Abstract
Bovine herpesvirus 4 (BoHV-4) is a gammaherpesvirus with no clear disease association. Previous studies have demonstrated that macrophages can harbor persistent BoHV-4. We found that the addition of prostaglandin E2 (PGE2) to bovine macrophage cells persistently infected with BoHV-4 increases viral replication. Because opportunistic infection can increase PGE2 production, we propose a link between opportunistic infection, PGE2 production, and BoHV-4 replication.
Bovine herpesvirus 4 (BoHV-4), a member of the Gammaherpesvirinae subfamily, was first isolated in Europe from respiratory and ocular diseases by Bartha and colleagues (1) and later in the United States by Mohanty and colleagues (12). BoHV-4 has been isolated from a variety of samples and cells from healthy cattle and from cattle with abortion, metritis, pneumonia, diarrhea, respiratory infection, and mammary pustular dermatitis (1, 7, 8, 11, 21). However, only a few investigators have successfully produced experimental disease, and the pathogenic role of BoHV-4 remains unclear (21). Like other herpesviruses, BoHV-4 establishes persistent infection. Although BoHV-4 has been identified in many tissues during persistent infection, accumulated evidence suggests that one site of persistence in both the natural and experimental host is the cells of the monocyte/macrophage lineage (3, 4, 13, 14). We previously established an in vitro model of BoHV-4 persistent infection in a bovine macrophage cell line, in which persistent infection was compatible with cell survival and replication (3). Persistently infected cell lines produced low levels of infectious virus. In the present study, we used this in vitro system to examine a possible link between BoHV-4 persistent infection and potential pathogenicity.
Establishment of macrophage cell line persistently infected with BoHV-4.
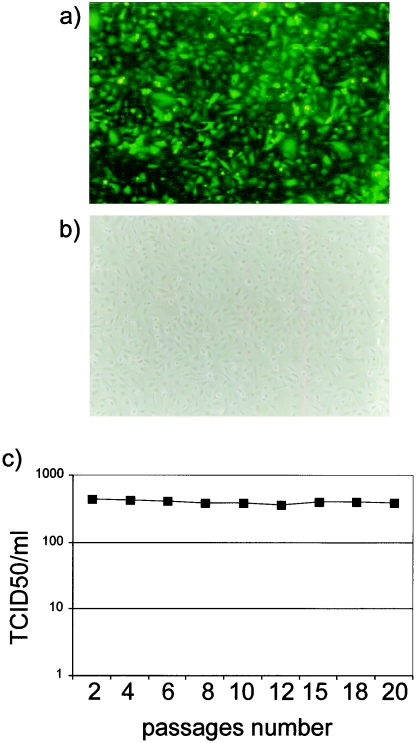
A persistently infected macrophage cell line (BOMAC/BoHV-4EGFPΔTK) was established as previously described (3) by infecting BOMAC cells, a cell line established from peritoneal macrophages by transformation with simian virus 40 DNA (18). Confluent monolayers of BOMAC cells were inoculated (multiplicity of infection [MOI] of 1 × 50% tissue culture infective dose [TCID50]/cell) with recombinant BoHV-4 (BoHV-4EGFPΔTK), which contains an enhanced green fluorescent protein (EGFP) gene inserted into the TK gene of BoHV-4 strain DN-599 (6). As we observed previously when we infected BOMAC cells with BoHV-4 (3), by the third day postinoculation, more than 95% of cells had detached from the monolayer, leaving behind a small number of cells that did not exhibit cytopathic effect (CPE). Confluent monolayers established from surviving cells showed 100% infection, as indicated by the strong fluorescent signal (Fig. 1a), but without apparent signs of CPE (Fig. 1b). Also consistent with our previous observations (3), the persistently infected macrophages produced infectious BoHV-4; medium recovered from BOMAC/BoHV-4EGFPΔTK cells inoculated onto susceptible BAE-7323 (bovine aortic endothelial cell line) or BEK (bovine embryo kidney cell line) cells produced green plaques typical of BoHV-4EGFPΔTK (data not shown). BOMAC/BoHV-4EGFPΔTK cells were subcultured at a dilution of 1:2 every 3 days and their growth medium was stored at −80°C for viral titration. The yield of virus in the culture medium on the day the cells were subcultured remained in the range of 4 × 102 TCID50/ml throughout the first 20 passages (Fig. 1c). The ability of infected cells to propagate as long-term virus-shedding cultures confirms that the culture represents a persistent infection, like the persistently infected cells we previously characterized (3).
FIG. 1.
Confluent monolayer of BOMAC/BoHV-4EGFPΔTK persistently infected cells expressing EGFP. Fluorescence (a) and phase-contrast (b) images are shown. (c) Amount of virus produced by BOMAC/BoHV-4EGFPΔTK persistently infected cells through the passages, expressed as TCID50/ml.
After the first 20 passages, if the cells were subcultured at a dilution greater than 1:4, a crisis developed that consisted of a wave of cell fusion followed by cytolysis, possibly due to a change to lytic infection in those cells. However, following the crisis, the remaining cells continued to grow, allowing the culture to become confluent within roughly 3 days. Fluorescence indicated that these cells remained persistently infected.
Although these data are generated in vitro, a theoretical model of the strategy used by BoHV-4 to establish a persistent infection in vivo can be formulated: following an acute replicative infection, macrophages become a reservoir for BoHV-4, where very low levels of virus are released unless insults causing reactivation of acute viral replication take place. Because establishment of persistent infections involves close interactions and adjustments in both host and virus, it would be informative to establish a paradigm whereby a normally cytolytic viral infection can be easily converted to persistent infection, so that the different stages in developing persistent infection can be examined. That what we have seen was a bona fide persistent infection was demonstrated by the ability of infected cells to propagate as long-term virus-shedding cultures.
BOMAC cells persistently infected with BoHV-4 are resistant to reinfection.
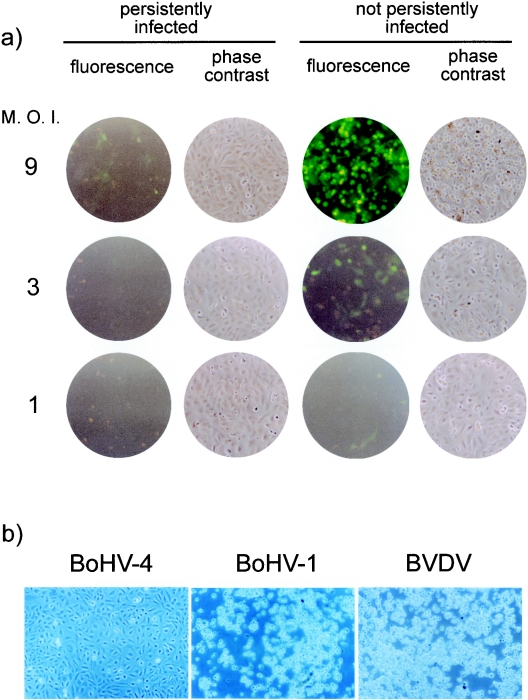
Because persistently infected macrophages produce infectious virus, persistently infected cells could potentially be reinfected with released virus. To determine whether persistently infected cells can be reinfected and to determine the outcome of reinfection, we started from BOMAC cells infected with a recombinant BoHV-4 (BoHV-4 26A34neo) (5) strain which carries the neomycin-resistance gene and allowed us, by drug selection, to ensure that all cells were persistently infected with BoHV-4 (5). These cells were reinfected with different doses (MOIs of 1, 3, and 9) of BoHV-4EGFPΔTK, and infection was monitored by fluorescence microscopy at 24 (data not shown) and 48 h (Fig. 2a) postinfection. As expected, BOMAC cells persistently infected with BoHV-4 were resistant to reinfection, as shown by lack of EGFP expression, in contrast to the readily observable EGFP expression upon infection of previously uninfected control BOMAC cells. At the highest dose of BoHV-4EGFPΔTK, a few fluorescent cells were observed in the persistently infected cultures, but clearly with lower frequency and lower intensity than those in the previously uninfected BOMAC cultures. Furthermore, the persistently infected BOMAC cells did not exhibit the CPE evident in the previously uninfected BOMAC cells infected with the highest dose of BoHV-4. Specificity of resistance to BoHV-4 infection was confirmed by the observation that BOMAC cells persistently infected with BoHV-4 were susceptible to infection with other bovine viruses: BoHV-1 (Colorado strain, ATCC VR-188) and bovine viral diarrhea virus (NADL strain, ATCC VR-1422) (Fig. 2b). Both viruses produced a strong CPE, even when used at a dose as low as an MOI of 0.1; in contrast, no CPE was observed at the highest dose of BoHV-4 (data not shown).
FIG. 2.
(a) Fluorescence and phase-contrast images of monolayers of BOMAC cells persistently infected with BoHV-4/26A3neo and uninfected BOMAC control cells, both reinfected at different MOIs (TCID50/cell) with BoHV-4EGFPΔTK. (b) Phase-contrast images of monolayers of BOMAC cells persistently infected with BoHV-4/26A3neo and reinfected with 1 × TCID50/cell of BoHV-4, BoHV-1, and bovine viral diarrhea virus (BVDV).
We did not investigate the mechanisms involved in resistance to reinfection and CPE. Resistance likely involves epigenetic receptor interference, as shown for other viruses. This would require that viral attachment proteins be expressed in the persistently infected cells rather than a state of latency. However, this simple but clear observation gives rise to the model we are building, which considers persistently infected macrophage cells as not only the site of persistent infection but also a safe viral reservoir, protected from the CPE resulting from reinfection with BoHV-4.
Prostaglandin E2 (PGE2) induces BoHV-4 lytic replication in persistently infected BOMAC cells.
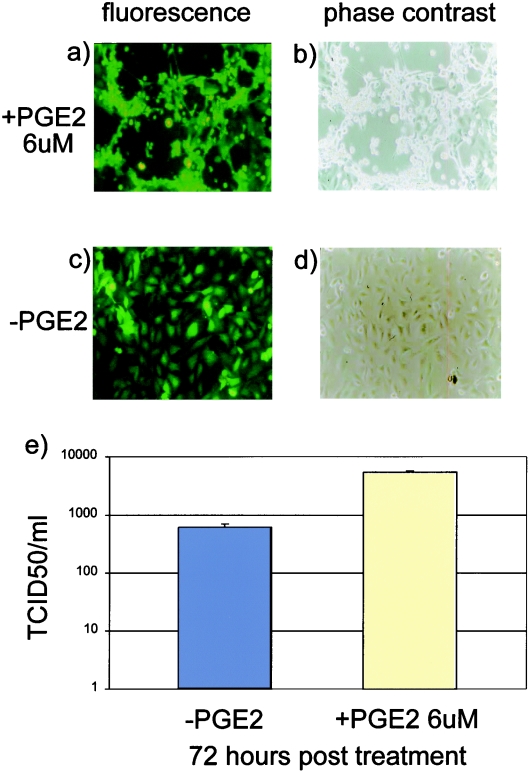
We envisioned a scenario whereby a soluble factor produced at sites of inflammation would be able to activate BoHV-4 lytic replication in persistently infected macrophages, based on the following premises: (i) BoHV-4 has been isolated from animals with a variety of inflammatory lesions such as metritis, pneumonia, diarrhea, respiratory infections, mammary pustular dermatitis, interdigital dermatitis, vaginitis, and so on (1, 21); (ii) persistently infected macrophages can serve as a reservoir of BoHV-4; and (iii) persistently infected macrophages can easily reach sites of inflammation through the bloodstream. PGE2, a key mediator in the inflammatory response, has been shown to be induced by bacterial infection and to perform an important function in supporting herpesvirus replication (20, 22). Thus, we determined whether PGE2 is a general activator of BoHV-4 lytic replication in persistently infected macrophages by testing the effect of exogenous PGE2 on BOMAC/BoHV-4EGFPΔTK persistently infected cells in vitro. Persistently infected BOMAC/BoHV-4EGFPΔTK cells and BOMAC uninfected control cells were treated with medium containing increasing concentrations of PGE2 (1, 3, and 6 μM). At 72 h posttreatment, a strong CPE appeared in BOMAC/BoHV-4EGFPΔTK persistently infected cells treated with 6 μM PGE2 (Fig. 3a, c); in contrast, no sign of CPE could be observed in BOMAC uninfected control cells treated with 6 μM PGE2 or in BOMAC/BoHV-4EGFPΔTK persistently infected cells treated with lower concentrations of PGE2 (data not shown). The activation of BoHV-4 lytic replication by PGE2 treatment in BOMAC/BoHV-4EGFPΔTK persistently infected cells was confirmed by determining the titer of virus in the culture medium, which was approximately 10-fold higher (103 TCID50/ml) than in medium from untreated cells (102 TCID50/ml) (Fig. 3e).
FIG. 3.
Induction of BoHV-4 lytic replication in BOMAC/BoHV-4EGFPΔTK persistently infected cells by PGE2 treatment. Fluorescence (a) and phase-contrast (b) images of BOMAC/BoHV-4EGFPΔTK persistently infected cells treated with 6 μM PGE2 at 72 h posttreatment, showing a strong CPE. Fluorescence (c) and phase-contrast (d) images of untreated control BOMAC/BoHV-4EGFPΔTK persistently infected cells without signs of CPE. (e) Difference in virus production in BOMAC cells persistently infected with BoHV-4EGFPΔTK and treated with PGE2 or untreated.
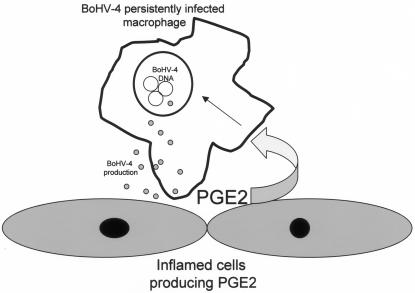
The association of BoHV-4 with other pathogens in cases of metritis has been consistently reported in the past (2, 9, 10, 15, 19), and bacterially induced metritis in cattle persistently infected with BoHV-4 could possibly be exacerbated or become chronic following the recruitment from the bloodstream to the site of inflammation of macrophages persistently infected with BoHV-4. Production of COX2 and PGE2 in murine deciduas is well known to increase rapidly in response to a number of cell activators and inflammatory signals such as lipopolysaccharide (16, 17). Therefore, these results together suggest that PGE2 produced from a preinflammatory decidua through lipopolysaccharide stimulation could activate BoHV-4 lytic replication in macrophages persistently infected with BoHV-4. Therefore, newly produced viral particles could infect the decidua, which could further increase PG production, as has been shown to occur in herpesvirus-infected cells in vitro (20, 22). Thus, a positive-feedback loop between PGE2 production and viral replication could be established (Fig. 4). This model suggests that BoHV-4 could contribute to disease by increasing inflammation. Obviously, the scenario described is just an example, related to the bacterial inflammatory state of the uterus, but the same mechanism could be applied to any site of inflammation in an animal persistently infected with BoHV-4, which is consistent with the fact that BoHV-4 can be easily isolated from a wide variety of lesions.
FIG. 4.
Diagram showing the potential linkage between PGE2 production from an inflamed tissue and BoHV-4 replication.
This is an in vitro study, which clearly needs to be corroborated through an in vivo approach. Therefore, we would consider this paper a simple starting point to shed light on the presently obscure pathogenic role of BoHV-4 in inducing disease.
REFERENCES
- 1.Bartha, A., M. Juhasz, and H. Liebermann. 1966. Isolation of a bovine herpesvirus from calves with respiratory disease and keratoconjuntivitis. Acta Vet. Acad. Sci. Hung. 16:357-358. [PubMed] [Google Scholar]
- 2.Czaplicki, G., and E. Thiry. 1998. An association exists between bovine herpesvirus-4 seropositivity and abortion in cows. Prev. Vet. Med. 33:235-240. [DOI] [PubMed] [Google Scholar]
- 3.Donofrio, G., and V. L. van Santen. 2001. A bovine macrophage cell line supports bovine herpesvirus 4 persistent infection. J. Gen. Virol. 82:1181-1185. [DOI] [PubMed] [Google Scholar]
- 4.Donofrio, G., C. F. Flammini, F. Scatozza, and S. Cavirani. 2000. Detection of bovine herpesvirus 4 (BoHV-4) DNA in the cell fraction of milk of dairy cattle with history of BoHV-4 infection. J. Clin. Microbiol. 38:4668-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donofrio, G., S. Cavirani, and V. L. van Santen. 2000. Establishment of a cell line persistently infected with bovine herpesvirus 4 using a recombinant virus. J. Gen. Virol. 81:1807-1814. [DOI] [PubMed] [Google Scholar]
- 6.Donofrio, G., S. Cavirani, S. Taddei, and V. L. van Santen. 2002. Bovine herpesvirus 4 as a gene delivery vector. J. Virol. Methods 101:49-61. [DOI] [PubMed] [Google Scholar]
- 7.Dubuisson, J., E. Thiry, F. Thalasso, M. Bublot, and P. P. Pastoret. 1988. Biological and biochemical comparison of bovid herpesvirus-4 strains. Vet. Microbiol. 16:339-349. [DOI] [PubMed] [Google Scholar]
- 8.Ehlers, B., H. J. Buhk, and H. Ludwig. 1985. Analysis of bovine cytomegalovirus genome structure: cloning and mapping of the monomeric polyrepetitive DNA unit, and comparison of European and American strains. J. Gen. Virol. 66:55-68. [DOI] [PubMed] [Google Scholar]
- 9.Frazier, K., M. Pence, M. J. Mauel, A. Liggett, M. E. Hines 2nd, L. Sangster, H. D. Lehmkuhl, D. Miller, E. Styer, J. West, and C. A. Baldwin. 2001. Endometritis in postparturient cattle associated with bovine herpesvirus-4 infection: 15 cases. J. Vet. Diagn. Investig. 13:502-508. [DOI] [PubMed] [Google Scholar]
- 10.Frazier, K. S., C. A. Baldwin, M. Pence, J. West, J. Bernard, A. Liggett, D. Miller, and M. E. Hines II. 2002. Seroprevalence and comparison of isolates of endometriotropic bovine herpesvirus-4. J. Vet. Diagn. Investig. 14:457-462. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig, H. 1983. Bovine herpesviruses, p. 135-214. In B. Roizman (ed.), The herpesviruses, vol. 2. Plenum Press, New York, N.Y. [Google Scholar]
- 12.Mohanty, S. B., R. C. Hammond, and M. G. Lillie. 1971. A new bovine herpesvirus and its effect on experimentally infected calves. Arch. Gesamte Virusforsch. 34:394-395. [DOI] [PubMed] [Google Scholar]
- 13.Osorio, F. A., D. E. Reed, M. J. Van Der Maaten, and C. A. Metz. 1985. Comparison of the herpesviruses of cattle by restriction endonuclease analysis and serologic analysis. Am. J. Vet. Res. 46:2104-2109. [PubMed] [Google Scholar]
- 14.Osorio, F. A., D. L. Rock, and D. E. Reed. 1985. Studies on the pathogenesis of a bovine cytomegalo-like virus in an experimental host. J. Gen. Virol. 66:1941-1951. [DOI] [PubMed] [Google Scholar]
- 15.Reed, D. E., T. J. Langpap, and M. E. Bergeland. 1979. Bovine abortion associated with mixed Movar 33/63 type herpesvirus and bovine viral diarrhoea virus infection. Cornell Vet. 69:54-66. [PubMed] [Google Scholar]
- 16.Sato, T. A., J. A. Keelan, D. K. Gupta, M. D. Mitchell. 2001. Gestational age-dependent effects of lipopolysaccharide on prostaglandin production by murine deciduas caps. Prostaglandins Leukot. Essent. Fatty Acids 64:135-138. [DOI] [PubMed] [Google Scholar]
- 17.Silver, R. M., S. S. Edwin, M. S. Trautman, D. L. Simmons, D. W. Branch, D. J. Dudley, and M. D. Mitchell. 1995. Bacterial lipopolysaccharide-mediated fetal death. Production of a newly recognized form of inducible cyclooxygenase (COX-2) in murine decidua in response to lipopolysaccharide. J. Clin. Investig. 95:725-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stabel, J. R., and Stabel, T. J. 1995. Immortalization and characterization of bovine peritoneal macrophages transfected with SV40 plasmid DNA. Vet. Immunol. Immunopathol. 45:211-220. [DOI] [PubMed] [Google Scholar]
- 19.Storz, J., B. Ehlers, V. J. Todd, and H. Ludwig. 1984. Bovine cytomegaloviruses: identification and differential properties. J. Gen. Virol. 65:697-706. [DOI] [PubMed] [Google Scholar]
- 20.Symensma, T. L., D. Martinez-Guzman, Q. Jia, E. Bortz, T. T. Wu, N. Rudra-Ganguly, S. Cole, H. Herschman, and R. Sun. 2003. COX-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression. J. Virol. 77:12753-12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiry, E., M. Bublot, J. Dubuisson, and P. P. Pastoret. 1989. Bovine herpesvirus-4 (BHV-4) infection in cattle, p. 96-115. In G. Wittmann (ed.), Herpesvirus diseases of cattle, horses and pigs. Kluwer, Boston, Mass.
- 22.Zhu, H., J. P. Cong, D. Yu, W. A. Bresnahan, and T. E. Shenk. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 99:3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]