Abstract
A yellow-pigmented rod- to coccoid-shaped coryneform microorganism was isolated from the blood of a patient with acute myeloid leukemia. It was identified by 16S rRNA gene sequencing as a previously undescribed species of Janibacter. The isolate was susceptible to penicillins, aminoglycosides, fluoroquinolones, and glycopeptides.
CASE REPORT
In October 2001, an acute myeloid leukemia was diagnosed in a 51-year-old man. Karyotype analysis confirmed the presence of the Philadelphia chromosome, resulting from a reciprocal translocation between chromosomes 9 and 22 that leads to the production of an abnormal fusion protein (b2a2) with tyrosine kinase activity. The patient was treated with oral hydroxyurea (50 mg/kg of body weight/day for 3 days) followed by a combination of intravenous cytarabine (200 mg/m2/day for 7 days) and intravenous idarubicin (8 mg/m2/day for 5 days). When complete remission was achieved, as shown by the normalization of blood cell counts and karyotype, the patient was treated with imatinib (400 mg/day per os), a specific inhibitor of the abnormal tyrosine kinase protein. On 15 January 2002, the patient was admitted at the University Hospital of Hôtel-Dieu (Paris, France) to undergo an allogeneic hematopoietic stem cell transplantation. On day 0 (D0; hereafter, all days are designated with reference to D0, with days before D0 being assigned negative values [e.g., D−9 is 9 days before D0] and days after D0 being assigned positive values [e.g., D+3 is 3 days after D0), 8 February 2002, bone marrow cells were collected from his full-matched HLA-identical brother and infused. Before the transplantation, a myeloablative conditioning regimen consisting of oral busulfan (1 mg/kg/6 h from D−9 to D−6) in combination with cyclophosphamide (60 mg/kg/day from D−4 to D−3) through a central venous catheter was administrated. The catheter had been introduced in January and kept in place for the following weeks. Graft-versus-host disease (GVHD) prophylaxis consisted of intravenous first and then oral cyclosporine (4 mg/kg/day from D−1 to D+180) in combination with intravenous methotrexate (15 mg/m2 at D+1 and 10 mg/m2 at D+3 and D+6). Prevention of veno-occlusive disease was obtained with intravenous heparin (100 IU/kg/day). To limit the risk of bacterial infection, the patient underwent a gut decontamination with oral antibiotics (colistin and gentamicin) and a daily skin disinfection. The patient was hospitalized in a laminar airflow room until recovery from severe aplasia. During the aplastic period (D0 to D+20), the patient developed grade 4 mucositis that needed morphine analgesia. On D+7, the patient presented with fever (38.5°C) that led to an antibiotherapy consisting of piperacillin-tazobactam (200 mg/kg/day) and tobramycin (4 mg/kg/day) for 14 days. The fever subsided in 24 h. The hematopoietic recovery started at D+18, as shown by the increase of white blood cell count (103 cells per μl), and was obtained at D+20 (0.5 × 103 neutrophils per μl and 50 × 103 platelets per μl). Thus, on D+20, the patient left the sterile room. Neither acute nor chronic GVHD were seen. A complete chimerism was obtained, as shown by the total replacement of the patient's bone marrow cells by his brother's cells. Cultures from blood samples taken both from the catheter and from a peripheral vein were performed. Interestingly, a strain of a gram-positive bacillus was isolated from five blood samples, including one taken from a peripheral vein at D−1 and four from the catheter at D+3, D+6, D+10, and D+13. Bacterial cultures of the catheter, removed at D+27, remained negative. At present (December 2004), the patient is in complete remission, and his Karnofsky score performance status is 100%.
Blood samples were inoculated in aerobic and anaerobic blood culture vials (BACTEC PLUS AEROBIC/F and PLUS ANAEROBIC/F; BD Diagnostic Systems, Sparks, Md.). The gram-positive bacillus was isolated from aerobic vials that were subcultured on nutrient agar. After 24 h of incubation at 37°C, colonies were cream-colored or yellowish, circular, opaque, glistening, and convex with entire margins. Staining of the bacteria at the early stage of growth showed gram-positive bacilli that occur singly, in pairs, or in irregular clumps. In older cultures, the shape of cells changed from short rods to coccoid cells. The microorganism (isolate 03-238) was nonmotile, non-spore-forming, strictly aerobic, catalase positive, and oxidase negative. Gelatin was hydrolyzed. No acid was produced from carbohydrates. Nitrate was not reduced to nitrite. Urease was not produced. Indole was not produced from tryptophan. In an attempt to identify this isolate, API Coryne strip (bioMérieux, Marcy l'Etoile, France) was used as recommended by the manufacturer. The repeated bacterial identification obtained with API Coryne strip was Arthrobacter sp. (code no. 2112004; percentage of identification of 37.5%; index of typicity of 0.95). Positive results were for pyrazinamidase, alkaline phosphatase, α-glucosidase, and gelatin hydrolysis. These results prompted us to determine the 16S rRNA gene sequence of the isolate as previously described (3). Briefly, the 16S rRNA gene was amplified by PCR with the primers Al (5′-AGRGTTYGATYCTGGCTCAGGAYG-3′) and rJ (5′-GGTTACCTTGTTACGACTT-3′) (5). A total of 1,405 continuous nucleotides were determined. The complete 16S rRNA gene sequence of the isolate was compared to all bacterial sequences available from the GenBank database by using the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/BLAST.cgi) and showed 98% similarity to each of the sequences of the type strains of Janibacter brevis (GenBank accession no. AJ310085), Janibacter terrae (GenBank accession no. AF176948), and Janibacter limosus (GenBank accession no. Y08539). It also showed 97% similarity to the sequence of the type strain of Janibacter melonis (GenBank accession no. AY522568). These five 16S rRNA gene sequences were aligned with ClustalV, and a phylogenetic tree was constructed by using MegAlign, a program of the Lasergene package (DNASTAR, Madison, WI). Moreover, 13 type species of related genera were included in this phylogenetic study. Bootstrap analysis (1,000 resamplings) was performed with the use of PAUP software (9) (Fig. 1). Antimicrobial susceptibility of the isolate was determined by the agar diffusion method using the Epsilometer test (E test; AB Biodisk, Solna, Sweden) on Mueller-Hinton agar as recommended by the manufacturer and according to the guidelines of the Antibiogram Committee of the French Society for Microbiology (http://www.sfm.asso.fr) (8). MIC results were as follows: penicillin G, 0.5 μg/ml; amoxicillin, 0.38 μg/ml; piperacillin, 0.38 μg/ml; cefotaxime, 4 μg/ml; cefepime, 8 μg/ml; gentamicin, 0.75 μg/ml; tobramycin, 0.5 μg/ml; ciprofloxacin, 0.25 μg/ml; vancomycin, 0.38 μg/ml; and teicoplanin, 0.25 μg/ml.
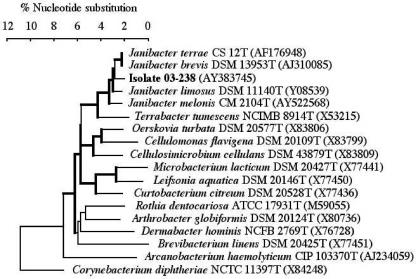
FIG. 1.
Phylogenetic tree of isolate 03-238 of Janibacter sp. and the type strains of related coryneform species based on comparative analysis of 16S rRNA gene sequences. For genera other than Janibacter, only type species were included. Sequence alignment and tree construction were with ClustalV and by use of MegAlign, a program of the Lasergene package (DNASTAR). Clusters with bootstrap values above 80% are indicated with thick lines. Other values were below 52%.
The genus Janibacter has been recently proposed with the description of J. limosus, isolated from sludge (7). Janus, a god in roman mythology, is said to have had two faces, and thus the name Janibacter refers to the changing morphology of the microorganisms during growth. It should be noted that other related genera of gram-positive rods, such as Arthrobacter and Brevibacterium, show also a rod-coccus cycle (1). The genus Janibacter is included in the family Intrasporangiaceae, order Actinomycetales, which belongs to the lineage of the gram-positive bacteria with high guanine-plus-cytosine (G+C) content. This group of microorganisms encompasses aerobically growing, asporogenous, irregularly shaped, non-partially acid-fast, gram-positive rods generally called coryneforms. The term “coryneform” is actually somewhat misleading, since only true Corynebacterium spp. exhibit a typical club-shaped morphology, whereas other bacteria such as Janibacter spp. show an irregular morphology. Besides J. limosus, three species of Janibacter have been described. J. terrae and J. brevis have been isolated from environmentally polluted samples (4, 10). Actually, J. terrae and J. brevis belong to the same species and J. brevis is a later heterotypic synonym of J. terrae (6). More recently, J. melonis has been isolated from abnormally spoiled oriental melon (11). Thus, all of the previously described species of Janibacter have been isolated from the environment. To our knowledge, strain 03-238 is the first to have been isolated from human blood. Preliminary phenotypic tests indicated that this strain, responsible for repeated bacteremia, may belong to the group of coryneform bacteria. In clinical laboratories, the identification of this group of bacteria is usually carried out by using identification systems such as the API Coryne strip (bioMérieux) (1, 2). However, in the present case, the identification obtained with API Coryne strip was not satisfactory. Thus, the 16S rRNA gene sequence of the isolate was determined. The comparison of the sequence obtained to all bacterial sequences available from the GenBank database showed that isolate 03-238 belongs to the genus Janibacter but is distinct from all of the species of Janibacter previously described (Fig. 1). Phenotypic tests, such as H2S production, nitrate reduction, esculin hydrolysis, and gelatin hydrolysis, may help to differentiate isolate 03-238 from the previously described Janibacter species (Table 1).
TABLE 1.
Phenotypic tests for differentiating between isolate 03-238 of Janibacter sp. and related species of Janibactera
| Test | Resultb for:
|
|||
|---|---|---|---|---|
| Isolate 03-238 | J. limosus | J. melonis | J. terraec | |
| Color of colonies | Cream or yellowish | White or yellow | Cream | White, cream, or yellowish |
| Morphology | Rods or cocci | Rods or cocci | Cocci | Rods or cocci |
| H2S production | − | + | − | V |
| Nitrate reduction | − | + | + | + |
| Esculin hydrolysis | − | − | + | − |
| Gelatin hydrolysis | + | + | − | + |
This case report shows that Janibacter species are not only present in the environment but may also be isolated from clinical specimens. The leukemia and the anticancerous chemotherapy administration were probably major risk factors for the dissemination of isolate 03-238 in blood. Thus, this species of Janibacter may be considered an opportunistic pathogen responsible for bacteremia in immunocompromised hosts. We assume that the intravascular catheterization triggered the penetration of the bacteria through the patient's skin and its spread to the bloodstream. As for other coryneform bacteria, Janibacter species may be present on human skin. However, the natural habitat of this human strain of Janibacter is at present unknown and remains to be determined. Antimicrobial susceptibility of previous isolates of Janibacter has not been studied according to current guidelines used in clinical laboratories. MIC results obtained for isolate 03-238 indicate that penicillins may be more active in vitro than cephalosporins. Such a result has been reported previously for Arthrobacter spp. (1). Isolate 03-238 was also susceptible to the tested aminosides, fluoroquinolones, and glycopeptides. Thus, the antibiotherapy including piperacillin and tobramycin was an adequate treatment and may have contributed to the control of the infection. In conclusion, the human source, the phenotypic tests, and the 16S rRNA gene sequence of isolate 03-238 suggest that this isolate may belong to a novel species of Janibacter. We refrained from naming this probable novel species, since a single strain is presently available. Strain 03-238 has been deposited in two collections (DSM 15959 and CIP 108123). Moreover, this case highlights the value of PCR amplification, sequencing, and comparison to 16S rRNA gene sequence databases for the identification of uncommon or novel pathogens. Clinical microbiologists must be aware of the opportunistic pathogenicity of this newly described species, which deserves further studies to confirm that it constitutes a novel species of Janibacter.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of isolate 03-238 of Janibacter sp. has been deposited in the GenBank database under accession number AY383745.
REFERENCES
- 1.Funke, G., A. von Graevenitz, J. E. Clarridge III, and K. A. Bernard. 1997. Clinical microbiology of coryneform bacteria. Clin. Microbiol. Rev. 10:125-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funke, G., and K. A. Bernard. 2003. Coryneform gram-positive rods, p. 472-501. In P. R. Murray, E. J. Baron, M. A. Pfaller, J. H. Jorgensen, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 3.Godreuil, S., M.-N. Didelot, C. Perez, A. Le Flèche, P. Boiron, J. Reynes, F. Laurent, H. Jean-Pierre, and H. Marchandin. 2003. Nocardia veterana isolated from ascitic fluid of a patient with human immunodeficiency virus infection. J. Clin. Microbiol. 41:2768-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imamura, Y., M. Ikeda, S.-I. Yoshida, and H. Kuraishi. 2000. Janibacter brevis sp. nov., a new trichloroethylene-degrading bacterium isolated from polluted environments. Int. J. Syst. Evol. Microbiol. 50:1899-1904. [DOI] [PubMed] [Google Scholar]
- 5.Janvier, M., and P. A. D. Grimont. 1995. The genus Methylophaga, a new line of descent within phylogenetic branch γ of Proteobacteria. Res. Microbiol. 146:543-550. [DOI] [PubMed] [Google Scholar]
- 6.Lang, E., R. M. Kroppenstedt, J. Swiderski, P. Schumann, W. Ludwig, A. Schmid, and N. Weiss. 2003. Emended description of Janibacter terrae, including ten dibenzofuran-degrading strains and Janibacter brevis as its later heterotypic synonym. Int. J. Syst. Evol. Microbiol. 53:1999-2005. [DOI] [PubMed] [Google Scholar]
- 7.Martin, K., P. Schumann, F. A. Rainey, B. Schuetze, and I. Groth. 1997. Janibacter limosus gen. nov., sp. nov., a new actinomycete with meso-diaminopimelic acid in the cell wall. Int. J. Syst. Bacteriol. 47:529-534. [DOI] [PubMed] [Google Scholar]
- 8.Members of the SFM Antibiogram Committee. 2003. Comite de l'antibiogramme de la Societe Francaise de Microbiologie report 2003. Int. J. Antimicrob. Agents 21:364-391. [DOI] [PubMed] [Google Scholar]
- 9.Swofford, D. L. 1998. PAUP. Phylogenetic analysis using parsimony (and other methods). Version 4. Sinauer Associates, Sunderland, Mass.
- 10.Yoon, J.-H., K.-C. Lee, S.-S. Kang, Y. H. Kho, K. H. Kang, and Y.-H. Park. 2000. Janibacter terrae sp. nov., a bacterium isolated from soil around a wastewater treatment plant. Int. J. Syst. Evol. Microbiol. 50:1821-1827. [DOI] [PubMed] [Google Scholar]
- 11.Yoon, J.-H., H. B. Lee, S.-H. Yeo, and J.-E. Choi. 2004. Janibacter melonis sp. nov., isolated from abnormally spoiled oriental melon in Korea. Int. J. Syst. Evol. Microbiol. 54:1975-1980. [DOI] [PubMed] [Google Scholar]