Abstract
Aims
Liver fibrosis predisposes patients to liver failure and hepatocellular carcinoma. Various markers, which can be calculated easily from serum parameters, have been reported to predict liver fibrosis accurately. This study investigated the prognostic factors, including blood‐based markers for liver fibrosis of patients with hepatocellular carcinoma following initial curative hepatectomy.
Methods
This retrospective study included 407 patients with hepatocellular carcinoma who underwent initial curative hepatectomy between April 2010 and December 2017. We investigated prognosis‐associated variables in these patients.
Results
Among the blood‐based markers for liver fibrosis examined in this study, the steatosis‐associated fibrosis estimator score demonstrated the best predictive capabilities. This score was revealed as a poor prognostic factor for both overall survival and recurrence‐free survival in patients with hepatocellular carcinoma following initial curative hepatectomy. A high steatosis‐associated fibrosis estimator score was independently associated with poor overall survival and recurrence‐free survival. After propensity score‐matching to minimize bias between high‐ and low‐steatosis‐associated fibrosis estimator score groups, the high steatosis‐associated fibrosis estimator score remained associated with poor overall survival and recurrence‐free survival.
Conclusions
The steatosis‐associated fibrosis estimator score is an independent predictor of long‐term prognosis in patients with hepatocellular carcinoma following initial curative hepatectomy.
Keywords: blood‐based liver fibrosis markers, hepatocellular carcinoma, initial curative hepatectomy, liver fibrosis, steatosis‐associated fibrosis estimator score
A high preoperative SAFE score was found to be independently associated with a poor prognosis in patients with HCC after initial curative hepatectomy. The preoperative SAFE score reflects the degree of liver fibrosis and is a useful assessment index for predicting the prognosis of patients who have undergone curative hepatectomy of HCC.
1. INTRODUCTION
Primary liver cancer is the third leading cause of cancer death. Hepatocellular carcinoma (HCC) accounts for 75%–80% of all liver cancers. 1 Although hepatectomy remains the main treatment for patients with early stage HCC and good liver function, 2 the postoperative intrahepatic recurrence rate is high, 3 , 4 and survival is poor after recurrence. 5 Certain studies indicate that hepatectomy for intrahepatic recurrence subsequent to HCC surgery enhances prognostic outcomes, 6 , 7 accurate postoperative prognostic markers need to be identified in order to plan the appropriate follow‐up strategies for HCC.
Recently, the number of cases of HCC related to hepatitis C virus (HCV) infection has decreased, while that of non‐viral HCC, such as those associated with diabetes mellitus (DM), dyslipidemia, and fatty liver, has increased. 8 Some patients with non‐alcoholic fatty liver disease (NAFLD), a non‐viral disease, as well as patients with viral disease develop liver fibrosis, with a certain proportion progressing to cirrhosis and liver failure, portal hypertension, and HCC. 9 , 10 Recently, metabolic dysfunction‐associated steatotic liver disease (MASLD) was defined as a new concept to replace NAFLD. 11 Liver fibrosis has been reported to be a risk factor for HCC recurrence after curative hepatectomy. 12 , 13 Thus, fibrosis serves as a crucial indicator for identifying patients at elevated risk of HCC. However, a conclusive diagnosis of liver fibrosis necessitates pathological evaluation. Liver biopsy, an invasive technique, is difficult to perform in all patients.
In contrast, various blood‐based markers for liver fibrosis have been reported as a means to detect clinically significant liver fibrosis in a noninvasive manner. The fibrosis‐4 (FIB‐4) index and the aspartate aminotransferase (AST)‐to‐platelet ratio index (APRI) were developed as non‐invasive markers, involving blood examinations, such as AST and alanine aminotransferase (ALT), for predicting liver fibrosis in patients with HCV. 14 , 15 The NAFLD fibrosis score (NFS) was developed to identify advanced liver fibrosis in NAFLD patients. 16 These scores can predict fibrosis in various liver backgrounds and have also been reported as prognostic factors after hepatic resection. 17 , 18 , 19 , 20 Recently, the steatosis‐associated fibrosis estimator (SAFE) score was reported as a new liver fibrosis marker in patients with NAFLD, 21 and is increasingly being recognized for its utility in stratifying degrees of liver fibrosis. To date, no studies have detailed the prognostic impact of the SAFE score after hepatectomy for HCC.
This retrospective study therefore compared blood‐based markers for liver fibrosis as prognostic indicators for HCC after initial curative hepatectomy, and investigated prognostic factors, including preoperative liver fibrosis markers, which affect the prognosis of patients who underwent initial curative hepatectomy for HCC.
2. MATERIALS AND METHODS
2.1. Study population
This retrospective study evaluated 713 patients with HCC who underwent hepatectomy between April 2010 and December 2017 at the Hiroshima University Hospital. Patients with repeat hepatectomy, distant metastasis, R1 resection, or who died within 30 days after surgery were excluded. Finally, 407 patients who underwent curative hepatectomy for the first time were enrolled in this study (Figure 1).
FIGURE 1.
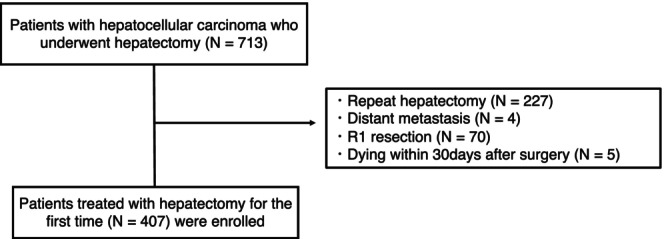
Flowchart of the study design.
This study was conducted in accordance with the guidelines of the Declaration of Helsinki (Fortaleza, Brazil; October 2013) and was approved by the Institutional Review Board of Hiroshima University Hospital (Approval no. E‐1580).
2.2. Definition of HBV‐positive and HCV‐positive patients
The definition of hepatitis B virus (HBV) positive and HCV positive is as follows: HBV‐positive patients are patients with a positive HBs antigen and positive HBV‐deoxyribonucleic acid, while HCV‐positive are patients with positive HCV‐ribonucleic acid or who have achieved sustained virological response following hepatitis treatment.
2.3. Definition of fibrosis stage and blood‐based markers for liver fibrosis
The stage of liver fibrosis (F0–F4) was diagnosed from pathological findings based on the scoring system proposed by Ichida et al. 22 The definition of each fibrosis stage is as follows: F0, no fibrosis; F1, fibrous portal expansion; F2, bridging fibrosis (portal–portal or portal–central linkage); F3, bridging fibrosis with lobular distortion; F4, cirrhosis. 22 Each blood‐based marker for liver fibrosis was calculated by preoperative blood examination. The SAFE score, FIB‐4 index, APRI, and NFS were calculated according to their formulas, as shown below 14 , 15 , 16 , 21 :
Age was expressed in years, BMI in kg/m2, AST and ALT in U/L, platelets as 109/L, and Alb and globulin in g/dL.
Globulin was calculated as serum total protein level (g/dL) − serum Alb level (g/dL). For APRI, the upper limit of normal range for AST was set as 40.
2.4. Definition of post hepatectomy liver failure
Post hepatectomy liver failure (PHLF) was defined according to International Study Group of Liver Surgery (ISGLS) diagnostic criteria, using international normalized ratio (INR) and total bilirubin (T‐Bil) levels on or after hepatectomy day 5. 23 Using this diagnostic guide, no specific treatment was required for patients with grade A PHLF, noninvasive treatments such as fresh‐frozen plasma, Alb, and daily diuretics were required for patients with grade B PHLF, and invasive procedures were required for patients with grade C PHLF.
2.5. Treatment and follow‐up
A follow‐up blood examination to identify tumor markers was performed every 3 months after surgery, for 5 years. Enhanced abdominal computed tomography was performed to rule out recurrence for 6 months. When HCC recurrence was suspected, magnetic resonance imaging was performed.
2.6. Statistical analysis
Continuous variables are presented as medians and interquartile ranges (only tumor number is range). Nominal variables are expressed as numbers (%). Nonparametric quantitative data were analyzed using the Mann–Whitney U‐test. The chi‐square test or Fisher's exact test was performed to determine the relationships among nominal variables. For continuous variables such as operative time and intraoperative blood loss, median values were used as cutoff values. The performance of the prognostic systems of blood‐based markers for liver fibrosis outcomes was separately evaluated in terms of the area under the curve receiver operating characteristic curve (AUROC) obtained in the receiver operating characteristic (ROC) curve analysis. The Kaplan–Meier method was used to analyze overall survival (OS) and recurrence‐free survival (RFS), and the log‐rank test was used to compare different groups. The cutoff values for the groups being compared using ROC curve analysis were set using Youden's J statistics. Yuden's J statistic determines the optimal cutoff value by maximizing the Se (true positive rate) and Sp (true negative rates) using the Yuden index. 24 Multivariate analyses were performed to assess the factors influencing OS and RFS using the Cox regression model. The backward‐elimination method with a removal criterion of p = 0.10 was used to select covariates. The multivariate analysis included the variables sex, liver background, SAFE score, T‐Bil, prothrombin time (PT), indocyanine green dye retention rate at 15 min (ICG‐R15), α‐fetoprotein (AFP), des‐gamma‐carboxy prothrombin (DCP), tumor number, tumor diameter, histology, vascular invasion, intrahepatic metastasis (IM), operative time, intraoperative blood loss, intraoperative blood transfusion, and postoperative complications. Age, BMI, and DM were excluded from the multivariate analysis, considering multicollinearity of the relevant clinical variables associated with the SAFE score.
To overcome the bias caused by different distributions of covariates among patients from the high‐ and low‐SAFE score groups, propensity score‐matched (PSM) analysis was performed using a multiple logistic regression model based on the clinicopathological variables. PSM analysis was performed according to baseline characteristics, such as sex, liver background, PT, ICG‐R15, tumor number, tumor histology, intraoperative blood loss, and postoperative complications, which were variables that differed significantly (p‐values of <0.05). p‐values <0.05 were considered statistically significant. Calculations were performed using JMP v17 (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Characteristics of patients in this study
Among 713 patients with HCC who underwent hepatectomy between April 2010 and December 2018 at our institute, 407 patients who underwent initial hepatectomy were enrolled in this study, after excluding 306 patients who met the exclusion criteria (Figure 1). Table 1 summarizes the characteristics of the patients included in this study. The median age of the patients was 70 years, with more men than women among the patients enrolled. The most common liver background was HCV infection (50.9%). The median AFP and DCP levels were 9.1 ng/mL and 42.0 mAU/mL. The median values of each blood‐based marker for liver fibrosis assessed were relatively high (SAFE score: 167.4, FIB‐4 index: 2.84, APRI: 0.53, NFS: 0.01). Overall, 225 patients (55.3%) experienced recurrence, with recurrence occurring within 2 years in 59.1% (133 patients).
TABLE 1.
Patient characteristics in this study.
| Characteristics | Patients (n = 407) |
|---|---|
| Male/Female | 318/89 |
| Age (years) | 70 (64–77) |
| BMI (kg/m2) | 23.1 (21.0–25.1) |
| Liver background | |
| HCV | 203 (49.9%) |
| HBV | 75 (18.4%) |
| HCV + HBV | 4 (1.0%) |
| NAFLD | 30 (7.4%) |
| Alcohol | 42 (10.3%) |
| Others | 53 (13.0%) |
| DM (+) | 136 (33.4%) |
| T‐Bil (mg/dL) | 0.8 (0.6–1.0) |
| PT (%) | 86.5 (79–95) |
| Alb (g/dL) | 4.0 (3.7–4.4) |
| ICG‐R15 (%) | 13.7 (8.6–19.2) |
| AFP (ng/mL) | 9.1 (4.3–57.8) |
| DCP (mAU/mL) | 42.0 (21.0–349.5) |
| SAFE score | 167.4 (78.7–249.4) |
| FIB‐4 index | 2.84 (1.83–4.68) |
| APRI | 0.53 (0.34–0.90) |
| NFS | 0.01 (−0.91–0.95) |
| Tumor number | 1 (1–13) |
| Tumor diameter (mm) | 25 (16–40) |
| Histology: poorly differentiation /other | 42/365 |
| Vascular invasion: Vp (+) | 64 (15.8%) |
| Vascular invasion: Vv (+) | 24 (6.0%) |
| IM (+) | 36 (8.9%) |
Note: Continuous variables are expressed as medians (interquartile ranges: only tumor number is range). Qualitative variables are expressed as numbers (%).
Abbreviations: AFP, α‐fetoprotein; Alb, albumin; APRI, aspartate aminotransferase‐to‐platelet ratio index; BMI, body mass index; DCP, des‐gamma‐carboxy prothrombin; DM, diabetes mellitus; FIB‐4 index, fibrosis‐4 index; HBV, hepatitis B virus; HCV, hepatitis C virus; ICG‐R15, indocyanine green dye retention rate at 15 min; IM, intrahepatic metastasis; NAFLD, non‐alcoholic fatty liver disease; NFS, NAFLD fibrosis score; PT, prothrombin time; SAFE score, steatosis‐associated fibrosis estimator score; T‐Bil, total‐bilirubin; Vp, portal vein invasion; Vv, hepatic venous invasion.
3.2. Comparison of blood‐based markers for liver fibrosis
Figure S1 shows the relationship between the degree of liver fibrosis and the new Inuyama classification, which was fibrosis classification revealed from postoperative pathological diagnosis. Risk categories for each liver fibrosis marker were defined based on the two cutoff points described in the original publications. 14 , 15 , 16 , 21 For the SAFE score, the degree of fibrosis was significantly worse (p = 0.002) in the group of patients with a score exceeding 100, defined as the high‐risk group in a previous study. The high‐risk group was almost exclusively composed of patients with liver fibrosis over F2, a trend that was similar for other blood‐based markers for liver fibrosis. Figure 2 presents a comparison of ROC curve analysis for each blood‐based marker for liver fibrosis with reference to OS after surgery. The AUROC value of the SAFE score was 0.6395, which was higher than that of the other indices (FIB‐4 index, 0.6234; APRI, 0.6208; NFS, 0.6125).
FIGURE 2.
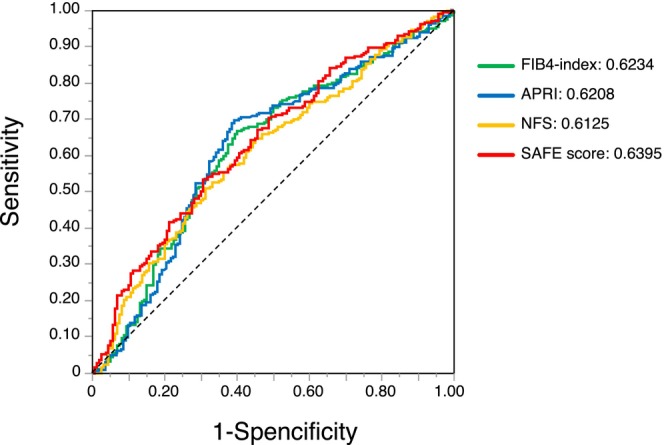
Comparison of the areas under receiver operating characteristic curves for survival prediction among the blood‐based markers for liver fibrosis.
3.3. Comparison of univariate and multivariate analyses of prognostic factors for OS and RFS in patients with hepatocellular carcinoma following first hepatectomy
Table 2 summarizes the results of the univariate and multivariate analyses of prognostic factors for OS. Among the blood‐based markers for liver fibrosis, the SAFE score, which demonstrated most adequate predictive capabilities for prognosis in ROC analysis, was included in the analysis. In univariate analyses, statistically significant prognostic factors for poor OS were age (p < 0.001), DM (+) (p = 0.036), SAFE score (p < 0.001), PT (p = 0.006), ICG‐R15 (p = 0.017), DCP (p = 0.003), tumor number (p = 0.011), tumor diameter (p < 0.001), portal vein invasion (Vp) (+) (p < 0.001), hepatic venous invasion (Vv) (+) (p = 0.002), IM (+) (p = 0.006), operative time (p = 0.013), intraoperative blood loss (p < 0.001), intraoperative blood transfusion (+) (p < 0.001), and postoperative complications (Clavien–Dindo [CD]) ≥ 3 (p < 0.001). In the multivariate analysis, the following five factors were identified as prognostic factors for poor OS in patients: SAFE score (hazard ratio [HR] = 1.33; 95% confidence interval [CI] = 1.19–1.48, p < 0.001), PT (HR = 0.90; 95% CI = 0.81–0.99, p = 0.031), tumor diameter (HR = 1.10; 95% CI = 1.05–1.15, p < 0.001), Vp (+) (HR = 1.95; 95% CI = 1.32–2.88, p < 0.001), and postoperative complications: CD ≥3 (HR = 2.31; 95% CI = 1.52–3.51, p < 0.001).
TABLE 2.
Univariate and multivariate analyses of prognostic factor for overall survival.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p‐Value | HR | 95% CI | p‐Value | |
| Male | 0.91 | 0.62–1.31 | 0.599 | |||
| Age (year) per increase of 10 | 1.35 | 1.13–1.61 | <0.001 | — | — | — |
| BMI (kg/m2) per increase of 10 | 0.62 | 0.37–1.01 | 0.056 | — | — | — |
| Liver background: HCV | 0.93 | 0.68–1.27 | 0.667 | |||
| Liver background: HBV | 0.54 | 0.34–0.86 | 0.009 | |||
| DM (+) | 1.40 | 1.02–1.93 | 0.036 | — | — | — |
| SAFE score per increase of 100 | 1.34 | 1.21–1.47 | <0.001 | 1.33 | 1.19–1.48 | <0.001 |
| T‐Bil (mg/dL) | 1.22 | 0.85–1.68 | 0.273 | |||
| PT (%) per increase of 10 | 0.87 | 0.80–0.96 | 0.006 | 0.90 | 0.81–0.99 | 0.031 |
| ICG‐R15 (%) per increase of 10 | 1.18 | 1.03–1.33 | 0.017 | |||
| AFP (ng/mL) per increase of 100 | 1.04 | 0.94–1.09 | 0.330 | |||
| DCP (mAU/mL) per increase of 100 | 1.11 | 1.04–1.17 | 0.003 | |||
| Tumor number | 1.13 | 1.03–1.21 | 0.011 | |||
| Tumor diameter (mm) per increase of 10 | 1.13 | 1.08–1.18 | <0.001 | 1.10 | 1.05–1.15 | <0.001 |
| Histology: poorly differentiation | 1.52 | 0.96–2.41 | 0.072 | |||
| Vascular invasion: Vp (+) | 2.39 | 1.65–3.46 | <0.001 | 1.95 | 1.32–2.88 | <0.001 |
| Vascular invasion: Vv (+) | 2.34 | 1.37–3.98 | 0.002 | |||
| IM (+) | 1.88 | 1.20–2.95 | 0.006 | |||
| Operative time (min) per increase of 100 | 1.20 | 1.04–1.38 | 0.013 | |||
| Intraoperative blood loss (mL) per increase of 1000 | 1.37 | 1.17–1.56 | <0.001 | |||
| Intraoperative blood transfusion (+) | 2.53 | 1.53–4.20 | <0.001 | |||
| Postoperative complications CD ≥3 | 2.86 | 1.91–4.28 | <0.001 | 2.31 | 1.52–3.51 | <0.001 |
Note: The variables in bold are statistically significant (p < 0.05).
Abbreviations: AFP, α‐fetoprotein; BMI, body mass index; CD, Clavien–Dindo; CI, confidence interval; DCP, des‐gamma‐carboxy prothrombin; DM, diabetes mellitus; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; ICG‐R15, indocyanine green dye retention rate at 15 min; IM, intrahepatic metastasis; PT, prothrombin time; SAFE score, steatosis‐associated fibrosis estimator score; T‐Bil, total‐bilirubin; Vp, portal vein invasion; Vv, hepatic venous invasion.
Table 3 summarizes the results of the univariate and multivariate analyses of prognostic factors for RFS. In the univariate analysis, statistically significant prognostic factors for poor RFS were age (p = 0.046), SAFE score (p < 0.001), ICG‐R15 (p = 0.008), tumor number (p = 0.002), tumor diameter (p < 0.001), Vp (+) (p < 0.001), Vv (+) (p = 0.005), IM (+) (p = 0.034), operative time (p = 0.002), intraoperative blood loss (p < 0.001), intraoperative blood transfusion (+) (p = 0.001), and postoperative complications: CD ≥ 3 (p < 0.001). In the multivariate analysis, the following seven factors were identified as prognostic factors for poor RFS in patients: male (HR = 1.40; 95% CI = 1.02–1.90, p = 0.035), HCV (HR = 1.32; 95% CI = 1.01–1.71, p = 0.043), SAFE score (HR = 1.26; 95% CI = 1.14–1.37, p < 0.001), tumor number (HR = 1.13; 95% CI = 1.04–1.21, p = 0.003), tumor diameter (HR = 1.10; 95% CI = 1.02–1.11, p = 0.005), Vp (+) (HR = 1.65; 95% CI = 1.17–2.31, p = 0.004), and intraoperative blood loss (HR = 1.23; 95% CI = 1.05–1.41, p = 0.015).
TABLE 3.
Univariate and multivariate analyses of prognostic factor for recurrence‐free survival.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p‐Value | HR | 95% CI | p‐Value | |
| Male | 1.15 | 0.86–1.54 | 0.358 | 1.40 | 1.02–1.90 | 0.035 |
| Age (year) per increase of 10 | 1.13 | 1.00–1.28 | 0.046 | — | — | — |
| BMI (kg/m2) per increase of 10 | 0.87 | 0.60–1.26 | 0.467 | — | — | — |
| Liver background: HCV | 1.11 | 0.88–1.41 | 0.370 | 1.32 | 1.01–1.71 | 0.043 |
| Liver background: HBV | 0.73 | 0.53–0.99 | 0.046 | |||
| DM (+) | 1.23 | 0.96–1.57 | 0.097 | — | — | — |
| SAFE score per increase of 100 | 1.28 | 1.17–1.39 | <0.001 | 1.26 | 1.14–1.37 | <0.001 |
| T‐Bil (mg/dL) | 1.10 | 0.83–1.40 | 0.505 | |||
| PT (%) per increase of 10 | 0.94 | 0.87–1.02 | 0.123 | |||
| ICG‐R15 (%) per increase of 10 | 1.15 | 1.04–1.26 | 0.008 | |||
| AFP (ng/mL) per increase of 100 | 1.07 | 0.99–1.11 | 0.064 | |||
| DCP (mAU/mL) per increase of 100 | 1.05 | 0.99–1.10 | 0.070 | |||
| Tumor number | 1.13 | 1.05–1.19 | 0.002 | 1.13 | 1.04–1.21 | 0.003 |
| Tumor diameter (mm) per increase of 10 | 1.10 | 1.05–1.14 | <0.001 | 1.10 | 1.02–1.11 | 0.005 |
| Histology: poorly differentiation | 1.33 | 0.92–1.92 | 0.131 | |||
| Vascular invasion: Vp (+) | 1.96 | 1.44–2.68 | <0.001 | 1.65 | 1.17–2.31 | 0.004 |
| Vascular invasion: Vv (+) | 1.97 | 1.23–3.17 | 0.005 | |||
| IM (+) | 1.523 | 1.03–2.26 | 0.034 | |||
| Operative time (min) per increase of 100 | 1.20 | 1.07–1.34 | 0.002 | |||
| Intraoperative blood loss (mL) per increase of 1000 | 1.35 | 1.18–1.50 | <0.001 | 1.23 | 1.05–1.41 | 0.015 |
| Intraoperative blood transfusion (+) | 2.14 | 1.39–3.30 | 0.001 | |||
| Postoperative complications CD ≥3 | 2.09 | 1.46–2.98 | <0.001 | |||
Note: The variables in bold are statistically significant (p < 0.05).
Abbreviations: AFP, α‐fetoprotein; BMI, body mass index; CD, Clavien–Dindo; CI, confidence interval; DCP, des‐gamma‐carboxy prothrombin; DM, diabetes mellitus; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; ICG‐R15, indocyanine green dye retention rate at 15 min; IM, intrahepatic metastasis; PT, prothrombin time; SAFE score, steatosis‐associated fibrosis estimator score; T‐Bil, total‐bilirubin; Vp, portal vein invasion; Vv, hepatic venous invasion.
Figure 3 summarizes the Kaplan–Meier analysis showing OS and RFS using the SAFE score. The cutoff value of the SAFE score was calculated from the ROC curve for OS after surgery using Youden index (cutoff value: 150). A high SAFE score was associated with poor OS and RFS (OS: p < 0.001; log‐rank test; Figure 3A, RFS: p < 0.001; log‐rank test; Figure 3B). The SAFE score has been reported as a liver fibrosis marker, which is designed for detecting fibrosis at F2 stage in patients with NAFLD. 21 However, nearly 70% of the patients had viral liver diseases in this study. Therefore, patients were classified based on the presence or absence of hepatitis virus infection to assess the SAFE score's prognostic impact across different liver backgrounds. The Kaplan–Meier analysis indicated the OS and RFS using the SAFE score in patients with different liver backgrounds (Figures S2 and S3). A high SAFE score was associated with poor OS only among patients with a viral liver background (p = 0.203; log‐rank test; Figure S2a, p < 0.001, log‐rank test; Figure S2b). For RFS, a high SAFE score was associated with poor RFS among patients in the viral and non‐viral groups (p = 0.032; log‐rank test; Figure S3a, p < 0.001; log‐rank test; Figure S3b).
FIGURE 3.
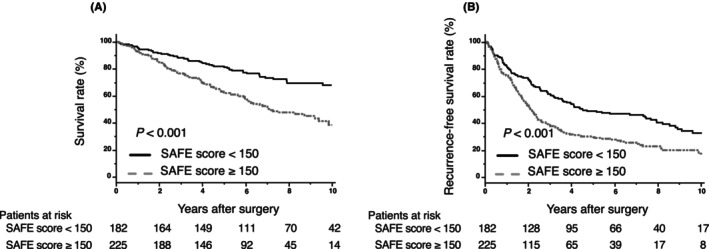
(A, B) Kaplan–Meier curves for overall survival and recurrence‐free survival used to compare the high‐ and low‐SAFE score groups.
3.4. Comparison of backgrounds by SAFE score
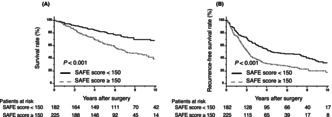
Table 4 summarizes the characteristics of patients in the high‐ and low‐SAFE score groups (cutoff value: 150). Compared with the low‐SAFE score group, the high‐SAFE score group included more females (p = 0.009), more patients with a liver background positive for HCV (p = 0.001), fewer patients with a liver background positive for HBV (p < 0.001), lower tumor numbers (p = 0.016), and lower numbers of patients with poorly differentiation (p = 0.042). ICG‐R15 (p < 0.001), intraoperative blood loss (p = 0.017), and the number of patients with postoperative complications. CD ≥ 3 (p = 0.040) were higher in the high‐SAFE score group than in the low‐SAFE score group. PT (p < 0.001) was lower in the high‐SAFE score group. No significant differences in tumor markers, vascular invasion, and IM were observed between the two groups. Table S1 summarizes the association between post PHLF and SAFE score. There was no significant association between PHLF ≥ grade B after curative hepatectomy and the degree of SAFE score (17.8% vs. 17.6%; p = 0.959). After PSM analysis, no significant differences were found between the two groups. Figure 4 summarizes the Kaplan–Meier analysis for OS and RFS using the SAFE score after PSM. A high SAFE score was associated with poor OS and RFS (OS: p = 0.006; log‐rank test; Figure 4A, RFS: p = 0.005; log‐rank test; Figure 4B).
TABLE 4.
Characteristics of patients according to SAFE score with the whole study series and for propensity score‐matched study.
| Whole study series | Propensity score‐matched series | |||||
|---|---|---|---|---|---|---|
| High‐SAFE score group (n = 225) | Low‐SAFE score group (n = 182) | p‐Value | High‐SAFE score group (n = 108) | Low‐SAFE score group (n = 108) | p‐Value | |
| Sex (M/F) | 165/60 | 153/29 | 0.009 | 89/19 | 87/21 | 0.726 |
| Liver background: HCV | 131 (58.2%) | 76 (41.8%) | 0.001 | 55 (50.9%) | 56 (51.9%) | 0.892 |
| Liver background: HBV | 17 (7.5%) | 62 (34.1%) | <0.001 | 15 (13.9%) | 14 (13.0%) | 0.842 |
| T‐Bil (mg/dL) | 0.8 (0.6–1.0) | 0.7 (0.6–1.0) | 0.103 | 0.7 (0.6–1.0) | 0.7 (0.6–0.9) | 0.549 |
| PT (%) | 83 (75–91) | 90 (84–98) | <0.001 | 87 (79–95) | 89 (81–96) | 0.489 |
| ICG‐R15 (%) | 16.5 (11.2–22.8) | 9.8 (6.6–15.7) | <0.001 | 13.7 (9.4–18.5) | 12.3 (8.3–17.7) | 0.192 |
| AFP (ng/mL) | 9.7 (4.8–45.1) | 7.7 (3.8–84.5) | 0.660 | 9.8 (3.6–70.2) | 6.8 (3.7–50.9) | 0.573 |
| DCP (mAU/mL) | 41.5 (20.0–344) | 44.0 (21.0–425) | 0.459 | 46.5 (20.0–408) | 51 (22.0–705) | 0.497 |
| Tumor number | 1 (1–10) | 1 (1–13) | 0.016 | 1 (1–5) | 1 (1–7) | 0.677 |
| Tumor diameter (mm) | 24.0 (16.0–35.0) | 25.0 (16.8–44.3) | 0.347 | 28.5 (20.0–44.8) | 26.0 (19.0–51.0) | 0.925 |
| Histology: poorly differentiation | 17 (7.6%) | 25 (13.7%) | 0.042 | 12 (11.1%) | 13 (12.0%) | 0.832 |
| Vascular invasion: Vp (+) | 34 (15.2%) | 30 (16.6%) | 0.702 | 20 (18.7%) | 15 (14.0%) | 0.355 |
| Vascular invasion: Vv (+) | 13 (5.9%) | 11 (6.1%) | 0.926 | 10 (7.6%) | 9 (6.8%) | 0.825 |
| IM (+) | 16 (7.1%) | 20 (11.1%) | 0.170 | 6 (5.6%) | 11 (10.3%) | 0.206 |
| Operative time (min) | 315 (247–391) | 301 (245–372) | 0.584 | 329 (266–408) | 302 (251–370) | 0.086 |
| Intraoperative blood loss (mL) | 380 (178–650) | 279 (129–551) | 0.017 | 390 (173–648) | 311 (133–574) | 0.167 |
| Intraoperative blood transfusion (+) | 20 (8.9%) | 8 (4.4%) | 0.069 | 8 (7.4%) | 6 (5.6%) | 0.580 |
| Postoperative complications CD ≥3 | 30 (13.3%) | 13 (7.1%) | 0.040 | 8 (7.4%) | 10 (9.3%) | 0.623 |
Note: The variables in bold are statistically significant (p < 0.05). Continuous variables are expressed as medians (interquartile ranges: only tumor number is range). Qualitative variables are expressed as numbers (%).
Abbreviations: AFP, α‐fetoprotein; CD, Clavien–Dindo; DCP, des‐gamma‐carboxy prothrombin; F, female; HBV, hepatitis B virus; HCV, hepatitis C virus; ICG‐R15, indocyanine green dye retention rate at 15 min; IM, intrahepatic metastasis; M, male; PT, prothrombin time; SAFE score, steatosis‐associated fibrosis estimator score; T‐Bil, total‐bilirubin; Vp, portal vein invasion; Vv, hepatic venous invasion.
FIGURE 4.
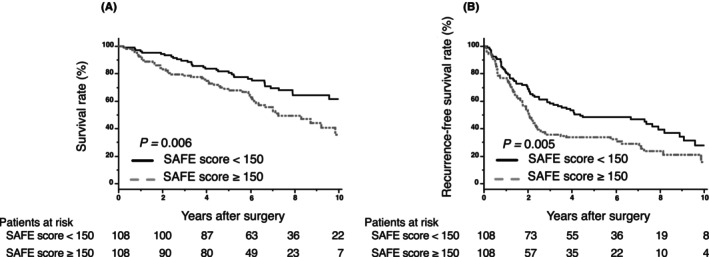
(A, B) Kaplan–Meier curves for overall survival and recurrence‐free survival after propensity score‐matched analysis, used to compare the high‐ and low‐SAFE score groups.
4. DISCUSSION
In this study, a high SAFE score was associated with severe liver fibrosis in the new Inuyama classification, and compared to other blood‐based markers for liver fibrosis, the SAFE score was shown to be an accurate prognostic indicator. In addition to tumor factors, such as tumor number, tumor diameter, vascular invasion, and postoperative complications, the preoperative high SAFE score, a liver fibrosis marker, was associated with poor long‐term prognostic factors of HCC following first curative hepatectomy, and the importance of assessing the degree of liver fibrosis was demonstrated. While liver histology remains essential for the accurate diagnosis of liver fibrosis, various markers and modalities have been identified that make it possible to determine liver fibrosis status noninvasively.
As imaging based‐markers, ultrasound‐based modality (vibration‐controlled transient elastography, Fibroscan 25 ) and magnetic resonance imaging‐based modality (magnetic resonance elastography, MRE 26 ) are used to evaluate liver fibrosis, which are reported to offer greater diagnostic accuracy than blood‐based biomarkers. 27 , 28 However, their applicability is limited due to the necessity for specialized expertise and costly apparatus. Thus, blood‐based markers for liver fibrosis, easily calculated from serum data, are clinically significant. Among the blood‐based markers for liver fibrosis evaluated in this study, the SAFE score demonstrated the best predictive capabilities for OS. The reasons for the good predictive value of the SAFE score for long‐term prognosis after initial curative hepatectomy for HCC may be as follows. Compared to other markers, the SAFE score is calculated from factors associated with liver fibrosis, such as BMI, as an indicator of obesity, DM, and globulin. One of the most significant features of the SAFE score is the inclusion of globulin, which has been used to predict fibrosis in HBV infection. 29 In addition, the SAFE score is designed to detect F2 stage fibrosis, unlike other blood‐based markers for liver fibrosis, which were designed to detect F3 stage fibrosis. Patients with liver fibrosis stage 2 or higher have been reported to have increased risks of liver‐related morbidity and mortality as compared to patients with lower stages of liver fibrosis. 30 Accordingly, the SAFE score could more accurately identify patients with poor prognosis after curative hepatectomy for HCC. Although no previous study has reported that the SAFE score can be a prognostic factor after curative hepatectomy for HCC, other liver fibrosis markers, such as FIB‐4 index and APRI, have already been reported as factors affecting postoperative outcomes in these patients. 17 , 18 , 19 , 20
Although the FIB‐4 index and the APRI were developed for predicting liver fibrosis in patients with HCV infection, 14 , 15 these markers have also been reported as prognostic factors after hepatectomy for HCC in liver backgrounds other than HCV. 20 , 31 In this study, HCV infection was the most common liver background, and only 30 patients (7.4%) had NAFLD. However, the relationship between the degree of liver fibrosis and the SAFE score in the new Inuyama classification on pathological examination was similar to that of other blood‐based markers for liver fibrosis, and more than 85% of high‐risk patients (SAFE score > 100), as defined in the original paper, were identified as stage F2 or above. These results suggested that the SAFE score can reflect the degree of liver fibrosis in patients with liver backgrounds other than NAFLD and can also be used as a prognostic factor after initial curative hepatectomy for HCC. While the SAFE score was initially formulated for European and American cohorts, a previous study has suggested that it is also efficacious in discriminating liver fibrosis among Asian patients. 32 Further cases of Asian patients should be accumulated in the future.
Liver fibrosis is a wound‐healing response to liver damage from some etiologies, and the degree of fibrosis affects liver functional reserve. Among the blood‐based markers for liver fibrosis, FIB4‐index and APRI have been reported to be associated with PHLF. 33 , 34 In this study, there was no significant association between PHLF ≥ grade B after curative hepatectomy and the degree of SAFE score. One of the reasons for these results may be that an appropriate amount of hepatectomies were performed in our institute according to preoperative liver functional reserve. The high‐SAFE score group was selected more for partial hepatectomy (data not shown) than the low‐SAFE score group. Recently, we developed a volume‐associated indocyanine green retention rate at 15 min, platelet, and prothrombin time index (VIPP) score, calculated from resection liver rate, remnant liver rate, ICG‐R15 levels, platelet counts, and the PT, to predict the development of severe PHLF and found that a high VIPP score was associated with a severe PHLF. 35 The VIPP score includes platelet counts, one of the indicators of liver fibrosis, which may help decide the amount of liver resection according to liver fibrosis to prevent the development of PHLF. It is desirable to accumulate cases to reveal whether determining the resection liver volume by VIPP score leads to improving the postoperative prognosis for patients with strong liver fibrosis.
This study had several limitations. This was a retrospective, single‐center study with a limited sample size, and all common postoperative prognostic factors for HCC indicated in existing reports could not be included in the analysis. Although a report has indicated that the SAFE score is useful in assessing the degree of liver fibrosis in Asians, this score is an index created from a population with a high BMI. As the number of patients with NAFLD and NASH was limited in this study, it was challenging to conduct the analysis only for these patients. Although a high SAFE score was not associated with poor OS among patients with a non‐viral liver background, this may be influenced by its small sample size. Recently, the number of non‐viral HCC cases caused by NASH has increased, and further analyses are warranted for confirming whether these results are similar to those of the present study after assessing biological markers, including blood‐based markers for liver fibrosis.
5. CONCLUSIONS
A high preoperative SAFE score was found to be independently associated with a poor prognosis in patients with HCC after initial curative hepatectomy. The preoperative SAFE score reflects the degree of liver fibrosis and is a useful assessment index for predicting the prognosis of patients who have undergone curative hepatectomy of HCC.
AUTHOR CONTRIBUTIONS
Drafting of article: TB, YI, and MO. Study conception and design: TB, YI, MO, and HO. Acquisition of data: TB, YI, RN, HS, SK, HT, MO, KI, and TK. Analysis and interpretation of data: TB, YI, MO. and MH. Funding acquisition of this article: YI, RN, MO, and HO. Critical revision of article: all authors.
FUNDING INFORMATION
This work was supported in part by JSPS KAKENHI (JP22H00479, JP23H02981, JP22K16534, and JP22K16535) and AMED (JP24fk0210108). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest for this article. Hideki Ohdan is an editorial board member of Annals of Gastroenterological Surgery.
ETHICS STATEMENTS
Approval of the research protocol: This study was approved by the Institutional Review Board of Hiroshima University Hospital (Approval no. E‐1580).
Informed consent: This study was conducted in accordance with the guidelines of Declaration of Helsinki. There was no need for consent to participate due to this being a retrospective study. The opt‐out method to obtain patient consent was utilized.
Registry and the registration no. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Figures S1–S3.
Table S1.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for the English language editing.
Bekki T, Ohira M, Imaoka Y, Hattori M, Nakano R, Sakai H, et al. The steatosis‐associated fibrosis estimator score is a useful indicator of recurrence and survival after initial curative hepatectomy for hepatocellular carcinoma. Ann Gastroenterol Surg. 2025;9:178–187. 10.1002/ags3.12846
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Reig M, Forner A, Rimola J, Ferrer‐Fàbrega J, Burrel M, Garcia‐Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, et al. Appropriate treatment strategy for intrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. J Gastrointest Surg. 2011;15:1182–1187. [DOI] [PubMed] [Google Scholar]
- 4. Harimoto N, Tsukagoshi M, Seki T, Hoshino K, Hagiwara K, Ishii N, et al. Predictors for early recurrence beyond up‐to‐7 or distant metastasis after hepatocellular carcinoma resection: proposal for borderline resectable HCC. Int J Clin Oncol. 2024;29:195–204. [DOI] [PubMed] [Google Scholar]
- 5. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 6. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long‐term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoh T, Seo S, Taura K, Iguchi K, Ogiso S, Fukumitsu K, et al. Surgery for recurrent hepatocellular carcinoma: achieving long‐term survival. Ann Surg. 2021;273:792–799. [DOI] [PubMed] [Google Scholar]
- 8. Tateishi R, Uchino K, Fujiwara N, Takehara T, Okanoue T, Seike M, et al. A nationwide survey on non‐B, non‐C hepatocellular carcinoma in Japan: 2011–2015 update. J Gastroenterol. 2019;54:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendes FD, Suzuki A, Sanderson SO, Lindor KD, Angulo P. Prevalence and indicators of portal hypertension in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:1028–1033.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non‐alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–1391. [DOI] [PubMed] [Google Scholar]
- 11. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542–1556. [DOI] [PubMed] [Google Scholar]
- 12. Ko S, Kanehiro H, Hisanaga M, Nagao M, Ikeda N, Nakajima Y. Liver fibrosis increases the risk of intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Br J Surg. 2002;89:57–62. [DOI] [PubMed] [Google Scholar]
- 13. Gassmann P, Spieker T, Haier J, Schmidt F, Mardin WA, Senninger N. Prognostic impact of underlying liver fibrosis and cirrhosis after curative resection of hepatocellular carcinoma. World J Surg. 2010;34:2442–2451. [DOI] [PubMed] [Google Scholar]
- 14. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 15. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. [DOI] [PubMed] [Google Scholar]
- 16. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 17. Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, et al. Fibrosis and AST to platelet ratio index predict post‐operative prognosis for solitary small hepatitis B‐related hepatocellular carcinoma. Hepatol Int. 2010;4:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen SL, Fu SJ, Chen B, Kuang M, Li SQ, Hua YP, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B‐induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. 2014;21:3802–3809. [DOI] [PubMed] [Google Scholar]
- 19. Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A. A laboratory marker, FIB‐4 index, as a predictor for long‐term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery. 2015;157:699–707. [DOI] [PubMed] [Google Scholar]
- 20. Okamura Y, Ashida R, Yamamoto Y, Ito T, Sugiura T, Uesaka K. FIB‐4 index is a predictor of background liver fibrosis and long‐term outcomes after curative resection of hepatocellular carcinoma. Ann Surg Oncol. 2016;23:467–474. [DOI] [PubMed] [Google Scholar]
- 21. Sripongpun P, Kim WR, Mannalithara A, Charu V, Vidovszky A, Asch S, et al. The steatosis‐associated fibrosis estimator (SAFE) score: a tool to detect low‐risk NAFLD in primary care. Hepatology. 2023;77(1):256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ichida F, Tsuji T, Omata M, Ichida T, Inoue K, Kamimura T, et al. New Inuyama classification; new criteria for histological assessment of chronic hepatitis. Int Hepatol Commun. 1996;6:112–119. [Google Scholar]
- 23. Rahbari NN, Garden OJ, Padbury R, Brooke‐Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713–724. [DOI] [PubMed] [Google Scholar]
- 24. Hassanzad M, Hajian‐Tilaki K. Methods of determining optimal cut‐point of diagnostic biomarkers with application of clinical data in ROC analysis: an update review. BMC Med Res Methodol. 2024;24:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. [DOI] [PubMed] [Google Scholar]
- 26. Imajo K, Honda Y, Yoneda M, Saito S, Nakajima A. Magnetic resonance imaging for the assessment of pathological hepatic findings in nonalcoholic fatty liver disease. J Med Ultrason. 2020;47:535–548. [DOI] [PubMed] [Google Scholar]
- 27. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta‐analysis. Hepatology. 2017;66:1486–1501. [DOI] [PubMed] [Google Scholar]
- 28. Tamaki N, Kurosaki M, Huang DQ, Loomba R. Noninvasive assessment of liver fibrosis and its clinical significance in nonalcoholic fatty liver disease. Hepatol Res. 2022;52:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu X‐D, Wu J‐L, Liang J, Zhang T, Sheng Q‐S. Globulin‐platelet model predicts minimal fibrosis and cirrhosis in chronic hepatitis B virus infected patients. World J Gastroenterol. 2012;18:2784–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ng CH, Lim WH, Hui Lim GE, Hao Tan DJ, Syn N, Muthiah MD, et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2023;21:931–939.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang J, Yang Y, Xia Y, Liu FC, Liu L, Zhu P, et al. Prediction of patient survival following hepatic resection in early‐stage hepatocellular carcinoma with indexed ratios of aspartate aminotransferase to platelets: a retrospective cohort study. Cancer Manag Res. 2021;13:1733–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li G, Lin H, Sripongpun P, Liang LY, Zhang X, Wong VWS, et al. Diagnostic and prognostic performance of the SAFE score in non‐alcoholic fatty liver disease. Liver Int. 2024;44:15–26. [DOI] [PubMed] [Google Scholar]
- 33. Mai RY, Ye JZ, Long ZR, Shi XM, Bai T, Chen J, et al. Preoperative aspartate aminotransferase‐to‐platelet‐ratio index as a predictor of posthepatectomy liver failure for resectable hepatocellular carcinoma. Cancer Manag Res. 2019;11:1401–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yugawa K, Maeda T, Nagata S, Shiraishi J, Sakai A, Yamaguchi S, et al. Impact of aspartate aminotransferase‐to‐platelet ratio index based score to assess posthepatectomy liver failure in patients with hepatocellular carcninoma. World J Surg Oncol. 2022;20:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Honmyo N, Kobayashi T, Kuroda S, Oshita A, Onoe T, Kohashi T, et al. A novel model for predicting posthepatectomy liver failure based on liver function and degree of liver resection in patients with hepatocellular carcinoma. HPB. 2021;23:134–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S3.
Table S1.


