Abstract
Among 3,027 nontyphoidal Salmonella enterica isolates identified between January 1999 and December 2002 in a medical center in northern Taiwan, 31 were resistant to the extended-spectrum cephalosporin ceftriaxone (1.02% [31/3,027]), including 2 in 1999 (0.36% [2/549]), 13 in 2000 (1.49% [13/870]), 7 in 2001 (0.78% [7/893]), and 9 in 2002 (1.26% [9/715]). Sixteen of these isolates belonged to Salmonella serogroup B, nine belonged to serogroup C, four belonged to serogroup D, and two belonged to serogroup E. The majority were from stool cultures. The mechanism of resistance was investigated for eight isolates, including three S. enterica serovar Typhimurium, one S. enterica serovar Wagenia, one S. enterica serovar Senftenberg, one S. enterica serovar Derby, one S. enterica serovar Panama, and one S. enterica serovar Duesseldorf isolate. All eight patients from whom these isolates were recovered had community-acquired infections. All eight isolates were resistant to ampicillin, ceftriaxone, and cefotaxime but susceptible to imipenem and ciprofloxacin. Ceftriaxone resistance was due to the production of the CMY-2 AmpC β-lactamase by seven isolates and the CTX-M-14 β-lactamase by the remaining isolate. Both β-lactamase genes were carried on conjugative plasmids. In a 2.5-kb region encompassing the blaCMY-2 gene, at nucleotide 49 upstream of the start codon of blaCMY-2, three of the seven blaCMY-2-positive isolates had an A nucleotide and four had a G nucleotide. In conclusion, the ceftriaxone resistance of nontyphoidal Salmonella isolates in our hospital was attributed to the CTX-M-14 and CMY-2 β-lactamases.
Nontyphoidal Salmonella species are among the most common enteric bacterial pathogens isolated from children (11). Most cause uncomplicated gastroenteritis, and antimicrobial treatment is usually unnecessary (1). However, invasive infections requiring antibiotics occasionally occur in children, particularly in infants (30). In a previous study, we found that the incidence of bacteremia was 7.6% among pediatric patients with Salmonella-induced enterocolitis and an even higher 11.5% among infants of <3 months of age (20).
Extended-spectrum cephalosporins, especially ceftriaxone, are commonly used to treat children with invasive infections or severe diarrhea caused by Salmonella enterica (19). In recent years, however, ceftriaxone-resistant Salmonella in humans as well as animals has frequently been reported worldwide, including reports from the United States, Europe, South America, Asia, and Russia (3, 7, 9, 12, 13, 17, 21, 26, 32-34, 36-38). In Taiwan, a high percentage of Salmonella isolates have been resistant to ampicillin, chloramphenicol, tetracycline, and trimethoprim-sulfamethoxazole since the 1990s (18, 22, 31, 40, 41). In addition, fluoroquinolone resistance emerged in 2000 (10). Ceftriaxone-resistant Salmonella, however, was not reported in the literature until 2001 (31).
The expression of a plasmid-mediated blaCMY-2 gene has been responsible for most ceftriaxone resistance in Salmonella spp. (7, 9, 12, 13, 15, 17, 36-38). This gene was first identified in a multiresistant S. enterica serovar Senftenberg strain isolated in 1994 (21). The blaCMY-2 gene encodes an AmpC-type β-lactamase and was first described as being carried by a plasmid in a cefoxitin-resistant Klebsiella pneumoniae strain isolated in 1990 (2). In Taiwan, this gene was first detected in Escherichia coli isolates collected in 1999 (39). Since then, it has been reported to be carried by various ceftriaxone-resistant Salmonella strains (9, 37, 38).
In contrast to CMY-2, the CTX-M-type β-lactamases have rarely been reported as present in ceftriaxone-resistant Salmonella strains. However, many strains that were originally identified as cefotaxime resistant because of the production of CTX-M-type β-lactamases (e.g., CTX-M-2, CTX-M-5, and CTX-M-9) have been found to be ceftriaxone resistant as well (3, 5, 26, 28, 33), and a ceftriaxone-resistant S. enterica serovar Anatum strain carrying the blaCTX-M-3 gene on a plasmid was isolated in Taiwan (32).
The horizontal gene transfer and interspecies spread of the blaCMY-2 gene carried by a plasmid were reported for S. enterica serovar Hadar and E. coli (37); they have also been suggested to occur among Salmonella, E. coli, and K. pneumoniae isolates (38). The in vivo acquisition of plasmids carrying the blaCTX-M-3 gene by originally susceptible isolates was reported for S. enterica serovar Anatum and E. coli (32). These data suggest the likelihood and ease of spread of the determinants of ceftriaxone resistance.
In our hospital, we have isolated ceftriaxone-resistant Salmonella strains in the clinical microbiology laboratory since 1999. For this study, we investigated the mechanisms of resistance in a sample of these organisms isolated between January 1999 and December 2002.
MATERIALS AND METHODS
Bacterial isolates and patients.
All Salmonella isolates from clinical specimens were identified and serotyped between January 1999 and December 2002 in the clinical microbiology laboratory of Mackay Memorial Hospital, a 2,000-bed medical center in northern Taiwan with about 6,000 outpatient visits per day. Thirty-one ceftriaxone-resistant isolates were recovered during this period, including 27 from stool samples, 3 from pus or wounds, and 1 from blood. These isolates were serotyped by standard methods (4) and then stored at −70°C in tryptic soy broth (Difco Laboratories, Detroit, Mich.) supplemented with 20% glycerol until further use. The medical records of patients from whom ceftriaxone-resistant organisms were isolated were reviewed.
Susceptibility testing.
MICs were determined by the agar dilution method (25). The antimicrobial agents tested included ampicillin, cefotaxime, ceftazidime, ceftriaxone, cefepime, imipenem, piperacillin-tazobactam, minocycline, ciprofloxacin, trimethoprim-sulfamethoxazole, and chloramphenicol. E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as reference strains.
IEF of β-lactamase.
Crude cell homogenates for the detection of β-lactamases were prepared from fresh overnight cultures by sonication (34). The cell extracts were subjected to isoelectric focusing (IEF) as described by Matthew et al. (24) by use of a PhastSystem apparatus (Amersham Biosciences Ltd., Taipei, Taiwan) and Phastgels with a pH range of 3 to 9. β-Lactamase activity was detected by using 0.5 mM nitrocefin in 0.01 M phosphate buffer as the substrate.
Pulsed-field gel electrophoresis (PFGE) of genomic DNA.
Genomic DNA was prepared in 0.5% SeaPlaque GTG agarose (FMC BioProducts, Rockland, Maine) as previously described (35) and incubated at 37°C for 4 h with 20 units of NotI (BioLabs, Beverly, Mass.) in enzyme buffer. The digested DNA fragments were separated in a 1% SeaKem GTG agarose gel (FMC BioProducts) on a CHEF-DRII apparatus (Bio-Rad Laboratories, Hercules, Calif.). Electrophoresis was performed with 0.5× Tris-borate-EDTA buffer at 6 V/cm at 14°C. The run time was 20 h, with the pulse time increasing from 5 to 9 s. DNA fragments were then visualized with ethidium bromide. A lambda DNA ladder (Amersham Biosciences Ltd.) was used as the molecular size marker.
Plasmid DNA preparation.
Plasmid DNA was purified by a modified alkaline lysis method as described by Sinnett et al. (29). An overnight culture of 3 ml in Luria broth (LB) was resuspended in 100 μl of chilled solution I (25 mM Tris-HCl, 50 mM glucose, 10 mM EDTA, pH 8.0, 2.5 mg/ml lysozyme, and 100 μg/ml RNase A), followed by 200 μl of solution II (0.2 N NaOH and 1% sodium N-lauroylsarcosine) and then 150 μl of solution III (5 M potassium acetate, pH 4.8). After centrifugation, the DNA in the supernatant was precipitated with ethanol, washed, and dried. The DNA was then resuspended in 100 μl of TE buffer (1 mM EDTA, 10 mM Tris-HCl, pH 7.5)-0.1% sodium N-lauroylsarcosine containing 100 μg/ml proteinase K and incubated at 37°C for 1 h to further purify it and to remove contaminants. The aqueous DNA solution was extracted several times with an equal volume of phenol-chloroform mixture and once with an equal volume of chloroform before being precipitated with ethanol.
The plasmid DNA (5 μg) was treated with 2.5 units of S1 nuclease (Roche Diagnostics, Germany) for 40 min at 37°C in 20 μl of incubation buffer (33 mM sodium acetate, 50 mM NaCl, 0.033 mM ZnSO4, pH 4.5). The DNA was separated in a 1.2% SeaKem GTG agarose gel on a CHEF-DRII apparatus at 6 V/cm at 14°C. The run time was 18 h, with the pulse time increasing from 5 to 10 s. The DNA was then visualized with ethidium bromide.
Southern blotting.
Southern blotting was performed as previously described (23). The chromosomal or plasmid DNA in agarose gels was transferred to N+ Hybond nylon membranes, followed by UV light cross-linking. A blaCMY-2 DNA probe was prepared from isolate L4 by a PCR with ampC-type gene primers by use of a PCR DIG probe synthesis kit (Roche Diagnostics). A blaCTX-M-14 DNA probe was prepared from isolate L8 by a PCR with blaCTX-M-type gene primers. Hybridization, washing, and detection were performed by the use of DIG Easy Hyb, DIG Wash and Block buffer set, and DIG luminescent detection kits (all from Roche Diagnostics), respectively, according to the manufacturer's instructions.
Conjugation and transformation.
Ceftriaxone-resistant isolates were incubated with the recipient E. coli HB101 strain on LB agar for 18 h at 37°C. Transconjugants were selected on LB agar supplemented with streptomycin (750 μg/ml) to inhibit the growth of the donor stains and with ceftriaxone (5 μg/ml) to inhibit the growth of the recipient strain.
Plasmid DNAs isolated from the ceftriaxone-resistant isolates were used to transform E. coli strain DH5α by electroporation. The transformants were selected on LB agar supplemented with ceftriaxone (5 μg/ml).
PCR.
The oligonucleotide primers used were as follows: for blaTEM-type genes, 5′-TCG GGG AAA TGT GCG CGG AA-3′ and 5′-TTA CCA ATG CTT AAT CAG TG-3′; for blaSHV-type genes, 5′-ATG CGT TAT ATT CGC CTG TG-3′ and 5′-TTA GCG TTG CCA GTG CTC G-3′; for blaCTX-M-type genes, 5′-TTT GCG ATG TGC AGT/C ACC AG-3′ and 5′-GAT ATC GTT GGT GGT GCC-3′; and for ampC-type genes, 5′-GCA CCA TCA CAC CAC TGA TG-3′ and 5′-TTT GCT GTC GCT GCC GTT GA-3′. Each PCR mixture contained 10 pmol of each primer, a 0.2 mM concentration of each deoxynucleoside triphosphate, and 2.5 U of Taq DNA polymerase (Amersham Biosciences Ltd.) in a total volume of 50 μl containing 1× PCR buffer (10 mM Tris-HCl, pH 8.8, at room temperature, with 50 mM KCl and 0.1% Triton X-100) and 1.5 mM, 2.5 mM, 2 mM, or 1.5 mM MgCl2 for blaTEM-, blaSHV-, blaCTX-M-, and ampC-type genes, respectively. Either heat-lysed Salmonella isolates or isolated plasmid DNAs were used as substrates. The PCR mixtures were subjected to 40 cycles in a DNA thermal cycler (Perkin-Elmer) as follows: for blaTEM, denaturation for 30 s at 94°C, annealing for 30 s at 50°C, and extension for 1 min at 72°C; for blaSHV, denaturation for 2 min at 94°C, annealing for 1 min at 50°C, and extension for 2 min at 72°C; and for blaCTX-M or ampC, denaturation for 1 min at 94°C, annealing for 1 min at 55°C, and extension for 1 min at 72°C. The amplification products were visualized by agarose gel electrophoresis and ethidium bromide staining. Clinical isolates of E. coli, P. aeruginosa, K. pneumoniae, and Citrobacter freundii carrying various β-lactamase genes were used as controls.
Cloning of the blaCMY-2 gene.
The plasmid DNA of isolate L4 was digested with the restriction enzyme EcoRI. The resulting DNA fragments were cloned into the vector pCS33, a plasmid derived from pBR322 that is tetracycline resistant and ampicillin sensitive, and selected with ceftriaxone (5 μg/ml) or ampicillin (100 μg/ml) according to the method described by Sambrook et al. (27).
DNA sequencing.
The PCR products were purified with a GFX PCR DNA and gel band purification kit (Amersham Biosciences Ltd.) and sequenced by using the primers used for PCR amplification on an ABI PRISM 377 sequence analyzer (Applied Biosystems, Foster City, Calif.). The EcoRI DNA fragment containing the ceftriaxone-resistant determinant from isolate L4 inserted into the plasmid pCS33 was sequenced by using primers designed for the vector and then primers designed for the newly revealed sequence. The region surrounding the blaCTX-M gene of isolate L8 was sequenced as well, using primers for blaCTX-M-type genes and then primers designed for the newly revealed sequence.
Nucleotide sequence accession numbers.
The sequences determined for this study were submitted to GenBank under the following accession numbers: isolate L4, AY899923; isolate L5, AY899924; isolate L6, AY899925; isolate L9, AY899926; isolate L10, AY899927; isolate B11, AY899928; isolate B25, AY899929; and isolate L8, AY899930.
RESULTS
Prevalence of ceftriaxone resistance among Salmonella isolates.
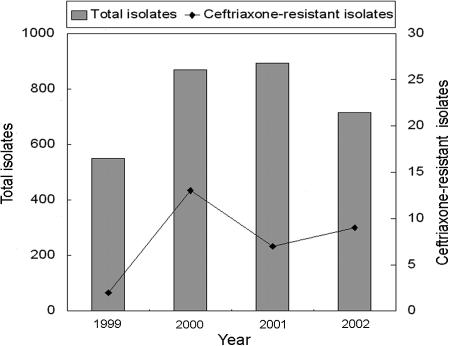
There were 3,027 nontyphoidal Salmonella isolates identified in our hospital between January 1999 and December 2002, including 549 isolates in 1999, 870 in 2000, 893 in 2001, and 715 in 2002 (Fig. 1). Among them, 1,623 belonged to serogroup B (53.62% [1,623/3,027]), 658 belonged to serogroup C (21.74%), 597 belonged to serogroup D (19.72%), 77 belonged to serogroup E (2.54%), and 72 belonged to other serogroups (2.38%). Thirty-one of the isolates (1.02% [31/3,027]) were ceftriaxone resistant, including 2 in 1999 (0.36% [2/549]), 13 in 2000 (1.49% [13/870]), 7 in 2001 (0.78% [7/893]), and 9 in 2002 (1.26% [9/715]). The prevalence of ceftriaxone resistance in each Salmonella serogroup was 0.99% for serogroup B (16/1,623), 1.37% for serogroup C (9/658), 0.67% for serogroup D (4/597), and 2.60% for serogroup E (2/77).
FIG. 1.
Distribution of total and ceftriaxone-resistant Salmonella isolates.
For the years 1999 through 2002, the annual ceftriaxone use was 11,117, 9,094, 14,354, and 13,147 g. For the same years, the numbers of stool cultures that were positive for Salmonella strains, which are rough estimates of the numbers of cases of gastrointestinal Salmonella infections, were 499, 598, 604, and 465. Both the most ceftriaxone use and the largest number of stool cultures that were positive for Salmonella occurred in 2001. The marked increase in ceftriaxone resistance in 2000 did not seem to correlate with ceftriaxone use or the number of gastrointestinal Salmonella infections.
Ceftriaxone-resistant Salmonella isolates.
The 31 ceftriaxone-resistant isolates came from 24 patients, with one patient harboring 4 isolates, one having 3 isolates, two patients having 2 isolates each, and the remaining 20 each harboring 1 isolate. Of these 31 strains, 15 ceftriaxone-resistant isolates from stool specimens obtained from eight patients were available for this study. The patients included all four from whom multiple isolates were obtained. According to PFGE analysis and susceptibility testing, the multiple isolates from each of the four patients who had them were considered identical. Therefore, only one isolate from each of the eight patients was studied further. These isolates were serotyped and found to include three S. enterica serovar Typhimurium, 1 S. enterica serovar Wagenia, 1 S. enterica serovar Senftenberg, 1 S. enterica serovar Derby, 1 S. enterica serovar Panama, and 1 S. enterica serovar Duesseldorf strain (Table 1).
TABLE 1.
Clinical data for patients and serotypes and β-lactamase genes of the eight ceftriaxone-resistant Salmonella isolates
| Isolate no. | Sex of patienta | Age of patient | Isolation date (yr/mo/day) | Underlying disease | Cause of hospitalizationb | Previous antibiotic use | S. enterica serotype (serogroup) | pI | Encoded β-lactamase |
|---|---|---|---|---|---|---|---|---|---|
| B11 | M | 1 yr 5 mos | 00/06/08 | None | AGE | Unknown | Wagenia (B) | 9.0, 5.4 | CMY-2, TEM-1 |
| B25 | M | 10 mos | 00/07/19 | None | AGE | None | Typhimurium (B) | 9.0, 7.6 | CMY-2, unidentified |
| L4 | F | 1 yr 1 mo | 00/07/18 | None | AGE | Unknown | Typhimurium (B) | 9.0, 7.6 | CMY-2, unidentified |
| L5 | F | 33 yrs | 01/10/20 | None | AGE, PID, vaginitis | Cephalexin | Senftenberg (E4) | 9.0 | CMY-2 |
| L6 | M | 7 mos | 01/11/16 | Cerebral palsy | AGE, bronchiolitis | None | Derby (B) | 9.0 | CMY-2 |
| L8 | M | 1 yr 4 mos | 02/06/07 | None | Salmonella group C sepsis | Ampicillin, ceftriaxone | Panama (D1) | 8.4 | CTX-M-14 |
| L9 | F | 2 mos | 02/06/08 | None | AGE, UTI (E. coli) | None | Typhimurium (B) | 9.0, 7.6 | CMY-2, unidentified |
| L10 | F | 65 yrs | 00/10/26 | Ovarian cancer | AGE | Ceftazidime, cefazolin, gentamicin | Duesseldorf (C2) | 9.0 | CMY-2 |
M, male; F, female.
AGE, acute gastroenteritis; PID, pelvic inflammatory disease; UTI, urinary tract infection.
Patient data.
The clinical data for the eight patients are listed in Table 1. Six patients were under the age of 2 years. One 7-month-old baby had cerebral palsy and epilepsy, and one 65-year-old female had ovarian cancer; the remaining six patients were generally in good health before the episode of salmonellosis. All eight patients acquired ceftriaxone-resistant Salmonella in the community and had symptomatic acute gastroenteritis. Three patients had been given antimicrobial therapy before the isolation of the resistant strains. One 33-year-old female had been treated for pelvic inflammatory disease with oral cephalexin 20 days prior to the isolation of the strain. The woman with ovarian cancer had been treated with ceftazidime, cephazolin, and gentamicin for suspected sepsis 10 days prior to the isolation of the strain. The third patient with previous antimicrobial therapy was a 1-year-old boy who was treated with ampicillin followed by ceftriaxone for sepsis caused by serogroup C Salmonella. Seventeen days later, after discharge from that episode, he developed diarrhea and his stool culture contained ceftriaxone-resistant S. enterica serovar Panama.
Susceptibility testing.
The MICs of various antibiotics for these eight isolates are listed in Table 2. All isolates were intermediate/resistant to ampicillin, ceftriaxone, and cefotaxime but susceptible to imipenem and ciprofloxacin. The MICs for isolate L8 were very different from the others in that this isolate was highly resistant to ceftriaxone, cefotaxime, and cefepime but was susceptible to ceftazidime.
TABLE 2.
Antimicrobial susceptibilities of eight ceftriaxone-resistant Salmonella isolates
| Isolate no. | MICa (μg/ml)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | CRO | CTX | CAZ | FEP | IPM | TZP | CHL | MIN | SXT | CIP | |
| B11 | >128 | 32 | 16 | 32 | 0.5 | 0.25 | 128 | >128 | 64 | >128 | 0.03 |
| B25 | >128 | 64 | 32 | 64 | 4 | 0.25 | 128 | >128 | 32 | >128 | 0.03 |
| L4 | >128 | 64 | 32 | 64 | 4 | 0.25 | 128 | >128 | 64 | >128 | 0.5 |
| L5 | >128 | 32 | 16 | 64 | 0.25 | 0.5 | 16 | 4 | 2 | 0.12 | 0.03 |
| L6 | >128 | 32 | 16 | 64 | 0.5 | 0.25 | 32 | 4 | 4 | >128 | 0.03 |
| L8 | >128 | >128 | >128 | 4 | >128 | 0.25 | 16 | >128 | 32 | >128 | 0.06 |
| L9 | >128 | 64 | 16 | 64 | 8 | 0.5 | 128 | >128 | 32 | >128 | 0.5 |
| L10 | >128 | 64 | 16 | 64 | 1 | 0.25 | 32 | >128 | 8 | >128 | 0.5 |
AMP, ampicillin; CRO, ceftriaxone; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; IPM, imipenem; TZP, piperacillin-tozobactam; CHL, chloramphenicol; MIN, minocycline; SXT, trimethoprim-sulfamethoxazole; CIP, ciprofloxacin.
IEF.
All eight isolates produced one or two bands corresponding to a β-lactamase(s) on IEF gels (Table 1). All isolates, with the exception of L8, produced a β-lactamase with a pI value of 9.0, which was cotransconjugated and cotransformed with ceftriaxone resistance and shown to be CMY-2. Isolate B11 produced a second β-lactamase with a pI value of 5.4, which was shown to be TEM-1. Isolates B25, L4, and L9 produced a second β-lactamase with a pI value of 7.6 which could not be further identified. Isolate L8 produced only one β-lactamase, with a pI value of 8.4, which was shown to be CTX-M-14.
PFGE.
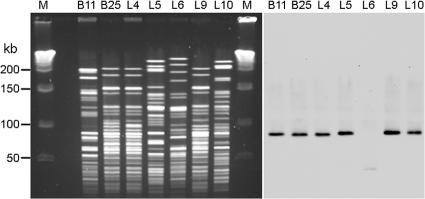
Seven of the eight isolates had unique, discernible PFGE patterns (Fig. 2, left panel). Isolates B25, L4, and L9, all of which were S. enterica serovar Typhimurium strains, were the most similar but were not identical. Isolate L8, an S. enterica serovar Panama strain, resulted in a smear on the PFGE gel (data not shown). PFGE of S. enterica serovar Panama isolates has never been successful and always results in a smear (C. S. Chiang, unpublished data).
FIG. 2.
PFGE patterns and Southern blotting of chromosomal DNAs. (A) PFGE of ceftriaxone-resistant Salmonella isolates after digestion of chromosomal DNAs with NotI. Lane M, molecular size marker; lanes B11 to L10, isolates B11 to L10. (B) Southern blotting of the PFGE gel with blaCMY-2 DNA as the probe. The two photographs were aligned so that the size marker applies to both.
Plasmid profiles.
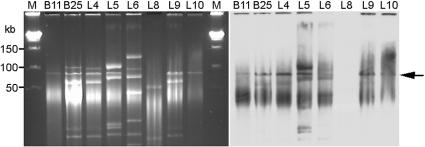
More than one plasmid was isolated from most of the eight isolates (Fig. 3, left panel). Several plasmids present in some isolates were of similar molecular sizes, e.g., 90 and 100 kb, in addition to some that were much smaller.
FIG. 3.
Plasmid profiles and Southern blotting. (A) PFGE of plasmid DNAs prepared from ceftriaxone-resistant Salmonella isolates. Lane M, molecular size marker; lanes B11 to L10, isolates B11 to L10. (B) Southern blotting of the plasmid DNAs in panel A with blaCMY-2 DNA as the probe. The arrow indicates the hybridized band. The two photographs were aligned so that the size marker applies to both.
Transconjugation and transformation.
The ceftriaxone resistance of the eight isolates was transconjugated into E. coli HB101 at similar frequencies of 10−3 to 10−4. The plasmid DNAs of all eight isolates successfully transformed E. coli, indicating that the ceftriaxone resistance genes are carried by a plasmid.
Southern blotting.
A 90-kb NotI fragment in the PFGE gel hybridized with a blaCMY-2 DNA probe for six of the seven isolates that carried the blaCMY-2 gene (Fig. 2, right panel). A fragment of <50 kb was hybridized for isolate L6. No hybridization signal was observed for isolate L8 (data not shown). When a similar gel including isolate L8 was hybridized with a blaCTX-M-14 DNA probe, only a smear with no specific bands was observed for L8 (data not shown), probably due to degradation of the DNA in PFGE. No signal was observed for the other seven isolates.
When Southern blotting using a blaCMY-2 DNA probe was performed on plasmid DNA, one major signal of 90 kb was present for the seven isolates that carried the blaCMY-2 gene (Fig. 3, right panel). Again, no hybridization signal was observed for isolate L8. When the gel was hybridized with a blaCTX-M-14 DNA probe, a smear with no specific bands was observed for L8, and no signal was observed for the other seven isolates (data not shown).
PCR amplification of β-lactamase genes and DNA sequencing.
All isolates except L8 had positive signals in a PCR using primers specific for ampC-type genes. Upon nucleotide sequencing, the PCR products from these seven isolates were found to be identical to each other and to the published blaCMY-2 gene (2). L8 had a positive signal in a PCR using primers specific for blaCTX-M-type genes, and nucleotide sequencing demonstrated that the β-lactamase was a product of the blaCTX-M-14 gene (8). In addition, isolate B11 demonstrated the blaTEM-1 gene. None of the eight isolates showed evidence of blaSHV-type genes.
Cloning of the region surrounding the blaCMY-2 gene.
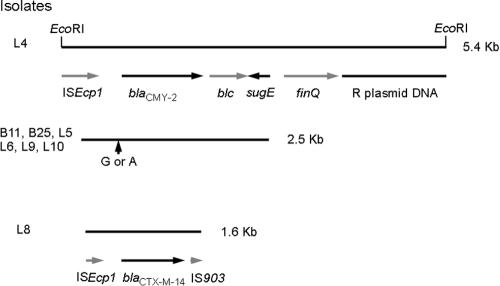
A 5,429-bp EcoRI fragment surrounding the blaCMY-2 gene from isolate L4 was cloned and sequenced. As shown in Fig. 4, one end of this fragment contained the ISEcp1, blaCMY-2, blc, and sugE genes. The organization was the same as that of pSCR1, a plasmid carrying blaCMY-2 from S. enterica serovar Choleraesuis (GenBank accession number AY253913), and of pNF4565, a plasmid carrying blaCMY-2 from S. enterica serovar Typhimurium (15). The sequence homology of isolate L4 to the corresponding regions in pSCR1 was 100% and that to the corresponding regions in pNF4565 was 99.97%. At nucleotide 49 upstream of the start codon of blaCMY-2, isolate L4 had an A, whereas pNF4565 has a G.
FIG. 4.
Map of blaCMY-2 and blaCTX-M-14 regions from ceftriaxone-resistant Salmonella isolates. Black lines indicate the DNA fragments sequenced. The restriction enzyme sites of EcoRI used for cloning are shown at the top. Arrows below the black lines indicate various genes. The vertical arrow indicates the only nucleotide difference, which occurred at nucleotide 49 upstream of the start codon of blaCMY-2, resulting in either an A or a G.
The other end of the EcoRI fragment from isolate L4 contained sequences homologous to the finQ gene of E. coli (16) and to R64 plasmid DNA from Salmonella (14). The homologies were 94.01% and 97.96%, respectively.
For the remaining six blaCMY-2-positive isolates, a 2.5-kb region encompassing the entire blaCMY-2 gene was also sequenced. All six isolates had sequences within this region identical to that of isolate L4, except for nucleotide 49 upstream of the start codon of blaCMY-2 (Fig. 4). Isolates B11 and B25 had an A nucleotide at this position, the same as isolate L4 and plasmid pSCR1, but L5, L6, L9, and L10 had a G, the same as plasmid pNF4565.
Region surrounding the blaCTX-M-14 gene.
A region of 1,569 bp surrounding the blaCTX-M-14 gene from isolate L8 was sequenced (Fig. 4) and was 100% homologous to the sequence reported by Chanawong et al. (8). It contained the 3′ end of ISEcp1, blaCTX-M-14, and the 5′ end of the insertion sequence IS903 transposase gene (6).
DISCUSSION
We have demonstrated the mechanism of ceftriaxone resistance for eight community-acquired nontyphoidal Salmonella strains isolated from January 1999 to December 2002. All eight strains carried a β-lactamase gene on a conjugative plasmid, with seven isolates carrying the blaCMY-2 gene and one carrying blaCTX-M-14. At nucleotide 49 upstream of the start codon of blaCMY-2 in a 2.5-kb region encompassing the blaCMY-2 gene, three of the seven blaCMY-2-positive isolates had an A nucleotide and four had a G.
The first ceftriaxone-resistant Salmonella strain isolated in our hospital in 1999 was from the stool of a child with uncomplicated gastroenteritis. There were 2 isolates recovered in 1999 and 13 in 2000, but only 7 and 9 in the next 2 years, so the prevalence does not appear to be increasing rapidly. The highest rate of ceftriaxone resistance was in 2000, whereas both the highest ceftriaxone use and the largest number of gastrointestinal Salmonella infections occurred in 2001. During our study period, there seemed to be no correlation between ceftriaxone resistance and either ceftriaxone use or the number of gastrointestinal Salmonella infections.
Sixteen of the 31 resistant isolates belonged to serogroup B, and the remaining 15 isolates were from three other serogroups. The eight isolates we studied for this report belonged to six different serotypes among four serogroups. That fact, in addition to their acquisition in the community rather than nosocomially, makes it unlikely that they were produced by the clonal spread of a particular ceftriaxone-resistant strain. Furthermore, the blaCMY-2 genes in all seven blaCMY-2-positive isolates were carried by plasmids of the same molecular weight, which suggests that the dissemination of a blaCMY-2-carrying plasmid was responsible for the spread of ceftriaxone resistance in our series.
In southern Taiwan, plasmids carrying the blaCMY-2 genes of Salmonella, E. coli, and K. pneumoniae have shown identical restriction patterns, indicating the interspecies spread and horizontal transfer of the resistant gene (37, 38). In our series, the blaCMY-2 genes resided on plasmids that could be transconjugated as well as transformed into E. coli, making interspecies transfer from other Enterobacteriaceae a possibility. The carriage of both the blaCMY-2 and blaCTX-M-14 genes on conjugal plasmids supports the possibility of horizontal transfer within as well as between species, aiding in an increasingly wide spread of resistance.
Su et al. reported that S. enterica serovar Anatum and E. coli strains isolated from wound cultures of a diabetic patient were initially ceftriaxone susceptible but became ceftriaxone resistant 2 weeks after the initiation of ceftriaxone therapy (32). Because the susceptible and resistant strains were genetically indistinguishable, the authors concluded that these isolates acquired the ceftriaxone-resistant blaCTX-M-3 gene in vivo. In our study, the blaCTX-M-14 gene-carrying isolate L8 was isolated from a 1-year-old boy who had been treated for sepsis with ceftriaxone for 10 days. In both cases, the CTX-M-type β-lactamases appeared after the use of antibiotics. However, it was not clear whether blaCTX-M was already present and its expression was induced by the pressure of antibiotic use or if the blaCTX-M gene was actually acquired during antibiotic use.
The difference at nucleotide 49 upstream of the start codon of blaCMY-2 was interesting, as three of our isolates had an A, as does plasmid pSCR1, which was isolated from S. enterica serovar Choleraesuis isolates in Taiwan (GenBank accession number AY253913), and the remaining four had a G, as does plasmid pNF4565, which was isolated from S. enterica serovar Typhimurium in the United States (15). It is unclear if this difference indicates that two different DNA fragments carry the same blaCMY-2 gene or that this particular nucleotide mutates easily.
In conclusion, in the majority of ceftriaxone-resistant Salmonella isolates studied, resistance was conferred by the expression of the plasmid-mediated blaCMY-2 gene. In only one of eight isolates, the resistance was due to the plasmid-mediated blaCTX-M-14 gene.
Acknowledgments
We thank Chun-Yan Yeung and Hung-Chang Lee for some of the clinical isolates.
This study was supported by grant MMH9014 from Mackay Memorial Hospital, Taipei, Taiwan. This work was performed at the Mackay Memorial Hospital, Taipei, Taiwan.
REFERENCES
- 1.Aserkoff, B., and J. V. Bennett. 1969. Effect of antibiotic therapy in acute salmonellosis on the fecal excretion of Salmonellae. N. Engl. J. Med. 281:636-640. [DOI] [PubMed] [Google Scholar]
- 2.Bauernfeind, A., I. Stemplinger, R. Jungwirth, and H. Giamarellou. 1996. Characterization of the plasmidic β-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob. Agents Chemother. 40:221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauernfeind, A., J. M. Casellas, M. Goldberg, M. Holley, R. Jungwirth, P. Mangold, T. Röhnisch, S. Schweighart, and R. Wilhelm. 1992. A new plasmidic cefotaximase from patients infected with Salmonella typhimurium. Infection 20:158-162. [DOI] [PubMed] [Google Scholar]
- 4.Bopp, C. A., F. W. Brenner, J. G. Wells, and N. A. Strockbine. 1999. Escherichia, Shigella, and Salmonella, p. 459-474. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 5.Bradford, P. A., Y. Yang, D. Sahm, I. Grope, D. Gardovska, and G. Storch. 1998. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob. Agents Chemother. 42:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, V., T. Lambert, and P. Courvalin. 2002. ColE1-like plasmid pIP843 of Klebsiella pneumoniae encoding extended-spectrum β-lactamase CTX-M-17. Antimicrob. Agents Chemother. 46:1212-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carattoli, A., F. Tosini, W. P. Giles, M. E. Rupp, S. H. Hinrichs, F. J. Angulo, T. J. Barrett, and P. D. Fey. 2002. Characterization of plasmids carrying CMY-2 from expanded-spectrum cephalosporin-resistant Salmonella strains isolated in the United States between 1996 and 1998. Antimicrob. Agents Chemother. 46:1269-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanawong, A., F. H. M'Zali, J. Heritage, J. H. Xiong, and P. M. Hawkey. 2002. Three cefotaximases, CTX-M-9, CTX-M-13, and CTX-M-14, among Enterobacteriaceae in the People's Republic of China. Antimicrob. Agents Chemother. 46:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu, C. H., L. H. Su, C. Chu, J. H. Chia, T. L. Wu, T. Y. Lin, Y. S. Lee, and J. T. Ou. 2004. Isolation of Salmonella enterica serotype Choleraesuis resistant to ceftriaxone and ciprofloxacin. Lancet 363:1285-1286. [DOI] [PubMed] [Google Scholar]
- 10.Chiu, C. H., T. L. Wu, L. H. Su, C. S. Chu, J. H. Chia, A. J. Kuo, M. S. Chien, and T. Y. Lin. 2002. The emergence in Taiwan of fluoroquinolone resistance in Salmonella enterica serotype Choleraesuis. N. Engl. J. Med. 346:413-419. [DOI] [PubMed] [Google Scholar]
- 11.Chyou, S. C., Y. J. Leu, F. Y. Huang, H. C. Lee, and D. I. Yang. 1988. An etiological study of infectious diarrhea in infants and children in Taipei area. Acta Paediatr. Sinica 29:213-219. [PubMed] [Google Scholar]
- 12.Dunne, E. F., P. D. Fey, P. Kludt, R. Reporter, F. Mostashari, P. Shillam, J. Wicklund, C. Miller, B. Holland, K. Stamey, T. J. Barrett, J. K. Rasheed, F. C. Tenover, E. M. Ribot, and F. J. Angulo. 2000. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with Amp C β-lactamase. JAMA 284:3151-3156. [DOI] [PubMed] [Google Scholar]
- 13.Fey, P. D., T. J. Safranek, M. E. Rupp, E. F. Dunne, E. Ribot, P. C. Iwen, P. A Bradford, F. J. Angulo, and S. H. Hinrichs. 2000. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. N. Engl. J. Med. 342:1242-1249. [DOI] [PubMed] [Google Scholar]
- 14.Furuya, N., and T. Komano. 1996. Nucleotide sequence and characterization of the trbABC region of the IncI1 plasmid R64: existence of the pnd gene for plasmid maintenance within the transfer region. J. Bacteriol. 178:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giles, W. P., A. K. Benson, M. E. Olson, R. W. Hutkins, J. M. Whichard, P. L. Winokur, and P. D. Fey. 2004. DNA sequence analysis of regions surrounding blaCMY-2 from multiple Salmonella plasmid backbones. Antimicrob. Agents Chemother. 48:2845-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ham, L. M., and R. Skurray. 1989. Molecular analysis and nucleotide sequence of finQ, a transcriptional inhibitor of the F plasmid transfer genes. Mol. Gen. Genet. 216:99-105. [DOI] [PubMed] [Google Scholar]
- 17.Herikstad, H., P. S. Hayes, J. Hogan, P. Floyd, L. Snyder, and F. J. Angulo. 1997. Ceftriaxone-resistant Salmonella in the United States. Pediatr. Infect. Dis. J. 16:904-905. [DOI] [PubMed] [Google Scholar]
- 18.Ho, M., L. C. McDonald, T. L. Lauderdale, L. L. Yeh, P. C. Chen, and Y. R. Shiau. 1999. Surveillance of antibiotic resistance in Taiwan, 1998. J. Microbiol. Immunol. Infect. 32:239-349. [PubMed] [Google Scholar]
- 19.Hohmann, E. L. 2001. Nontyphoidal salmonellosis. Clin. Infect. Dis. 32:263-269. [DOI] [PubMed] [Google Scholar]
- 20.Huang, F. Y., S. H. Huang, S. H. Chen, Y. C. Hsu, and C. H. Lin. 1991. Bacteremia in infants with Salmonella enterocolitis. Acta Paediatr. Sinica 32:358-364. [PubMed] [Google Scholar]
- 21.Koeck, J. L., G. Arlet, A. Philippon, S. Basmaciogullari, H. V. Thien, Y. Buisson, and J. D. Cavallo. 1997. A plasmid-mediated CMY-2 β-lactamase from an Algerian clinical isolate of Salmonella senftenberg. FEMS Microbiol. Lett. 152:255-260. [DOI] [PubMed] [Google Scholar]
- 22.Lee, C. Y., C. H. Chiu, Y. Y. Chuang, L. H. Su, T. L. Wu, L. Y. Chang, Y. C. Huang, and T. Y. Lin. 2002. Multidrug-resistant non-typhoid Salmonella infections in a medical center. J. Microbiol. Immunol. Infect. 35:78-84. [PubMed] [Google Scholar]
- 23.Liu, C. P., N. Y. Wang, C. M. Lee, L. C. Weng, H. K. Tseng, C. W. Liu, C. S. Chiang, and F. Y. Huang. 2004. Nosocomial and community-acquired Enterobacter cloacae bloodstream infection: risk factors for and prevalence of SHV-12 in multiresistant isolates in a medical centre. J. Hosp. Infect. 58:63-77. [DOI] [PubMed] [Google Scholar]
- 24.Matthew, M., A. M. Harris, M. J. Marshall, and G. W. Ross. 1975. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88:169-178. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Rossi, A., H. Lopardo, M. Woloj, A. M. Picandet, M. Marino, M. Galds, M. Radice, and G. Gutkind. 1995. Non-typhoid Salmonella spp. resistant to cefotaxime. J. Antimicrob. Chemother. 36:697-702. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Simarro, E., F. Navarro, J. Ruiz, E. Miró, J. Gómez, and B. Mirelis. 2000. Salmonella enterica serovar Virchow with CTX-M-like β-lactamase in Spain. J. Clin. Microbiol. 38:4676-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinnett, D., C. Richer, and A. Baccichet. 1998. Isolation of stable bacterial artificial chromosome DNA using a modified alkaline lysis method. BioTechniques 24:752-754. [DOI] [PubMed] [Google Scholar]
- 30.Sirinavin, S., P. Jayanetra, and A. Thakkinstian. 1999. Clinical and prognostic categorization of extraintestinal nontyphoid Salmonella infections in infants and children. Clin. Infect. Dis. 29:1151-1156. [DOI] [PubMed] [Google Scholar]
- 31.Su, L. H., C. H. Chiu, A. J. Kuo, J. H. Chia, C. F. Sun, H. S. Leu, and T. L. Wu. 2001. Secular trends in incidence and antimicrobial resistance among clinical isolates of Salmonella at a university hospital in Taiwan, 1983-1999. Epidemiol. Infect. 27:207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su, L. H., C. H. Chiu, C. Chu, M. H. Wang, J. H. Chia, and T. L. Wu. 2003. In vivo acquisition of ceftriaxone resistance in Salmonella enterica serotype Anatum. Antimicrob. Agents Chemother. 47:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tassios, P. T., M. Gazouli, E. Tzelepi, H. Milch, N. Kozlova, S. Sidorenko, N. J. Legakis, and L. S. Tzouvelekis. 1999. Spread of a Salmonella typhimurium clone resistant to expanded-spectrum cephalosporins in three European countries. J. Clin. Microbiol. 37:3774-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vahaboglu, H., L. M. C. Hall, L. Mulazimoglu, S. Dodanli, I. Yildirim, and D. M. Livermore. 1995. Resistance to extended-spectrum cephalosporins, caused by PER-1 β-lactamase, in Salmonella typhimurium from Istanbul, Turkey. J. Med. Microbiol. 43:294-299. [DOI] [PubMed] [Google Scholar]
- 35.Weng, L. C., G. J. Liaw, N. Y. Wang, S. F. Wang, C. M. Lee, F. Y. Huang, D. I. Yang, and C. S. Chiang. 1999. Investigation of an outbreak of Pseudomonas putida using antimicrobial susceptibility patterns, pulsed-field gel electrophoresis of genomic DNA and restriction fragment length polymorphism of PCR-amplified rRNA operons. J. Microbiol. Immunol. Infect. 32:187-193. [PubMed] [Google Scholar]
- 36.Winokur, P. L., A. Bruggemann, D. L. DeSalvo, L. Hoffmann, M. D. Apley, E. K. Uhlenhopp, M. A. Pfaller, and G. V. Doern. 2000. Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC β-lactamase. Antimicrob. Agents Chemother. 44:2777-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan, J. J., C. H. Chiu, W. C. Ko, C. L. Chuang, and J. J. Wu. 2002. Ceftriaxone-resistant Salmonella enterica serovar Hadar: evidence for interspecies transfer of blaCMY-2 in a Taiwanese university hospital. J. Formos Med. Assoc. 101:665-668. [PubMed] [Google Scholar]
- 38.Yan, J. J., W. C. Ko, C. H. Chiu, S. H. Tsai, H. M. Wu, and J. J. Wu. 2003. Emergence of ceftriaxone-resistant Salmonella isolates and rapid spread of plasmid-encoded CMY-2-like cephalosporinase, Taiwan. Emerg. Infect. Dis. 9:323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan, J. J., W. C. Ko, S. H. Tsai, H. M. Wu, Y. T. Jin, and J. J. Wu. 2000. Dissemination of CTX-M-3 and CMY-2 beta-lactamases among clinical isolates of Escherichia coli in southern Taiwan. J. Clin. Microbiol. 38:4320-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, C. H., H. H. Tseng, K. J. Chen, and J. D. Liu. 1996. Salmonella infections: a retrospective 10-year analysis of 134 cases in a regional hospital in Taiwan. Scand. J. Infect. Dis. 28:171-175. [DOI] [PubMed] [Google Scholar]
- 41.Yang, Y. J., C. C. Liu, S. M. Wang, J. J. Wu, A. H. Huang, and C. P. Cheng. 1998. High rates of antimicrobial resistance among clinical isolates of nontyphoidal Salmonella in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 17:880-883. [DOI] [PubMed] [Google Scholar]