Abstract
We report a case of pulseless electrical activity (PEA) associated with profound hypermagnesemia immediately after cementation of a novel magnesium-based cement in spine surgery. During T8 to T12 posterior instrumentation and decompression laminectomy for vertebral metastasis secondary to lung cancer, a 61-year-old Chinese woman developed sudden hypotension and went into PEA immediately after injection of a novel magnesium-based cement. Intraoperative fluoroscopic imaging did not show any notable cement extravasation. Resuscitation using intravenous epinephrine with five doses of 1-mg epinephrine in 1:10,000 dilution was instituted, and the patient had return of spontaneous circulation after 5 minutes. After successful resuscitation, surgery was expedited and completed. Intraoperative and postoperative investigations were notable for profound hypermagnesemia and hyperphosphatemia requiring diuresis. No echocardiographic or computerized tomographic evidence of pulmonary embolism was found. The patient was transferred to the surgical intensive care unit and remained on dual inotropic support over the next few days. She subsequently weaned off inotropic support and electrolyte imbalances resolved before making a full recovery. This case report demonstrates the severe magnesium toxicity and PEA related to the use of novel magnesium-based cement in spine surgery. Further studies need to be conducted to understand the potential complications related to its use and compare them to the standard bone cement implantation syndrome.
Novel magnesium-based bone cement aims to create an osteogenic and dissolvable cement that will provide the necessary tensile strength and resistance to the complex mechanical environments in the context of orthopaedic applications.1,2 Compared with standard calcium phosphate cement (CMC) and polymethyl methacrylate (PMMA)–based cement, magnesium-based cement is shown to have faster absorption time, have higher tensile strength at 24h after insertion, and is more resistant to complex mechanical environments.2-6 Given the benefits of magnesium-based cement, a special committee of senior officials and physicians approved a GN-27 application under the Health Sciences Authority Special Access Routes for the use of this novel magnesium-based cement. Despite the proposed material benefits of magnesium-based cement, there has been no studies on the complications related to its use. Bone cement implantation syndrome (BCIS) is one of the most feared complications associated with cement use in orthopaedic surgery and is associated with high mortality.7-10 This case study alerts early adopters of magnesium-based cement in orthopaedic surgery to the potential complication of acute magnesium toxicity postcementation in spine surgery and attempts to delineate the differences as compared with the standard BCIS. This case study also serves to demonstrate the prompt investigations and management of pulseless electrical activity (PEA) in the setting of acute hypermagnesemia immediately after insertion of magnesium-based cement.
Case Description
We present a case of a 61-year-old Chinese woman from Singapore presenting to the oncology clinic with a history of worsening thoracic back pain for a 3-month duration with notable night pain acutely worsening over the past 2 weeks. She has a background history of left-sided stage two-B non–small cell lung cancer of adenosqamous subtype with broad contact with the aorta on active surveillance with CT of thorax, abdomen, and pelvis (CT-TAP) once every 3 months. She had initially declined exploratory surgery and lobectomy because of the high risk associated with the surgery and was also trialed on chemotherapy but was unable to tolerate the adverse effects. At the time of consultation, she had a prognosis of longer than 6 months. Other medical conditions include hypertension on medications, hyperlipidemia, and recovered shingles over left T10 and T11 dermatome 1 month before presentation. Surveillance CT-TAP done and reviewed in clinic showed a new lytic, permeative T10 bony metastasis with circumferential epidural component with severe spinal narrowing of the spinal canal at T10. No previous trauma, falls, or constitutional symptoms such as loss of weight or appetite were noted. She did not complain of any lower limb weakness or numbness and also did not complain of any bowel or urinary incontinence. She was admitted to the ward for further investigations in view of the worsening thoracic back pain and impending cord compromise.
On general examination, the patient's vitals were within normal limits. She had thoracic midline spinal tenderness over T10 vertebrae but was able to ambulate without assistance. Presence of dried erythematous lesions was found over T10 and T11 dermatome relating to the previously resolving zoster infection. No associated step deformities or paraspinal tenderness was observed. Neurological examination as charted by the America Spinal Injury Association scoring system showed full motor and sensory subscores, with an overall score of 112 of 112. No thoracic spinal sensory levels were detected, and no signs of an upper motor neuron lesion were found in the lower limbs. She had no palpable bladder or no saddle anesthesia, and digital rectal examination showed a normal anal tone.
Preoperative Investigations
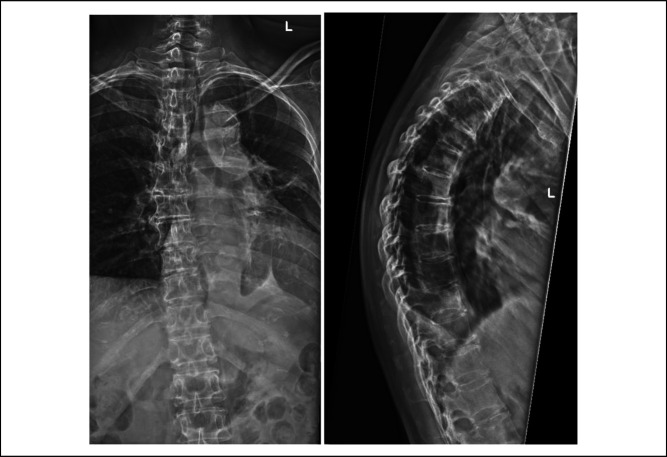
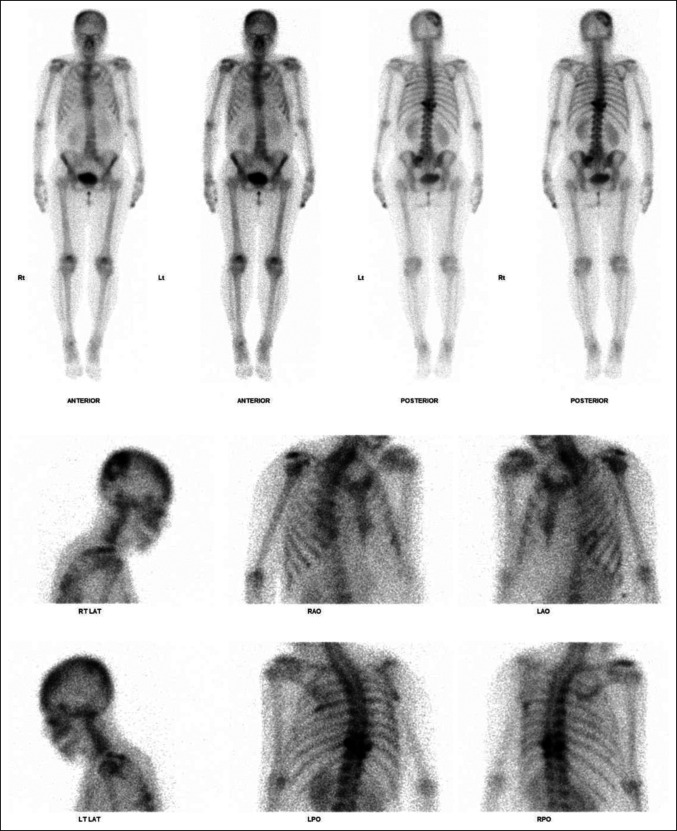
She underwent radiography and magnetic resonance imaging of the entire spine (Figures 1 and 2). Initial radiographs of the thoracic spine demonstrated loss of T10 pedicles with the preservation of thoracic vertebral alignment and vertebral body height. No acute fractures of the vertebral body were reported. MRI of the thoracic spine revealed metastatic disease affecting T9 to T11. At the level of T9, abnormal marrow signal and enhancement were found involving the posterior vertebral body, bilateral pedicles, bilateral lamina, spinous process and bilateral ninth ribs. At the level of T10, abnormal marrow signal and enhancement were found involving the mid and posterior vertebral body, right pedicle, bilateral lamina, spinous process and bilateral 10th ribs. At the level of T11, abnormal marrow signal and enhancement were found involving the posterior vertebral body, right pedicle, bilateral lamina, spinous process, and bilateral 11th ribs. Pathological soft-tissue involvement of the right T9-10 and T10-11 neural foramina was found, causing severe neural foraminal stenosis. In addition, spinal epidural soft tissue suspicious for metastasis was found extending from T9-10 down to the T10 level, causing severe spinal canal stenosis and cord compression. No evidence of metastases to both the cervical and lumbar spine was found. Subsequently, a bone scan (Figure 3) was done for the patient who showed increased tracer uptake in the known lesions in T9-11 vertebral bodies and ribs. In addition, increased tracer uptake was observed in the left sacral ala, left posterior sixth rib, and right parieto-occipital skull, suspicious for metastases. No preoperative bone mineral density scan was done for the above patient. Preoperative blood tests revealed normal serum electrolyte levels, including sodium, potassium, chloride, calcium, phosphate, and magnesium.
Figure 1.
Radiographs showing preoperative coronal and sagittal views of the thoracic spine.
Figure 2.
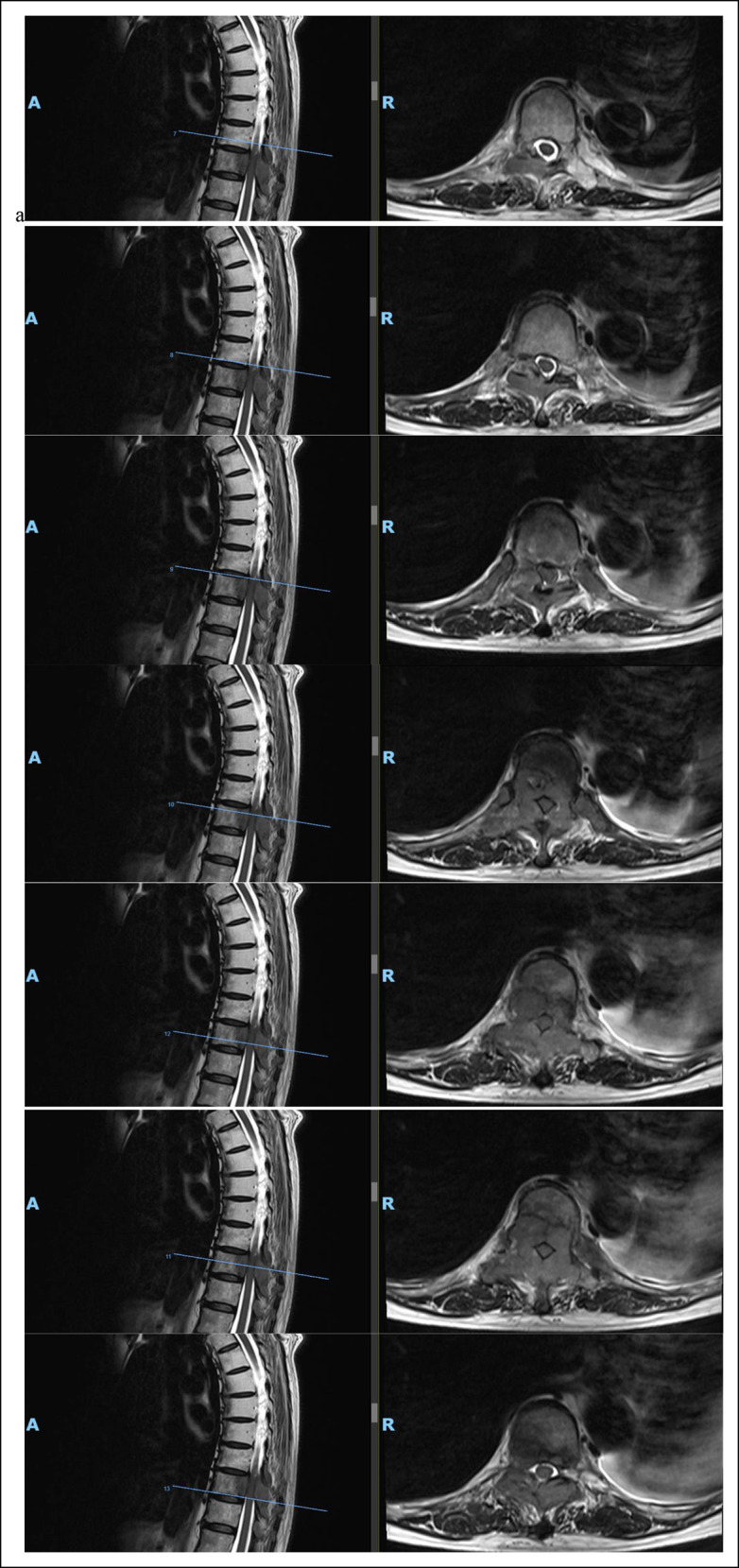
Radiographs showing preoperative magnetic resonance imaging (MRI) in coronal and axial cuts of the thoracic spine from T9 to T10, revealing severe cord compression at T10.
Figure 3.
Radiographs showing preoperative bone.
Surgical Plan
She was subsequently counselled for T8-T12 posterior instrumentation, decompression laminectomy, and fusion keep in view cement augmentation keep in view more levels.
Intraoperative Events
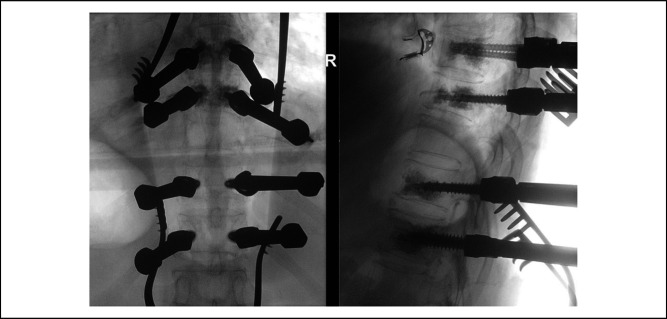
The patient underwent general anesthesia and was placed in prone position on a Jackson table. She was catheterized to monitor urine output hourly and also placed on neuromonitoring intraoperatively. A posterior midline incision was used. Pedicles of T8, T9, T11, and T12 were identified and marked. Intraoperative fluoroscopy showed satisfactory positioning, and fenestrated screws were inserted. Bilateral 4.5 mm by 35 mm, 5.5 mm by 35 mm, 6.5 mm by 35 mm, and 7.5 mm by 35 mm screws were inserted to T8, T9, T11, and T12, respectively. Novel magnesium-based cement was prepared intraoperatively by the surgical assistant as per product descriptions and instruction, with the aid of a circulating vendor. Twenty milliliter of cement was then prepared in total and injected into bilateral pedicle screws of T8, T9, T11, and T12, with 2.5 mL of cement injected into each pedicle screw. A plunger was then used to ensure all cement was injected into the pedicle screw. Placement of the cement into the vertebral body was confirmed on intraoperative fluoroscopy, showing no extravasation of cement (Figure 4).
Figure 4.
Intraoperative fluoroscopy of T8-T12 instrumentation, with evidence of cement inserted through bilateral pedicle screws of T8-9 and T11-12, and no evidence of extrusion of cement.
Immediately after cement injection, profound hypotension was observed with systolic blood pressure dropping to 50 to 60 noted on the intraoperative patient monitors. No arterial waveform was noted on the intra-arterial line, and the patient went into PEA arrest. The patient was subsequently immediately resuscitated in accordance with the advanced cardiac life support principles, and surgery was expedited. Five doses of intravenous epinephrine 1 mg diluted in 1:10000 each were given over five minutes. Cardiopulmonary resuscitation could not be initiated because the patient was in prone position at the time of PEA arrest. Subsequently, return of spontaneous circulation was observed after five minutes of intravenous resuscitation.
Arterial blood gas done immediately after return of spontaneous circulation showed pH 7.33, partial pressure of oxygen 443 (on 100% oxygen), partial pressure of carbon dioxide 38.5, base excess −6, bicarbonate 20.2, hemoglobin (Hb) 8.5, potassium 6.5, ionized calcium 1.08, and blood glucose 4.6. Immediately, the patient received treatment for her hyperkalemia and anemia with insulin-dextrose, calcium gluconate, and packed red blood cell transfusion. Her mean arterial blood pressure was maintained at greater than 65mmhg on dual inotropic support with noradrenaline (max 0.2mcg/kg/min) and epinephrine (max 0.5mcg/kg/min) through a peripheral 16-gauge intravenous cannula. She also received 100 mg of intravenous hydrocortisone. Surgery was resumed and expedited with close intraoperative monitoring of vital signs. She subsequently had another episode of profound hypotension with systolic blood pressure dropping to 50 s. She required three more doses of intravenous epinephrine 1 mg diluted in 1:10000 to maintain a mean arterial blood pressure greater than 65 mmHg. She underwent completion of T8-T12 posterior instrumentation, stabilization, and fusion and decompression laminectomy of T9-T11. Intraoperative histology was sent from the bone and epidural metastases. Cancellous bone chips were laid over the lateral gutters along with vancomycin powder. A drain was inserted with suction pressure calibrated to 75 mmHg. Closure was then performed in layers. The surgery was completed two hours and six minutes from the time of incision.
Postoperative Recovery
Immediately after closure, two doses of intravenous epinephrine 1 mg each was given preemptively before turning patient supine. A central venous catheter was then inserted into the right internal jugular vein, and the patient was started on noradrenaline and epinephrine infusions through the central venous catheter port. A bedside echocardiogram was done, showing good biventricular contractility, no debris or air in the cardiac chambers, and the inferior vena cava was well filled. She was subsequently sent to the surgical intensive care unit (SICU) intubated.
Postoperative Investigations
A formal portable transthoracic echocardiogram was done, which showed a dilated right ventricle. She also underwent a CT pulmonary angiogram, which showed no evidence of pulmonary embolism but showed evidence of heart strain. Postoperative troponin levels returned as 1,612 ng/L and 1,166 ng/L, and cardiology was consulted with initial impressions of type II myocardial infarction likely due to intraoperative PEA with plans to start single antiplatelet therapy and transition to dual antiplatelet therapy subsequently. Notable hypermagnesemia, hyperphosphatemia, and hypocalcemia were recorded postoperatively in the SICU. Initial magnesium, phosphate, and calcium levels recorded on admission to SICU were 2.46 mmol/L (normal range: 0.75 to 1.05 mmol/L), 3.83 mmol/L (normal range: 0.80 to 1.50 mmol/L), and 2.02 mmol/L (normal range: 2.20 to 2.60 mmol/L), respectively. Immediate infusion of intravenous calcium gluconate and subsequent diuresis with intravenous frusemide was initiated. The intravenous frusemide infusion was continued for 48 hours for diuresis as magnesium, phosphate, and calcium levels began to normalize. Serum tryptase at 0h, 1h, and 24h were sent off and returned as 2.0 ng/L, 4.8 ng/L and 1.8 ng/L, respectively (normal range: <11.4 ng/L).
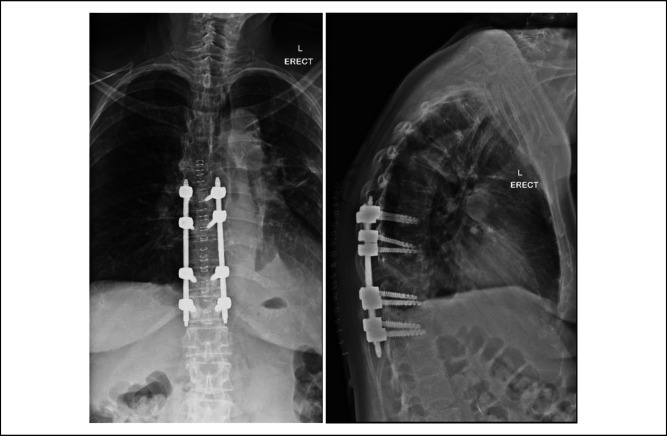
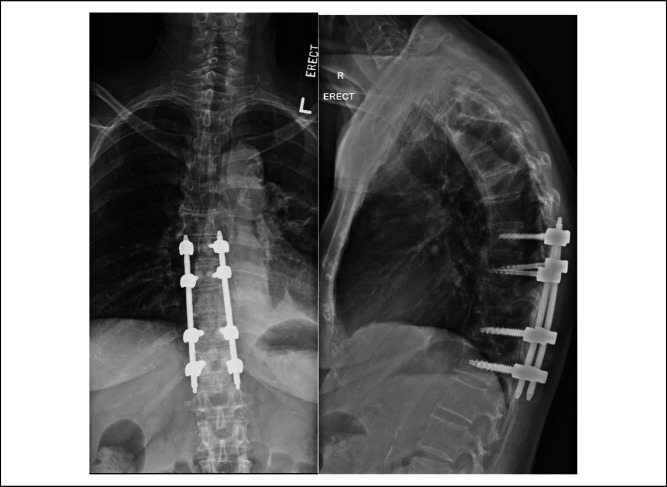
She remained in the SICU for 72 hours and was subsequently weaned off inotropes and sent to the high dependency unit for further monitoring. In the high-dependency unit, she began ambulating 4 m with a rotator frame, 4 days after her surgery. She was then transferred to the general ward and subsequently went to the community hospital for slow stream rehabilitation and made an uneventful recovery. Postoperative radiographs done 6 days and 60 days after the surgery showed stable implant positioning and fully resorbed cement (Figures 5 and 6).
Figure 5.
Radiographs showing coronal and sagittal views of the thoracic spine on postoperation day 6.
Figure 6.
Radiographs showing coronal and sagittal views of the thoracic spine on post-operation day 60.
Discussion
Polymethyl methacrylate–based cement and CMC are both widely used clinical bone substitutes.2,11 However, their drawbacks include toxic organic monomers, high condensation temperatures, long setting time, poor initial mechanical properties, and lack of biocompatibility and degradability.11-14 Magnesium-based cement is a newer alternative inorganic cement exhibiting good biocompatibility and degradability, while also being absorbed faster than PMMA-based cement and CMC in animal studies.3,4,15,16 Moreover, magnesium-based cement is superior to PMMA bone cement in terms of higher initial strength in the first 24h, rapid setting time, and better resistance to complex mechanical environments.2 Magnesium ions released from the cement during degradation not only is antimicrobial but also aids in mineral metabolization and triggers osteoblastic adhesion, proliferation, and stability.5,6,17 Although these characteristics of magnesium-based cement make it highly suitable for orthopaedic-related applications, to date, there are no clinical trials to demonstrate its superiority to the standard PMMA-based cement and CMC. There has also not been any studies demonstrating complications related to magnesium-based cement in comparison to PMMA-based cement and CMC.
Bone cement implantation syndrome is the most feared complication of the cementing process, and intraoperative mortality has been reported since the introduction of PMMA-based cement in the 1960s.9,10,18 BCIS is characterized by clinical features including but not limited to cardiac arrest, hypoxia, hypotension, and cardiac arrythmias.8,19,20 The postulated pathophysiology behind BCIS include anaphylactic reactions to the acrylic monomer, systemic toxicity to the unpolymerized methylacrylate (MMA) mixed with MMA polymer, or additives used in cement and microembolization of cement particles.7,21
However, this case report suggests that the intraoperative PEA occurred as a direct result to systemic dissemination of the novel magnesium-based cement rather than the standard pathophysiology of BCIS. The authors postulate that a direct causal relationship was found between the hypermagnesemia associated with the systemic dissemination of the cement and the intraoperative PEA. We postulate that the spine, being a highly vascular structure, enables higher concentration of magnesium to leak out into the surrounding blood vessels and blood stream directly from the use of magnesium-based cement.22,23 Moreover, non–small cell adenosquamous lung carcinoma metastases to the spine resulted in angiogenesis and hence potentially resulting in increased uptake of magnesium-based cement from the spine.22 There is also limited literature regarding the rate of uptake of phosphate and magnesium from the use of this cement into the blood stream. Furthermore, the lack of evidence of pulmonary emboli in the postoperative CT pulmonary angiogram and transthoracic echocardiogram suggest that pulmonary emboli played an ancillary role in the causation of the intraoperative PEA. The echocardiographic evidence of embolic showers with notable cardiac dysfunction in the pathophysiology of standard BCIS have been well demonstrated in the right atrium, right ventricle, and the pulmonary vasculature.24,25
Although there are some studies to suggest serum hypomagnesemia is related to sudden cardiac death,26,27 the relationship between serum hypermagnesemia and cardiac mortality is not well studied and poorly understood mainly due to its rarity. Magnesium toxicity has been observed in several case reports with iatrogenic overdose of magnesium sulphate intravenously,28,29 with major life-threatening complications, including cardiac conduction delays, hypotension, apnea, and cardiac arrest. The authors postulate that our patient had an acute magnesium toxicity affecting cardiac contractility and cardiac output, as evidenced by the profound hypermagnesemia requiring diuresis with the lack of evidence of pulmonary emboli. Prompt resuscitation intraoperatively and early recognition of electrolyte imbalances were crucial in the management of the patient's PEA. Although the treatment for both BCIS-related and magnesium-based cement–related cardiac arrest is generally supportive,7,10,21 early recognition of hypermagnesemia intraoperatively and postoperatively will allow prompt administration of diuresis and calcium supplementation due to the depletional effects of hypermagnesemia on circulating calcium levels, hence stabilizing the cardiac membrane.27,30 Early adopters of magnesium-based cement in orthopaedic-related surgeries should be aware of both BCIS and acute magnesium toxicity in the context of the cementing process and should work together with the anesthetists to recognize these devastating complications early and communicate effectively to manage them. There is currently a paucity of literature in the study of complications related to magnesium-based cement and how it compares to the complications arising from CMC- and PMMA-based cement. This case report serves to alert users of magnesium-based cement on its potential complications such that further studies can be done to determine its pathophysiology, risk factors, and surgical implications.
Conclusion
This case report serves to demonstrate the potential severe complication of magnesium toxicity and PEA related to the use of novel magnesium-based cement in spine surgery. Despite the proposed material benefits of magnesium-based cement, such as the good biocompatibility and degradability, its safety profile is not well understood and studied, especially in spine surgery. Before adoption of magnesium-based cement in spine surgery, further studies need to be conducted to understand the potential complications related to its use and compare such complications to the standard BCIS related to CMC- and PMMA-based cement. Understanding the pathophysiology of magnesium toxicity in magnesium-based cementing will not only allow anesthetists to promptly assess the intraoperative complications and immediately start treatment but potentially also to prevent such complications from arising. Understanding the potential risks and benefits of using magnesium-based cement will allow surgeons to refine indications for its use and counsel patients accordingly. Currently, we do not recommend the use of novel magnesium-based cement especially for tumor-related spine surgery.
Contributor Information
Jia Yi Loh, Email: dr.lohjiayi@gmail.com.
Zhixing Marcus Ling, Email: marcus.ling.z.x@sgh.com.sg.
Lei Jiang, Email: jiang.lei@singhealth.com.sg.
Qing Yuan Goh, Email: goh.qing.yuan@singhealth.com.sg.
Yee Gen Lim, Email: lim.yee.gen@singhealth.com.sg.
References
- 1.Nabiyouni M, Brückner T, Zhou H, Gbureck U, Bhaduri SB: Magnesium-based bioceramics in orthopedic applications. Acta Biomater 2018;66:23-43. [DOI] [PubMed] [Google Scholar]
- 2.Han Z, Wang B, Ren B, et al. : Characterization and biomechanical study of a novel magnesium potassium phosphate cement. Life 2022;12:997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanter B, Vikman A, Brückner T, Schamel M, Gbureck U, Ignatius A: Bone regeneration capacity of magnesium phosphate cements in a large animal model. Acta Biomater 2018;69:352-361. [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Xu C, Dai H: Preparation and characterization of a degradable magnesium phosphate bone cement. Regen Biomater 2016;3:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshizawa S, Brown A, Barchowsky A, Sfeir C: Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater 2014;10:2834-2842. [DOI] [PubMed] [Google Scholar]
- 6.Wu L, Feyerabend F, Schilling AF, Willumeit-Römer R, Luthringer BJC: Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater 2015;27:294-304. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson AJ, Thomson HE, Harper NJ, Kenny NW: Bone cement implantation syndrome. Br J Anaesth 2009;102:12-22. [DOI] [PubMed] [Google Scholar]
- 8.Govil P, Kakar PN, Arora D, et al. : Bone cement implantation syndrome: A report of four cases. Indian J Anaesth 2009;53:214-218. [PMC free article] [PubMed] [Google Scholar]
- 9.Bonfait H, Delaunay C, De Thomasson E, Tracol P, Werther J-R, Orthorisq: Bone cement implantation syndrome in hip arthroplasty: Frequency, severity and prevention. Orthop Traumatol Surg Res 2022;108:103139. [DOI] [PubMed] [Google Scholar]
- 10.Lamadé WR, Friedl W, Schmid B, Meeder PJ: Bone cement implantation syndrome. A prospective randomised trial for use of antihistamine blockade. Arch Orthop Trauma Surg 1995;114:335-339. [DOI] [PubMed] [Google Scholar]
- 11.Saab M, Hildebrand F, Martel B, Blanchemain N: Osteoinductive bone morphogenic protein, collagen scaffold, calcium phosphate cement, and magnesium-based fixation enhance anterior cruciate ligament tendon graft to bone healing in animal models: A systematic review. Arthroscopy 2023;39:529-548.e9. [DOI] [PubMed] [Google Scholar]
- 12.Sa Y, Yang F, Wang Y, Wolke JGC, Jansen JA: Modifications of poly(methyl methacrylate) cement for application in orthopedic surgery. Adv Exp Med Biol 2018;1078:119-134. [DOI] [PubMed] [Google Scholar]
- 13.Phakatkar AH, Shirdar MR, Qi M, et al. : Novel PMMA bone cement nanocomposites containing magnesium phosphate nanosheets and hydroxyapatite nanofibers. Mater Sci Eng C, Mater Biol Appl 2020;109:110497. [DOI] [PubMed] [Google Scholar]
- 14.Kühn K-D, Höntzsch D: Augmentation mit PMMA-Zement. Unfallchirurg 2015;118:737-748. [DOI] [PubMed] [Google Scholar]
- 15.Waselau M, Samii VF, Weisbrode SE, Litsky AS, Bertone AL: Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am J Vet Res 2007;68:370-378. [DOI] [PubMed] [Google Scholar]
- 16.Ewald A, Kreczy D, Brückner T, et al. : Development and bone regeneration capacity of premixed magnesium phosphate cement pastes. Materials 2019;12:2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mestres G, Fernandez-Yague MA, Pastorino D, et al. : In vivo efficiency of antimicrobial inorganic bone grafts in osteomyelitis treatments. Mater Sci Eng C, Mater Biol Appl 2019;97:84-95. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe JD, Edwards CJ, Hamzi R, Khanna AK, Olsen F. Bone cement implantation syndrome: Incidence and associated factors in a United States setting. Cureus. 2022;14(11):Article e31908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kallos T: Impaired arterial oxygenation associated with use of bone cement in the femoral shaft. Anesthesiology 1975;42:210-215. [DOI] [PubMed] [Google Scholar]
- 20.Sasano N, Ishida S, Tetsu S, et al. : Cerebral fat embolism diagnosed by magnetic resonance imaging at one, eight, and 50 days after hip arthroplasty: A case report. Can J Anesth 2004;51:875-879. [DOI] [PubMed] [Google Scholar]
- 21.Kalra A, Sharma A, Palaniswamy C, et al. : Diagnosis and management of bone cement implantation syndrome: Case report and brief review. Am J Ther 2013;20:121-125. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Xie X, Wu J: Mechanism of lung adenocarcinoma spine metastasis induced by CXCL17. Cell Oncol 2020;43:311-320. [DOI] [PubMed] [Google Scholar]
- 23.Griessenauer CJ, Raborn J, Foreman P, Shoja MM, Loukas M, Tubbs RS: Venous drainage of the spine and spinal cord: A comprehensive review of its history, embryology, anatomy, physiology, and pathology. Clin Anat 2015;28:75-87. [DOI] [PubMed] [Google Scholar]
- 24.Lafont ND, Kalonji MK, Barre J, Guillaume C, Boogaerts JG: Clinical features and echocardiography of embolism during cemented hip arthroplasty. Can J Anaesth 1997;44:112-117. [DOI] [PubMed] [Google Scholar]
- 25.Chen HL, Wong CS, Ho ST, Chang FL, Hsu CH, Wu CT: A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg 2002;95:1060. [DOI] [PubMed] [Google Scholar]
- 26.Kieboom BCT, Niemeijer MN, Leening MJG, et al. : Serum magnesium and the risk of death from coronary heart disease and sudden cardiac death. J Am Heart Assoc 2016;5:e002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR: Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2010;160:464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vissers RJ, Purssell R: Iatrogenic magnesium overdose: Two case reports. J Emerg Med 1996;14:187-191. [DOI] [PubMed] [Google Scholar]
- 29.Cavell GF, Bryant C, Jheeta S: Iatrogenic magnesium toxicity following intravenous infusion of magnesium sulfate: Risks and strategies for prevention. BMJ Case Rep 2015;2015:bcr2015209499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aal-Hamad AH, Al-Alawi AM, Kashoub MS, Falhammar H: Hypermagnesemia in clinical practice. Medicina (Mex) 2023;59:1190. [DOI] [PMC free article] [PubMed] [Google Scholar]