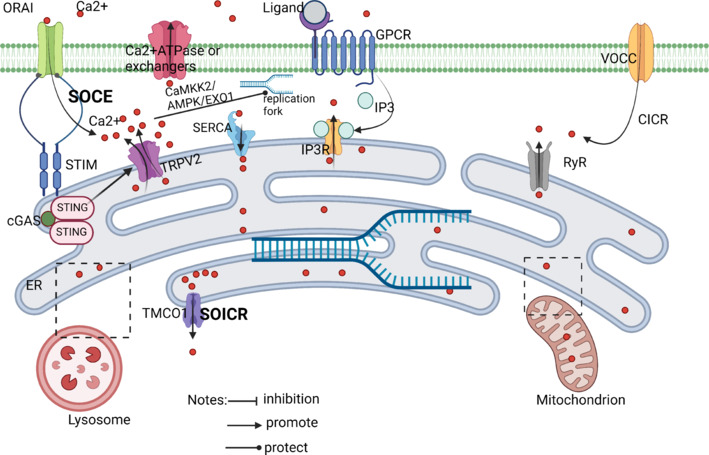
How does STING regulate calcium homeostasis?
STIM1 controls Ca2+ entry into the ER through calcium channels, and STIM1 also directly binds STING to enable STING to reside on the ER. Stimulation using 2,3-cGAMp disrupts this interaction, allowing STING to withdraw from the ER. Activation of STING signaling pathway can increase intracellular calcium ion concentration and cause calcium overload. Created with BioRender.com. AMPK: Adenosine 5’-monophosphate-activated protein kinase; CaMKK2: calcium/calmodulin-dependent protein kinase 2; cGAS: cyclic GMP-AMP synthase; CICR: calcium induced calcium release; EXO1: exonuclease 1; GPCR: G protein-coupled receptor; IP3: inositol triphosphate; IP3R: inositol 1,4,5-triphate receptor; RyR: ryanodine receptor; SERCA: sarcoplasmic/endoplasmic reticulum Ca2+ ATPase; SOCE: store-operated calcium entry; SOICR: store-overload induced calcium release; STING: stimulator of interferon genes; TMCO1: transmembrane and coiled-coil domains 1; VOCC: voltage-gated calcium channel.
As a result, it is critical to consider the consequences of disrupting calcium homeostasis and the signaling function of calcium as a second messenger while studying calcium homeostasis. CIRI activates sodium-calcium exchange proteins, which disturb the ER and impact calcium homeostasis by generating intracellular and extracellular ion misalignment. The condition causes intracellular calcium excretion by allowing Ca2+ to flood cells. Cellular organelles are subjected to irreparable damage from the excess calcium, which causes energy problems and widespread calcium absorption by all organelles. Cell death is the ultimate outcome of this cascade of events. The store-operated calcium entry (SOCE) mechanism is responsible for restocking intracellular Ca2+ reserves. STIM in the ER and the channel ORAI protein in the plasma membrane are two proteins required for SOCE. (Daverkausen-Fischer and Pröls, 2022). There are two isoforms of STIM: STIM1 and STIM2. STIM1 activates SOCE in response to substantial store depletion, whereas STIM2 keeps basal Ca2+ homeostasis in check in response to mild store depletion (Chen et al., 2019c). From a mechanistic standpoint, it seems that STIM1 binds preferentially to the ER monomer of STING in order to inhibit its spontaneous activation, as STIM1 deficiency amplifies STING translocation and dimerization. Conversely, SOCE is increased, and STIM1 translocation and the Ca2+ entry driven by ER Ca2+ store depletion are exacerbated by STING deficiency (Srikanth et al., 2019). Thus, STIM1 is an essential component in CIRI-induced severe calcium overload. To stop STING from being translocated and activated, STIM1 keeps some STING localized in the ER. Nevertheless, STING may regulate calcium homeostasis, improve SOCE, and boost STIM1 translocation if blocked early. During CIRI, STING activation is enhanced due to the direct effect of cGAS activation on the STING produced by cytoplasmic DNA breakage. The free translocation of STIM1 and its interaction with calcium-release-activated calcium modulator 1 might result in a brief inward influx of cytoplasmic Ca2+ (Mathavarajah et al., 2019). Furthermore, calcium homeostasis is disrupted and SOCE is restricted by excessive STING activity, which also significantly increases Ca2+ inward flow. A recent study found that cytosolic DNA is recognized by cGAS under replication stress, resulting in self-activation and cGAMP production. cGAMP then binds to STING, resulting in the separation of the latter from transient receptor potential vanilloid type 2 (TRPV2) and ending with the loss of TRPV2 inhibition and the release of Ca2+ into the cytoplasm (Li et al., 2023b; Figure 6).
Mathavarajah et al. (2019) found that STING activation is additionally influenced by Ca2+ signal. The cGAS-STING signalling pathway has been discovered to be regulated by Ca2+ fluxes that come from the mitochondrial and endoplasmic reticulum Ca2+ pools, as well as important Ca2+ signalling proteins such as calmodulin, CAMKII, and CAMKIV (Mathavarajah et al., 2019). Furthermore, the primary recipients of calcium signaling are autophagy and AMP-activated protein kinase (Mathavarajah et al., 2019). Therefore, it is clear that STING and Ca2+ are functionally related. Calcium excess impacts STING activity during CIRI. However, STING activation undermines calcium homeostasis control, creating a self-perpetuating cycle that amplifies CIRI’s harming of cells.
The cGAS–STING pathway and many forms of cell death such as apoptosis, necrosis, ferroptosis, and pyroptosis in cerebral ischemia-reperfusion injury
To maintain homeostasis in vivo, ER stress becomes a pro-apoptotic stimulator after CIRI, activating caspase family members and driving the initiation of apoptosis (D’Arcy, 2019). The hallmarks of cell death include DNA degradation inside the cell, nuclear sequestration, nuclear fragmentation, and the formation of apoptotic vesicles (Xiong et al., 2021). Numerous cysteine proteases are required for cell death to begin, including those that initiate cell death (caspase-2, caspase-8, caspase-9, and caspase-10), as well as those that carry out cell death (caspase-3, caspase-6, caspase-7, and caspase-14) (Yuan et al., 2018). New research has shown that caspase enzymes and the cGAS-STING pathway communicate with one another (Xiong et al., 2021). Caspase-4, caspase-11, caspase-3, and caspase-7 may all be activated by cGAS, as seen in Figure 7. Additionally, the cGAS has the ability to activate caspase-1, which results in the generation of IL-1β and IL-8. Caspases-3 and caspase-7 may be activated by IL-1β and IL-8. At the same time, caspase-9 and its downstream executors, caspase-3 and caspase-7, are stimulated by STING and the IRF3 production it mediates (Figure 7).
Figure 7.
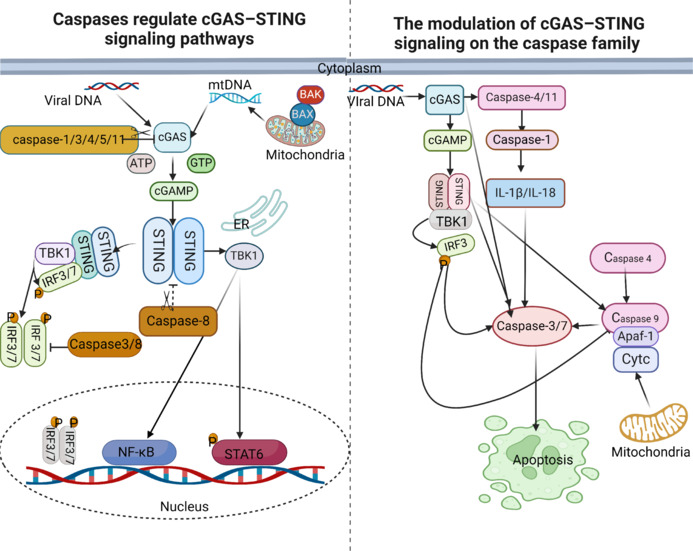
Crosstalk between cGAS-STING and caspases family.
Left: (1) Apoptotic caspase-1, caspase-3, caspase-4, caspase-5, and caspase-11 can directly cut cGAS; (2) Apoptotic caspase-3, caspase-7, caspase-8, and caspase-9 can inhibit the production of IFN-a/b mediated by cGAS/STING/TBK1/IRF3; (3) caspase-3 and caspase-8 negatively regulate the CGAS-STING pathway; and (4) caspase-8 can also directly cut STING. Right: As shown in the figure, cGAS can activate Caspase-4, Caspase-11, Caspase-3, and Caspase-7. Moreover, it can also activate caspase-1 by activating the activation of caspase-4 and caspase-11, thus activating IL-1b and IL-8; IL-1b and IL-8 also have the function of activating caspase-3 and caspase-7. At the same time, STING and its mediated generation of IRF3 can stimulate the activation of caspase-9 and downstream actuators caspase-3 and caspase-7. Created with BioRender.com. Apaf-1: Apoptotic protease activating factor-1; Caspase: cysteinyl aspartate specific proteinase; cGAMP: cyclic GMP-AMP; cGAS: cyclic GMP-AMP synthase; IL-1β: interleukin-1β; IRF3/7: interferon regulatory factor 3/7; NF-κB: nuclear factor kappa-B; P: phosphorylation; STAT6: signal transducer and activator of transcription 6; STING: stimulator of interferon genes; TBK1: TANK-binding kinase 1.
Most cells undergo cellular necrosis following CIRI. Cell necrosis is regulated by receptor-interacting kinase 1 (RIP1 or RIPK1), receptor-interacting kinase 3 (RIPK3 or RIP3), and pseudokinase mixed-spectrum kinase structural domain-like protein (MLKL) (He and Wang, 2018). Increased cellular vulnerability to necrosis is caused by the transcriptional induction of mixed-spectrum kinase structural domain-like protein and the upregulation of receptor-interacting kinase 3 in response to IFN stimulation (Chen et al., 2019a; Ingram et al., 2019; Knuth et al., 2019; Sarhan et al., 2019). In the event that caspase-8 inhibition occurs, the phosphorylation of mixed-spectrum kinase structural domain-like protein and subsequent necrotic apoptosis might result from TNF binding to the tumor necrosis factor receptor 1 trimer, which in turn activates receptor-interacting kinase 1/receptor-interacting kinase 3. Research by Murthy et al. (2020) supports the idea that the cGAS-STING pathway is involved in necrosis initiation. Inhibiting caspase-8 causes necrosis by activating the TNF and IFN signaling pathways, which are reliant on cGAS-STING and react to cytoplasmic DNA (Brault et al., 2018). Thus, cell necrosis necessitates the activation of receptor-interacting kinase 1/receptor-interacting kinase 3 via a TNF–tumor necrosis factor receptor 1 interaction, as well as mutual and synergistic signaling across the two STING-activated pathways. This is consistent with what we mentioned above: cGAS-STING can activate TNF by activating NF-κB, providing a “primer” for the subsequent reaction.
Recent research has shown that an imbalance in iron metabolism is a key factor leading to the worsening of early brain damage in CIRI (Li et al., 2021a). Ferroptosis, a kind of controlled cell death related to an iron metabolism imbalance, is characterized by a fatal buildup of lipid peroxides caused by increased intracellular iron and reduced antioxidant capability. Iron mortality is specifically caused by a deficiency in anti-lipid oxidative activity (Dodson et al., 2019). Nuclear receptor coactivator 4 (NCOA4), a cargo receptor, promotes ferritin-selective autophagy, resulting in elevated intracellular Fe2+ levels and lipid peroxidation, which causes iron death. According to research, NCOA4 controls ferritin iron phagocytosis and modulates iron death in ischemic stroke (Li et al., 2021a). It is worth noting that ferroptosis occurs via the glutathione peroxidase 4 (GPX4) inactivation caused by glutathione deficiency as well as direct GPX4 inactivation. As a result, GPX4 functional loss, whether direct or indirect, is a critical event that leads to iron death events (Ingold et al., 2018). In other words, cells with lower GPX4 levels are more susceptible to iron death, and GPX4 knockdown may cause ferroptosis.
An experimental model of sepsis in knockout C57BL/6 mice revealed a significant discovery: the direct interaction of STING and NCOA4 results in the liberation of free Fe2+. The CDN-binding domain of STING binds to the CC structural domain of NCOA4 for autophagic degradation, enhancing the stability of the STING dimer, reducing the nuclear coactivator function of NCOA4, and leading to decreased protective effects. The nuclear localization capacity of NCOA4 is diminished as a consequence (Wu et al., 2022). These results indicate that STING activation is necessary for the formation of iron mutations, with NCOA4 playing a role. NOCA4 enhances STING-mediated downstream reactions, which is pertinent not only to sepsis, as shown by this research, but also to iron mortality in stroke, as shown by prior studies.
A recent study has shown the critical functions of GPX4 in controlling lipid peroxidation and STING activation. When GPX4 was inhibited, lipid peroxidation increased, resulting in STING carbonylation at C88 and transport blockage from the ER to the Golgi complex (Jia et al., 2020). This study’s findings imply that GPX4 activation is required for the cGAS-STING pathway to function in CIRI. Furthermore, without external energy replenishment, CIRI produces a progressive drop in energy levels over time, leading to the downregulation of glutathione and GPX4. Interestingly, the STING pathway remains elevated despite lower energy levels, indicating a strong reliance on GPX4 activation (Gamdzyk et al., 2020). Under normal settings, ferroptosis needs coordination between GPX4, STING, and NACO4 to maintain dynamic equilibrium. However, the ferroptosis caused by CIRI or other external sources disturbs this coordination, resulting in a decrease in GPX4 and STING overactivation, therefore increasing ferroptosis.
Pyroptosis is a form of programmed cell death induced by the inflammatory vesicle-mediated activation of caspase-1. An exaggerated inflammatory response ensues as a consequence of gasdermin-D-induced cellular lysis, cytoplasmic release, and the generation of pro-inflammatory cytokines, including IL-1β and IL-18 (He et al., 2015; Poh et al., 2019). The localized death-associated inflammatory vesicles encapsulate various pattern recognition receptors from the NOD-like receptor family, including nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing 1-3 (NLRP1-3), NLR family CARD domain-containing 4, and melanoma 2 (AIM2), as well as pro-caspase-1 and apoptosis-associated speck-like protein, among other components (Chen et al., 2019b; Akbal et al., 2022). The process of pyroptosis is initiated when inflammatory vesicles containing proteins, such as NLRP3 and AIM2, activate caspase-1, resulting in the release of the pro-inflammatory cytokines IL-1γ and IL-18. The ischemic semidark zone is the primary site of pyroptosis, a powerful instigator of pro-inflammatory pathways in ischemic stroke (Dong et al., 2018). CIRI damage is associated with pyroptosis, inflammation, autophagy, and mitochondrial dysfunction, according to recent research (Zhang et al., 2022). Excessive autophagy contributes to cell death and cytoplasmic content release in the late phases of reperfusion damage, which in turn causes adjacent cells to die and worsens CIRI (Shi et al., 2012; Strowig et al., 2012). STING aids pyroptosis by increasing caspase-1 activity. To facilitate the production of inflammatory vesicles, STING recruits NLRP3 and encourages its ER location (Wang et al., 2020b). An additional mechanism that might enhance AIM2 protein levels is the activation of the cGAS-STING-IFN1 pathway (Swanson et al., 2017). When DNA is present, AIM2’s function is replaced by the cGAS-STING-NLRP3 pathway in human myeloid cells. This pathway is responsible for lysosomal cell death, K+ efflux, and the activation of NLRP3 inflammatory vesicles (Gaidt et al., 2017). The number of danger signals is proportional to the DNA level and its location in the cytoplasm, which in turn governs which DNA sensor is triggered.
The development of gasdermin D holes causes the outflow of potassium ions, according to ion channel research. Consequently, this reduces IFN production by blocking the activation of the dsDNA-mediated cGAS-STING pathway (Feltham and Vince, 2018). To further restrict cytoplasmic DNA-mediated cGAS-STING activation, caspase-1 may directly cleave cGAS (Wang et al., 2017). On the other hand, IFN has the ability to suppress the activation of NLRP3 inflammatory vesicles in a STAT1-dependent way, which means it can prevent the release of IL-1β that is reliant on caspase-1 (Guarda et al., 2011). These results demonstrate the role of the intricate relationship between cGAS-STING and components of inflammatory vesicles in controlling inflammation and avoiding its excess (Murthy et al., 2020; Figure 8).
Figure 8.
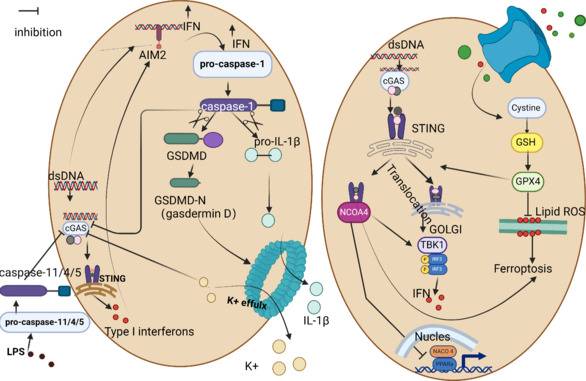
STING with two different modes of cell death.
Left: The crosstalk between the STING pathway and pyroptosis. When cGAS-STING-IFN1 amplifies AIM2 protein levels, AIM2 can activate caspase-1, which in turn triggers the secretion of IL-1β and IL-18 from cells, leading to pyroptosis. Caspase-1 has the ability to shear GSDMD and release its N-terminal domain. In addition to GSDMD, Caspase-1 can also shear IL-1β precursors and IL-18 precursors. Right: The crosstalk between the STING pathway and ferroptosis. GPX4 activates STING, and STING directly interacts with NCOA4 to release free Fe2+ and activate iron death. Created with BioRender.com. Caspase-1: Cysteinyl aspartate specific proteinase 1; Caspase-11/4/5: cysteinyl aspartate specific proteinase-11/4/5; cGAS: cyclic GMP-AMP synthase; DNA: double-stranded DNA; GPX4: glutathione peroxidase 4; GSDMD: gasdermin; GSH: glutathione, reduced; IFN: interferon; IL-1β: interleukin-1β; IRF3: interferon regulatory factor 3; NCOA 4: nuclear receptor coactivator 4; PPARa: peroxisome proliferator-activated receptor-alpha; STING: stimulator of interferon genes; TBK1: TANK-binding kinase 1.
Relationship between cGAS–STING and time following cerebral ischemia-reperfusion injury
When researching therapy and prognosis, the connection between CIRI and the time frame are crucial considerations. Current attention to the relationship between CIRI and time has focused more on the timing of thrombolysis and intervention, with fewer studies at longer time points such as 24 and 48 hours after the onset of CIRI. Nevertheless, cerebral edema usually begins to form within 24 hours after CIRI and reaches its peak 72–96 hours later. Researchers discovered that levels of cGAS and STING transcripts were much higher 72 hours after hypoxia-ischemia, suggesting that STING-induced autophagy is often harmful, as it is at its highest at the late stage of reperfusion (Gamdzyk et al., 2020). It is worth noting that the use of small-interfering RNA to silence STING resulted in a smaller infarct, less cortical neurodegeneration, and better neurobehavior after 48 hours.
In a study by Rong et al. (2022), it was found that in an environment of adequate nutrition and unactivated innate immunity, STING molecules could inhibit the autophagosome-lysosome fusion process involved in STX17 and down-regulate the autophagic flux by binding to STX17. But when CIRI starts, along with hypoxia and ischemia, mitochondria are damaged and lose energy over time, which reduces the binding of STING and STX17 and eventually triggers autophagy. In CIRI, expression of the cGAS-STING signaling pathway reaches a peak, and the sooner it is inhibited before then, the greater the benefit. It is therefore important to know exactly when the cGAS-STING signalling pathway begins to be highly expressed. The likelihood of a positive CIRI prognosis increases the sooner negative the regulation of cGAS-STING signaling begins.
In another study, STING-mRNA levels in the hippocampus, cortex, liver, and spleen tissues of adult rats were higher than those of 10-day-old neonatal rats (Gamdzyk et al., 2020); thus there appears to be a relationship between STING and the age of an individual. Sladitschek-Martens et al. (2022) discovered that stromal cell Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) activity regulated cGAS-STING, which in turn halted senescence. The negative regulation of cGAS-STING may be used as a therapeutic signaling route that could be integrated into regular preventative strategies, as aging is also intricately linked to CIRI.
cGAS–STING and complement system
An important part of the body’s innate immune system is the complement system, which includes the cGAS–STING pathway, and there is evidence these systems are related, according to studies conducted on human immunodeficiency virus infection (Posch et al., 2021). Complement-enabled human immunodeficiency virus RNA was used to induce the generation of type I IFN downstream of STING in the absence of cGAS. Another study that looked at Listeria infection also obtained the same results, finding that wildtype bone marrow-derived dendritic cells produced fewer IFN-β signaling molecules, such as dead-box helicase 41, STING, phosphorylated TBK1, and phosphorylated p38 mitogen-activated protein kinase, when cells were pre-treated with C5a and C3a (Mueller-Ortiz et al., 2017).
Inhibitors to cGAS–STING Pathway
According to the findings, the cGAS–STING pathway seems to be significantly involved in CIRI deterioration. That being said, the path and the course of the illness may be related. However, STX17 seems to have a multi-faceted function in CIRI formation, impacting autophagy and mitochondrial dynamics in ways that are at odds with one another. Thus, cGAS–STING pathway inhibitors have been investigated as a means to modify disease progression.
Inhibitors of cGAS
The DNA-binding and pathway-starting processes of the CGAS-STING pathway are dependent on cGAS. Therefore, inhibiting cGAS activity or preventing it from attaching to DNA may be a means of disrupting the cGAS-STING pathway. Table 1 summarizes the various cGAS inhibitors found in the literature.
Table 1.
Inhibitors of cGAS–STING pathway
| Target | Inhibitor | Mechanism | Reference |
|---|---|---|---|
| Cytoplasmic DNA | Metformin or rapamycin | Autophagy can be induced by causing an increase in intracellular reactive oxygen species, and the presence of extracellular DNA can be reduced by autophagy. | Han et al., 2020 |
| Hydroxychloroquine | Prevents DNA from binding to cGAS and replaces the bound DNA | An et al., 2015 | |
| Quinacrine | Prevents DNA from binding to cGAS | An et al., 2015 | |
| X6 | Blocking DNA binding of DNA to cGAS inhibits its activity | An et al., 2018 | |
| Suramin sodium | Functions as a DNA imitator but does not trigger cGAS for GAMP production | Wang et al., 2018b | |
| cGAS | RU. 521 | Binding to the catalytic region of cGAS and decreasing its binding strength to ATP and GTP | Vincent et al., 2017 |
| G150 | Taking over cGAS’s active spot and making it less likely to bind to ATP and GTP | Lama et al., 2019 | |
| Aspirin | Acetylation of cGAS | Dai et al., 2019 | |
| pf-06928215 (highly inhibited but inactive intracellularly) | Inhibition of cGAS activity | Hall et al., 2017 | |
| S2/S3 | Inhibition of cGAS activity | Zhao et al., 2020 | |
| CU-32/CU-76 | Prevention of cGAS dimerization | Padilla-Salinas et al., 2020 | |
| A151 | Abrogation of cytoplasmic nucleic acid sensing cGAS and activation of AIM inflammatory vesicles by immunostimulatory DNA competition for binding | Li et al., 2020 | |
| Epigallocatechin gallate | Blocking the interaction of cGAS with DNA by inhibiting the activity of GTP-activated proteins | Liu et al., 2019 | |
| STING | C-178/C-176 | Blocking STING protein activation-induced palmitoylation and preventing palmitoylation-induced STING aggregation | Haag et al., 2018 |
| H-151 | Blocking palmitoylation caused by STING protein activation and preventing STING protein aggregation | Haag et al., 2018 | |
| NO2-FAs | Blocking STING protein activation-induced palmitoylation | Hansen et al., 2018 | |
| Astin C | Binding to STING and CDN, and preventing IRF3 from joining the STING signalosome | Li et al., 2018 | |
| Compound 18 | Binding to STING, and preventing IRF3 from joining the STING signalosome | Siu et al., 2019 | |
| Cyanocarbonyl-3-chlorophenylhydrazone | Disruption of the mitochondrial membrane potential stops STING and TBK-1 from talking to each other | Kwon et al., 2017 | |
| IFN | Ruxolitinib | Selective inhibition of IFN-γ/JAK/STAT signaling pathway | Qian et al., 2022 |
| Baricitinib | Blocking the IFN signal | Sanchez et al., 2018 | |
| Cyclo(L-Phe-L-Pro) | Inhibition of IFN-β production by interfering with the activation of retinoic acid-inducible gene-I (RIG-I) | Lee et al., 2018 |
Atg5: Autophagy related 5; cGAS: cyclic GMP-AMP synthase; IFN: interferon; Mfn2: Mitofusin 2; mito-LC3II: mitochondrial microtubule-associated proteins 1A/1B light chain 3B II; Smac: second mitochondria-derived activator of caspase; x-IAP: X-linked inhibitor of apoptosis protein.
On the other hand, certain inhibitors, such as antimalarials and anticancer medications, may not be viable options for CIRI therapy due to their potential for unwanted side effects. However, for some CIRI patients with malaria or cancer disease, there may be some benefits to treating CIRI with these drugs. Because many CIRI patients also have type 2 diabetes, hyperglycemia is a major concern when using the diabetic medication metformin, which increases the likelihood of ischemic stroke. It has also been discovered that aspirin, a key medication for CIRI therapy, inhibits cGAS (Elkon, 2019). Hence, the timing of aspirin administration is equally important.
Inhibitors of STING
As a key molecule in the cGAS–STING pathway, aberrant STING protein activation may be triggered by micronuclei, mtDNA, bacteria, viruses, and defective cytoplasmic chromatin, among others (Dou et al., 2017; Parkes et al., 2017; Hu et al., 2021; Liu et al., 2021). Therefore, to avoid the ensuing IFN-mediated response, it is crucial to find STING inhibitors. Table 1 summarizes the literature on STING inhibitors. Studies show that STING inhibitors significantly hinder palmitoylation, an essential step in protein membrane migration (Linder and Jennings, 2013; Jiang et al., 2018). These findings have provided further evidence that intercellular transport may be impacted by inhibitors of STING proteins. For this reason, CIRI mitigation relies heavily on the exact timing of STING blocking.
Inhibitors of downstream effectors of the cGAS–STING pathway
We have already shown that variables farther down the cGAS-STING pathway may regulate one another via inhibitory effects and negative feedback. Some studies have found inhibitors of these downstream effectors (Table 1). The cytosolic RNA innate sensor retinoic acid-inducible gene-I is the target of cyclo-L-phenylalanyl-L-proline, a cyclic dipeptide with antifungal properties (Wang et al., 2022). Therefore, it may be more pertinent when discussing fungal disease.
We found that RIG-1 has a similar effect to cGAS (Lee et al., 2019; Han et al., 2021), and this drug inhibits not only the recognition of endogenous nucleic acids by inhibiting RIG-1 but also IFN synthesis (Lee et al., 2018). Interferon acts downstream of cGAS-STING, and if IFN cannot play a role, it will reduce the inflammatory response, which will also reduce CIRI.
The CRISPR/CRISPR-associated nuclease 9 (CRISPR/Cas9) genome editing system is a powerful genome engineering tool. CRISPR/Cas9 technology has become the most examined gene editing technology in recent years due to its simple design, yet low cost, high efficiency, and simple operation. Using this method, it was found that sulfated glycosaminoglycans (sGAGs) in the Golgi apparatus were necessary and sufficient to drive STING polymerization (Fang et al., 2021), and inhibition of sGAGs should be effective for alleviating CIRI. Another study found that CRISPR transfection activated STING-dependent antiviral responses in keratinocytes, but CRISPR-generated KO keratinocytes permanently suppressed IFN-κand IFN-stimulated gene (ISG) expression (Sarkar et al., 2023), which would cause permanent damage if used as a target and is therefore not recommended. Genome-wide CRISPR-Cas9 screening confirmed that Armadillo-like helical domain-containing protein 3 is the key to STING activation. Moreover, STING activation was shown to be impaired by disrupting PI4P-dependent lipid transport through RNA interference with other PI4P-binding proteins (Fang et al., 2023). Therefore, RNA interference is also an option for treatment research.
The controlled activation of the cGAS–STING may be beneficial to individuals with CIRI, but overactivation can have adverse effects. Overstimulation of the immune system’s functions may cause autoimmune diseases. Timely intervention is therefore essential. Restoring the homeostasis of the organism and maximizing the beneficial effects of the cGAS–STING pathway would likely be a better therapeutic strategy.
Conclusion and Outlook
Physicians have had long-term difficulties treating CIRI due to the many mechanisms that eventually lead to nerve cell loss and patient dysfunction. However, there is a lot of hope for improving the clinical management of CIRI with the introduction of novel ideas such as therapeutic time windows and ischemic penumbra, as well as novel treatments such as mechanical thrombolysis and endovascular thrombolysis. Understanding the molecular underpinnings of CIRI is essential to maximizing neuronal cell salvage efforts and glial and neuronal cell survival in order to minimize neurological problems. Recent studies on CIRI have focused on modulating the cGAS–STING pathway. Some studies on the treatment of CIRI by modulating the cGAS–STING pathway have confirmed its beneficial effect on the alleviation of CIRI. However, we found that there is a certain correlation between the expression of cGAS–STING pathway and time, so it will be a challenge to choose the right time point for correct regulation.
The clinical etiology of stroke is complicated, which makes managing the convergence of pathogenic processes in the period after reperfusion and identifying the optimal time point for reperfusion recovery difficult. As CIRI causes severe damage and loss of neuronal cells, long-term therapy and future treatments depend on the negative regulation of the cGAS–STING pathway. The identification of pathogenic mechanisms of CIRI mediated by the STING pathway has been based on STING’s effects on inflammation and autophagy. As STING ultimately appears to be harmful to cellular autophagy in CIRI, neuronal cell survival may also be impacted by the surge that appears after 72 hours. Conversely, when therapies are given during the ischemic phase or within the time window, early activation of the cGAS-STING pathway may be required to reduce the effects on the nervous system and to preserve as many neuronal cells as possible. This begs the question of whether the scheduling of aspirin administration in clinical settings should be altered in order to optimize its effectiveness as a cGAS inhibitor. It is important to carefully consider the timing of cGAS-STING pathway regulation in order to achieve the intended therapeutic impact.
According to many recent studies, there is still a chance of cerebral bleeding with thrombolytic treatments (Whiteley et al., 2012; Powers et al., 2019). Thrombolytic medication infusion causes BBB damage to worsen, upregulates NETs, and elevates STING expression (Gao et al., 2023). It is possible that focusing on NETs or cGAS might lower the incidence of post-thrombolytic bleeding in ischemic stroke and provide novel treatment approaches to enhance thrombolysis safety (Wang et al., 2021).
The upregulation of STING expression in CIRI enhance STX17 protein activity, promotes NF-κβ activation, IRF3 release, and cellular autophagy. The patients with the illness benefit from the appropriate activation of STING, but in the pathological state of CIRI, the initial balance of STING control is upset, leading to the overactivation of many pathways. Numerous studies have discovered that STING pathway downregulation reduces neuronal damage and improves neuroinflammation. STING also modulates cellular autophagy and inflammation, but these effects are not independent of other mechanisms (such as the brain-gut axis, microglia activation, calcium regulation, and apoptosis). In order to promote post-stroke neurological healing, precision-targeted STING-based treatments are probably more promising therapeutic approaches than broad-based therapies such as anti-inflammatory and antioxidant therapies.
This review has some limitations: First, it is based on a comprehensive analysis of the literature within a specific time frame. Due to the constant updates to and growth of the literature, some recent studies may not have been taken into account. Second, many of the research subjects or models discussed in this paper are only theoretically related to CIRI, and some of the latest research on CIRI models is lacking. Moreover, the ratio of animal studies to clinical studies may be somewhat imbalanced in this paper, and there is currently a lack of STING-related CIRI clinical trials. After all, the results of animal experiments do not fully apply to humans. Finally, this scientific field is still in the exploration stage and at the whole-genome level, so the sorting of inhibitors is not comprehensive. Future inhibitor screening and validations of this signaling pathway and some of its related proteins should make use of more sophisticated technologies, including CRISPR, in order to expand the range of available therapy options.
The cGAS-STING pathway plays a significant role in the pathophysiology of CIRI, but there are several additional contributing elements. It would be incorrect to believe that treating or inhibiting CIRI should only be dependent on blocking or activating one particular protein or biological pathway. Consequently, considering the temporal link, combination treatments that concurrently target the cGAS-STING pathway may represent feasible therapeutic approaches. In the future, a thorough evaluation of autophagy with a refined or dynamic emphasis on the period after reperfusion is required, as autophagy is a dynamic process, and it is difficult to manage its moderate activation. As this review makes clear, further effort is required to adapt the existing findings to clinical practice and integrate time-based relationships into the therapy of CIRI. In conclusion, our knowledge of the roles of the STX17 protein and cGAS-STING pathway in CIRI will expand along with our comprehension of their pathogenicity and the interactions between them throughout time. This will undoubtedly aid in the creation of more effective CIRI therapies.
Funding Statement
Funding: This work was supported by Yuan Du Scholars, Clinical Research Center of Affiliated Hospital of Shandong Second Medical University, No. 2022WYFYLCYJ02; Weifang Key Laboratory, Weifang Science and Technology Development Plan Project Medical Category, No. 2022YX093.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editor: Wang L; T-Editor: Jia Y
Data availability statement:
Not applicable.
References
- Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Röhl I, Hopfner KP, Ludwig J, Hornung V. cGAS produces a 2’-5’-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbal A, Dernst A, Lovotti M, Mangan MSJ, McManus RM, Latz E. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell Mol Immunol. 2022;19:1201–1214. doi: 10.1038/s41423-022-00922-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Woodward JJ, Sasaki T, Minie M, Elkon KB. Cutting edge: Antimalarial drugs inhibit IFN-β production through blockade of cyclic GMP-AMP synthase-DNA interaction. J Immunol. 2015;194:4089–4093. doi: 10.4049/jimmunol.1402793. [DOI] [PubMed] [Google Scholar]
- An J, Woodward JJ, Lai W, Minie M, Sun X, Tanaka L, Snyder JM, Sasaki T, Elkon KB. Inhibition of cyclic GMP-AMP synthase using a novel antimalarial drug derivative in Trex1-deficient mice. Arthritis Rheumatol. 2018;70:1807–1819. doi: 10.1002/art.40559. [DOI] [PubMed] [Google Scholar]
- Andreeva L, Hiller B, Kostrewa D, Lässig C, de Oliveira Mann CC, Jan Drexler D, Maiser A, Gaidt M, Leonhardt H, Hornung V, Hopfner KP. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature. 2017;549:394–398. doi: 10.1038/nature23890. [DOI] [PubMed] [Google Scholar]
- Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13:661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Cui N, Liao Y, Guo C, Li L, Yin Y, Wen A, Wang J, Ye W, Ding Y. Astrocytes and microglia-targeted Danshensu liposomes enhance the therapeutic effects on cerebral ischemia-reperfusion injury. J Control Release. 2023;364:473–489. doi: 10.1016/j.jconrel.2023.11.002. [DOI] [PubMed] [Google Scholar]
- Bakhoum SF, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–472. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balka KR, Louis C, Saunders TL, Smith AM, Calleja DJ, D’Silva DB, Moghaddas F, Tailler M, Lawlor KE, Zhan Y, Burns CJ, Wicks IP, Miner JJ, Kile BT, Masters SL, De Nardo D. TBK1 and IKKε act redundantly to mediate STING-induced NF-κB responses in myeloid cells. Cell Rep. 2020;31:107492. doi: 10.1016/j.celrep.2020.03.056. [DOI] [PubMed] [Google Scholar]
- Baskaran S, Carlson LA, Stjepanovic G, Young LN, Kim DJ, Grob P, Stanley RE, Nogales E, Hurley JH. Architecture and dynamics of the autophagic phosphatidylinositol 3-kinase complex. Elife. 2014;3:e05115. doi: 10.7554/eLife.05115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Hong SH, Menghani H, Marcell SJ, Obenaus A, Freitas RS, Khoutorova L, Balaszczuk V, Jun B, Oriá RB, Bazan NG. Docosanoids promote neurogenesis and angiogenesis, blood-brain barrier integrity, penumbra protection, and neurobehavioral recovery after experimental ischemic stroke. Mol Neurobiol. 2018;55:7090–7106. doi: 10.1007/s12035-018-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerzoug S, Ryffel B, Togbe D, Quesniaux VFJ. Self-DNA sensing in lung inflammatory diseases. Trends Immunol. 2019;40:719–734. doi: 10.1016/j.it.2019.06.001. [DOI] [PubMed] [Google Scholar]
- Berge E, Cohen G, Roaldsen MB, Lundström E, Isaksson E, Rudberg AS, Slot KB, Forbes J, Smith J, Drever J, Wardlaw JM, Lindley RI, Sandercock PA, Whiteley WN, IST-3 Collaborative Group Effects of alteplase on survival after ischaemic stroke (IST-3): 3 year follow-up of a randomised, controlled, open-label trial. Lancet Neurol. 2016;15:1028–1034. doi: 10.1016/S1474-4422(16)30139-9. [DOI] [PubMed] [Google Scholar]
- Berke IC, Li Y, Modis Y. Structural basis of innate immune recognition of viral RNA. Cell Microbiol. 2013;15:386–394. doi: 10.1111/cmi.12061. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Brault M, Olsen TM, Martinez J, Stetson DB, Oberst A. Intracellular nucleic acid sensing triggers necroptosis through synergistic type I IFN and TNF signaling. J Immunol. 2018;200:2748–2756. doi: 10.4049/jimmunol.1701492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Hu M, Li C, Wu R, Lu D, Xie C, Zhang W, Li T, Shen S, Huang H, Qiu W, Liu Q, Lu Y, Lu Z. FOXP3+ macrophage represses acute ischemic stroke-induced neural inflammation. Autophagy. 2023;19:1144–1163. doi: 10.1080/15548627.2022.2116833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kuroki S, Someda M, Yonehara S. Interferon-γ induces the cell surface exposure of phosphatidylserine by activating the protein MLKL in the absence of caspase-8 activity. J Biol Chem. 2019;294:11994–12006. doi: 10.1074/jbc.RA118.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xia YF, Shen SF, Tang J, Chen JL, Qian K, Chen Z, Qin ZH, Sheng R. Syntaxin 17 inhibits ischemic neuronal injury by resuming autophagy flux and ameliorating endoplasmic reticulum stress. Free Radic Biol Med. 2020;160:319–333. doi: 10.1016/j.freeradbiomed.2020.08.010. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- Chen X, Yao Z, Peng X, Wu L, Wu H, Ou Y, Lai J. Eupafolin alleviates cerebral ischemia/reperfusion injury in rats via blocking the TLR4/NF-κB signaling pathway. Mol Med Rep. 2020;22:5135–5144. doi: 10.3892/mmr.2020.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yao N, Mao Y, Xiao D, Huang Y, Zhang X, Wang Y. Activation of the Wnt/β-catenin/CYP1B1 pathway alleviates oxidative stress and protects the blood-brain barrier under cerebral ischemia/reperfusion conditions. Neural Regen Res. 2024;19:1541–1547. doi: 10.4103/1673-5374.386398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Meng J, Bi F, Li H, Chang C, Ji C, Liu W. EK7 regulates NLRP3 inflammasome activation and neuroinflammation post-traumatic brain injury. Front Mol Neurosci. 2019;12:202. doi: 10.3389/fnmol.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Chen LH, Shen MR. The distinct role of STIM1 and STIM2 in the regulation of store-operated Ca(2+) entry and cellular function. J Cell Physiol. 2019;234:8727–8739. doi: 10.1002/jcp.27532. [DOI] [PubMed] [Google Scholar]
- Cui S, Ke L, Wang H, Li L. TSG-6 alleviates cerebral ischemia/reperfusion injury and blood-brain barrier disruption by suppressing ER stress-mediated inflammation. Brain Res. 2023;1817:148466. doi: 10.1016/j.brainres.2023.148466. [DOI] [PubMed] [Google Scholar]
- D’Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- Dai F, Hu C, Li X, Zhang Z, Wang H, Zhou W, Wang J, Geng Q, Dong Y, Tang C. Cav3.2 channel regulates cerebral ischemia/reperfusion injury: a promising target for intervention. Neural Regen Res. 2024;19:2480–2487. doi: 10.4103/1673-5374.390966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, et al. Acetylation blocks cGAS activity and inhibits Self-DNA-induced autoimmunity. Cell. 2019;176:1447–1460.e14. doi: 10.1016/j.cell.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daverkausen-Fischer L, Pröls F. Regulation of calcium homeostasis and flux between the endoplasmic reticulum and the cytosol. J Biol Chem. 2022;298:102061. doi: 10.1016/j.jbc.2022.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Mann CC, Orzalli MH, King DS, Kagan JC, Lee ASY, Kranzusch PJ. Modular architecture of the STING C-terminal tail allows interferon and NF-κB signaling adaptation. Cell Rep. 2019;27:1165–1175.e5. doi: 10.1016/j.celrep.2019.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548–569. doi: 10.1038/s41577-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, Khalessi AA, Levy EI, Palesch YY, Prabhakaran S, Saposnik G, Saver JL, Smith EE, American Heart Association Stroke Council and Council on Epidemiology and Prevention Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47:581–641. doi: 10.1161/STR.0000000000000086. [DOI] [PubMed] [Google Scholar]
- Deng Z, Chong Z, Law CS, Mukai K, Ho FO, Martinu T, Backes BJ, Eckalbar WL, Taguchi T, Shum AK. A defect in COPI-mediated transport of STING causes immune dysregulation in COPA syndrome. J Exp Med. 2020;217:e20201045. doi: 10.1084/jem.20201045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, Vance RE. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013;3:1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Han R, Jiang W, Xiao J, Liu H, Chen X, Li X, Hao J. Programmed death ligand 1 plays a neuroprotective role in experimental autoimmune neuritis by controlling peripheral nervous system inflammation of rats. J Immunol. 2016;197:3831–3840. doi: 10.4049/jimmunol.1601083. [DOI] [PubMed] [Google Scholar]
- Dobbs N, Burnaevskiy N, Chen D, Gonugunta VK, Alto NM, Yan N. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe. 2015;18:157–168. doi: 10.1016/j.chom.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Pan K, Pan J, Peng Q, Wang Y. The possibility and molecular mechanisms of cell pyroptosis after cerebral ischemia. Neurosci Bull. 2018;34:1131–1136. doi: 10.1007/s12264-018-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooyema SDR, Noto JM, Wroblewski LE, Piazuelo MB, Krishna U, Suarez G, Romero-Gallo J, Delgado AG, Peek RM. Helicobacter pylori actively suppresses innate immune nucleic acid receptors. Gut Microbes. 2022;14:2105102. doi: 10.1080/19490976.2022.2105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Chen ZJ. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–709. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas AA, Borst K, Prinz M. Current tools to interrogate microglial biology. Neuron. 2021;109:2805–2819. doi: 10.1016/j.neuron.2021.07.004. [DOI] [PubMed] [Google Scholar]
- El Amki M, Glück C, Binder N, Middleham W, Wyss MT, Weiss T, Meister H, Luft A, Weller M, Weber B, Wegener S. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep. 2020;33:108260. doi: 10.1016/j.celrep.2020.108260. [DOI] [PubMed] [Google Scholar]
- Elkon KB. Aspirin meets cGAS. Nat Rev Rheumatol. 2019;15:254–255. doi: 10.1038/s41584-019-0205-y. [DOI] [PubMed] [Google Scholar]
- Erttmann SF, Swacha P, Aung KM, Brindefalk B, Jiang H, Härtlova A, Uhlin BE, Wai SN, Gekara NO. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity. 2022;55:847–861.e10. doi: 10.1016/j.immuni.2022.04.006. [DOI] [PubMed] [Google Scholar]
- Fang R, Jiang Q, Guan Y, Gao P, Zhang R, Zhao Z, Jiang Z. Golgi apparatus-synthesized sulfated glycosaminoglycans mediate polymerization and activation of the cGAMP sensor STING. Immunity. 2021;54:962–975.e8. doi: 10.1016/j.immuni.2021.03.011. [DOI] [PubMed] [Google Scholar]
- Fang R, Jiang Q, Jia X, Jiang Z. ARMH3-mediated recruitment of PI4KB directs Golgi-to-endosome trafficking and activation of the antiviral effector STING. Immunity. 2023;56:500–515.e6. doi: 10.1016/j.immuni.2023.02.004. [DOI] [PubMed] [Google Scholar]
- Feltham R, Vince JE. Ion man: GSDMD punches pores to knock out cGAS. Immunity. 2018;49:379–381. doi: 10.1016/j.immuni.2018.08.026. [DOI] [PubMed] [Google Scholar]
- Feng Y, He X, Luo S, Chen X, Long S, Liang F, Shi T, Pei Z, Li Z. Chronic colitis induces meninges traffic of gut-derived T cells, unbalances M1 and M2 microglia/macrophage and increases ischemic brain injury in mice. Brain Res. 2019;1707:8–17. doi: 10.1016/j.brainres.2018.11.019. [DOI] [PubMed] [Google Scholar]
- Fu X, Cui J, Meng X, Jiang P, Zheng Q, Zhao W, Chen X. Endoplasmic reticulum stress, cell death and tumor: Association between endoplasmic reticulum stress and the apoptosis pathway in tumors (Review) Oncol Rep. 2021;45:801–808. doi: 10.3892/or.2021.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel S, Koncina E, Dorban G, Heurtaux T, Birck C, Glaab E, Michelucci A, Heuschling P, Grandbarbe L. Inflammation promotes a conversion of astrocytes into neural progenitor cells via NF-κB activation. Mol Neurobiol. 2016;53:5041–5055. doi: 10.1007/s12035-015-9428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, O’Duill F, Schmid-Burgk JL, Hoss F, Buhmann R, Wittmann G, Latz E, Subklewe M, Hornung V. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171:1110–1124.e18. doi: 10.1016/j.cell.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamdzyk M, Doycheva DM, Araujo C, Ocak U, Luo Y, Tang J, Zhang JH. cGAS/STING pathway activation contributes to delayed neurodegeneration in neonatal hypoxia-ischemia rat model: possible involvement of LINE-1. Mol Neurobiol. 2020;57:2600–2619. doi: 10.1007/s12035-020-01904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Hao JP, Li BY, Zheng CC, Miao BB, Zhang L, Li YL, Li L, Li XJ, Zhang L. Tetrahydroxy stilbene glycoside ameliorates neuroinflammation for Alzheimer’s disease via cGAS-STING. Eur J Pharmacol. 2023;953:175809. doi: 10.1016/j.ejphar.2023.175809. [DOI] [PubMed] [Google Scholar]
- Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, Tuschl T, Patel DJ. Cyclic [G(2’,5’)pA(3’,5’)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili M, Lahaye X, Nadalin F, Nader GPF, Puig Lombardi E, Herve S, De Silva NS, Rookhuizen DC, Zueva E, Goudot C, Maurin M, Bochnakian A, Amigorena S, Piel M, Fachinetti D, Londoño-Vallejo A, Manel N. The N-terminal domain of cGAS determines preferential association with centromeric DNA and innate immune activation in the nucleus. Cell Rep. 2019;26:2377–2393.e13. doi: 10.1016/j.celrep.2019.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud M, Cho TH, Nighoghossian N, Maucort-Boulch D, Deiana G, Østergaard L, Baron JC, Fiehler J, Pedraza S, Derex L, Berthezène Y. Early blood brain barrier changes in acute ischemic stroke: a sequential MRI study. J Neuroimaging. 2015;25:959–963. doi: 10.1111/jon.12225. [DOI] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Gui X, Yang H, Li T, Tan X, Shi P, Li M, Du F, Chen ZJ. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262–266. doi: 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Ma M, Yu H, Han Y, Zhang D. Dexmedetomidine enables copper homeostasis in cerebral ischemia/reperfusion via ferredoxin 1. Ann Med. 2023;55:2209735. doi: 10.1080/07853890.2023.2209735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Jiang F, Kong L, Li B, Yang Y, Zhang L, Liu B, Zheng Y, Gao C. Cutting edge: USP27X deubiquitinates and stabilizes the DNA sensor cGAS to regulate cytosolic DNA-mediated signaling. J Immunol. 2019;203:2049–2054. doi: 10.4049/jimmunol.1900514. [DOI] [PubMed] [Google Scholar]
- Haag SM, Gulen MF, Reymond L, Gibelin A, Abrami L, Decout A, Heymann M, van der Goot FG, Turcatti G, Behrendt R, Ablasser A. Targeting STING with covalent small-molecule inhibitors. Nature. 2018;559:269–273. doi: 10.1038/s41586-018-0287-8. [DOI] [PubMed] [Google Scholar]
- Hall J, et al. Discovery of PF-06928215 as a high affinity inhibitor of cGAS enabled by a novel fluorescence polarization assay. PLoS One. 2017;12:e0184843. doi: 10.1371/journal.pone.0184843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond MD, Taylor RA, Mullen MT, Ai Y, Aguila HL, Mack M, Kasner SE, McCullough LD, Sansing LH. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34:3901–3909. doi: 10.1523/JNEUROSCI.4070-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Zhuang MW, Deng J, Zheng Y, Zhang J, Nan ML, Zhang XJ, Gao C, Wang PH. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3–TRIF, and cGAS-STING signaling pathways. J Med Virol. 2021;93:5376–5389. doi: 10.1002/jmv.27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen H, Gong H, Tang X, Huang N, Xu W, Tai H, Zhang G, Zhao T, Gong C, Wang S, Yang Y, Xiao H. Autolysosomal degradation of cytosolic chromatin fragments antagonizes oxidative stress-induced senescence. J Biol Chem. 2020;295:4451–4463. doi: 10.1074/jbc.RA119.010734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AL, et al. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc Natl Acad Sci U S A. 2018;115:E7768–E7775. doi: 10.1073/pnas.1806239115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann G. Nucleic Acid Immunity. Adv Immunol. 2017;133:121–169. doi: 10.1016/bs.ai.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskew-Layton RE, Mongin AA, Kimelberg HK. Hydrogen peroxide potentiates volume-sensitive excitatory amino acid release via a mechanism involving Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:3548–3554. doi: 10.1074/jbc.M409803200. [DOI] [PubMed] [Google Scholar]
- He S, Wang X. RIP kinases as modulators of inflammation and immunity. Nat Immunol. 2018;19:912–922. doi: 10.1038/s41590-018-0188-x. [DOI] [PubMed] [Google Scholar]
- He T, Li W, Song Y, Li Z, Tang Y, Zhang Z, Yang GY. Sestrin2 regulates microglia polarization through mTOR-mediated autophagic flux to attenuate inflammation during experimental brain ischemia. J Neuroinflammation. 2020;17:329. doi: 10.1186/s12974-020-01987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21:501–521. doi: 10.1038/s41580-020-0244-x. [DOI] [PubMed] [Google Scholar]
- Horsch AD, Dankbaar JW, van Seeters T, Niesten JM, Luitse MJ, Vos PC, van der Schaaf IC, Biessels GJ, van der Graaf Y, Kappelle LJ, Mali WP, Velthuis BK. Relation between stroke severity, patient characteristics and CT-perfusion derived blood-brain barrier permeability measurements in acute ischemic stroke. Clin Neuroradiol. 2016;26:415–421. doi: 10.1007/s00062-015-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Liang H, Rao E, Zheng W, Huang X, Deng L, Zhang Y, Yu X, Xu M, Mauceri H, Arina A, Weichselbaum RR, Fu YX. Non-canonical NF-κB antagonizes STING sensor-mediated DNA sensing in radiotherapy. Immunity. 2018;49:490–503.e4. doi: 10.1016/j.immuni.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zhang H, Zhang Q, Yao X, Ni W, Zhou K. Emerging role of STING signalling in CNS injury: inflammation, autophagy, necroptosis, ferroptosis and pyroptosis. J Neuroinflammation. 2022;19:242. doi: 10.1186/s12974-022-02602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Deng H, Xu S, Zhang J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int J Mol Sci. 2015;16:24895–24917. doi: 10.3390/ijms161024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Manasrah BK, McGregor SM, Lera RF, Norman RX, Tucker JB, Scribano CM, Yan RE, Humayun M, Wisinski KB, Tevaarwerk AJ, O’Regan RM, Wilke LG, Weaver BA, Beebe DJ, Jin N, Burkard ME. Paclitaxel Induces micronucleation and activates pro-inflammatory cGAS-STING signaling in triple-negative breast cancer. Mol Cancer Ther. 2021;20:2553–2567. doi: 10.1158/1535-7163.MCT-21-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua R, Han S, Zhang N, Dai Q, Liu T, Li J. cPKCγ-modulated sequential reactivation of mTOR inhibited autophagic flux in neurons exposed to oxygen glucose deprivation/reperfusion. Int J Mol Sci. 2018;19:1380. doi: 10.3390/ijms19051380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua R, Wei H, Liu C, Shi Z, Xing Y. Phosphorylated mTORC1 represses autophagic-related mRNA translation in neurons exposed to ischemia-reperfusion injury. J Cell Biochem. 2019;120:15915–15923. doi: 10.1002/jcb.28865. [DOI] [PubMed] [Google Scholar]
- Huang Y, Chen S, Luo Y, Han Z. Crosstalk between Inflammation and the BBB in Stroke. Curr Neuropharmacol. 2020;18:1227–1236. doi: 10.2174/1570159X18666200620230321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CJ, Yun HM, Jung YY, Lee DH, Yoon NY, Seo HO, Han JY, Oh KW, Choi DY, Han SB, Yoon DY, Hong JT. Reducing effect of IL-32α in the development of stroke through blocking of NF-κB, but enhancement of STAT3 pathways. Mol Neurobiol. 2015;51:648–660. doi: 10.1007/s12035-014-8739-0. [DOI] [PubMed] [Google Scholar]
- Ingold I, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172:409–422.e421. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- Ingram JP, Thapa RJ, Fisher A, Tummers B, Zhang T, Yin C, Rodriguez DA, Guo H, Lane R, Williams R, Slifker MJ, Basagoudanavar SH, Rall GF, Dillon CP, Green DR, Kaiser WJ, Balachandran S. ZBP1/DAI drives RIPK3-mediated cell death induced by IFNs in the absence of RIPK1. J Immunol. 2019;203:1348–1355. doi: 10.4049/jimmunol.1900216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangholi E, Sharifi ZN, Hoseinian M, Zarrindast MR, Rahimi HR, Mowla A, Aryan H, Javidi MA, Parsa Y, Ghaffarpasand F, Yadollah-Damavandi S, Arani HZ, Shahi F, Movassaghi S. Verapamil inhibits mitochondria-induced reactive oxygen species and dependent apoptosis pathways in cerebral transient global ischemia/reperfusion. Oxid Med Cell Longev. 2020;2020:5872645. doi: 10.1155/2020/5872645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M, Qin D, Zhao C, Chai L, Yu Z, Wang W, Tong L, Lv L, Wang Y, Rehwinkel J, Yu J, Zhao W. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat Immunol. 2020;21:727–735. doi: 10.1038/s41590-020-0699-0. [DOI] [PubMed] [Google Scholar]
- Jia R, Solé-Guardia G, Kiliaan AJ. Blood-brain barrier pathology in cerebral small vessel disease. Neural Regen Res. 2024;19:1233–1240. doi: 10.4103/1673-5374.385864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CT, Wu WF, Deng YH, Ge JW. Modulators of microglia activation and polarization in ischemic stroke (Review) Mol Med Rep. 2020;21:2006–2018. doi: 10.3892/mmr.2020.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang GL, Yang XL, Zhou HJ, Long J, Liu B, Zhang LM, Lu D. cGAS knockdown promotes microglial M2 polarization to alleviate neuroinflammation by inhibiting cGAS-STING signaling pathway in cerebral ischemic stroke. Brain Res Bull. 2021;171:183–195. doi: 10.1016/j.brainresbull.2021.03.010. [DOI] [PubMed] [Google Scholar]
- Jiang H, Zhang X, Chen X, Aramsangtienchai P, Tong Z, Lin H. Protein lipidation: occurrence, mechanisms, biological functions, and enabling technologies. Chem Rev. 2018;118:919–988. doi: 10.1021/acs.chemrev.6b00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin MM, Wang F, Qi D, Liu WW, Gu C, Mao CJ, Yang YP, Zhao Z, Hu LF, Liu CF. A critical role of autophagy in regulating microglia polarization in neurodegeneration. Front Aging Neurosci. 2018;10:378. doi: 10.3389/fnagi.2018.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Guo P, Li X, Ke J, Wang Y, Wu H. Neuroprotective effects of irisin against cerebral ischemia/ reperfusion injury via Notch signaling pathway. Biomed Pharmacother. 2019;120:109452. doi: 10.1016/j.biopha.2019.109452. [DOI] [PubMed] [Google Scholar]
- Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, Cao Y, Xu H, Luo H, Lu L, Shi MJ, Tian Y, Fan W, Zhao BQ. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. 2020;11:2488. doi: 10.1038/s41467-020-16191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290.e1217. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee W, Cho K. P62 links the autophagy pathway and the ubiquitin-proteasome system in endothelial cells during atherosclerosis. Int J Mol Sci. 2021;22:7791. doi: 10.3390/ijms22157791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuth AK, Rösler S, Schenk B, Kowald L, van Wijk SJL, Fulda S. Interferons transcriptionally up-regulate MLKL expression in cancer cells. Neoplasia. 2019;21:74–81. doi: 10.1016/j.neo.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Li W, Chang E, Wang W, Shen N, Xu X, Wang X, Zhang Y, Sun W, Hu W, Xu P, Liu X. mtDNA-STING axis mediates microglial polarization via IRF3/NF-κB signaling after ischemic stroke. Front Immunol. 2022;13:860977. doi: 10.3389/fimmu.2022.860977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, et al. Mammalian hybrid pre-autophagosomal structure HyPAS generates autophagosomes. Cell. 2021;184:5950–5969.e22. doi: 10.1016/j.cell.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D, Park E, Sesaki H, Kang SJ. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) suppresses STING-mediated DNA sensing pathway through inducing mitochondrial fission. Biochem Biophys Res Commun. 2017;493:737–743. doi: 10.1016/j.bbrc.2017.08.121. [DOI] [PubMed] [Google Scholar]
- Lama L, et al. Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nat Commun. 2019;10:2261. doi: 10.1038/s41467-019-08620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Chiang C, Gack MU. Endogenous nucleic acid recognition by RIG-I-like receptors and cGAS. J Interferon Cytokine Res. 2019;39:450–458. doi: 10.1089/jir.2019.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Lee SH, Kim M, Moon JS, Kim GW, Jung HG, Kim IH, Oh JE, Jung HE, Lee HK, Ku KB, Ahn DG, Kim SJ, Kim KS, Oh JW. Vibrio vulnificus quorum-sensing molecule cyclo(Phe-Pro) inhibits RIG-I-mediated antiviral innate immunity. Nat Commun. 2018;9:1606. doi: 10.1038/s41467-018-04075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Concepcion K, Meng X, Zhang L. Brain-immune interactions in perinatal hypoxic-ischemic brain injury. Prog Neurobiol. 2017;159:50–68. doi: 10.1016/j.pneurobio.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Sun G, Chen B, Xu L, Ye Y, He J, Bao Z, Zhao P, Miao Z, Zhao L, Hu J, You Y, Liu N, Chao H, Ji J. Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res. 2021;174:105933. doi: 10.1016/j.phrs.2021.105933. [DOI] [PubMed] [Google Scholar]
- Li C, Ren J, Zhang M, Wang H, Yi F, Wu J, Tang Y. The heterogeneity of microglial activation and its epigenetic and non-coding RNA regulations in the immunopathogenesis of neurodegenerative diseases. Cell Mol Life Sci. 2022;79:511. doi: 10.1007/s00018-022-04536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu P, Zhang B, Yuan Z, Guo M, Zou X, Qian Y, Deng S, Zhu L, Cao X, Tao T, Xia S, Bao X, Xu Y. Acute ischemia induces spatially and transcriptionally distinct microglial subclusters. Genome Med. 2023;15:109. doi: 10.1186/s13073-023-01257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Cheng Z, Zhou L, Darmanis S, Neff NF, Okamoto J, Gulati G, Bennett ML, Sun LO, Clarke LE, Marschallinger J, Yu G, Quake SR, Wyss-Coray T, Barres BA. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron. 2019;101:207–223.e10. doi: 10.1016/j.neuron.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Cao Y, Dang C, Han B, Han R, Ma H, Hao J, Wang L. Inhibition of double-strand DNA-sensing cGAS ameliorates brain injury after ischemic stroke. EMBO Mol Med. 2020;12:e11002. doi: 10.15252/emmm.201911002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QQ, Ding DH, Wang XY, Sun YY, Wu J. Lipoxin A4 regulates microglial M1/M2 polarization after cerebral ischemia-reperfusion injury via the Notch signaling pathway. Exp Neurol. 2021;339:113645. doi: 10.1016/j.expneurol.2021.113645. [DOI] [PubMed] [Google Scholar]
- Li R, Si M, Jia HY, Ma Z, Li XW, Li XY, Dai XR, Gong P, Luo SY. Anfibatide alleviates inflammation and apoptosis via inhibiting NF-kappaB/NLRP3 axis in ischemic stroke. Eur J Pharmacol. 2022;926:175032. doi: 10.1016/j.ejphar.2022.175032. [DOI] [PubMed] [Google Scholar]
- Li S, et al. The cyclopeptide astin C specifically inhibits the innate immune CDN sensor STING. Cell Rep. 2018;25:3405–3421.e7. doi: 10.1016/j.celrep.2018.11.097. [DOI] [PubMed] [Google Scholar]
- Li S, Kong L, Meng Y, Cheng C, Lemacon DS, Yang Z, Tan K, Cheruiyot A, Lu Z, You Z. Cytosolic DNA sensing by cGAS/STING promotes TRPV2-mediated Ca(2+) release to protect stressed replication forks. Mol Cell. 2023;83:556–573.e7. doi: 10.1016/j.molcel.2022.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Han Z, Wang T, Ma C, Li H, Lei H, Yang Y, Wang Y, Pei Z, Liu Z, Cheng L, Chen G. Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials. 2022;291:121904. doi: 10.1016/j.biomaterials.2022.121904. [DOI] [PubMed] [Google Scholar]
- Li X, Yang Y, Xu S, Gui Y, Chen J, Xu J. Screening biomarkers for spinal cord injury using weighted gene co-expression network analysis and machine learning. Neural Regen Res. 2024;19:2723–2734. doi: 10.4103/1673-5374.391306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen H, Yang Q, Wan L, Zhao J, Wu Y, Wang J, Yang Y, Niu M, Liu H, Liu J, Yang H, Wan S, Wang Y, Bao D. Increased Drp1 promotes autophagy and ESCC progression by mtDNA stress mediated cGAS-STING pathway. J Exp Clin Cancer Res. 2022;41:76. doi: 10.1186/s13046-022-02262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Apaijai N, Chattipakorn N, Chattipakorn SC. The possible roles of necroptosis during cerebral ischemia and ischemia / reperfusion injury. Arch Biochem Biophys. 2020;695:108629. doi: 10.1016/j.abb.2020.108629. [DOI] [PubMed] [Google Scholar]
- Liao Y, Cheng J, Kong X, Li S, Li X, Zhang M, Zhang H, Yang T, Dong Y, Li J, Xu Y, Yuan Z. HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics. 2020;10:9644–9662. doi: 10.7150/thno.47651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder ME, Jennings BC. Mechanism and function of DHHC S-acyltransferases. Biochem Soc Trans. 2013;41:29–34. doi: 10.1042/BST20120328. [DOI] [PubMed] [Google Scholar]
- Lipinski MM, Wu J, Faden AI, Sarkar C. Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid Redox Signal. 2015;23:565–577. doi: 10.1089/ars.2015.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lisi L, Ciotti GM, Braun D, Kalinin S, Currò D, Dello Russo C, Coli A, Mangiola A, Anile C, Feinstein DL, Navarra P. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci Lett. 2017;645:106–112. doi: 10.1016/j.neulet.2017.02.076. [DOI] [PubMed] [Google Scholar]
- Liu M, Li Y, Han S, Wang H, Li J. Activin A alleviates neuronal injury through inhibiting cGAS-STING-mediated autophagy in mice with ischemic stroke. J Cereb Blood Flow Metab. 2023;43:736–748. doi: 10.1177/0271678X221147056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wu J, Zhang X, Li X, Wu X, Zhao Y, Ren J. Circulating mitochondrial DNA-triggered autophagy dysfunction via STING underlies sepsis-related acute lung injury. Cell Death Dis. 2021;12:673. doi: 10.1038/s41419-021-03961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, Du F, Ren J, Wu YT, Grishin NV, Chen ZJ. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- Liu S, Su Y, Sun B, Hao R, Pan S, Gao X, Dong X, Ismail AM, Han B. Luteolin protects against CIRI, potentially via regulation of the SIRT3/AMPK/mTOR signaling pathway. Neurochem Res. 2020;45:2499–2515. doi: 10.1007/s11064-020-03108-w. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, Ji X, Leak RK, Gao Y, Chen J, Hu X. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke. 2016;47:498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wen S, Zhao S, Yan F, Zhao S, Wu D, Ji X. Mild therapeutic hypothermia protects the brain from ischemia/reperfusion injury through upregulation of iASPP. Aging Dis. 2018;9:401–411. doi: 10.14336/AD.2017.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZS, et al. G3BP1 promotes DNA binding and activation of cGAS. Nat Immunol. 2019;20:18–28. doi: 10.1038/s41590-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J, Zhang J, Deng Q, Chen X. Neutrophil extracellular traps mediate neuro-immunothrombosis. Neural Regen Res. 2024;19:1734–1740. doi: 10.4103/1673-5374.389625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecke S, Holleufer A, Christensen MH, Jønsson KL, Boni GA, Sørensen LK, Johannsen M, Jakobsen MR, Hartmann R, Paludan SR. cGAS is activated by DNA in a length-dependent manner. EMBO Rep. 2017;18:1707–1715. doi: 10.15252/embr.201744017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Zhu X, Xing C, Chen Y, Bian H, Yin H, Gu X, Su L. Stimulator of interferon genes (STING): Key therapeutic targets in ischemia/reperfusion injury. Biomed Pharmacother. 2023;167:115458. doi: 10.1016/j.biopha.2023.115458. [DOI] [PubMed] [Google Scholar]
- Ma F, Li B, Yu Y, Iyer SS, Sun M, Cheng G. Positive feedback regulation of type I interferon by the interferon-stimulated gene STING. EMBO Rep. 2015;16:202–212. doi: 10.15252/embr.201439366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Li B, Liu SY, Iyer SS, Yu Y, Wu A, Cheng G. Positive feedback regulation of type I IFN production by the IFN-inducible DNA sensor cGAS. J Immunol. 2015;194:1545–1554. doi: 10.4049/jimmunol.1402066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Mathavarajah S, Salsman J, Dellaire G. An emerging role for calcium signalling in innate and autoimmunity via the cGAS-STING axis. Cytokine Growth Factor Rev. 2019;50:43–51. doi: 10.1016/j.cytogfr.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374:1070–1075. doi: 10.1126/science.abi5200. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Murphy LO. Autophagy assays for biological discovery and therapeutic development. Trends Biochem Sci. 2020;45:1080–1093. doi: 10.1016/j.tibs.2020.07.006. [DOI] [PubMed] [Google Scholar]
- Mueller-Ortiz SL, Calame DG, Shenoi N, Li YD, Wetsel RA. The complement anaphylatoxins C5a and C3a suppress IFN-β production in response to listeria monocytogenes by inhibition of the cyclic dinucleotide-activated cytosolic surveillance pathway. J Immunol. 2017;198:3237–3244. doi: 10.4049/jimmunol.1601420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K, Ogawa E, Uematsu R, Kuchitsu Y, Kiku F, Uemura T, Waguri S, Suzuki T, Dohmae N, Arai H, Shum AK, Taguchi T. Homeostatic regulation of STING by retrograde membrane traffic to the ER. Nat Commun. 2021;12:61. doi: 10.1038/s41467-020-20234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy AMV, Robinson N, Kumar S. Crosstalk between cGAS-STING signaling and cell death. Cell Death Differ. 2020;27:2989–3003. doi: 10.1038/s41418-020-00624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Cunningham C. Priming microglia for innate immune memory in the brain. Trends Immunol. 2019;40:358–374. doi: 10.1016/j.it.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Neumann J, Riek-Burchardt M, Herz J, Doeppner TR, König R, Hütten H, Etemire E, Männ L, Klingberg A, Fischer T, Görtler MW, Heinze HJ, Reichardt P, Schraven B, Hermann DM, Reymann KG, Gunzer M. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129:259–277. doi: 10.1007/s00401-014-1355-2. [DOI] [PubMed] [Google Scholar]
- Ni H, Zhang H, Li L, Huang H, Guo H, Zhang L, Li C, Xu JX, Nie CP, Li K, Zhang X, Xia X, Li J. T cell-intrinsic STING signaling promotes regulatory T cell induction and immunosuppression by upregulating FOXP3 transcription in cervical cancer. J Immunother Cancer. 2022;10:e005151. doi: 10.1136/jitc-2022-005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduro PK, Zheng X, Wei J, Yang Y, Wang Y, Zhang H, Liu E, Gao X, Du M, Wang Q. The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy. Acta Pharm Sin B. 2022;12:50–75. doi: 10.1016/j.apsb.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021;40:e104705. doi: 10.15252/embj.2020104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Salinas R, Sun L, Anderson R, Yang X, Zhang S, Chen ZJ, Yin H. Discovery of small-molecule cyclic GMP-AMP synthase inhibitors. J Org Chem. 2020;85:1579–1600. doi: 10.1021/acs.joc.9b02666. [DOI] [PubMed] [Google Scholar]
- Pandian JD, Gall SL, Kate MP, Silva GS, Akinyemi RO, Ovbiagele BI, Lavados PM, Gandhi DBC, Thrift AG. Prevention of stroke: a global perspective. Lancet. 2018;392:1269–1278. doi: 10.1016/S0140-6736(18)31269-8. [DOI] [PubMed] [Google Scholar]
- Parkes EE, Walker SM, Taggart LE, McCabe N, Knight LA, Wilkinson R, McCloskey KD, Buckley NE, Savage KI, Salto-Tellez M, McQuaid S, Harte MT, Mullan PB, Harkin DP, Kennedy RD. Activation of STING-Dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst. 2017;109:djw199. doi: 10.1093/jnci/djw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZF, Zhang NB, Meng J, Zhang JH. Early aerobic exercise promotes neurological function recovery of rats after cerebral ischemia/reperfusion by upregulating the expression of heat shock protein A5. Curr Med Sci. 2022;42:267–273. doi: 10.1007/s11596-022-2537-0. [DOI] [PubMed] [Google Scholar]
- Phuagkhaopong S, Ospondpant D, Kasemsuk T, Sibmooh N, Soodvilai S, Power C, Vivithanaporn P. Cadmium-induced IL-6 and IL-8 expression and release from astrocytes are mediated by MAPK and NF-κB pathways. Neurotoxicology. 2017;60:82–91. doi: 10.1016/j.neuro.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Poh L, Kang SW, Baik SH, Ng GYQ, She DT, Balaganapathy P, Dheen ST, Magnus T, Gelderblom M, Sobey CG, Koo EH, Fann DY, Arumugam TV. Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav Immun. 2019;75:34–47. doi: 10.1016/j.bbi.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Posch W, Bermejo-Jambrina M, Steger M, Witting C, Diem G, Hörtnagl P, Hackl H, Lass-Flörl C, Huber LA, Geijtenbeek TBH, Wilflingseder D. Complement potentiates immune sensing of HIV-1 and early type I interferon responses. mBio. 2021;12:e0240821. doi: 10.1128/mBio.02408-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Prabakaran T, et al. Attenuation of cGAS-STING signaling is mediated by a p62/SQSTM1-dependent autophagy pathway activated by TBK1. EMBO J. 2018;37:e97858. doi: 10.15252/embj.201797858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian ZY, Kong RY, Zhang S, Wang BY, Chang J, Cao J, Wu CQ, Huang ZY, Duan A, Li HJ, Yang L, Cao XJ. Ruxolitinib attenuates secondary injury after traumatic spinal cord injury. Neural Regen Res. 2022;17:2029–2035. doi: 10.4103/1673-5374.335165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Guo J, Liu L, Liu X, Sun X, Chen H. Vav1 promotes inflammation and neuronal apoptosis in cerebral ischemia/reperfusion injury by upregulating microglial and NLRP3 inflammasome activation. Neural Regen Res. 2023;18:2436–2442. doi: 10.4103/1673-5374.371368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YM, Zhang CL, Chen AQ, Wang HL, Zhou YF, Li YN, Hu B. Immune cells in the BBB disruption after acute ischemic stroke: targets for immune therapy? Front Immunol. 2021;12:678744. doi: 10.3389/fimmu.2021.678744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizwan H, Pal S, Sabnam S, Pal A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020;241:117148. doi: 10.1016/j.lfs.2019.117148. [DOI] [PubMed] [Google Scholar]
- Rong Y, Zhang S, Nandi N, Wu Z, Li L, Liu Y, Wei Y, Zhao Y, Yuan W, Zhou C, Xiao G, Levine B, Yan N, Mou S, Deng L, Tang Z, Liu X, Kramer H, Zhong Q. STING controls energy stress-induced autophagy and energy metabolism via STX17. J Cell Biol. 2022;221:e202202060. doi: 10.1083/jcb.202202060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan S, Besson-Girard S, Kaya T, Cantuti-Castelvetri L, Liu L, Ji H, Schifferer M, Gouna G, Usifo F, Kannaiyan N, Fitzner D, Xiang X, Rossner MJ, Brendel M, Gokce O, Simons M. White matter aging drives microglial diversity. Neuron. 2021;109:1100–1117.e10. doi: 10.1016/j.neuron.2021.01.027. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Fujita N, Hayashi T, Takahara K, Satoh T, Lee H, Matsunaga K, Kageyama S, Omori H, Noda T, Yamamoto N, Kawai T, Ishii K, Takeuchi O, Yoshimori T, Akira S. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez GAM, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. 2018;128:3041–3052. doi: 10.1172/JCI98814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankowski R, Böttcher C, Masuda T, Geirsdottir L, Sagar, Sindram E, Seredenina T, Muhs A, Scheiwe C, Shah MJ, Heiland DH, Schnell O, Grün D, Priller J, Prinz M. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci. 2019;22:2098–2110. doi: 10.1038/s41593-019-0532-y. [DOI] [PubMed] [Google Scholar]
- Saposnik G, Galanos LC, Guerrero R, Casagrande F, Adhamidhis E, Gao MMY, Grupper MF, Arsovska A. The World Stroke Academy: A World Stroke Organization global pathway to improve knowledge in stroke care. Int J Stroke. 2022;17:829–834. doi: 10.1177/17474930221085895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Weindel CG, Smirnova I, Tang AY, Ilyukha V, Sorokin M, Buzdin A, Fitzgerald KA, Poltorak A. Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ. 2019;26:332–347. doi: 10.1038/s41418-018-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar MK, Uppala R, Zeng C, Billi AC, Tsoi LC, Kidder A, Xing X, Perez White BE, Shao S, Plazyo O, Sirobhushanam S, Xing E, Jiang Y, Gallagher KA, Voorhees JJ, Kahlenberg JM, Gudjonsson JE. Keratinocytes sense and eliminate CRISPR DNA through STING/IFN-κ activation and APOBEC3G induction. J Clin Invest. 2023;133:e159393. doi: 10.1172/JCI159393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser H, Fey SB, Horowitz J, Fischer ER, Balinsky CA, Miyake K, Bekisz J, Snow AL, Zoon KC. Type I interferons induce autophagy in certain human cancer cell lines. Autophagy. 2013;9:683–696. doi: 10.4161/auto.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Gan Q, Yang Y, Reis C, Zhang Z, Xu S, Zhang T, Sun C. Mitophagy in cerebral ischemia and ischemia/reperfusion injury. Front Aging Neurosci. 2021;13:687246. doi: 10.3389/fnagi.2021.687246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18:1058–1066. doi: 10.1016/S1474-4422(19)30078-X. [DOI] [PubMed] [Google Scholar]
- Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther. 2012;18:250–260. doi: 10.1111/j.1755-5949.2012.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu T, Altman MD, Baltus GA, Childers M, Ellis JM, Gunaydin H, Hatch H, Ho T, Jewell J, Lacey BM, Lesburg CA, Pan BS, Sauvagnat B, Schroeder GK, Xu S. Discovery of a novel cGAMP competitive ligand of the inactive form of STING. ACS Med Chem Lett. 2019;10:92–97. doi: 10.1021/acsmedchemlett.8b00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladitschek-Martens HL, Guarnieri A, Brumana G, Zanconato F, Battilana G, Xiccato RL, Panciera T, Forcato M, Bicciato S, Guzzardo V, Fassan M, Ulliana L, Gandin A, Tripodo C, Foiani M, Brusatin G, Cordenonsi M, Piccolo S. YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING. Nature. 2022;607:790–798. doi: 10.1038/s41586-022-04924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xi Y, Dai M, Li T, Du S, Zhu Y, Li M, Li Y, Liu S, Ding X, Yao X, Lai Y, Liu X. STING guides the STX17-SNAP29-VAMP8 complex assembly to control autophagy. Cell Insight. 2024;3:100147. doi: 10.1016/j.cellin.2024.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Woo JS, Wu B, El-Sherbiny YM, Leung J, Chupradit K, Rice L, Seo GJ, Calmettes G, Ramakrishna C, Cantin E, An DS, Sun R, Wu TT, Jung JU, Savic S, Gwack Y. The Ca(2+) sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat Immunol. 2019;20:152–162. doi: 10.1038/s41590-018-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MS, Jin H, Sun X, Huang S, Zhang FL, Guo ZN, Yang Y. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018;2018:3804979. doi: 10.1155/2018/3804979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Li Y, Chen L, Chen H, You F, Zhou X, Zhou Y, Zhai Z, Chen D, Jiang Z. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci U S A. 2009;106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KV, Junkins RD, Kurkjian CJ, Holley-Guthrie E, Pendse AA, El Morabiti R, Petrucelli A, Barber GN, Benedict CA, Ting JP. A noncanonical function of cGAMP in inflammasome priming and activation. J Exp Med. 2017;214:3611–3626. doi: 10.1084/jem.20171749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5:ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Zhang X, Hao X, Dou H, Zou C, Zhou Y, Li B, Yue H, Wang D, Wang Y, Yang C, Fu J. Hepatocyte growth factor-modified hair follicle stem cells ameliorate cerebral ischemia/reperfusion injury in rats. Stem Cell Res Ther. 2023;14:25. doi: 10.1186/s13287-023-03251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Yan T, Wang S, Liu Q, Yang Q, Zhang Y, Li Y, Wu Y, Liu S, Ma Y, Yang L. Treatment with β-sitosterol ameliorates the effects of cerebral ischemia/reperfusion injury by suppressing cholesterol overload, endoplasmic reticulum stress, and apoptosis. Neural Regen Res. 2024;19:642–649. doi: 10.4103/1673-5374.380904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo QZ, Zhang ST, Lei P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med Res Rev. 2022;42:259–305. doi: 10.1002/med.21817. [DOI] [PubMed] [Google Scholar]
- Tuo QZ, et al. Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct Target Ther. 2022;7:59. doi: 10.1038/s41392-022-00917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriarte Huarte O, Kyriakis D, Heurtaux T, Pires-Afonso Y, Grzyb K, Halder R, Buttini M, Skupin A, Mittelbronn M, Michelucci A. Single-Cell transcriptomics and in situ morphological analyses reveal microglia heterogeneity across the nigrostriatal pathway. Front Immunol. 2021;12:639613. doi: 10.3389/fimmu.2021.639613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venketasubramanian N, Yoon BW, Pandian J, Navarro JC. Stroke epidemiology in south, east, and south-east Asia: A Review. J Stroke. 2017;19:286–294. doi: 10.5853/jos.2017.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J, Adura C, Gao P, Luz A, Lama L, Asano Y, Okamoto R, Imaeda T, Aida J, Rothamel K, Gogakos T, Steinberg J, Reasoner S, Aso K, Tuschl T, Patel DJ, Glickman JF, Ascano M. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat Commun. 2017;8:750. doi: 10.1038/s41467-017-00833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan D, Jiang W, Hao J. Research advances in how the cGAS-STING pathway controls the cellular inflammatory response. Front Immunol. 2020;11:615. doi: 10.3389/fimmu.2020.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Xiao X, Huang F, Liu R. Syntaxin-17-dependent mitochondrial dynamics is essential for protection against oxidative-stress-induced apoptosis. Antioxidants (Basel) 2019;8:522. doi: 10.3390/antiox8110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhou W, Liu Y, Xu Y, Zhang X, Jiang C, Jiang M, Cao X. Nuclear translocation of RIG-I promotes cellular apoptosis. J Autoimmun. 2022;130:102840. doi: 10.1016/j.jaut.2022.102840. [DOI] [PubMed] [Google Scholar]
- Wang D, Liu F, Zhu L, Lin P, Han F, Wang X, Tan X, Lin L, Xiong Y. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J Neuroinflammation. 2020;17:257. doi: 10.1186/s12974-020-01921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother. 2018;105:518–525. doi: 10.1016/j.biopha.2018.05.143. [DOI] [PubMed] [Google Scholar]
- Wang M, Sooreshjani MA, Mikek C, Opoku-Temeng C, Sintim HO. Suramin potently inhibits cGAMP synthase, cGAS, in THP1 cells to modulate IFN-β levels. Future Med Chem. 2018;10:1301–1317. doi: 10.4155/fmc-2017-0322. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhu Y, Liu Z, Chang L, Bai X, Kang L, Cao Y, Yang X, Yu H, Shi MJ, Hu Y, Fan W, Zhao BQ. Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke. Blood. 2021;138:91–103. doi: 10.1182/blood.2020008913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hu D, Wu C, Feng Y, Li A, Liu W, Wang Y, Chen K, Tian M, Xiao F, Zhang Q, Shereen MA, Chen W, Pan P, Wan P, Wu K, Wu J. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to activate the inflammasome upon HSV-1 infection. PLoS Pathog. 2020;16:e1008335. doi: 10.1371/journal.ppat.1008335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ning X, Gao P, Wu S, Sha M, Lv M, Zhou X, Gao J, Fang R, Meng G, Su X, Jiang Z. Inflammasome activation triggers Caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity. 2017;46:393–404. doi: 10.1016/j.immuni.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin H, Wang W, Wang F, Zhao H. Myosin1f-mediated neutrophil migration contributes to acute neuroinflammation and brain injury after stroke in mice. J Neuroinflammation. 2019;16:77. doi: 10.1186/s12974-019-1465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Li H, Deng Y, Zheng X, Zhou Y, Xue X. The combination of Alisma and Atractylodes ameliorates cerebral ischaemia/reperfusion injury by negatively regulating astrocyte-derived exosomal miR-200a-3p/141-3p by targeting SIRT1. J Ethnopharmacol. 2023;313:116597. doi: 10.1016/j.jep.2023.116597. [DOI] [PubMed] [Google Scholar]
- Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke. 2012;43:2904–2909. doi: 10.1161/STROKEAHA.112.665331. [DOI] [PubMed] [Google Scholar]
- Wollert T. Autophagy. Curr Biol. 2019;29:R671–R677. doi: 10.1016/j.cub.2019.06.014. [DOI] [PubMed] [Google Scholar]
- Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liu Q, Zhang X, Tan M, Li X, Liu P, Wu L, Jiao F, Lin Z, Wu X, Wang X, Zhao Y, Ren J. The interaction between STING and NCOA4 exacerbates lethal sepsis by orchestrating ferroptosis and inflammatory responses in macrophages. Cell Death Dis. 2022;13:653. doi: 10.1038/s41419-022-05115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Tang YD, Zheng C. The crosstalk between the caspase family and the cGAS‒STING signaling pathway. J Mol Cell Biol. 2021;13:739–747. doi: 10.1093/jmcb/mjab071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Kong T, Shao Z, Liu M, Zhang R, Zhang S, Kong Q, Chen J, Cheng B, Wang C. Orexin-A alleviates astrocytic apoptosis and inflammation via inhibiting OX1R-mediated NF-κB and MAPK signaling pathways in cerebral ischemia/reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166230. doi: 10.1016/j.bbadis.2021.166230. [DOI] [PubMed] [Google Scholar]
- Xu Q, Guohui M, Li D, Bai F, Fang J, Zhang G, Xing Y, Zhou J, Guo Y, Kan Y. lncRNA C2dat2 facilitates autophagy and apoptosis via the miR-30d-5p/DDIT4/mTOR axis in cerebral ischemia-reperfusion injury. Aging (Albany NY) 2021;13:11315–11335. doi: 10.18632/aging.202824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Bian HJ, Shu S, Xia SN, Gu Y, Zhang MJ, Xu Y, Cao X. AIM2 deletion enhances blood-brain barrier integrity in experimental ischemic stroke. CNS Neurosci Ther. 2021;27:1224–1237. doi: 10.1111/cns.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhang A, Zhu Y, He W, Di W, Fang Y, Shi X. MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κB and PI3K-Akt pathways. J Cell Physiol. 2018;234:904–914. doi: 10.1002/jcp.26918. [DOI] [PubMed] [Google Scholar]
- Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Liang S, Venketasubramanian N, Rathakrishnan R, Ahmad A, Ng KW, Loh PK, Ong JJ, Wakerley BR, Chong VF, Bathla G, Sharma VK. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol. 2013;70:353–358. doi: 10.1001/2013.jamaneurol.547. [DOI] [PubMed] [Google Scholar]
- Yu L, Zhang Y, Chen Q, He Y, Zhou H, Wan H, Yang J. Formononetin protects against inflammation associated with cerebral ischemia-reperfusion injury in rats by targeting the JAK2/STAT3 signaling pathway. Biomed Pharmacother. 2022;149:112836. doi: 10.1016/j.biopha.2022.112836. [DOI] [PubMed] [Google Scholar]
- Yu Y, Xue X, Tang W, Su L, Zhang L, Zhang Y. Cytosolic DNA‒mediated STING-dependent inflammation contributes to the progression of psoriasis. J Invest Dermatol. 2022;142:898–906.e4. doi: 10.1016/j.jid.2021.08.430. [DOI] [PubMed] [Google Scholar]
- Yuan J, Liu W, Zhu H, Chen Y, Zhang X, Li L, Chu W, Wen Z, Feng H, Lin J. Curcumin inhibits glial scar formation by suppressing astrocyte-induced inflammation and fibrosis in vitro and in vivo. Brain Res. 2017;1655:90–103. doi: 10.1016/j.brainres.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Yuan YY, Xie KX, Wang SL, Yuan LW. Inflammatory caspase-related pyroptosis: mechanism, regulation and therapeutic potential for inflammatory bowel disease. Gastroenterol Rep (Oxf) 2018;6:167–176. doi: 10.1093/gastro/goy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid A, Ismail H, Li B, Jin T. Molecular and structural basis of DNA sensors in antiviral innate immunity. Front Immunol. 2020;11:613039. doi: 10.3389/fimmu.2020.613039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Zhang YD, Ma RY, Chen YJ, Xiang XM, Hou DY, Li XH, Huang H, Li T, Duan CY. Activated Drp1 regulates p62-mediated autophagic flux and aggravates inflammation in cerebral ischemia-reperfusion via the ROS-RIP1/RIP3-exosome axis. Mil Med Res. 2022;9:25. doi: 10.1186/s40779-022-00383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Jia M, Wang Y, Wang Q, Wu J. Cell death mechanisms in cerebral ischemia-reperfusion injury. Neurochem Res. 2022;47:3525–3542. doi: 10.1007/s11064-022-03697-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wei M, Fan J, Yan W, Zha X, Song H, Wan R, Yin Y, Wang W. Ischemia-induced upregulation of autophagy preludes dysfunctional lysosomal storage and associated synaptic impairments in neurons. Autophagy. 2021;17:1519–1542. doi: 10.1080/15548627.2020.1840796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Xiong M, Yuan X, Li M, Sun H, Xu Y. In silico screening-based discovery of novel inhibitors of human cyclic GMP-AMP synthase: a cross-validation study of molecular docking and experimental testing. J Chem Inf Model. 2020;60:3265–3276. doi: 10.1021/acs.jcim.0c00171. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang S, Hu S, Chen Y, Ren J. ER-mitochondria microdomains in cardiac ischemia-reperfusion injury: a fresh perspective. Front Physiol. 2018;9:755. doi: 10.3389/fphys.2018.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


