Abstract
Introduction
Solitary fibrous bladder tumors are extremely uncommon, with only a few cases reported. These fibroblastic mesenchymal neoplasms are typically benign, indolent, and slow growing.
Case presentation
A 44‐year‐old male patient with obstructive uropathy was referred to our unit for workup. Ultrasonography and MRI of the pelvis showed a large, well‐circumscribed bladder mass, also visualized cystoscopically. This mass was excised en bloc using the Pfannenstiel approach. Histopathological and immunohistochemical analyses revealed a solitary fibrous tumor.
Conclusion
The management of SFTs can be challenging due to the lack of established guidelines. Hence, we offered our patient long‐term follow‐up. Twelve months postoperatively, no recurrence or metastases were found on the follow‐up imaging.
Keywords: bladder, bladder malignancies, solitary fibrous tumor
Abbreviations & Acronyms
- ART
antiretroviral therapy
- HIV
human immunodeficiency virus
- MDT
multidisciplinary team
- MRI
magnetic resonance imaging
- PCR
polymerase chain reaction
- SFT
solitary fibrous tumor
- TURBT
transurethral resection of the bladder tumor
Keynote message.
Bladder SFTs are exceedingly rare, requiring a multidisciplinary team approach for optimal patient outcomes. Complete surgical excision with clear margins is the cornerstone of treatment. However, due to the size (>10 cm) and high signal intensity on MRI, long‐term follow‐up was offered, given the risk of metastasis.
Introduction
SFT is a rare fibroblastic neoplasm occurring at any anatomical site. 1 It accounts for 2% of soft tissue tumors and is exceptionally rare in the genitourinary tract. 2 SFTs have been described in the retroperitoneum, kidney, prostate, and bladder. 3 We present a rare incident of a bladder SFT in a 44‐year‐old male patient and review the relevant literature.
Case presentation
A 44‐year‐old African male was referred to our unit for definitive management of obstructive uropathy. He presented with acute urinary retention and renal dysfunction, with a serum creatinine level of 149 μmol/L (1.69 mg/day), eGFR 47 ml/min, warranting transurethral catheterization. He was known with HIV, and was compliant with ART. He reported no bowel symptoms and had no risk factors for urological malignancies. Clinically, his vital signs were normal. A firm, smooth, fixed, and non‐tender mass was palpated suprapubically with a normal digital rectal examination. Ultrasonography revealed a large, well‐circumscribed, hypoechoic bladder floor mass and bilateral Grade IV (ONEN) hydronephrosis. A complete blood count revealed anemia (Hb 9.8 g/dL) and normal leukocyte and thrombocyte counts. His serum creatinine had decreased to 125 μmol/L (1.41 mg/dL, eGFR 58 mL/min) after transurethral catheterization. His blood glucose, PSA levels, and urine cytology were normal. T1‐weighted MRI of the pelvis showed an isointense bladder mass (9.2 × 7.1 × 8.2 cm) with rounded low to intermediate‐intensity foci with high signal intensity on T2‐weighted imaging and moderate enhancement after gadolinium injection (Fig. 1). A trabeculated bladder and unremarkable prostate were noted. Cystoscopy revealed a large, pedunculated, mobile, smooth bladder mass with a narrow base to the right and anterolateral from the bladder neck, sparing the trigone and ureteric orifices (Fig. 2). A decision was made to proceed with an open partial cystectomy to excise the lesion en bloc, with a safe surgical margin, for several reasons. First, the mass occupied most of the bladder, making it very difficult to completely resect endoscopically. Second, the mass was pedunculated, and while the narrow base of the lesion could be seen at the dome, it was not possible to maneuver around the mass and reach the base. Third, the mass did not have the typical appearance of bladder malignancy. It was a distinct smooth mass with regular borders that was freely mobile and free of the bladder mucosa, aside from its thin base. Forth, considering the atypical nature of the mass, there was a concern over the possible vascularity of the lesion. Thus, endoscopic resection may be unsafe for the patient. Macroscopic examination of the resected specimen showed a pedunculated nodular lesion approximately 10 × 8 × 6.5 cm (Fig. 3). Microscopic sections revealed a circumscribed, nodular, and cellular fibroblastic neoplasm. Gaping, “stag‐horn” shaped vascular channels demonstrating perivascular stromal edema and mural hyalinization were visualized (Fig. 4a). Cellular areas comprising short spindled, fusiform cells arranged haphazardly and fascicles were observed (Fig. 4b). The hypocellular areas showed fibrosis, edema, and stromal myoxid changes with no necrosis. Mitotic cell division was 2 per 10 high‐power fields with negative surgical margins. Immunohistochemistry results were positive for CD34 and STAT6. SMA, Desmin, S100, and EMA were negative. Overall, histological and immunohistochemical findings were typical of SFT.
Fig. 1.
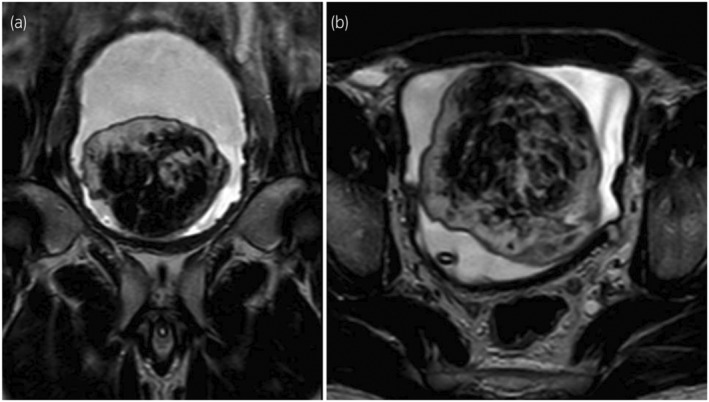
MRI of the pelvis (T2‐W) showing a large heterogeneous mass within the bladder lumen with high signal intensity on T2‐W imaging.
Fig. 2.
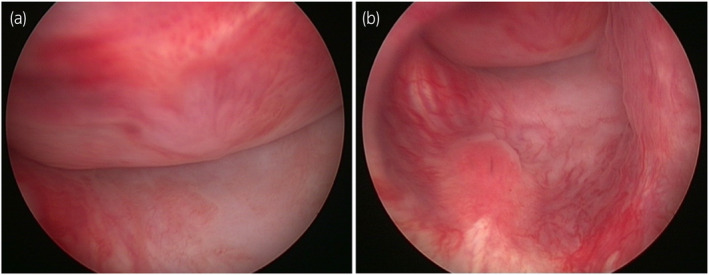
Cystoscopic images showing a large, pedunculated mass occupying most of the bladder that appeared free from the bladder trigone and ureteric orifices.
Fig. 3.
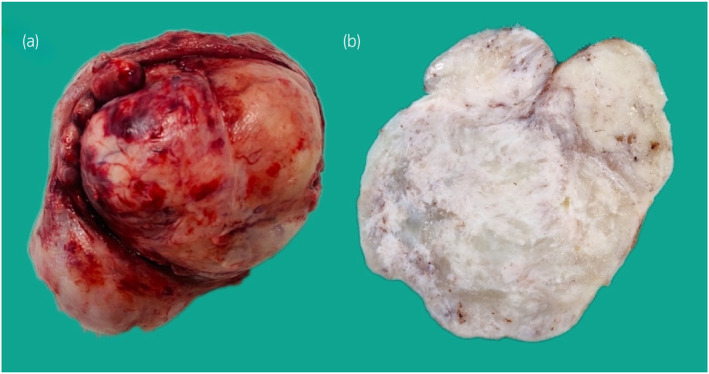
Macroscopic uncut (a) and cut (b) images showing a solid nodular tumor with pale‐to‐tan fibrous consistency and areas of myxoidal stromal change.
Fig. 4.
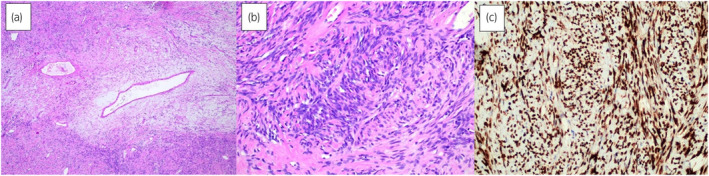
(a) Low‐power magnification showing conspicuous gaping and “stag‐horn”‐shaped vascular channels with variable mural hyalinization (Hematoxylin & Eosin, 100×); (b) Haphazardly arranged spindled and fusiform cells with hyperchromatic nuclei, eosinophilic cytoplasm and indistinct cellular borders (Hematoxylin & Eosin, 200×); (c) STAT6 immunohistochemistry is strongly and diffusely positive (200×).
His postoperative course remained symptom‐free, and renal function normalized. Due to the tumor size and high signal intensity on T2‐weighted imaging, a MDT outlined a follow‐up with 6‐monthly cross‐sectional abdominal and pelvic imaging and cystoscopy for the first 2 years. At his recent postoperative visit, 24 months later, no recurrence or metastases were identified, and he remained euglycemic. Further follow‐up, in the form of 6‐monthly ultrasonography and in‐office cystoscopy will now continue for the next 5 years. More invasive imaging will be used if any suspicion of recurrence arises.
Discussion
SFTs are rare mesenchymal neoplasms derived from CD34‐expressing fibroblastic cells, explaining their widespread distribution. 4 They are usually benign, indolent, and slow‐growing, but aggressive metastatic SFTs have been reported.
Most patients are asymptomatic but may present with lower abdominal pain, a palpable mass, urinary retention, hematuria, and lower urinary tract symptoms. 5 It can infrequently cause paraneoplastic syndromes, such as hypoinsulinemic hypoglycemia (Doege Potter syndrome). 6
Ultrasonography revealed a well‐circumscribed, hypoechoic mass. Contrast‐enhanced CT shows a circumscribed, smooth, and lobulated soft tissue mass. Smaller lesions enhance homogenously, while larger lesions are heterogeneous, with necrosis and cystic degeneration. 7 MRI findings of SFTs are variable. Benign lesions are homogeneous with low or intermediate signal intensity on T1‐weighted and T2‐weighted imaging. 7 , 8 Malignant SFTs demonstrate high signal intensity on T2‐weighted imaging with marked enhancement after contrast injection. 9 , 10
Radiological findings are nonspecific. Histopathology is diagnostic and can be confirmed by STAT6 immunohistochemistry. Variant morphological patterns, including lipomatous, giant cell‐rich, and dedifferentiated (anaplastic) SFT, were excluded by analysis. The NAB2‐STAT6 gene fusion is pathognomonic for SFT, but immunohistochemistry for STAT6 is preferred over conventional cytogenetics and PCR testing. 11
Complete surgical removal with clear margins is the mainstay of surgical management and the prognostic determinant; some authors approximate 100% survival with this approach. 12 TURBT, en bloc excision, and partial or radical cystectomy are documented approaches. 13 Chemotherapy for SFT of the bladder has been suggested for inoperable or metastatic disease. 8 , 14
A minority of patients develop distant metastasis or local recurrence, and long‐term monitoring is crucial. 14 , 15 , 16 Multivariate risk models are valuable prognostic indicators and two risk stratification systems have been validated for clinical use. Due to the rarity of bladder involvement, its relationship with the prognosis of SFT of the bladder is unclear. Therefore, we decided to offer long‐term surveillance with 6‐month cross‐sectional abdominal and pelvic imaging with cystoscopy for our patient considering the large size of the tumor (>10 cm) and high signal intensity on T2‐weighted imaging. Fortunately, his follow‐up identified no recurrence or distant lesions.
Conclusion
We report an uncommon instance of an STF of the bladder. Histopathological analysis and immunohistochemistry are crucial for diagnosis, as radiological and cystoscopic findings are typically nonspecific. Prognosis is favorable after complete surgical removal; however, long‐term monitoring is essential due to the potential for metastasis or relapse.
Author contributions
Návan van Jaarsveld: Validation; writing – original draft; writing – review and editing. Alessandro Pietro Aldera: Validation; writing – original draft; writing – review and editing. Jeff John: Supervision; validation; writing – original draft; writing – review and editing.
Conflict of interest
Regarding the research, authorship, and publication of this manuscript, the authors declare no competing interests.
Approval of the research protocol by an Institutional Reviewer Board
Obtaining ethical approval for individual case reports at our institution is not required.
Informed consent
The patient provided written informed consent for anonymous publication of information and images.
Registry and the Registration No. of the study/trial
Not applicable.
Funding information
This article's research, authorship, and publication did not receive any financial aid.
Acknowledgments
None.
Data availability statement
Not applicable.
References
- 1. Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. Am. J. Ind. Med. 1992; 22: 4–31. [DOI] [PubMed] [Google Scholar]
- 2. López Martín L, Calahorra Fernández FJ. Solitary fibrous tumor of the bladder. Actas Urol. Esp. 2010; 34: 206–208. [PubMed] [Google Scholar]
- 3. Gao C, Zhang Y, Li YY et al. Postoperative recurrence solitary fibrous tumor of the pelvic with malignant transformation. Int. J. Clin. Exp. Med. 2015; 8: 16827–16833. [PMC free article] [PubMed] [Google Scholar]
- 4. Tanaka EY, Buonfiglio VB, Manzano JP, Filippi RZ, Sadi MV. Two cases of solitary fibrous tumor involving urinary bladder and a review of the literature. Case Rep. Urol. 2016; 2016: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petracco G, Patriarca C. A small solitary fibrous tumor of the bladder. J. Cancer Metastasis Treat. 2016; 2: 388. [Google Scholar]
- 6. Urbina‐Lima ÁD, Román‐Martín AÁ, Crespo‐Santos A et al. Solitary fibrous tumor of the urinary bladder associated with hypoglycemia: an unusual case of Doege‐potter syndrome. Urol. Int. 2019; 103: 120–124. [DOI] [PubMed] [Google Scholar]
- 7. Hartung M, Niknejad M. Solitary fibrous tumour. Radiopaedia.org2013.
- 8. Vargas JF, Gandhi D, Bajaj D, Serhal M, Erazo IS, Singh J. Solitary fibrous tumor of the urinary bladder: an unusual case report with literature review. Radiol. Case Rep. 2021; 16: 3898–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagase T, Adachi I, Yamada T et al. Solitary fibrous tumor in the pelvic cavity with hypoglycemia: report of a case. Surg. Today 2005; 35: 181–184. [DOI] [PubMed] [Google Scholar]
- 10. Cheng SH, Wang SS, Lee CH, Ou YC, Cheng CL. Malignant solitary fibrous tumor of the urinary bladder. J. Chin. Med. Assoc. 2012; 75: 479–482. [DOI] [PubMed] [Google Scholar]
- 11. Doyle LA, Vivero M, Fletcher CDM, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod. Pathol. 2014; 27: 390–395. [DOI] [PubMed] [Google Scholar]
- 12. Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Zaheer A, Sandrasegaran K. Somatic and visceral solitary fibrous tumors in the abdomen and pelvis: cross‐sectional imaging spectrum. Radiographics 2011; 31: 393–408. [DOI] [PubMed] [Google Scholar]
- 13. Ong K, Singh S, Swarbrick N, Hayne D. Solitary fibrous tumour of the urinary bladder—a rare and potentially malignant neoplasm. Urol. Case Rep. 2023; 50: 102501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun S, Tang M, Dong H et al. Solitary fibrous tumor involving urinary bladder: a case report and literature review. Transl. Androl. Urol. 2020; 9: 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Evaristo G, Fiset PO, Kassouf W, Jung S, Brimo F, Ajise O. Metastatic malignant solitary fibrous tumor of urinary bladder. Hum Pathol: Case Rep. 2021; 25: 200527. [Google Scholar]
- 16. Kuramoto T, Iguchi T, Iba A. Solitary fibrous tumor of the urinary bladder which was incidentally found by MRI: a case report. Acta Urol Jpn 2021; 67: 289–385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


