Abstract
We report the first case of human cryptococcosis due to Cryptococcus neoformans var. gattii described in our country, which was presented as brain cryptococcoma in an immunocompetent patient. An extensive sampling of the patient's environment was carried out to find the source of infection.
CASE REPORT
A 60-year-old heterosexual Spanish farmer came to the Hospital General de Alicante in July 2003, having suffered for several days from cephalalgia and somnolence. He had never traveled abroad. A diabetes mellitus type 2 identified 2 years previously was the only clinical antecedent of interest. Human immunodeficiency virus serology was investigated with repeated negative results. General and neurological exploration included computerized tomography scanning, which disclosed a brain mass lesion in basal ganglions. Capsulated yeasts were seen in a stereotaxic brain puncture sample, and Cryptococcus neoformans was suspected to be the causative agent. C. neoformans capsular antigen was detected in blood and cerebrospinal fluid (CSF) several times during the process (maximum values detected, 1/256 and 1/32, respectively). The yeast was cultured from a surgical drainage sample of the brain abscess. Species identification was carried out on the basis of microscopic morphology, growth at 37°C, a urease test, phenoloxydase production, and the carbohydrate assimilation pattern (Auxacolor; Bio-Rad). Further testing such as canavanina glycine bromothimol blue agar growth, serotype determination (Cryptocheck test; Iatron), and genotype analysis revealed that the strain was C. neoformans var. gatti serotype B. The strain identification and serotype were confirmed in another mycology laboratory (IMIM, Barcelona, Spain). Two antifungal drug sensitivity tests were performed (Sensititre and Etest). Both tests showed low amphotericin B (AMB) and ketoconazole MICs but different results with fluconazole (MICsensititre, 8 μg/ml; MICEtest, 64 μg/ml) and 5-flucytosine (MICsensititre, 2 μg/ml; MICEtest, 32 μg/ml). Voriconazole was only tested with the Sensititre test (MICsensititre, 12 μg/ml). Genotype analysis consisted of the study of five molecular DNA targets: internal transcribed spacer-5.8S rRNA gene sequence analysis (36), 5S rRNA gene and URA5 gene restriction fragment length polymorphism (RFLP) analysis (24), and amplification patterns of two highly repeated minisatellite sequences (M13 and GACA4) (14, 24). Nucleotide sequence analysis of the internal transcribed spacer-5.8S rRNA gene confirmed the identification at a species level by comparing it to ribosomal sequence databases (EMBL and GenBank). RFLP of the URA5 gene showed a VGI molecular type (Fig. 1) which is characteristic of C. neoformans var. gattii. RFLP 5S rRNA gene and minisatellite amplification of M13 and GACA4 also displayed molecular patterns attributable to C. neoformans var. gattii strains (14, 24).
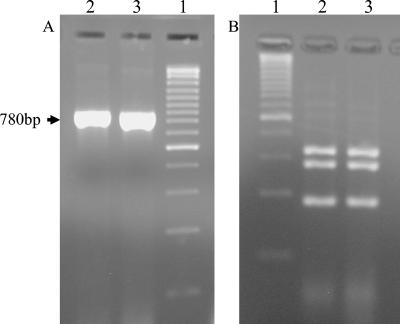
FIG. 1.
Agarose gel electrophoresis of DNA obtained by PCR amplification of the URA5 gene (A) and subsequent RFLP fingerprinting after DNA enzymatic digestion (B). Lanes 1, molecular ruler; lanes 2, DNA obtained from C. neoformans-cultured cells; lanes 3, DNA obtained from the clinical sample (cryptococcoma drainage).
Some different antifungal therapies were followed, depending on the clinical evolution and the antigen levels (serum and CSF). Intravenous AMB at 200 mg/day (Ambisome) was the first choice, but fluconazole (400 mg/12 h) and AMB at 400 mg/day combined with 5 flucytosine (2.5g/6 h) were also prescribed. Three months after diagnosis, neurological symptoms and serum and CSF positive antigen detection persisted. Therefore, intra-abscess amphotericin B deoxicolate was administrated by a surgical procedure, and a symptomatic improvement was rapidly detected. Although during the next 3 months serum antigen detection remained positive at low levels (1/16), 6 months after diagnosis, the serum level increased again to 1/64. Therefore, oral voriconazole was prescribed as a maintenance therapy. At present the patient is asymptomatic, and serum remained negative for C. neoformans antigen for more than 6 months.
An extensive sampling of the patient's area of work was carried out, including different soil samples, bird droppings, and vegetal tissues from all the tree species around; no eucalyptus was seen in the area. Some Cryptococcus and other yeast species were found, but, unfortunately, none of them corresponded to C. neoformans. Moreover, the patient's family reported the presence of a species of parrot in the house next door. Our interest in visiting the neighbors and taking samples of the parrot's feces was misunderstood, and they therefore got rid of the parrot, together with the possibility of studying a putative source of the infection (30, 32, 35).
Cryptococcosis is an infectious disease caused by the yeast C. neoformans. It presents different clinical manifestations and a wide range of severity, depending not only on the patient's risk factors but also on the yeast variety involved. This microorganism was traditionally described as a unique species, C. neoformans, that included two pathogenic varieties, C. neoformans var. neoformans and C. neoformans var. gattii. Important differences between the two varieties have recently raised C. neoformans var. gattii to species status as Cryptococcus gattii (20).
Cryptococcosis from C. neoformans var. neoformans infection is cosmopolite. Patients are commonly immunocompromised, mostly with a cellular immunity alteration, as in human immunodeficiency virus-infected individuals. C. neoformans var. gattii is believed to behave more aggressively than C. neoformans var. neoformans and to cause infections in immunocompetent patients more frequently (8). Until now, this yeast was considered to be restricted to warm areas (tropical and subtropical climates), but this statement is under discussion after the recent outbreak of cryptococcosis infection by C. gattii in the temperate climate of Vancouver Island (British Columbia, Canada) (18). Both pathogenic varieties also show different natural habitats. C. neoformans var. neoformans is widely associated with bird feces, with a strong presence in pigeon excreta (19). The association of C. neoformans var. gattii with eucalyptus trees has been demonstrated (13), and some strains have also been isolated from a wide range of different natural sources, including some tropical birds (2, 12, 16, 23, 26, 27). Cryptococcoma is not the usual clinical presentation of the disease, although a number of cases were described in countries all over the world, including Spain (28). They usually appear in the literature as case reports because of their low incidence. They are more common in immunocompetent hosts and usually have a better prognosis than disseminated cryptococcosis (17, 22).
In Spain, Cryptococcus and cryptococcosis have already been studied by some authors with various points of view (3, 25). The presence of the yeast in relation to birds has been demonstrated in different studies and in various locations (11, 15, 29). Some reports on human and animal cryptococcosis have also been reported since 1971 (1). Among these studies, the first report of the presence of C. neoformans var. gattii in our country is remarkable (4). In 1998, 13 strains of C. neoformans var. gattii were isolated from the tissues of different dead goats. All of them suffered from invasive disease. At that time, a national epidemiological study for human cryptococcosis had just started in Spain (10). During a 6-year period (1998 to 2003) all strains found in the human study (a total of 64) belonged to C. neoformans var. neoformans until July 2003, when the present case came to our laboratory.
Finding the source of the infection is very important to clarify whether it could be an autochthonous infection or an imported case. Some published cases of C. neoformans var. gattii cryptococcosis outside the area of endemicity (5, 6, 31, 33, 34) had a clear source of infection. Most of them occurred in patients who had traveled to Australia or South America. For some others, without a probable contact with eucalyptus trees and without any prior travel, the source remains uncertain. An exceptional situation has recently been described in Vancouver Island (Canada). An extended cryptococcosis outbreak of C. gattii involved some locations on the east coast of the island, and a large number of humans and animals were affected. The exhaustive environmental sampling carried out in the area allowed the detection of the possible natural reservoirs of the disease. The yeast was present in some tree hollows, soils, and other materials (18). This fact marks an important change in the understanding of the geographical distribution and natural life cycle of C. gattii and highlights the importance of finding the source of the infection. In order to demonstrate the presence of the yeast in our patient's environment, a total of 43 samples were taken all around his working area. Samples included vegetal material from different tree species (not including eucalyptus), different kinds of soils, and some pigeon droppings. As with other works focused on the isolation of C. neoformans var. gattii from eucalyptus and other trees outside the zones of endemicity (7, 9, 21), until now the presence of the yeast in the natural environment has not been proved. All these findings encouraged the search for a C. gattii environmental niche in Spain.
Acknowledgments
This work was supported by grants from “Generalitat Valenciana GV00-045-03,” “Fondos Europeos de Desarrollo Regional (Programa Operativo Integrado FEDER-FSE de Investigación, Desarrollo e Innovación Objetivo 1),” and “Fondos de Investigación Sanitaria” of Instituto de Salud Carlos III from the Ministerio de Sanidad y Consumo (02/1767).
REFERENCES
- 1.Aller, B., E. Santiago, A. Escudero, and M. Rey. 1971. Cryptococosis pulmonar en cabras. Rev. Patron. Biol. Anim. 15:387-397. [Google Scholar]
- 2.Argüero Licea, B., D. Garza Garza, and M. y Torres Zúñiga. 1996. Aislamiento de Crypotococcus neoformans var. gattii de Eucalyptus tereticornis. Rev. Iberoam. Micol. 13:27-28. [Google Scholar]
- 3.Baró, T., Y. Morera, J. M. Torres Rodríguez, C. Alía, and M. Lázera. 1998. Estudio de muestras de polvo doméstico para el aislamiento ambiental de Cryptococcus neoformans. Rev. Iberoam. Micol. 15:309. [PubMed] [Google Scholar]
- 4.Baró, T., J. M. Torres Rodríguez, M. Hermoso de Mendoza, Y. Morera, and C. Alía. 1998. First identification of autochthonous Cryptococcus neoformans var. gattii isloated from goats with predominantly severe pulmonary disease in Spain. J. Clin. Microbiol. 36:458-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodasing, N., R. A. Seaton, G. S. Shankland, and D. Kennedy. 2004. Cryptococcus neoformans var. gattii meningitis in an HIV-positive patient: first observation in the United Kingdom. J. Infect. 49:253-255. [DOI] [PubMed] [Google Scholar]
- 6.Bottone, E., J. Paul, A. Kirschner, and I. F. Salkin. 1986. Isolation of highly encapsulated Cryptococcus neoformans serotype B from a patient in New York City. J. Clin. Microbiol. 23:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campisi, E., F. Mancianti, G. Pini, E. Faggi, and G. Gargani. 2003. Investigation in Central Italy of the posible association between Cryptococcus neoformans var. gattii and Eucalyptus camaldulensis. Eur. J. Epidemiol. 18:357-362. [DOI] [PubMed] [Google Scholar]
- 8.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, D.C.
- 9.Chakrabarti, A., M. Jatana, P. Kumar, L. Chatha, A. Causal, and A. A. Padhye. 1997. Isolation of Cryptococcus neoformans var. gattii from Eucalyptus camaldulensis in India. J. Clin. Microbiol. 35:3340-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colom, M. F., S. Frasés, C. Ferrer, E. Martín-Mazuelos, M. Hermoso de Mendoza, J. M. Torres-Rodríguez, G. Quindós, y los colaboradores desde las instituciones Hospitalarias. 2001. Estudio epidemiológico de la criptococosis en España: primeros resultados. Rev. Iberoam. Micol. 18:99-104. [PubMed] [Google Scholar]
- 11.Colom-Valiente, M. F., M. Alberdi, I. Meseguer, and J. M. Torres-Rodriguez. 1997. Aislamiento de Cryptococcus neoformans de muestras de medio ambiente de Alicante. Rev. Iberoam. Micol. 14:63-64. [PubMed] [Google Scholar]
- 12.Davel, G., R. Abrantes, M. Brudny, S. Córdoba, L. Rodero, C. Canteros, and D. Perrota. 2003. 1st environmental isolation of Cryptococcus neoformans var. gattii in Argentina. Rev. Argent. Microbiol. 35:110-112. (In Spanish.) [PubMed] [Google Scholar]
- 13.Ellis, D. H., and T. J. Pfeifer. 1990. Natural habitat of Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 28:1642-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frasés, S. 2004. Epidemiología Molecular de Cryptococcus neoformans. Valoración de marcadores. Ph.D. thesis. University Miguel Hernández, Alicante, Spain.
- 15.Hermoso de Mendoza, M., A. Miranda, A. J. Perea, A. Arenas, J. B. Poveda, J. Carranza, and L. León. 1987. Criptococosis en paloma I. Frecuencia de portadores en buche en el area urbana de Córdoba. Rev. Iberoam. Micol. 4:121-127. [Google Scholar]
- 16.Hill, F. I., A. J. Woodgyer, and M. A. Lintott. 1995. Cryptococcosis in a North Island brown kiwi (Apteryx australis mantelli) in New Zealand. J. Med. Vet. Mycol. 33:305-309. [DOI] [PubMed] [Google Scholar]
- 17.Kesler, R., and P. Maertens. 1999. Pontine cryptococcoma in a nonimmunocompromised individual: MRI characteristics. J. Neuroimaging 9:118-121. [DOI] [PubMed] [Google Scholar]
- 18.Kidd, S. E., F. Hagen, R. L. Tscharke, M. Huynh, K. H. Bartlett, M. Fyfe, L. MacDougall, T. Boekhout, K. J. Kwon-Chung, and W. Meyer. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. USA 101:17258-17263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon-Chung, K. J., and J. E. Bennet. 1984. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am. J. Epidemiol. 120:123-130. [DOI] [PubMed] [Google Scholar]
- 20.Kwon-Chung, K. J., T. Boekhout, J. W. Fell, and M. Díaz. 2002. Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. bacillisporus (Basidiomycota, Hymenomycetes, Tremellomycetidae). Taxon 51:804-806. [Google Scholar]
- 21.Laurenson, I. F., D. G. Lalloo, S. Naraqi, R. A. Saetón, A. J. Trevett, A. Matuka, and I. H. Kevau. 1997. Cryptococcus neoformans in Papua New Guinea: a common pathogen but an elusive source. Med. Mycol. 35:437-440. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann, P. F., R. J. Morgan, and E. H. Frimer. 1984. Infection with Cryptococcus neoformans var. gattii leading to pulmonary cryptococcoma and meningitis. J. Infect. 9:301-306. [DOI] [PubMed] [Google Scholar]
- 23.Malik, R., M. B. Krockenberger, G. Cross, R. Doneley, D. N. Madill, D. Black. P. McWhirter, A. Rozenwax, K. Rose, M. Alley, D. Forshaw, I. Russell-Brown, A. C. Johnstone, P. Martin, C. R. O'Brien, and D. N. Love. 2003. Avian cryptococcosis. Med. Mycol. 41:115-124. [DOI] [PubMed] [Google Scholar]
- 24.Meyer, W., A. Castañeda, S. Jackson, M. Huynh, and E. Castañeda. 2003. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg. Infect. Dis. 9:189-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez Ramos, S. 1980. Aportaciones bioquímicas y ecológicas al género Cryptococcus. Ph.D. thesis. University of Cádiz, Cádiz, Spain.
- 26.Randhawa, H. S., T. Kowshik, and Z. U. Khan. 2003. Decayed wood of Syzygium cumini and Ficus religiosa living trees in Delhi/New Delhi metropolitan area as natural habitat of Cryptococcus neoformans. Med. Mycol. 41:199-209. [DOI] [PubMed] [Google Scholar]
- 27.Raso, T. F., K. Werther, E. T. Miranda, and M. J. Mendes-Giannini. 2004. Cryptococcosis outbreak in psittacine birds in Brazil. Med. Mycol. 42:355-362. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez, J. M., A. Andia, F. Carreras, A. Aberasturi, P. Anaut, and F. García. 2001. Meningitis criptocócica y criptococoma pulmonar en paciente con linfocitopenia CD4 idiopática. Enf. Infec. Micro. Clin. 19:236. [PubMed] [Google Scholar]
- 29.Rosario, I., B. Acosta, S. Déniz, D. Padilla, and G. Soro. 2002. Presencia de Cryptococcus spp. en buche y heces de palomas mensajeras (Columba livia) en la isla de Gran Canaria (España). Rev. Iberoam. Micol. 19:S30-S31. [Google Scholar]
- 30.Sherestha, R. K., J. K. Stoller, G. Honari, G. W. Procop, and S. M. Gordon. 2004. Pneumonia due to Cryptococcus neoformans in a patient receiving infliximab: possible zoonotic transmission from a pet cockatiel. Respir. Care 49:606-608. [PubMed] [Google Scholar]
- 31.Taylor, M. B., D. Chadwick, and T. Barkham. 2002. First reported isolation of Cryptococcus neoformans var. gattii from a patient in Singapore. J. Clin. Microbiol. 40:3098-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokojima, M., T. Ihi, Y. Kyoraku, T. Hiratsuka, K. Matsumoto, N. Matsumoto, S. Katoh, H. Mukae, and S. Matsukura. 2001. A case of bird fanciers' disease caused by parrot droppings. Nihon Kokyuki Gakkai Zasshi 39:739-743. (In Japanese.) [PubMed] [Google Scholar]
- 33.Tsunemi, T., T. Kamata, Y. Fumimura, M. Watanabe, M. Yamawaki, Y. Saito, T. Kanda, K. Ohashi, N. Suegara, S. Murayama, K. Makimura, H. Yamaguchi, and H. Mizusawa. 2001. Immunohistochemical diagnosis of Cryptococcus neoformans var. gattii infection in chronic meningoencephalitis: the first case in Japan. Intern. Med. 40:1241-1244. [DOI] [PubMed] [Google Scholar]
- 34.Velegraki, A., V. G. Kiosses, H. Pitsouni, D. Toukas, V. D. Daniilidis, and N. J. Legakis. 2001. First report of Cryptococcus neoformans var. gattii serotype B in Greece. Med. Mycol. 39:419-422. [DOI] [PubMed] [Google Scholar]
- 35.Wegener, H. H., and F. Staib. 1983. Fatal cryptococcosis in a bird fancier. A clinical case report on pathology, diagnosis and epidemiology of cryptococcosis. Zentbl. Bakteriol. Microbiol. Hyg. 256:231-238. [PubMed] [Google Scholar]
- 36.White, T. J., T. Bruns, S. Lee, and S. Taylor. 1990. Amplifications and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfland, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, Calif.