Abstract
Aim
In this study, we evaluated the difference in short‐term outcomes and postoperative nutritional status between subtotal gastrectomy (sTG) and proximal gastrectomy (PG) to determine the optimal surgical treatment for early gastric cancer in the upper third of the stomach.
Methods
Patients who underwent laparoscopic or robotic sTG or PG at the Shizuoka Cancer Center in Shizuoka between January 2014 and December 2020 were enrolled in this retrospective study. Patient characteristics, surgical outcomes, endoscopic findings, and postoperative nutritional changes, including blood tests, body weight, psoas muscle, and subcutaneous and visceral adipose tissue, were measured and compared between the two groups.
Results
A total of 110 patients were enrolled, including 42 in the sTG group and 68 in the PG group. Albumin and hemoglobin levels were comparable between the two groups. Changes in body weight and psoas mass index measured over 36 months postoperatively were favorable in the sTG group compared with the PG group (p = 0.005 and p = 0.002, respectively). There were no significant differences in subcutaneous or visceral adipose tissue between the two groups (p = 0.331 and 0.845, respectively).
Conclusion
sTG is the preferred function‐preserving gastrectomy procedure for early gastric cancer in the upper third of the stomach because it is associated with less postoperative body weight loss and psoas mass index loss.
Keywords: gastric cancer, laparoscopic surgery, proximal gastrectomy, robotic surgery, subtotal gastrectomy
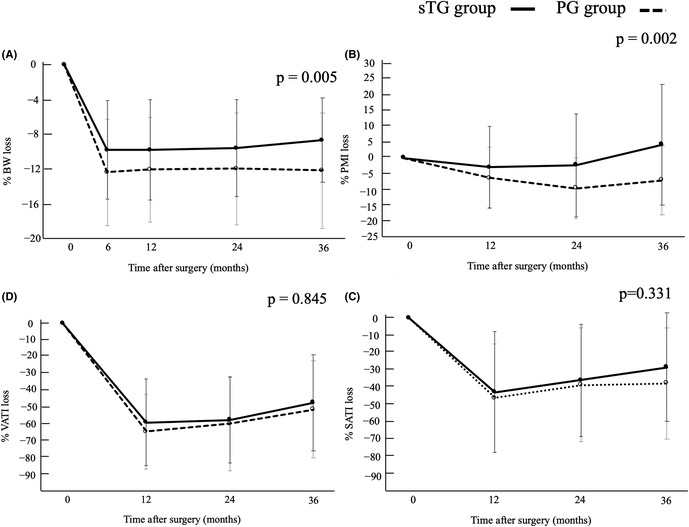
Changes in % BW and % psoas muscle index were significantly better in the subtotal gastrectomy group than in the proximal gastrectomy group.
1. INTRODUCTION
Gastric cancer is the fifth leading cause of cancer worldwide. 1 In Japan, with the increased availability of screening examinations, the proportion of early gastric cancer (EGC), including that in the upper third of the stomach, has increased in recent years. 2 Total gastrectomy (TG) used to be the standard treatment for EGC of the upper third of the stomach; however, function‐preserving gastrectomy (FPG) has recently been introduced to maintain long‐term nutritional status and quality of life following surgery without affecting oncological outcomes. 3 , 4
For FPG, subtotal gastrectomy (sTG), in which the upper part of the stomach is preserved, and proximal gastrectomy (PG), in which the upper part of the stomach is resected, are now routinely performed. 5 For EGC, laparoscopic gastrectomy is considered the standard treatment in Japan. Accordingly, laparoscopic subtotal gastrectomy (LsTG) and laparoscopic proximal gastrectomy (LPG) are common procedures. Furthermore, with the rapid spread of robotic gastrectomy, robotic sTG (RsTG) and robotic PG (RPG) are becoming more routine; however, there is little data comparing LsTG with LPG. It has been reported that survival is improved with LPG compared with LsTG. 6 There are few reports comparing PG and sTG with respect to short‐term outcomes and postoperative nutritional status. Kano et al. found that the postoperative nutritional status of sTG and PG were comparable. 7 Because the long‐term nutritional status following these procedures may depend on the different lesions and resected stomach size in sTG and PG, the long‐term complications may also vary between these procedures. There is a lack of evidence comparing short‐term outcomes and postoperative nutritional status between sTG and PG, which makes it difficult to select the optimal procedure for EGC for the upper third of the stomach.
The goal of this study was to determine the difference in short‐term outcomes and postoperative nutritional status between sTG and PG to improve the selection of the optimal surgical treatment for EGC in the upper third of the stomach.
2. PATIENTS AND METHODS
2.1. Patients
Between January 2014 and December 2020, in the division of gastric surgery at the Shizuoka Cancer Center, patients who underwent laparoscopic or robot‐assisted gastrectomy for clinical T1N0M0 gastric cancer in the upper third were enrolled. Cases involving surgical resection after endoscopic submucosal resection (ESD) were also included. Patients with esophageal invasion, concomitant surgery for multiple cancers, and intraoperative conversion to open surgery, were excluded. The clinicopathological diagnosis was based on the 8th edition of the Tumor‐Node‐Metastasis classification by the American Joint Committee on Cancer. The histological classification was based on the Lauren classification. 8
2.2. Selection of surgical procedure
FPG for EGC in the upper third includes sTG and PG. Both procedures are performed for cT1N0M0 gastric cancer located in the upper third of the stomach. The selection of sTG or PG was made based on the tumor location and size. sTG was the first choice when the proximal tumor margin was >3 cm from the esophagogastric junction. If this condition was not met, PG was performed. The approach was either laparoscopic or robotic based on the surgeon's preference or availability of equipment. The da Vinci Si and Xi Surgical System (INTUITIVE Surgical Inc, Sunnyvale, LA, USA) was used for robotic surgery.
2.3. Surgical procedure
2.3.1. LsTG and RsTG
The definition of sTG is as previously reported. 9 The difference from conventional distal gastrectomy is the distance from EGJ to the resected line, which is defined as sTG if it is <3 cm. LsTG was performed with a five‐port inverted trapezoidal arrangement and laparoscopic coagulation shears were used as the energetic device. After lymph node dissection of the greater curvature, the duodenum was transected using a linear stapler, and the suprapyloric and suprapancreatic lymph nodes were dissected. We usually performed a D1+ lymph node dissection for sTG, although D2 lymph node dissection was performed in patients at high risk of lymph node metastasis following ESD. 10 As previously reported, intraoperative endoscopic observation is routinely performed to ensure a secure oral resection margin. 11 The stomach was transected according to the marking clips that were placed the day before surgery and guided by intraoperative endoscopy. The proximal section of the resected specimen was submitted for frozen‐section analysis to confirm the histologically as cancer‐negative. In cases of resection after ESD with a negative horizontal margin, the frozen‐section analysis was not done. Roux‐en‐Y reconstruction was performed via the antecolic route. 9 A side‐to‐side jejunojejunostomy was created extracorporeally, and the gastrojejunostomy was performed intracorporeally at 30 cm to the jejunojejunostomy using a linear stapler and an overlap technique. The closure of Petersen's defect and the mesenterial defect was performed with nonabsorbable running sutures. For RsTG, the port was placed horizontally at the level of the umbilicus and the operation was performed in the same way as LsTG.
2.3.2. LPG and RPG
LPG was performed with the same port placement as LsTG, and the gastric body was transected intracorporeally with a linear stapler. After D1+ lymph node dissection, the abdominal esophagus was transected with a linear stapler. Intraoperative frozen‐section analysis of the distal margin was submitted for all patients except those undergoing ESD with negative horizontal margins. The reconstruction methods were selected according to the size of the remnant stomach. If more than two‐thirds of the remnant stomach remained, the double‐flap technique was performed. 12 , 13 A H‐shaped seromuscular flap (2.5 × 3.5 cm2) was created on the anterior wall of the remnant stomach, and a hand‐sewn esophagogastrostomy was performed laparoscopically. Finally, the seromuscular flap was sutured to provide an antireflux mechanism. If the remnant stomach was less than one‐half or present in patients with severe hiatal hernia, the double tract was selected. 14 Esophagojejunostomy was performed with an overlap technique using a linear stapler, and the entry hole was closed by hand‐sewn running sutures. Jejunogastrostomy was performed with a linear stapler at 8–10 cm anal from the esophagojejunostomy, and further jejunojejunostomy was performed at 20–30 cm anal from the jejunogastrostomy. For RPG, the operation was performed in the same way as LPG. For both procedures, a single drainage tube was placed in the suprapancreatic area.
2.4. Evaluation of outcome
Patient background and surgical outcomes were assessed using prospectively registered medical records. Early postoperative complications occurring within 30 days after surgery were defined as grade II or above based on the Clavien–Dindo classification. 15 Endoscopic observation 1 year after surgery was done to evaluate reflux esophagitis of grade B or above based on the Los Angeles classification 16 and gastric stasis was defined as food residue of grade 2 or above based on the residue gastritis, bile classification. 17 The case for anastomotic stenosis requiring endoscopic dilation within 1 year after surgery was recorded as anastomotic stenosis.
Indicators of nutritional status, albumin, hemoglobin levels, and body weight, were evaluated preoperatively, and at 6, 12, 24, and 36 months following surgery. Body composition based on computed tomography was acquired preoperatively, and at 12, 24, and 36 months following surgery. The SYNAPSE VINCENT Volume Analyzer (Fujifilm Medical Co., Japan) was used to analyze body composition. The psoas muscle was quantified by a Hounsfield unit threshold of −29 to 150 at the levels of the third lumbar vertebrae (L3), 18 and the subcutaneous adipose tissues (SAT) and visceral adipose tissues (VAT) were quantified by Hounsfield unit thresholds of −190 to −30 and −150 to −50 at the level of the umbilicus. 19 Tissue boundaries were manually modified as needed. Tissue cross‐sectional area (cm2) was automatically calculated, and subsequently standardized according to stature (m2). The psoas muscle index (PMI) (cm2/m2), the SAT index (SATI) (cm2/m2), and the VAT index (VATI) (cm2/m2) were used to define the data. Postoperative changes in BW, PMI, SATI, and VATI were evaluated for each patient.
2.5. Statistical analysis
To evaluate postoperative changes, the percent loss was determined. The percentage of BW loss (%BW loss) was calculated as follows:
The %PMI loss, %SATI loss, and %VATI loss were all defined in the same way as the %BW loss. Descriptive statistics were expressed as the median with interquartile range (IQR) for continuous variables and percentages for categorical variables. Comparisons between the study groups were done using the Mann–Whitney test and Fischer's exact test for continuous variables and categorical variables. A repeated measures analysis of variance was used to compare the longitudinal changes of the serological indices as well as %BW loss, %PMI loss, %SATI loss, and %VATI loss between the sTG and PG groups. All statistical analyses were done using EZR 20 and p‐values of <0.05 were considered statistically significant.
3. RESULTS
3.1. Patient characteristics
A total of 158 patients were included in this study. Of these, TG was performed on 44 patients, including four patients with planned sTG and two patients with planned PG, but changed to TG were excluded Abecause of a positive resection margin. Of 114 patients, excluding patients who underwent TG, a patient who had concurrent surgery for another cancer (n = 1), patients switching to open surgery because of tumor progression (n = 2), and patients with esophageal invasion (n = 1) were also excluded. As a result, 42 patients who underwent sTG and 68 patients who underwent PG were analyzed (Figure 1).
FIGURE 1.
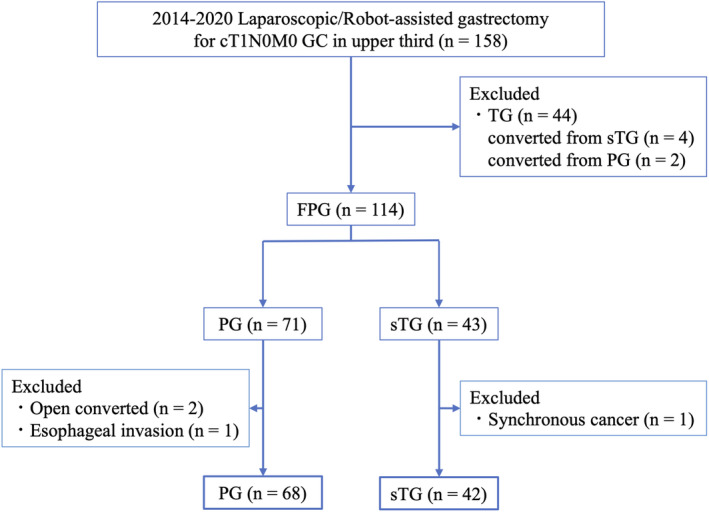
Patient flow diagram for the study. GC, gastric cancer; FPG, function‐preserving gastrectomy; PG, proximal gastrectomy; sTG, subtotal gastrectomy; TG, total gastrectomy.
The clinicopathological characteristics of the patients in each group are shown in Table 1. There were no significant differences in age, sex, BMI, tumor size, tumor circumference, and proportion of preoperative ESD between the two groups. There was a significantly higher percentage of patients with concurrent multiple gastric cancers in the sTG group. There were no significant differences in other background parameters, including pathological findings between the two groups. Four sTG cases were pStage II or higher, and three received postoperative adjuvant therapy.
TABLE 1.
Patient characteristics.
| sTG (n = 42) | PG (n = 68) | p‐value | ||
|---|---|---|---|---|
| Age (years) | Median (IQR) | 68.5 (63.3–76.8) | 70.5 (60.8–75) | 0.798 |
| Sex | Male/Female | 33/9 | 60/8 | 0.186 |
| BMI (kg/m2) | Median (IQR) | 22.8 (20.3–24.7) | 23.2 ± 3.0 | 0.358 |
| Clinical tumor depth | 1a | 8 (19.0%) | 13 (19.1%) | 0.954 |
| 1b | 34 (81.0%) | 55 (80.9%) | ||
| Tumor size (mm) | Median (IQR) | 28 (20–36) | 28 (19–40) | 0.938 |
| Circumference | Less | 16 (38.1%) | 28 (41.1%) | 0.893 |
| Ant | 5 (11.9%) | 7 (10.3%) | ||
| Post | 12 (28.6%) | 22 (32.3%) | ||
| Gre | 9 (21.4%) | 10 (14.7%) | ||
| Circ | 0 (0%) | 1 (1.5%) | ||
| ESD before surgery | 18 (42.8%) | 34 (50.0%) | 0.856 | |
| Multiple lesions | 7 (16.7%) | 3 (4.4%) | 0.041 | |
| Histological type | Intestinal | 16 (38.1%) | 21 (30.9%) | 0.139 |
| Diffuse | 14 (33.3%) | 13 (19.1%) | ||
| Mixed | 11 (26.2%) | 29 (42.6%) | ||
| Indeterminate | 1 (2.4%) | 5 (7.4%) | ||
| Preoperative Hb (g/dL) | Median (IQR) | 13.8 (12.5–15.0) | 13.8 (12.6–14.5) | 0.794 |
| Preoperative Alb (g/dL) | Median (IQR) | 4.2 (3.9–4.4) | 4.3 (4.0–4.5) | 0.134 |
| Preoperative PNI | Median (IQR) | 50.4 (46.7–52.6) | 51.6 (47.3–54.5) | 0.272 |
| Oral margin (mm) | Median (IQR) | 10 (8–22.5) | 17 (8.8–28.5) | 0.139 |
| Pathological T | 1a | 10 (23.8%) | 9 (13.2%) | 0.153 |
| 1b | 26 (61.9%) | 54 (79.4%) | ||
| 2 | 4 (9.5%) | 3 (4.4%) | ||
| 3 | 1 (2.4%) | 2 (2.9%) | ||
| Pathological stage (AJCC 8th) | IA | 33 (78.6%) | 58 (85.3%) | 0.291 |
| IB | 5 (11.9%) | 8 (11.8%) | ||
| IIA | 1 (2.4%) | 2 (2.9%) | ||
| IIB | 2 (4.7%) | 0 (0%) | ||
| III | 1 (2.4%) | 0 (0%) |
Abbreviations: AJCC, American Joint Commission on Cancer; Alb, albumin; BMI, body mass index; ESD, endoscopic submucosal resection; Hb; hemoglobin; IQR, interquartile range; PG, proximal gastrectomy; PNI, prognostic nutritional index; sTG, subtotal gastrectomy.
3.2. Surgical outcomes and postoperative complications
The surgical outcomes for each group are listed in Table 2. All patients in the PG group underwent D1+ lymph node dissection, whereas approximately 14% of the patients received D2 lymph node dissection in the sTG group. The duration of the surgery was significantly shorter and intraoperative blood loss was significantly less in the sTG group compared with that in the PG group. In the sTG group, all cases underwent RY reconstruction, whereas in the PG group, 23 (33.8%) and 45 (66.2%) cases underwent the double‐flap technique (DFT) and double‐tract reconstruction (DT), respectively. The choice of reconstruction method in the PG group was determined by the surgeon. DFT reconstruction and DT reconstruction were performed in patients with a relatively larger and smaller remnant stomach, respectively. There was no significant difference in postoperative complications between the two groups.
TABLE 2.
Surgical outcomes.
| sTG (n = 42) | PG (n = 68) | p‐value | ||
|---|---|---|---|---|
| Approach | Laparoscopic | 25 (59.5%) | 38 (55.9%) | 0.843 |
| Robot‐assisted | 17 (40.5%) | 30 (44.1%) | ||
| LN dissection | D1+ | 36 (85.7%) | 68 (100%) | 0.002 |
| D2 | 6 (14.3%) | 0 (0%) | ||
| Reconstruction | RY | 42 (100%) | ‐ | ‐ |
| DF | ‐ | 23 (33.8%) | ||
| DT | ‐ | 45 (66.2%) | ||
| Operative time (min) | Median (IQR) | 309 (259.5–374.3) | 345.5 (302.3–404) | 0.013 |
| Blood loss (mL) | Median (IQR) | 10.0 (5–37.5) | 28.5 (1–88) | 0.002 |
| Hospital stays (day) | Median (IQR) | 11 (10–13) | 11 (10–13) | 0.711 |
| 30 days mortality | 0 (0%) | 0 (0%) | ‐ | |
| Postoperative complications | ||||
| All complications | ≧CD Grade2 | 6 (14.3%) | 16 (23.5%) | 0.328 |
| Anastomotic leakage | ≧CD Grade2 | 1 (2.4%) | 3 (4.4%) | 1.000 |
| Abdominal abscess | ≧CD Grade2 | 3 (7.1%) | 5 (7.4%) | 1.000 |
| Pancreatic fistula | ≧CD Grade2 | 1 (2.4%) | 1 (1.5%) | 1.000 |
| SSI | ≧CD Grade2 | 0 (0%) | 2 (2.9%) | 0.524 |
| Postoperative bleeding | ≧CD Grade2 | 0 (0%) | 2 (2.9%) | 0.524 |
Abbreviations: IQR, interquartile range; LN, lymph node; PG, proximal gastrectomy; SSI, surgical site infection; sTG, subtotal gastrectomy.
A comparison of endoscopic findings 1 year after operation is listed in Table 3. There were no significant differences between the groups regarding the occurrence of reflex esophagitis or gastric stasis; however, postoperative anastomotic stenosis was observed in approximately 15% of the patients in the PG group and the incidence was significantly higher compared with that in the sTG group.
TABLE 3.
Endoscopically findings at 1 year after operation.
| sTG (n = 42) | PG (n = 68) | p‐value | ||
|---|---|---|---|---|
| Reflux esophagitis | ≧LA Grade B | 0 (0%) | 0 (0%) | ‐ |
| Gastric stasis | ≧RGB score 2 | 4 (9.5%) | 10 (14.7%) | 0.730 |
| Gastric ulcer | 0 (0%) | 2 (2.9%) | 0.518 | |
| Anastomotic stenosis | ≧CD Grade 2 | 0 (0%) | 10 (14.7%) | 0.006 |
Abbreviations: CD, Clavien–Dindo classification; LA, Los Angeles classification; PG, proximal gastrectomy; RGB, food residual, gastritis, and bile reflux; sTG, subtotal gastrectomy.
3.3. Postoperative nutritional outcomes
Figure 2 shows the postoperative albumin and Hb levels. Postoperative albumin levels were significantly higher in the PG group (Figure 2A; p = 0.047), whereas there was no significant difference in hemoglobin levels between the two groups (Figure 2B; p = 0.960).
FIGURE 2.
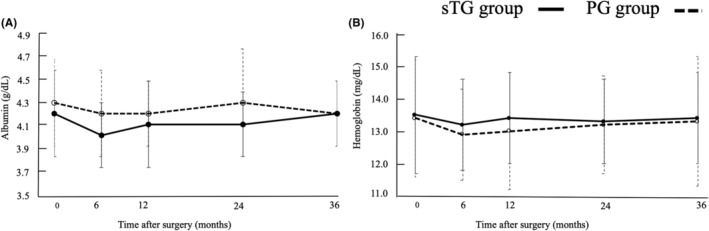
Comparison of nutritional status between subtotal gastrectomy and proximal gastrectomy: (A) albumin and (b) hemoglobin. PG, proximal gastrectomy; sTG, subtotal gastrectomy.
Figure 3 shows the changes in %BW, %PMI, %SATI, and %VATI postoperatively over 3 years. The patients in the sTG group had a significantly lower %BW compared with that in the PG group (Figure 3A; −9.9% vs. −12.5% at 6 months, −9.9% vs. −12.2% at 12 months, −9.7% vs. −12.1% at 24 months, −8.8% vs. −12.3% at 36 months, p = 0.005). Changes in %PMI in the sTG group were significantly smaller compared with that in the PG group (Figure 3B; −2.9% vs. −6.4% at 12 months, −2.3% vs. −9.6% at 24 months, 4.3% vs. −7.1% at 36 months, p = 0.002). Notably, the %PMI loss at 3 years was 4.3% higher in the sTG group compared with preoperative levels. Changes in %SATI and %VATI did not differ between the two groups (Figure 3C; p = 0.331) (Figure 3D; p = 0.845).
FIGURE 3.
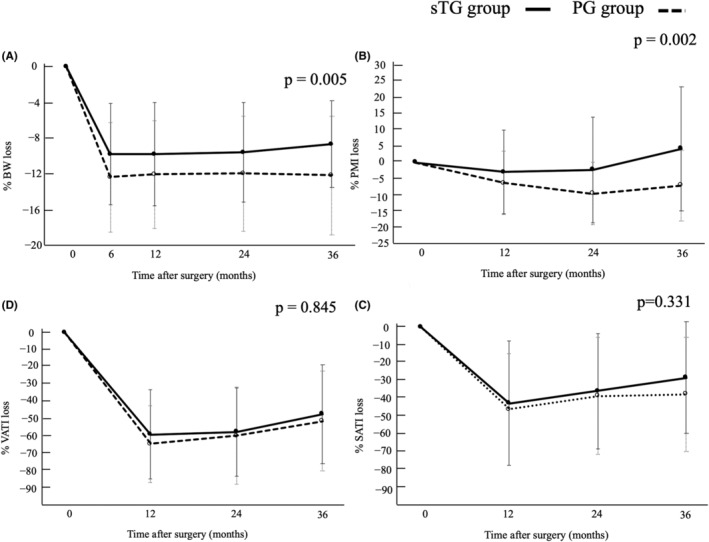
Comparison of longitudinal changes of body composition between sTG and PG subtotal gastrectomy and proximal gastrectomy: (A) body weight, (B) psoas mass index, (C) subcutaneous adipose tissue index, and (D) visceral adipose tissue index. PG, proximal gastrectomy; sTG, subtotal gastrectomy.
As a secondary analysis, we assessed changes in nutritional status in the PG group (n = 68) with or without postoperative anastomotic stenosis (Figure S1). In the PG group, postoperative anastomotic stenosis was observed in 10 patients; of these, three underwent DT reconstruction. Anastomotic stenosis was observed during esophagojejunostomy and jejunogastrostomy in two and one patient. There was no significant difference in %BW loss between patients with and without anastomotic stenosis (Figure S1A; p = 0.965). A decreasing trend was observed in %PMI loss in the group with anastomotic stenosis; however, this did not reach statistical significance (Figure S1B; p = 0.259). In addition, Figure S2 shows the postoperative nutritional outcomes of the PG group based on the reconstruction method. A comparison between the DFT and DT reconstructions showed no significant differences in %BW loss (Figure S2A; p = 0.473), %PMI loss (Figure S2B; p = 0.930), %SATI loss (Figure S2C; p = 0.085), and %VATI loss (Figure S2D; p = 0.406).
4. DISCUSSION
In this retrospective study, we assessed changes in postoperative nutritional status following sTG and PG for EGC in the upper stomach. Two important findings were obtained in this study. First, sTG and PG did not differ in short‐term postoperative complications; however, PG has a high risk of anastomotic stenosis 1 year after operation. Second, there is an advantage of postoperative nutritional status of %BW and %PMI in the sTG group over the PG group at 3 years postoperation.
There were limitations to the selection of surgical procedures in this retrospective study. Some cases of EGC in the upper region of the stomach, resulting from the circumferential involvement of the wall, were technically challenging for sTG. Thus, some of these cases were included in the PG group for analysis. Therefore, the surgical indications for sTG and PG were not strictly the same. sTG has previously been shown to reduce the deterioration of postoperative quality of life and weight loss compared with TG. 11 , 21 In contrast, PG showed better results compared with TG in terms of postoperative nutritional status, including the frequency of postoperative complications and weight changes. 22 TG is a risk factor for loss of skeletal muscle mass, compared with the other gastrectomy procedures. 23 Skeletal muscle wasting was associated with a poorer QOL 24 and poor compliance during adjuvant chemotherapy and is a risk factor for survival. 25 Therefore, avoiding TG if possible and undergoing sTG or PG is the best option for patients with EGC in the upper third of the stomach.
It remains unclear whether sTG or PG is the superior procedure. A previous study comparing LsTG and LPG found that LPG was superior with respect to Hb levels; however, postoperative BW, serum albumin, and PNI were comparable. 7 In the present study, esophagogastrostomy with DFT was used for reconstruction after PG, which caused differences in iron absorption kinetics because the acid secretory area is preserved in PG. The reconstruction method allows all food to pass through the remnant stomach. In a study including DT reconstruction, postoperative serum albumin, and PNI values in LsTG were superior compared with that in LPG; however, postoperative weight change was comparable between the two procedures. 11 In the present study, reconstruction methods included both DFT and DT. DFT was shown to be superior to DT in reducing postoperative BW loss 26 ; however, the subgroup analysis showed no difference in postoperative nutritional status by the reconstruction method in the present study. We concluded that there is no difference in postoperative nutritional index regardless of the reconstruction method used. In addition, the sTG group included four cases of advanced cancer based on postoperative pathological diagnosis. Postoperative adjuvant chemotherapy was administered to three of four patients, considered while evaluating the postoperative nutritional status. However, the average rate of weight loss (−7.9%) and the rate of change in PMI (+2.4%) at 3 years postoperation did not significantly deviate from the overall sTG group (−8.8%, +4.3%), indicating that the effect of adjuvant chemotherapy was minimal and negligible.
In addition to laparoscopic surgery, robot‐assisted surgery was also included in this analysis as a method in the present study. The role of robotic surgery on postoperative nutritional status has not yet been reported. To our knowledge, this is the first study comparing the postoperative nutritional status of sTG and PG in minimally invasive surgery, including robot‐assisted surgery. There appears to be no difference between laparoscopic and robotic surgical approaches in terms of short‐term outcomes and postoperative nutritional status. The results of this study, which showed that sTG reduced BW and PMI loss compared with PG at 3 years postoperatively, may provide a basis for making surgical decisions for the treatment of EGC in the upper third of the stomach. The PGSAS‐NEXT Study 27 demonstrated that proximal gastrectomy is superior to TG concerning postoperative symptoms and quality of life (QOL) in patients with proximal gastric cancer. To prevent the deterioration of symptoms and QOL postgastrectomy, avoiding TG is necessary; however, for upper gastric cancer, further studies are needed to determine how the preservation of the stomach can result in improved QOL.
The results of the present study revealed a difference in postoperative BW changes between sTG and PG, suggesting that the main reason for this difference is not because of body fat mass, but rather the suppression of skeletal muscle loss. The reason for the difference in skeletal muscle between sTG and PG was initially believed to be the shortcoming of PG in maintaining dietary intake because of the higher rate of postoperative anastomotic stenosis. However, the subgroup analysis of the PG group showed no difference in postoperative BW loss or PMI loss between patients with and without anastomotic stenosis. This suggests that the influence of anastomotic stenosis was not substantial. Early postoperative weight loss is primarily the result of skeletal muscle mass loss, 28 and a strong correlation has been found between the rate of lean body weight loss and the rate of weight loss. 29 The body weight loss after gastrectomy was attributed to a variety of factors, including anatomical changes resulting from reconstruction, decreased food intake due to decreased gastric retention, or a combination of these factors may have contributed to the results of the present study. In addition, the postgastrectomy decrease in ghrelin, a peptide hormone responsible for pleiotropic functions including appetite stimulation, is also considered to be a major cause. Ghrelin‐producing cells have been identified immunohistochemically in the fundic gland of the stomach. 30 A persistent decline of serum ghrelin and BW was frequently observed after TG. 31 The biggest difference between the sTG and PG procedures is the preservation of the fundus. In sTG, ghrelin secretion was preserved postoperatively by preserving the fundus, which may have resulted in preventing decreased appetite, suppression of protein catabolism associated with growth hormone secretion, suppression of weight loss, and muscle mass loss. There are no reports describe the differences in postoperative ghrelin secretion between PG and sTG and their relationship with food intake. This relationship will potentially be examined in future studies.
A detailed evaluation of the distance between the proximal tumor margins and the esophagogastric junction must be made when selecting a surgical procedure, and preoperative negative biopsy and simulation of the dissection line are essential.
The present study has several limitations. First, this is a single‐center, retrospective study, and the number of cases analyzed was not large. Second, this study did not evaluate the relationship between the size of the remnant stomach and postoperative nutritional indicators. Ri et al. indicated that the size of the remnant stomach is crucial for maintaining SMI in PG cases undergoing DFT reconstruction. 32 Similarly, the size of the remnant stomach influences the comparison between sTG and PG. Third, the assessment of patient QOL survey to assess postoperative nutritional status was subjective. In conclusion, sTG is considered a desirable FPG for EGC in the upper third of the stomach, with less postoperative weight loss and PMI loss.
AUTHOR CONTRIBUTIONS
W. Soneda: Conceptualization (lead); writing—original draft (lead); formal analysis (lead); writing—review and editing (equal). M. Terashima: Conceptualization (lead); writing—original draft (lead); formal analysis (lead); writing—review and editing (equal). Y. Koseki, K. Furukawa, K. Fujiya, Y. Tanizawa, H. Takeuchi, and E. Bando: Provision of study materials and patients (equal); writing—review and editing (equal). All authors approved the final version of the manuscript.
FUNDING INFORMATION
No external funding was received for this study.
CONFLICT OF INTEREST STATEMENT
Author M. T. has received personal fees from Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol Myers Squib Japan, Yakult Honsha, Takeda Pharmaceutical, Eli Lilly Japan, Pfizer Japan, Daiichi Sankyo, Johnson and Johnson, Medtronic Japan, Intuitive Japan, and Olympus outside of the submitted work. Author E. B. has received personal fees from Daiichi Sankyo, Johnson and Johnson, Medtronic Japan, Intuitive Japan outside of the submitted work. Author H. T. is an editorial board member of Annals of Gastroenterological Surgery. Authors declare no conflicts of interest for this article.
ETHICS STATEMENT
Approval of the research protocol: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki (Fortaleza, Brazil, October 2013).
Informed Consent: Informed consent or an appropriate substitute was obtained.
Registry and the Registration No. of the study/trial: The ethics committees of SCC (Shizuoka, Japan) reviewed and approved this study (approval number: J2023‐96).
Animal Studies: N/A.
PATIENT CONSENT STATEMENT
Informed consent was obtained from all the participants.
Supporting information
Figure S1.
Figure S2.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the cooperation of every participant in this study.
Soneda W, Terashima M, Koseki Y, Furukawa K, Fujiya K, Tanizawa Y, et al. Comparison of surgical outcomes and postoperative nutritional parameters between subtotal and proximal gastrectomy in patients with proximal early gastric cancer. Ann Gastroenterol Surg. 2025;9:89–97. 10.1002/ags3.12856
REFERENCES
- 1. Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Global cancer observatory: cancer today. International Agency for Research on Cancer. https://gco.iarc.who.int/today
- 2. Fujishiro M, Yoshida S, Matsuda R, Narita A, Yamashita H, Seto Y. Updated evidence on endoscopic resection of early gastric cancer from Japan. Gastric Cancer. 2017;20(suppl 1):39–44. [DOI] [PubMed] [Google Scholar]
- 3. Terayama M, Ohashi M, Ida S, Hayami M, Makuuchi R, Kumagai K, et al. Advantages of function‐preserving gastrectomy for older patients with upper‐third early gastric cancer: maintenance of nutritional status and favorable survival. J Gastric Cancer. 2023;23(2):303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee I, Oh Y, Park SH, Kwon Y, Park S. Postoperative nutritional outcomes and quality of life‐related complications of proximal versus total gastrectomy for upper‐third early gastric cancer: a meta‐analysis. Sci Rep. 2020;10(1):21460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2021. Gastric Cancer. 2023;26(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kano Y, Ohashi M, Ida S, Kumagai K, Nunobe S, Sano T, et al. Oncological feasibility of laparoscopic subtotal gastrectomy compared with laparoscopic proximal or total gastrectomy for cT1N0M0 gastric cancer in the upper gastric body. Gastric Cancer. 2019;22(5):1060–1068. [DOI] [PubMed] [Google Scholar]
- 7. Kano Y, Ohashi M, Ida S, Kumagai K, Sano T, Hiki N, et al. Laparoscopic proximal gastrectomy with double‐flap technique versus laparoscopic subtotal gastrectomy for proximal early gastric cancer. BJS Open. 2020;4(2):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand. 1965. Apr;64:31–49. [DOI] [PubMed] [Google Scholar]
- 9. Jiang X, Hiki N, Nunobe S, Nohara K, Kumagai K, Sano T, et al. Laparoscopy‐assisted subtotal gastrectomy with very small remnant stomach: a novel surgical procedure for selected early gastric cancer in the upper stomach. Gastric Cancer. 2011;14(2):194–199. [DOI] [PubMed] [Google Scholar]
- 10. Furukawa K, Kamiya S, Sugino T, Aizawa D, Kawabata T, Notsu A, et al. Optimal extent of lymph node dissection in patients with gastric cancer who underwent non‐curative endoscopic submucosal dissection with a positive vertical margin. Eur J Surg Oncol. 2020;46(12):2229–2235. [DOI] [PubMed] [Google Scholar]
- 11. Kawakatsu S, Ohashi M, Hiki N, Nunobe S, Nagino M, Sano T. Use of endoscopy to determine the resection margin during laparoscopic gastrectomy for cancer. Br J Surg. 2017;104(13):1829–1836. [DOI] [PubMed] [Google Scholar]
- 12. Kuroda S, Nishizaki M, Kikuchi S, Noma K, Tanabe S, Kagawa S, et al. Double‐flap technique as an antireflux procedure in esophagogastrostomy after proximal gastrectomy. J Am Coll Surg. 2016;223(2):e7–e13. [DOI] [PubMed] [Google Scholar]
- 13. Hayami M, Hiki N, Nunobe S, Mine S, Ohashi M, Kumagai K, et al. Clinical outcomes and evaluation of laparoscopic proximal gastrectomy with double‐flap technique for early gastric cancer in the upper third of the stomach. Ann Surg Oncol. 2017;24(6):1635–1642. [DOI] [PubMed] [Google Scholar]
- 14. Lewis TS, Feng Y. A review on double tract reconstruction after proximal gastrectomy for proximal gastric cancer. Ann Med Surg (Lond). 2022;79:103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Ann Surg. 2009;250(2):187–196. [DOI] [PubMed] [Google Scholar]
- 16. Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D, et al. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer. 2002;5(2):83–89. [DOI] [PubMed] [Google Scholar]
- 18. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85(1):115–122. [DOI] [PubMed] [Google Scholar]
- 19. Kvist H, Sjöström L, Tylén U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes. 1986;10(1):53–67. [PubMed] [Google Scholar]
- 20. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, et al. Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg. 2012;256(6):1008–1013. [DOI] [PubMed] [Google Scholar]
- 22. Huh YJ, Lee HJ, Oh SY, Lee KG, Yang JY, Ahn HS, et al. Clinical outcome of modified laparoscopy‐assisted proximal gastrectomy compared to conventional proximal gastrectomy or total gastrectomy for upper‐third early gastric cancer with special references to postoperative reflux esophagitis. J Gastric Cancer. 2015;15(3):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshida S, Nishigori T, Maekawa H, Hoshino N, Hisamori S, Tsunoda S, et al. Total gastrectomy as a risk factor for postoperative loss of skeletal muscle in minimally invasive surgery for patients with gastric cancer. Asian J Endosc Surg. 2023;16(4):715–723. [DOI] [PubMed] [Google Scholar]
- 24. Huang DD, Ji YB, Zhou DL, Li B, Wang SL, Chen XL, et al. Effect of surgery‐induced acute muscle wasting on postoperative outcomes and quality of life. J Surg Res. 2017;218:58–66. [DOI] [PubMed] [Google Scholar]
- 25. Aoyama T, Yoshikawa T, Maezawa Y, Kano K, Numata M, Hara K, et al. The postoperative lean body mass loss at one month leads to a poor survival in patients with locally advanced gastric cancer. J Cancer. 2019;10(11):2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu B, Park KB, Park JY, Lee SS, Kwon OK, Chung HY, et al. Double tract reconstruction versus double flap technique: short‐term clinical outcomes after laparoscopic proximal gastrectomy for early gastric cancer. Surg Endosc. 2022;36(7):5243–5256. [DOI] [PubMed] [Google Scholar]
- 27. Kunisaki C, Yoshida K, Yoshida M, Matsumoto S, Arigami T, Sugiyama Y, et al. Effects of proximal gastrectomy and various clinical factors on postoperative quality of life for upper‐third gastric cancer assessed using the postgastrectomy syndrome assessment scale‐ 45 (PGSAS‐45): a PGSAS next study. Ann Surg Oncol. 2022;29(6):3899–3908. [DOI] [PubMed] [Google Scholar]
- 28. Abdiev S, Kodera Y, Fujiwara M, Koike M, Nakayama G, Ohashi N, et al. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer. 2011;14(2):144–149. [DOI] [PubMed] [Google Scholar]
- 29. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. [DOI] [PubMed] [Google Scholar]
- 30. Takiguchi S, Adachi S, Yamamoto K, Morii E, Miyata H, Nakajima K, et al. Mapping analysis of ghrelin producing cells in the human stomach associated with chronic gastritis and early cancers. Dig Dis Sci. 2012;57(5):1238–1246. [DOI] [PubMed] [Google Scholar]
- 31. Takachi K, Doki Y, Ishikawa O, Miyashiro I, Sasaki Y, Ohigashi H, et al. Postoperative ghrelin levels and delayed recovery from body weight loss after distal or total gastrectomy. J Surg Res. 2006;130(1):1–7. [DOI] [PubMed] [Google Scholar]
- 32. Ri M, Nunobe S, Makuuchi R, Ida S, Kumagai K, Ohashi M, et al. Key factors for maintaining postoperative skeletal muscle mass after laparoscopic proximal gastrectomy with double‐flap technique reconstruction for early gastric cancer. J Gastrointest Surg. 2021;25(6):1569–1572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.


