Abstract
Between 1 February and 15 April 2002, 95 patients were admitted to Gaston Bourret Territorial Hospital (New Caledonia, France) for drainage of community-acquired soft tissue abscesses. Staphylococcus aureus was detected in 68 cases (72%). Two-thirds of the patients with S. aureus infection had furuncles, which were located on the limbs in 82% of cases. The median interval between symptom onset and hospital admission was 5.7 days. Three-quarters of the patients were Melanesians living in tribes. Fifty-four S. aureus isolates were screened for toxin genes. Panton-Valentine leucocidin (PVL) genes were detected in 48 isolates (89%), the exfoliative toxin A gene was detected in 1 isolate, and no toxin genes were detected in 4 isolates. S. aureus nasal carriage was detected in 39.7% of patients with S. aureus infections. Two infecting S. aureus strains and two nasal carriage strains were resistant to methicillin. Comparative pulsed-field gel electrophoresis, performed in 16 cases, showed that five of six patients with PVL-positive nasal carriage strains were infected by the same strains. In contrast, 8 of 10 patients with PVL-negative nasal carriage strains were infected by PVL-positive strains. PVL genes thus appear to be a major virulence factor in both primary and secondary S. aureus skin infections.
Skin and soft tissue infections caused by Staphylococcus aureus are frequent worldwide. These infections are considered “primary” in patients with no preexisting skin lesion and “secondary” in other cases (25). Folliculitis, furuncles, and impetigo are examples of primary S. aureus infections. Furuncles are associated with S. aureus strains that produce the Panton-Valentine leucocidin (PVL) (10, 18), while bullous impetigo is associated with strains that produce exfoliative toxins. Secondary skin infections can occur in patients with skin trauma, surgical incisions, skin ulcers, or eczema. These secondary infections are not generally associated with specific toxins, but the proinflammatory effects of superantigenic toxins (e.g., toxic shock syndrome toxin and staphylococcal enterotoxins) may contribute to the clinical severity of the secondary infection, especially in patients with eczema (21).
Less than 2% of the consecutive strains isolated in Strasbourg General Hospital Microbiology Laboratory, Strasbourg, France, were PVL positive (28), but that study included isolates from patients with community- and hospital-acquired infections, primary and secondary skin and soft tissue infections, and systemic infections.
Soft tissue infections are responsible for about 500 stays per annum in Gaston Bourret Territorial Hospital, New Caledonia, France. Most are abscesses that require surgical incision and drainage. This high prevalence allowed us to conduct a large prospective clinical and microbiological study of PVL in primary and secondary soft tissue abscesses caused by S. aureus.
MATERIALS AND METHODS
Study design.
Between 1 February and 15 April 2002 we prospectively collected standard data on patients admitted to Gaston Bourret Territorial Hospital (New Caledonia, France) for drainage of soft tissue abscesses, defined with the MeSH terms “soft tissue infection” and “abscess” (see the National Center for Biotechnology Information MeSH database at http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=mesh). Between 1991 and 2002, the annual number of admissions at this hospital fluctuated from 14,255 to 16,669, including 483 to 537 admissions for surgical drainage of soft tissue infection per year.
Patients with abscesses that occurred after surgery, intravenous or intramuscular injections, or indwelling catheter placement were excluded, together with patients who lived in institutions or who were hospitalized during the month preceding the current infection. Infections were considered primary when the patient noted no skin damage prior to the appearance of the abscess and secondary in other cases.
The following data were recorded in each case: demographic characteristics, underlying conditions (diabetes mellitus, alcoholism, progressive neoplasia, steroid therapy, intravenous drug addiction, or a personal or familial history of furunculosis or abscess), hospitalization in the previous 3 months, antibiotic treatment before admission, recent skin damage (trauma, superficial wound, or puncture), clinical presentation, length of hospital stay, and number of surgical procedures. Abscess fluid was collected by swabbing drained material in the operating room. Blood cultures were performed in some cases. The anterior nares were swabbed to detect S. aureus carriage.
S. aureus identification, gene detection, and genotyping.
S. aureus was identified on the basis of colony and microscopic morphology, coagulase testing with rabbit plasma (bioMérieux, Marcy l'Etoile, France), and the Staphyslide agglutination test (bioMérieux). Antimicrobial susceptibility was tested with the API ATB Staph system (bioMérieux). Strains were sent to the French National Reference Centre for Staphylococci and were grown on brain heart infusion agar or in brain heart infusion broth at 37°C overnight. Genomic DNA was used as the PCR target after extraction by a standard procedure (1); the DNA concentration was estimated spectrophotometrically (19). Sequences specific for exfoliative toxin genes (eta, etb), PVL genes (lukS-PV--lukF-PV), and accessory gene regulator alleles (agr-1 to agr-4) were detected by PCR, as described previously (13). The mecA gene, which codes for methicillin resistance, was detected by PCR, as described by Murakami et al. (22). Amplification of gyrA was used to confirm the quality of each DNA extract and the absence of PCR inhibitors (8). All PCR products were analyzed by electrophoresis through 1% agarose gels (Sigma, Saint Quentin Fallavier, France). Isolates were genotyped by pulsed-field gel electrophoresis (PFGE) by using the restriction enzyme SmaI, as described previously (11). The PFGE patterns were digitized and analyzed by using the Taxotron typing system (Institut Pasteur, France). Strain relatedness was interpreted by using published guidelines (30). Isolates that differed by no more than three fragments were considered subtypes of a given clonal type.
Statistical analysis.
Categorical data were compared by the chi-square test or Fisher's exact test, while the equality of means was compared by the t test with SPSS software version 10.0 (SPSS Inc., Chicago, Ill.). P values of <0.05 were considered statistically significant.
RESULTS
Patient characteristics.
Ninety-five patients were enrolled in the study. The initial symptom was usually pruritus in patients with primary infections (P = 0.021; odds ratio [OR], 4.4 [95% confidence interval {CI}, 1.3 to 14.1]) and pain in patients with secondary infection (P = 0.051; OR, 2.64 [95% CI, 0.98 to 7.15]). Furuncles were frequently associated with furunculosis in friends or family members (P = 0.006; OR, 9.8 [95% CI, 1.8 to 52.7]). S. aureus was isolated from abscess fluid in 68 cases (72%). The other 27 cases consisted of Streptococcus pyogenes abscesses (n = 7), Nocardia asteroides tendon sheath infection (n = 1), and abscesses with no positive bacterial culture (n = 19). The clinical and epidemiological characteristics differed significantly between patients with and without S. aureus infection (Table 1): the S. aureus group had a higher frequency of S. aureus nasal carriage (P = 0.049), a possible shorter interval between symptom onset and hospitalization (5.7 days versus 10 days; P = 0.117), and a higher frequency of primary infection (furuncles), while the non-S. aureus group had a higher frequency of secondary infection (P = 0.001).
TABLE 1.
Epidemiological and clinical characteristics of patients with soft tissue abscesses in New Caledonia, France
| Characteristic | Patients witha:
|
P value | |
|---|---|---|---|
| S. aureus abscess (n = 68) | Non-S.aureus abscess (n = 27) | ||
| Demographics | |||
| Men | 43 (63.2) | 17 (63) | 0.58 |
| Mean age (yr) | 29.5 | 29.4 | |
| Melanesian | 51 (75) | 18 (67.7) | 0.28 |
| Tribal living | 28 (55) | 9 (50) | 0.45 |
| Potential predisposing factors | |||
| Diabetes mellitus | 5 (7.4) | 4 (14.8) | 0.23 |
| S. aureus nasal carriage | 27 (39.7) | 5 (18.5) | 0.39 |
| Personal history of furunculosis | 8 (11.8) | 3 (11.1) | 0.62 |
| Familial history of furunculosis | 6 (8.8) | 2 (7.4) | 0.59 |
| Personal history of abscess | 13 (19.1) | 5 (18.5) | 0.60 |
| Familial history of abscess | 6 (8.8) | 3 (11.1) | 0.50 |
| Disease presentation | |||
| Fever (temp, >38°C) on admission | 19 (27.9) | 6 (22.2) | 0.38 |
| Mean days (SD) between symptom onset and hospitalization | 5.7 (3.4) | 10 (15.6) | 0.177 |
| “Primary” infections | 46 (67.6) | 8 (29.6) | 0.001 |
| Onset as a furuncle | 25 (36.8) | 2 (7.4) | 0.003 |
| None | 21 (30.9) | 6 (22.2) | 0.28 |
| “Secondary” infections | 22 (32.4) | 19 (70.4) | 0.001 |
| Superficial wound injury | 12 (17.7) | 12 (44.4) | 0.008 |
| Trauma | 1 (1.5) | 3 (1.1) | 0.068 |
| Puncture | 9 (13.2) | 2 (7.4) | 0.34 |
| Other | 0 | 2 (7.4) | 0.08 |
The data are presented as the number (percent) of patients unless indicated otherwise.
Clinical characteristics of patients with S. aureus skin abscesses.
The 68 patients with S. aureus infections comprised 43 males and 25 females, with a mean age of 29.5 years (range, 1 to 80 years) (Table 1). The patients were mainly Melanesians (51 cases; 75%) living in tribes (28 cases). Thirty-three patients (48.5%) had either a personal (21 cases) or a familial (12 cases) history of skin infections (furunculosis or abscess). Twenty-two patients noted a skin lesion before onset. Nineteen patients (27.9%) were febrile (temperature, >38°C) on hospital admission. The first sign of infection (known in 66 cases) was pain (35 cases; 52%), swelling (19 cases; 28%), or pruritus (12 cases; 18%). The mean interval between symptom onset and hospital admission was 5.7 days (median, 5 days; range, 1 to 15 days). Most infections were located on the limbs (56 cases [82%]; an upper limb in 32 cases and a lower limb in 24 cases). Three patients had multiple abscesses. S. aureus bacteremia was diagnosed in three cases; one case involved a young patient with myositis of the thigh and the right arm who required intensive care. All the infections healed after surgical drainage. More than one drainage procedure was necessary in five cases.
Bacteriology of S. aureus abscesses.
Among the 68 abscesses from which S. aureus was isolated, S. aureus was the only potential pathogen in 56 cases, while it was associated with Streptococcus pyogenes in 7 cases and with a gram-negative bacillus in 4 cases (Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Enterobacter cloacae) and with Streptococcus agalactiae in 1 case. Only two S. aureus isolates (2.1%) were resistant to methicillin. Fifty-four of the 68 S. aureus isolates (38 from primary infections and 16 from secondary infections) were forwarded to the French Reference Centre for Staphylococci (the remaining 14 had not been stored frozen in New Caledonia). Comparative studies of the toxin gene profiles (PVL and exfoliative toxin genes) (Table 2) showed no significant differences between isolates from primary and secondary infections, and the 54 strains are therefore considered below as a single group. The PVL genes were detected in 48 strains (89%), none of which was methicillin resistant. The PVL-positive isolates had a diverse genetic background: 9 strains (19%) were agr group 1, 13 strains (27%) were agr group 3, and 26 strains (54%) were agr group 4. The exfoliative toxin A (eta) gene was detected in only 1 of the 54 strains, which was from a patient with an abscess of the toe and no signs of exfoliation; this isolate was susceptible to methicillin. The remaining five isolates did not harbor either the PVL genes or exfoliative toxin genes, but two were resistant to methicillin.
TABLE 2.
PCR detection of PVL and exfoliative toxin genes in S. aureus isolates from 54 patients with skin abscesses
| Toxin gene | No. (%) of patients with:
|
P value | |
|---|---|---|---|
| Primary abscesses (n = 38) | Secondary abscesses (n = 16) | ||
| Panton-Valentine leucocidin | 33 (86.8) | 15 (93.8) | 0.657 |
| Exfoliative toxin A | 0 | 1 (6.3) | |
| Exfoliative toxin B | 0 | 0 | |
| None detected | 5 (13.2) | 0 | |
S. aureus nasal carriage.
S. aureus nasal carriage was found in 32 of the patients (33.7%), including 27 patients with S. aureus abscesses (39.7%). When the carriage population as a whole is considered, S. aureus carriage was significantly less frequent in patients recently exposed to antibiotics (44.5% versus 55.5%; P = 0.017). Two of the S. aureus nasal isolates were resistant to methicillin; the two corresponding infections were not due to the same strain as the nasal isolate and occurred in patients with no classical risk factors for methicillin-resistant S. aureus infection. PVL genes were less frequently detected in nasal carriage strains (6 of 21 cases tested; 28.6%) than in abscess isolates (48 of 54 cases; 89%) (P < 0.0001).
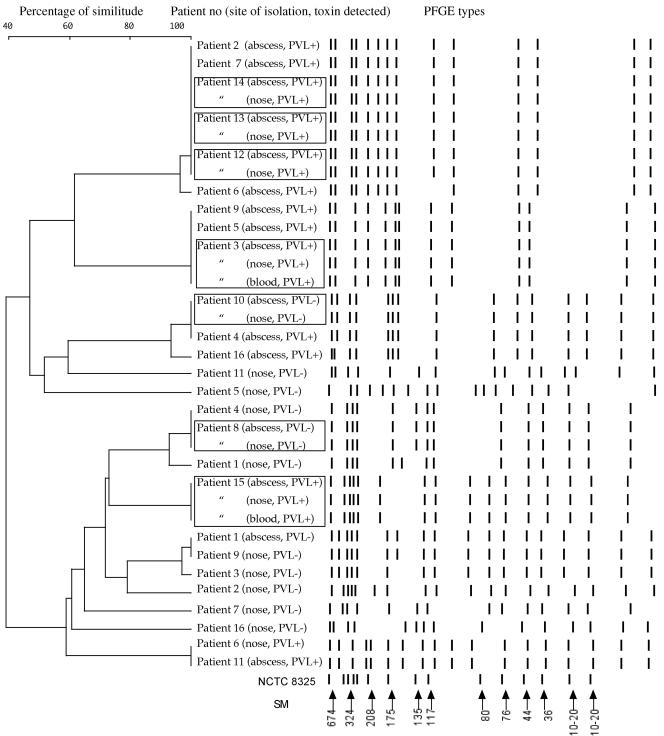
PFGE comparison of infecting and nasal carriage strains was possible in 16 cases (Fig. 1). The PFGE patterns of the infecting and the nasal carriage strains were identical in seven cases (43.7%), five of which were PVL positive. In the nine remaining cases, the PFGE patterns of the infecting and the nasal carriage strains were different: in seven cases (patients 2, 4, 5, 7, 9, 11, and 16) the nasal isolates were PVL negative and the infecting isolates were PVL positive; in one case (patient 6) the nasal and the infecting isolates were two different PVL-positive isolates; the last case (patient 1) had different PVL-negative strains from both sites. Hence, patients with nasal carriage of PVL-positive strains were frequently infected by the same strain, whereas patients carrying PVL-negative strains tended to be infected by PVL-positive strains (P = 0.035).
FIG. 1.
Unweighted pair group method with averages dendrogram of PFGE results based on the Dice distance matrix and schematic representation of the PFGE types (SmaI restriction enzyme) of PVL-positive and PVL-negative S. aureus isolates. NCTC 8325 is the reference strain for size markers (SM), expressed in kilobases. Square boxes indicate patients with identical isolates in the nose and abscess or blood. Two different isolates (PVL positive and PVL negative) were detected in the nose of patient 3.
DISCUSSION
This study involved 95 patients with community-acquired soft tissue abscesses requiring surgical drainage. Only surgically treated patients were studied, in order to avoid the risk of contamination associated with superficial sampling. S. aureus was detected in 72% of cases. Most patients had rapidly progressive primary infections (furuncles), with a median interval of only 5.7 days between symptom onset and hospital admission. PVL genes were detected in 89% of S. aureus isolates. The discrepancy with reports describing PVL as a very infrequent toxin (<2%) in S. aureus (28) is probably due to differences in patient selection (random patients versus patients with community-acquired soft tissue abscesses requiring surgical drainage) and in geographic area.
The link between PVL and severe abscesses was first described more than 70 years ago. PVL toxin was discovered by Van de Velde in 1894 (33) but was only clearly distinguished from hemolysins by its leukotoxic activity in 1932 in studies by Panton and Valentine (27), who noted an association between leucocidin (PVL) production and styes, carbuncles, and “pyaemic” infections. In 1936, Valentine reported that “S. aureus [isolates] which have succeeded in invading human tissue were found capable of producing in vitro considerable amounts of leucocidin” (31). Interest in staphylococcal leucocidin subsided for several decades, until Cribier et al. (7) demonstrated in 1992 that PVL was mainly associated with primary cutaneous infections and especially furuncles and abscesses. Cribier et al. (7) detected PVL by immunoassay in 43 S. aureus isolates from patients with skin infections. In 1999, Lina et al. (18) used PCR to screen 172 S. aureus isolates for PVL genes and confirmed the strong association with furunculosis and with necrotizing pneumonia in children and young adults. Clinical investigation made by Yamasaki et al. (36) revealed that PVL-positive S. aureus strains are involved in the development of multiple furuncles with more intense erythema, particularly in healthy young adults. The only report on the association of PVL with cutaneous abscesses included only six isolates (of which three were PVL positive) and offered no clinical information (18).
We found no difference in the prevalence of PVL genes between S. aureus isolates from primary and secondary skin infections. PVL has a dermonecrotic effect on both intact and damaged skin (35), damaging host cell membranes by the synergistic action of two classes of secretory proteins designated S and F. PVL-producing isolates produce the PVL-specific class S and F proteins (LUKS-PV and LUKF-PV). Injection of both components intradermally into rabbits induces severe inflammatory lesions, capillary dilation, chemotaxis, skin infiltration, polymorphonuclear leukocyte PMN karyorrhexis, and skin necrosis (6, 35). PVL is also leukotoxic, by pore induction, for rabbit and human polymorphonuclear leukocytes and macrophages. The large number of secondary skin infections in our series may be due to frequent minor skin trauma among individuals in tribes.
The PVL genes are mainly associated with pandemic clones of true community-acquired methicillin-resistant S. aureus. This combination of PVL genes and methicillin resistance has not yet been detected in New Caledonia, even though multiple highly epidemic clones have been described on all the continents (9, 10, 32).
The overall nasal carriage rate of S. aureus found here (33.7%) is similar to that observed in the general population of industrialized countries (15). We found that patients with severe S. aureus skin infections were more likely than patients with non-S. aureus infections to be nasal carriers (39% and 18%, respectively), in keeping with findings presented in previous reports (12, 17, 23). Nasal carriage is considered a risk factor for S. aureus infection in patients undergoing surgery (34) and hemodialysis (20). In our study, in the 16 cases in which genotyping of paired nasal carriage and infecting strains was possible, genetic identity was found in seven cases, of which five were PVL positive, suggesting that subjects with nasal carriage of PVL-positive S. aureus isolates might be at risk of cutaneous infection.
Genotyping of the strains also revealed that several patients were infected with the same strain. We suspect that the transmission of strains between these patients in the closed community formed by tribes, as has been shown for other closed communities, such as an Alaskan village or sports teams (4, 16, 29). Moreover, PFGE typing has revealed a higher diversity among the PVL-negative strains than among the PVL-positive strains. We speculate that PVL-positive strains are more successful at spreading, as observed with PVL-positive community-acquired methicillin-resistant S. aureus (2, 3, 5, 14, 26, 37). Lastly, in one case, the PFGE pattern of PVL-negative isolates (patient 10) was identical to that of a PVL-positive isolate (patient 4), suggesting that acquisition or deletion of the PVL phage occurred, in accordance with the known mobility of such elements (24).
The colonizing and infecting isolates differed genetically in the other nine patients, in keeping with another study of community-acquired S. aureus pyoderma, in which the antibiotic susceptibility profiles of the infecting isolates differed from those of the nasal isolates in 51% of cases (23). In such cases, nasal carriage may simply reflect individual susceptibility to staphylococcal colonization and/or infection rather than a specific characteristic of certain strains.
Acknowledgments
We thank S. Gervolinet-Ballivet from the Département d'Information Médicale and all the surgeons and intensivists from the Gaston Bourret hospital for their help in collecting data, Angèle Gayet for statistical analysis, and David Young for editing the manuscript.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, B. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
- 2.Baggett, H. C., T. W. Hennessy, K. Rudolph, D. Bruden, A. Reasonover, A. Parkinson, R. Sparks, R. M. Donlan, P. Martinez, K. Mongkolrattanothai, and J. C. Butler. 2004. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska. J. Infect. Dis. 189:1565-1573. [DOI] [PubMed] [Google Scholar]
- 3.Baillargeon, J., M. F. Kelley, C. T. Leach, G. Baillargeon, and B. H. Pollock. 2004. Methicillin-resistant Staphylococcus aureus infection in the Texas prison system. Clin. Infect. Dis. 38:e92-e95. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett, P. C., R. J. Martin, and B. R. Cahill. 1982. Furunculosis in a high school football team. Am. J. Sports Med. 10:371-374. [DOI] [PubMed] [Google Scholar]
- 5.Begier, E. M., K. Frenette, N. L. Barrett, P. Mshar, S. Petit, D. J. Boxrud, K. Watkins-Colwell, S. Wheeler, E. A. Cebelinski, A. Glennen, D. Nguyen, and J. L. Hadler. 2004. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin. Infect. Dis. 39:1446-1453. [DOI] [PubMed] [Google Scholar]
- 6.Couppié, P., B. Cribier, G. Prévost, E. Grosshans, and Y. Piémont. 1994. Leukocidin from Staphylococcus aureus and cutaneous infections: an epidemiologic study. Arch. Dermatol. 130:1208-1209. [DOI] [PubMed] [Google Scholar]
- 7.Cribier, B., G. Prévost, P. Couppié, V. Finck-Barbançon, E. Gosshans, and Y. Piémont. 1992. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology 185:175-185. [DOI] [PubMed] [Google Scholar]
- 8.De Buyser, M. L., A. Morvan, F. Grimont, and N. el Solh. 1989. Characterization of Staphylococcus species by ribosomal RNA gene restriction patterns. J. Gen. Microbiol. 135:989-999. [DOI] [PubMed] [Google Scholar]
- 9.Diep, B. A., G. F. Sensabaugh, N. S. Somboona, H. A. Carleton, and F. Perdreau-Remington. 2004. Widespread skin and soft tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J. Clin. Microbiol. 42:2080-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufour, P., Y. Gillet, M. Bes, G. Lina, F. Vandenesch, D. Floret, J. Etienne, and H. Richet. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin. Infect. Dis. 35:819-824. [DOI] [PubMed] [Google Scholar]
- 11.Goering, R. V., and M. A. Winters. 1992. Rapid method for epidemiological evaluation of gram-positive cocci by field inversion gel electrophoresis. J. Clin. Microbiol. 30:577-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedstrom, S. A. 1981. Recurrent staphylococcal furunculosis. Bacteriological findings and epidemiology in 100 cases. Scand. J. Infect. Dis. 13:115-119. [DOI] [PubMed] [Google Scholar]
- 13.Jarraud, S., C. Mougel, J. Thioulouse, G. Lina, H. Meugnier, F. Forey, X. Nesme, J. Etienne, and F. Vandenesch. 2002. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazakova, S. V., J. C. Hageman, M. Matava, A. Srinivasan, L. Phelan, B. Garfinkel, T. Boo, S. McAllister, J. Anderson, B. Jensen, D. Dodson, D. Lonsway, L. K. McDougal, M. Arduino, V. J. Fraser, G. Killgore, F. C. Tenover, S. Cody, and D. B. Jernigan. 2005. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 352:468-475. [DOI] [PubMed] [Google Scholar]
- 15.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landen, M. G., B. J. McCumber, E. D. Asam, and G. M. Egeland. 2000. Outbreak of boils in an Alaskan village: a case-control study. West. J. Med. 172:235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leigh, D. A., and G. Joy. 1993. Treatment of familial staphylococcal infection—comparison of mupirocin nasal ointment and chlorhexidine/neomycin (Naseptin) cream in eradication of nasal carriage. J. Antimicrob. Chemother. 31:909-917. [DOI] [PubMed] [Google Scholar]
- 18.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 19.Lina, G., A. Quaglia, M. E. Reverdy, R. Leclercq, F. Vandenesch, and J. Etienne. 1999. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 43:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marr, K. A. 2000. Staphylococcus aureus bacteremia in patients undergoing hemodialysis. Semin. Dial. 13:23-29. [DOI] [PubMed] [Google Scholar]
- 21.Mempel, M., G. Lina, M. Hojka, C. Schnopp, H. P. Seidl, T. Schafer, J. Ring, F. Vandenesch, and D. Abeck. 2003. High prevalence of superantigens associated with the egc locus in Staphylococcus aureus isolates from patients with atopic eczema. Eur. J. Clin. Microbiol. Infect. Dis. 22:306-309. [DOI] [PubMed] [Google Scholar]
- 22.Murakami, K., W. Minamide, K. Wada, E. Nakamura, and H. Teraoka. 1991. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J. Clin. Microbiol. 29:2240-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaraju, U., G. Bhat, M. Kuruvila, G. S. Pai, R. P. Babu, Jayalakshmi, and P. B. Ravindra. 2004. Methicillin-resistant Staphylococcus aureus in community-acquired pyoderma. Int. J. Dermatol. 43:412-414. [DOI] [PubMed] [Google Scholar]
- 24.Narita, S., J. Kaneko, J. Chiba, Y. Piemont, S. Jarraud, J. Etienne, and Y. Kamio. 2001. Phage conversion of Panton-Valentine leukocidin in Staphylococcus aureus: molecular analysis of a PVL-converting phage, phiSLT. Gene 268:195-206. [DOI] [PubMed] [Google Scholar]
- 25.Noble, W. C. 1998. Skin bacteriology and the role of Staphylococcus aureus in infection. Br. J. Dermatol. 139(Suppl. 53):9-12. [DOI] [PubMed] [Google Scholar]
- 26.Pan, E. S., B. A. Diep, H. A. Carleton, E. D. Charlebois, G. F. Sensabaugh, B. L. Haller, and F. Perdreau-Remington. 2003. Increasing prevalence of methicillin-resistant Staphylococcus aureus infection in California jails. Clin. Infect. Dis. 37:1384-1388. [DOI] [PubMed] [Google Scholar]
- 27.Panton, P. N., and F. C. O. Valentine. 1932. Staphylococcal toxin. Lancet i:506-508. [Google Scholar]
- 28.Prévost, G., P. Couppié, P. Prévost, S. Gayet, S. Petiau, B. Cribier, H. Monteil, and Y. Piémont. 1995. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J. Med. Microbiol. 42:237-245. [DOI] [PubMed] [Google Scholar]
- 29.Sosin, D. M., R. A. Gunn, W. L. Ford, and J. W. Skaggs. 1989. An outbreak of furunculosis among high school athletes. Am. J. Sports Med. 17:828-832. [DOI] [PubMed] [Google Scholar]
- 30.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentine, F. C. O. 1936. Further observations on the role of the toxin in staphylococcal infection. Lancet i:526-531. [Google Scholar]
- 32.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van de Velde, H. 1894. Etude sur le mécanisme de la virulence du Staphylocoque pyogène. La Cellule 10:401-410. [Google Scholar]
- 34.von Eiff, C., K. Becker, K. Machka, H. Stammer, G. Peters, et al. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 35.Ward, P. D., and W. H. Turner. 1980. Identification of staphylococcal Panton-Valentine leukocidin as a potent dermonecrotic toxin. Infect. Immun. 28:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamasaki, O., J. Kaneko, S. Morizane, H. Akiyama, J. Arata, S. Narita, J. Chiba, Y. Kamio, and K. Iwatsuki. 2005. The association between Staphylococcus aureus strains carrying Panton-Valentine leukocidin genes and the development of deep-seated follicular infection. Clin. Infect. Dis. 40:381-385. [DOI] [PubMed] [Google Scholar]
- 37.Zinderman, C. E., B. Conner, M. A. Malakooti, J. E. LaMar, A. Armstrong, and B. K. Bohnker. 2004. Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg. Infect. Dis. 10:941-944. [DOI] [PMC free article] [PubMed] [Google Scholar]