Abstract
Background
There are some common pathophysiological risk factors between myocardial infarction and osteoporosis, and the exact relationship between the two is not yet clear. Our study aims to provide evidence on the relationship between myocardial infarction and osteoporosis through the analysis of data from the National Health and Nutrition Examination Survey (NHANES) and Mendelian Randomization (MR) analysis from 2015 to 2018.
Methods
A two-sample MR study using summary statistics from genome-wide association studies (GWAS) was conducted to determine the causal relationship between myocardial infarction and osteoporosis. The Inverse Variance Weighted (IVW) method and other supplementary MR methods were used to validate the causal relationship between myocardial infarction and osteoporosis. Sensitivity analysis was performed to verify the robustness of the results. Weighted multivariable adjusted logistic regression was used on the NHANES 2015–2018 data to evaluate the relationship between HDL, LDL, and BMD factors closely related to myocardial infarction.
Results
An observational study conducted in NHANES included a total of 2516 participants. Weighted multivariable adjusted logistic regression analysis showed that HDL was positively correlated with BMD, with OR and 95 % CI of 0.051 and 0.013–0.088, respectively. LDL was negatively correlated with BMD. The MR analysis also indicated a causal relationship between myocardial infarction and osteoporosis (IVW (OR = 1.16, 95 % CI = 1.02–1.32, P = 0.03)). Sensitivity analysis further confirmed the robustness and reliability of these study results (all P > 0.05).
Conclusion
There is a causal relationship between myocardial infarction and osteoporosis.
Keywords: National Health and Nutrition Examination Survey, Mendelian randomization, Myocardial infarction, Osteoporosis, Inflammation
1. Introduction
Fractures are mainly caused by trauma or bone density loss, with osteoporosis being a significant factor [1]. With the global aging trend, osteoporosis has become one of the most concerning public health issues worldwide [2]. Its pathological characteristic is reduced bone strength, leading to an increased risk of fractures, primarily due to an imbalance in bone turnover, where the rate of bone resorption by osteoclasts exceeds the rate of bone formation by osteoblasts, resulting in prolonged and progressive bone loss [3]. Osteoporosis is also closely related to the occurrence of cardiovascular events. Studies have shown that patients with heart failure are at a higher risk of osteoporosis [4]. Coronary artery disease and peripheral arterial atherosclerosis are associated with an increased risk of osteoporotic fractures [5]. Additionally, bone loss is also a significant factor in initiating inflammation within the cardiovascular system [6]. Research has shown a close relationship between osteoporosis and inflammation [7]. Myocardial infarction occurs due to sudden blood vessel blockage leading to myocardial ischemia and cell damage [8]. Substantial myocardial cell death triggers an inflammatory response, resulting in the extravasation of neutrophils and monocytes [9], indicating a connection between myocardial infarction development and inflammation. However, further exploration is needed to determine whether myocardial infarction affects the risk of osteoporosis and the genetic relationship between them.
Mendelian Randomization is a method used to explore the genetic associations in genetic processes, widely utilized in investigating the genetic links to fractures and various exposure factors. In this study, we used data from the Genome-Wide Association Studies (GWAS) and conducted a two-sample Mendelian Randomization (MR) analysis to explore the relationship between osteoporosis and myocardial infarction.
Additionally, based on the monitoring data from the 2015–2018 National Health and Nutrition Examination Survey (NHANES), we conducted a large cross-sectional study to examine the potential relationship between myocardial infarction and bone density.
2. Methods and materials
2.1. Mendelian randomization
2.1.1. Study design
Two-sample Mendelian randomization is a commonly used randomization method in the field of bioinformatics, used to process data from two groups of samples in order to ensure the randomization of grouping and the reliability of experimental results. By using randomization to group and process the two sets of samples, non-random factors in the experimental design and implementation are eliminated, and the experimental group and control group are randomly assigned to ensure fairness and balance of the samples under experimental conditions. The principle of randomization can reduce bias and errors in the experimental data, ensuring the objectivity and scientific nature of the results. In this study, GWAS database data was used to reveal the causal relationship between exposure factors and the main outcomes [10].
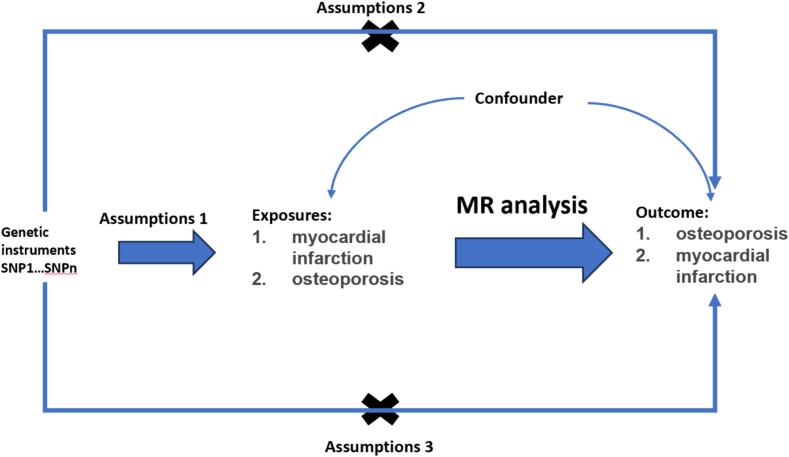
The Mendelian randomization in this research is based on three conditions: 1. The assumption of relevance: the selected independent variables are directly related to the exposure factors. 2. The assumption of independence: the selected independent variables are unrelated to confounding variables. 3. The exclusion restriction assumption: the selected independent variables should not directly affect the results, unless it is through their association with the exposure factors [11], [12] (Fig. 1).
Fig. 1.
Schematic diagram design of two-sample Mendelian randomization study on myocardial infarction and osteoporosis.
In this study, four GWAS datasets were selected to investigate the significantly genetic meaning of SNPs between myocardial infarction and osteoporosis.
2.1.2. Data source
In this study, we obtained four summary datasets from the GWAS database for two-sample MR analysis. The dataset related to myocardial infarction is ebi-a-GCST90038610 and finn-b-I9_MI_COMPLICATIONS_EXNONE, including 484,598 European participants and 218,795 Asian participants; the dataset related to osteoporosis is finn-b-OSTEOPOROSIS_FRACTURE_FG and bbj-a-137, including 173,519 European participants and 212,453 Asian participants. The diagnosis of myocardial infarction and osteoporosis patients was conducted according to the standards set by the American Heart Association and the American Orthopaedic Association. Detailed study design and data control processes have been published elsewhere.
2.1.3. Statistical analysis
In this study, R (version 4.2.1) was used for data analysis through the TwoSampleMR (0.5.6) package and MRPRESSO (1.0) (1.0). To avoid linkage disequilibrium, we used the standard kb = 10,000 and r2 = 0.001 when aggregating SNPs. We also set the threshold at p < 5 × 10^-5 as the genome-wide significance level to select the highly correlated SNPs with myocardial infarction and osteoporosis. We removed palindromic SNPs because it is difficult to determine if they are arranged in the same direction for myocardial infarction and osteoporosis. Finally, we used the r2 value of each SNP to calculate the proportion of variance in exposure and used the f-statistic to estimate the strength of the instrumental variable, to avoid weak instrument bias.
MR analysis mainly utilizes the classic Inverse Variance Weighted (IVW) method. IVW weights the effect size of each genetic variant by the size and standard error (or the inverse variance) of each variant, and reduces the bias in estimation caused by heterogeneity through the allocation of relative weights. This method combines the effect size estimates from multiple variants [13].
Additionally, we applied the weighted median estimator model (WME), weighted model-based method (WM), MR-Egger regression model (MER), and Simple mode (SE). WME calculates the median effect size of the variants and applies a weighted average based on the standard errors to address outliers, providing a comprehensive estimate of the combined effect size. WM calculates the median effect size of multiple genetic variant loci and combines them using weighted scoring to obtain an estimate of the overall effect. MER integrates the concept of Egger regression to assess bias and symmetry in causal effect estimates, evaluating the stability and consistency of estimates. SE extracts basic genetic information and association patterns, providing an intuitive understanding of the frequency distribution of genotypes, genetic relationships with phenotypes, and other fundamental genetic characteristics.
We simultaneously used Harmonize to remove incompatible SNP and SNP with palindromic allele frequencies. Considering the differences in the extracted SNPs in different experimental environments and protocols, the two-sample MR analysis may be heterogeneous, leading to errors in the final calculation of causal relationships. Therefore, this study adopted heterogeneity tests and MR-Egger regression tests for the main IVW analysis method. The P values of the test results were 0.1957 and 0.2183, respectively, and therefore, no heterogeneity was considered to exist.
We also examined the horizontal pleiotropy in the MR analysis, using the intercept value in MR-Egger to assess pleiotropy. We used the P value of the pleiotropy test to analyze the presence of pleiotropy. If the P value is greater than 0.05, pleiotropy in the causal analysis can be ignored. Finally, we tested the consistency of the results through leave-one-out.
2.2. NHANES
2.2.1. Study population
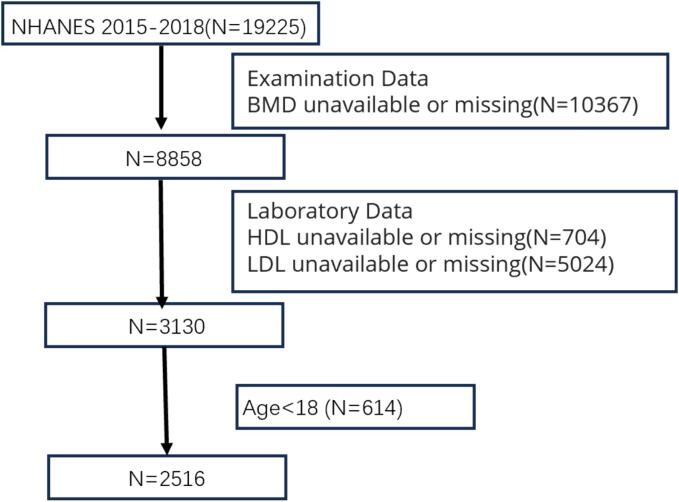
The data used in this study can be obtained from the NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm). The research data of NHANES have been approved by the NCHS Research Ethics Review Board. All NHANES participants have given informed consent. This study has been approved by an institutional review board as it uses de-identified, publicly available data. In this analysis, data from two cycles, NHANES (2015–2016, 2017–2018), were used to accumulate an appropriate sample size. If key analytical variables were missing or if participants were under 18 years of age, they were excluded (as shown in the Fig. 2).
Fig. 2.
Flowchart of participants in NHANES 2015–2018.BMD,Bone Mineral Density;HDL, high-density lipoprotein; LDL, low-density lipoprotein.
2.2.2. Exposure and outcome definition
We used HDL and LDL, which have been proven to be closely related to myocardial infarction, as exposures. The blood serum collection for HDL and LDL followed the guidelines set forth by the American Heart Association and the American Heart Association Working Group. The serum specimens were processed, stored, and transported to the University of Minnesota, Minneapolis for analysis. Meanwhile, the small vials were stored under appropriate freezing conditions (−30 °C) until they were transported to the University of Minnesota for testing. We simultaneously used bone density as an indicator directly related to osteoporosis as the outcome. We used dual-energy X-ray absorptiometry (DXA) to observe bone density. DXA scans provided measurements of bone and soft tissue in the whole body, arms and legs, trunk, and head. The scans and phantom scans for each participant were reviewed and analyzed by the University of California, San Francisco, using standard radiological techniques and research-specific protocols developed for NHANES.
2.2.3. Other covariates used in NHANES
Based on existing literature and data, this study collected potential variables related to myocardial infarction and cardiovascular events. The covariates included sex (male, female); race/ethnicity/ethnicity (Mexican American, other Hispanic, non-Hispanic white, or non-Hispanic black); poverty income ratio (PIR; <1.2 or ≥ 1.2); diabetes (yes, no, or unknown); hypertension (yes, no, or unknown); hypercholesterolemia (yes, no, or unknown); systolic and diastolic blood pressure (mmHg); and triglycerides (mg/dl). The systolic and diastolic blood pressure followed the guidelines of the American Heart Association (mmHg) and were the average of three static measurements. Triglycerides in serum were measured using a series of coupled reactions (mg/dl).
2.2.4. Data analysis
For the NHANES analysis, we used multivariable adjusted logistic regression to assess the relationship between HDL, LDL, BMD. We evaluated three models adjusted for covariates: Model 1 without adjustment; Model 2 including sex, age, and race/ethnicity; Model 3 further adjusted for age, sex, race/ethnicity, PIR, triglycerides, diabetes, hypertension, hyperlipidemia, systolic blood pressure, and diastolic blood pressure. The results were presented as odds ratios (ORs) or beta coefficients (95 % confidence intervals [CI]). Given the complex probability cluster design of NHANES, this study considered weights in the statistical analysis.
3. Results
3.1. Mendelian randomization results
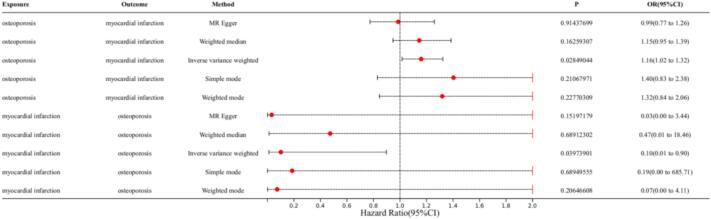
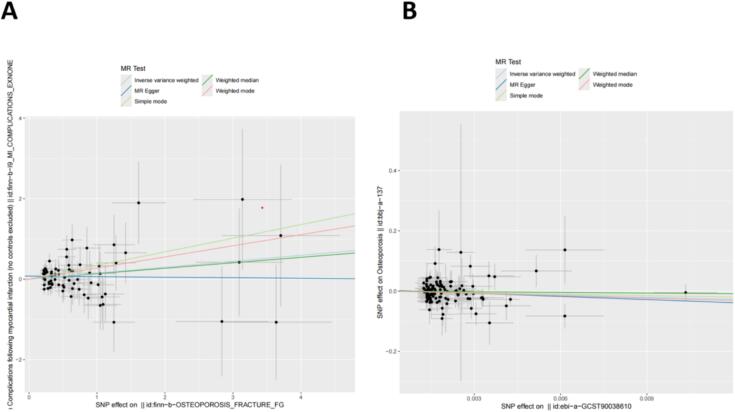
In the MR analysis with osteoporosis as the exposure factor and myocardial infarction as the outcome factor, we extracted 112 SNPs. The results showed significant effects in IVW (OR = 1.16, 95 % CI = 1.02–1.32, P = 0.03), MR-Egger (OR = 0.99, 95 % CI = 0.77–1.26, P = 0.91), weighted median (OR = 1.15, 95 % CI = 0.95–1.39, P = 0.16), simple mode (OR = 1.40, 95 % CI = 0.83–2.38, P = 0.21), and weighted mode (OR = 1.32, 95 % CI = 0.84–2.06, P = 0.23) (Fig. 3). In the MR analysis with myocardial infarction as the exposure factor and osteoporosis as the outcome factor, we extracted 75 SNPs. The results showed significant effects in IVW (OR = 0.1, 95 % CI = 0.01–0.90, P = 0.04), MR-Egger (OR = 0.03, 95 % CI = 0.0003–3.44, P = 0.15), weighted median (OR = 0.47, 95 % CI = 0.01–18.46, P = 0.69), simple mode (OR = 0.19, 95 % CI = 0.0005–685.71, P = 0.69), and weighted mode (OR = 0.07, 95 % CI = 0.0013–4.11, P = 0.20) (Fig. 3). The results indicate that myocardial infarction has a significant impact on osteoporosis, and osteoporosis also has a significant impact on myocardial infarction (Fig. 3). Cochran's Q test shows no heterogeneity between myocardial infarction and osteoporosis (P > 0.05). MR-Egger analysis shows no horizontal pleiotropy between myocardial infarction and osteoporosis, with an intercept of 0.075, P = 0.127 and 0.0027, P = 0.5853 (Table 1). The forest plot of the causal relationship between myocardial infarction and osteoporosis predicted by genes is shown in Fig. 4, and the scatter plot of SNP expression for myocardial infarction and osteoporosis is shown in Fig. 5. The total sample sizes for myocardial infarction were 484,598 and 218,795 cases, and for osteoporosis were 173,519 and 212,453 cases. When an effective allele frequency (EAF) value existed, we used EAF and effect estimate (BETA) to calculate R2 and the F-statistic to estimate the strength of the instrumental variables. The F-statistic values were all greater than 10.
Fig. 3.
The forest plot shows the relationship between myocardial infarction and osteoporosis in MR.
Table 1.
Heterogeneity of directional pleiotropy and MR-Egger test for directional pleiotropy.
| Heterogeneity | ||||
|---|---|---|---|---|
| outcome | exposure | Q | Q_df | Q_pval |
| Myocardial infarction | Osteoporosis | 114.96 | 111 | 0.38 |
| Osteoporosis | Myocardial infarction | 70.91 | 74 | 0.58 |
| MR-Egger pleiotropy test | ||||
| outcome | exposure | intercept | SE | pval |
| Osteoporosis | Myocardial infarction | 0.075 | 0.049 | 0.127 |
| Myocardial infarction | Osteoporosis | 0.0027 | 0.005 | 0.5853 |
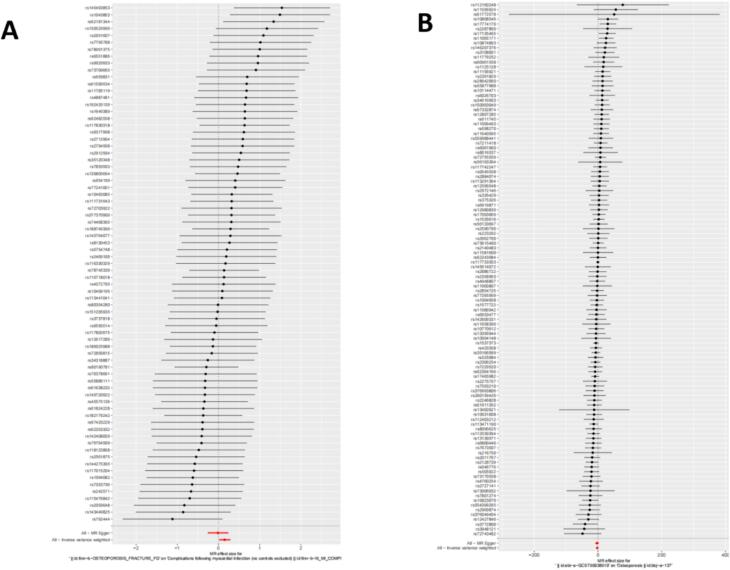
Fig. 4.
Forest plot of the causal effects of single nucleotide polymorphisms (SNPs). (A) Causal effects of single nucleotide polymorphisms (SNPs) with exposure to osteoporosis and outcome of myocardial infarction. (B) Causal effects of single nucleotide polymorphisms (SNPs) with exposure to myocardial infarction and outcome of osteoporosis.
Fig. 5.
Scatter plot of the genetic association between myocardial infarction and osteoporosis. (A) Scatter plot with exposure to osteoporosis and outcome of myocardial infarction. (B) Scatter plot with exposure to myocardial infarction and outcome of osteoporosis.
3.2. NHANES study results
3.2.1. Baseline characteristics Baseline characteristics
Our analysis included 2516 participants, of which 47.2 % were male (weighted proportion). The BMD ranges for quartiles 1–4 were 8.68–46.01, 46.03–53.59, 53.6–62.58, and 62.63–151.26, respectively. Overall, the weighted average HDL of participants decreased with increasing BMD quartiles (Q1: 55.13 ± 16.40; Q2: 53.50 ± 15.39; Q3: 53.59 ± 15.58; Q4: 53.28 ± 15.64, P = 0.18), while LDL increased with increasing BMD quartiles (Q1: 114.42 ± 35.04; Q2: 115.34 ± 32.94; Q3: 113.13 ± 34.23; Q4: 108.71 ± 32.49, P = 0.0016). We also found statistically significant differences in Age, PIR, SEX, RACE/ETHNICITY, and Hypertension (all < 0.05) among the population (TABLE 2).
Table 2.
Baseline characteristics of the participants, weighted.
| Characteristic | BMD | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P value | |
| Age(years) | 39.78 ± 12.46 | 38.89 ± 12.37 | 37.85 ± 12.05 | 38.24 ± 12.35 | 0.0423 |
| HDL | 55.13 ± 16.40 | 53.50 ± 15.39 | 53.59 ± 15.58 | 53.28 ± 15.64 | 0.18 |
| TG | 100.61 ± 63.49 | 110.24 ± 62.79 | 105.02 ± 68.59 | 106.62 ± 68.34 | 0.1042 |
| LDL | 114.42 ± 35.04 | 115.34 ± 32.94 | 113.13 ± 34.23 | 108.71 ± 32.49 | 0.0016 |
| SBP | 118.80 ± 14.34 | 119.33 ± 14.99 | 118.97 ± 15.30 | 120.13 ± 14.46 | 0.4045 |
| DBP | 71.36 ± 10.77 | 71.75 ± 11.41 | 70.74 ± 11.79 | 72.04 ± 11.43 | 0.2035 |
| PIR | 2.51 ± 1.57 | 2.78 ± 1.62 | 2.94 ± 1.58 | 3.01 ± 1.58 | <0.0001 |
| SEX | <0.0001 | ||||
| Male | 35.32 | 49.91 | 54.25 | 68.76 | |
| Female | 64.68 | 50.09 | 45.75 | 31.24 | |
| RACE/ETHNICITY | <0.0001 | ||||
| Mexican American | 17.04 | 12.84 | 8.22 | 5.5 | |
| Other Hispanic | 11.07 | 9.82 | 7.53 | 4.27 | |
| Non-Hispanic White | 47.17 | 54.44 | 61.72 | 69.7 | |
| Non-Hispanic Black | 12.12 | 9.51 | 12.21 | 13.08 | |
| Other race/ethnicity − Including Multi-Racial | 12.6 | 13.4 | 10.31 | 7.45 | |
| Hypertension | 0.0811 | ||||
| Yes | 22.56 | 23.03 | 18.53 | 24.81 | |
| No | 77.44 | 76.81 | 81.47 | 74.94 | |
| Don't know | 0.16 | 0.26 | |||
| Hypercholesteremia | 0.1429 | ||||
| Yes | 25.89 | 23.42 | 19.49 | 22.53 | |
| No | 73.78 | 76.43 | 80.38 | 77.47 | |
| Don't know | 0.33 | 0.15 | 0.13 | ||
| DIABETES | 0.1783 | ||||
| Yes | 4.92 | 6.47 | 4.73 | 6.89 | |
| No | 91.7 | 91.89 | 93.71 | 91.63 | |
| Borderline | 3.04 | 1.57 | 1.56 | 1.35 | |
| Don't know | 0.34 | 0.08 | 0.12 |
Mean ± standard error (SE) for continuous variables and percentage (%) for categorical variables. PIR, poverty to income ratio; HDL, high-density lipoprotein; LDL, low Density Lipoprotein;DBP, diastolic blood pressure; SBP, systolic blood pressure; TG,triglyceride.
3.2.2. Relationship between HDL, LDL, and BMD
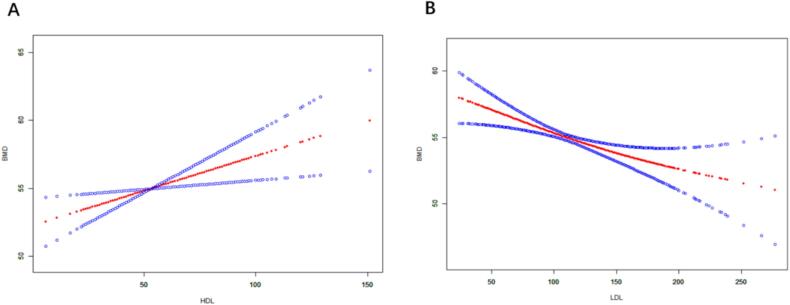
Table 3 shows the association between HDL, LDL, and BMD. We found that higher HDL was positively correlated with increased BMD scores in both crude and adjusted models. In the unadjusted model 1, OR = -0.018, 95 % CI: −0.050 to 0.013. After adjusting for age, sex, and race/ethnicity, the OR in model 2 was 0.033, with a 95 % CI of 0.002 to 0.065. In the fully adjusted model 3, the OR and 95 % CI were 0.051 and 0.013 to 0.088, indicating a 5.1 % higher likelihood of increased bone density in participants with higher HDL compared to those with lower HDL. We also found that higher LDL was negatively correlated with BMD scores in both the crude and adjusted models. In the unadjusted model 1, OR = -0.035, 95 % CI: −0.050 to −0.020. After adjusting for age, sex, and race/ethnicity, the OR in model 2 was −0.035, with a 95 % CI of −0.049 to −0.020. In the fully adjusted model 3, the OR and 95 % CI were −0.035 and −0.051 to −0.019, indicating a 3.5 % higher likelihood of increased bone density in participants with lower LDL compared to those with higher LDL. We further used smooth curve fitting to explore the relationship between HDL, LDL, and BMD. The results showed a positive correlation between HDL and BMD (Fig. 6A) and a negative correlation between LDL and BMD (Fig. 6B).
Table 3.
Multivariable logistic regression analysis results for HDL, LDL, and BMD, weighted.
| Exposure | Non-adjusted | Adjust I | Adjust II |
|---|---|---|---|
| HDL | −0.018 (−0.050, 0.013) 0.25828 | 0.033 (0.002, 0.065) 0.03885 | 0.051 (0.013, 0.088) 0.00834 |
| LDL | −0.035 (−0.050, −0.020) < 0.00001 | −0.035 (−0.049, −0.020) < 0.00001 | −0.035 (−0.051, −0.019) 0.00002 |
OR, odds ratio; 95 % CI, 95 % confidence interval.
Model 1: Adjusted for no covariates.
Model 2: Adjusted for age, sex, and race/ethnicity.
Model 3: Adjusted for age; sex; race/ethnicity; PIR; systolic blood pressure; diastolic blood pressure; triglyceride; diabetes; hypertension; hypercholesterolemia.
Fig. 6.
The association between the probabilities of HDL, LDL, and BMD. Adjusting for potential confounding variables (age; sex; race/ethnicity; PIR; systolic blood pressure; diastolic blood pressure; triglyceride; diabetes; hypertension; hypercholesterolemia). The red dotted line represents the fitted spline curve. The blue dotted line represents the 95% confidence interval. (A) The association between HDL and the probability of BMD; (B) The association between LDL and the probability of BMD.
3.2.3. Subgroup analysis
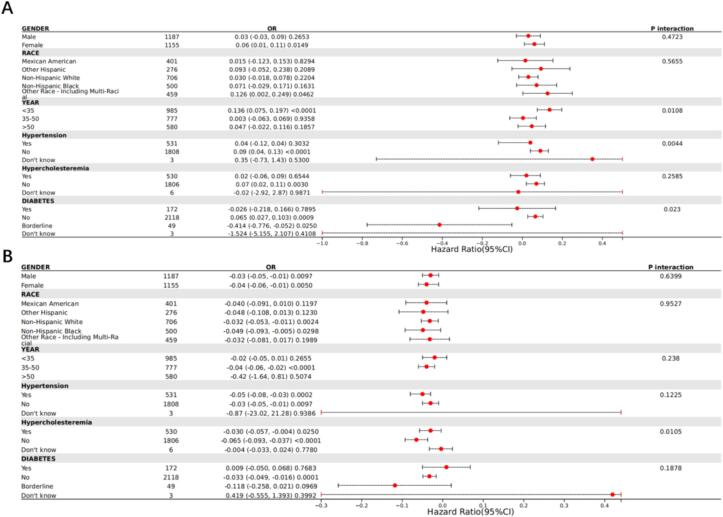
To evaluate whether the relationship between HDL, LDL, and BMD is consistent across different population subgroups, we conducted subgroup analyses based on sex, race/ethnicity, age, diabetes, hypertension, and hypercholesterolemia. The results indicated inconsistent associations. We found significant interactions for HDL with sex, age, and hypertension (P for interaction < 0.05), suggesting that the correlation between HDL and BMD depends on hypertension and may apply to patients without hypertension. For LDL, there was a significant interaction with hypertension (P for interaction < 0.05), similarly indicating that the correlation between LDL and BMD depends on hypertension and may apply to patients without hypertension Fig. 7..
Fig. 7.
Subgroup analysis of the correlation between HDL and BMD scores (A) and the correlation between LDL and BMD scores (B).
4. Discussion
Our MR study revealed a causal relationship between myocardial infarction and osteoporosis. The MR results demonstrated a close relationship between myocardial infarction and osteoporosis (with all IVW analysis results having p-values less than 0.05). Myocardial infarction was identified as a detriment factor for osteoporosis (OR = 0.1 in IVW analysis), while osteoporosis was beneficial for myocardial infarction (OR = 1.16 in IVW analysis).
In our NHANES study, following the standards for clinical and preclinical research [14], [15], we established a myocardial infarction model with HDL and LDL as the dependent variables, and triglycerides, blood pressure, hyperlipidemia, diabetes, age, sex, race/ethnicity, and poverty income ratio as covariates. We investigated the relationship between this model and BMD, which reflects osteoporosis. The results indicated that in the myocardial infarction model, compared to participants with lower HDL, those with higher HDL had a 5.1 % higher likelihood of increased bone density, showing a positive correlation between HDL and BMD. Similarly, in the myocardial infarction model, compared to participants with higher LDL, those with lower LDL had a 3.5 % higher likelihood of increased bone density, indicating a negative correlation between LDL and BMD. Patients with myocardial infarction typically had decreased HDL levels and increased LDL levels, suggesting a decreased bone density and increased risk of osteoporosis, consistent with our MR results.
Osteoporosis can be triggered by various inflammatory factors. The inflammatory cytokine TNF-alpha (Tumor necrosis factor alpha) is a key factor in the inflammatory response, leading to the production of a series of cell and inflammatory factors like IL-17A, IL-17F, IL-12, or IL-23 [16]. In mice, TNF-alpha can activate the NF-κB pathway, which then activates c-Fos, leading to the activation of NFATc1, ultimately inducing the development and activation of osteoclasts [17], resulting in excessive osteoclast activity and osteoporosis. IL-8, a member of the IL-1 family, is produced mainly by monocytes, macrophages, lymphocytes, and endothelial cells, attracting neutrophils, monocytes, and other inflammatory cells and promoting the production of inflammatory mediators [18]. In COPD patients, IL-8 can promote RANKL expression via the STAT3 pathway [19], [20], and increased RANKL expression can elevate the expression of neutrophils in peripheral blood, affecting the development of osteoporosis [21]. IL-10 is primarily produced by monocytes, macrophages, and T cells. It can inhibit the inflammatory response and attenuate immune responses [22]. With the progression of myocardial ischemia–reperfusion after myocardial infarction, the release of IL-10 significantly increases [23]. Additionally, IL-10 can weaken the calcium pathway to inhibit the RANK signal's co-stimulation, thereby reducing RANKL-induced osteoclast generation, inhibiting human osteoclast formation, slowing down osteoporosis development. Moreover, high concentrations of IL-10 in the blood can significantly reduce TNF-alpha levels and inhibit osteoclast apoptosis, lowering the risk of osteoporosis occurrence [24]. We believe that the relationship between myocardial infarction and osteoporosis is likely initiated by inflammation. Inflammatory factors released post-myocardial infarction to counteract reperfusion injury, notably TNF-alpha, IL-1α, and IL-8, elicit the production of anti-inflammatory factors like IL-10, TGF-β, IL-4, or PGE2. These anti-inflammatory factors can weaken the onset and progression of osteoporosis, and inflammation triggered by osteoporosis may exacerbate damage to cardiac vascular endothelium, leading to clot formation and subsequent myocardial infarction.
Among the biomarkers in myocardial infarction, inhibiting galectin-3 can alleviate fibrosis and dysfunction post-myocardial infarction [25]. Experimental results indicate that mice lacking galectin-3 exhibit increased osteoclasts in bone tissue and decreased trabecular bone volume. Galectin-3 may interfere with the RANKL/OPG signaling pathway, suppressing osteoclast generation [26]. Galectin-3 can form a complex with TRIM16 and other autophagy-related proteins like ULK1 and Beclin1 to induce autophagy and promote osteogenic differentiation of bone marrow mesenchymal stem cells, potentially influencing the occurrence and development of osteoporosis through regulating the autophagic process [27]. Adiponectin exerts antioxidant effects, neutralizing free radicals, reducing oxidative stress on myocardial cells, aiding nitric oxide synthesis, lowering levels of angiotensin II, protecting myocardial cells from oxidative stress, and can function as a biomarker for myocardial infarction [28]. Additionally, Adiponectin can activate AMPK, which plays a significant role in bone metabolism. AMPK activation assists in inhibiting fat cell formation and proliferation while promoting osteoblast function, aiding in increased bone density [29]. Adiponectin enhances bone formation by influencing the Wnt signaling pathway. In the Wnt signaling pathway, the stability and accumulation of β-catenin protein are critical for osteoblast proliferation and differentiation. Adiponectin can increase the expression of β-catenin, promoting osteoblast function, enhancing bone formation [30], thus affecting osteoporosis. In the context of myocardial infarction, ischemia-induced cardiac cell energy metabolism disruption and internal calcium ion imbalance due to cellular hypoxia result in damaged cell membrane and endoplasmic reticulum membranes, leading to troponin T release into the bloodstream as a biomarker for myocardial infarction [31]. Troponin T is directly related to bone formation; when muscles contract, they secrete muscle growth factors like IGF-1, which promote osteoblast (bone-forming cell) activity and inhibit bone resorption (induced by osteoclasts). Furthermore, troponin T isoforms, especially the isoform associated with fast muscle fibers, may directly correlate with bone formation and metabolism. It may enhance osteoblast activity (through the Wnt/β-catenin signaling pathway), increase bone matrix synthesis and mineralization, improve bone density, and alleviate osteoporosis [32].
Within our study, the implications of the positive correlation between HDL and BMD include: a positive relationship signifies that as HDL levels increase, BMD typically also increases. This relationship suggests that HDL may play a beneficial role in bone metabolism. Studies indicate that HDL may enhance bone tissue formation by activating the PI3K/Akt pathway to inhibit apoptotic signaling pathways, thereby increasing osteoblast survival rates and enhancing bone tissue formation. HDL can also promote osteoblast differentiation and function by regulating the Wnt/β-catenin signaling pathway [33]. In contrast, the implications of the negative correlation between LDL and BMD include: a negative relationship signifies that as LDL levels increase, BMD typically decreases. This relationship suggests that LDL may play a detrimental role in bone metabolism. LDL may lead to increased RANKL (receptor activator of nuclear factor-κB ligand) expression, stimulating osteoclast formation and activity by binding to its receptor RANK, thereby increasing bone resorption. High LDL levels are generally associated with chronic inflammation, which can impact bone metabolism, suppressing osteoblast function [34].
5. Conclusion
We conducted bidirectional MR analyses and NHANES analyses on osteoporosis and myocardial infarction, revealing a causal relationship between the two. It is noteworthy to consider the role of inflammatory factors in osteoporosis. However, the specific mechanisms by which myocardial infarction is beneficial for osteoporosis and osteoporosis is detrimental for myocardial infarction still require further in-depth research.
Author Contributions
GL and DL were responsible for designing the entire study. BP and XF performed data analysis and website operation. GL wrote the manuscript. RZ,KH,JF were responsible for review and editing. All authors have read and agreed to the published version of the manuscript.
6. Funding
This work was supported by National Natural Science Foundation of China (NO.82300315; NO.82374240), Guangdong Province Basic and Applied Basic Research Fund Project (No. 2024A1515012174; No.2024A1515013184). National Administration of Traditional Chinese Medicine Research Project (No. 0102023703), Project of the State Key Laboratory of Dampness Syndrome of Traditional Chinese Medicine jointly established by the province and the ministry (No.SZ2022KF10), Scientific Research Initiation Project of Guangdong Provincial Hospital of Traditional Chinese Medicine (No.2021KT1709), Research Project of Guangdong Provincial Bureau of Traditional Chinese Medicine (No.20241120), Guangdong Provincial Key Laboratory of Research on Emergency in TCM (No. 2023B1212060062; 2023KT15450).
CRediT authorship contribution statement
Guanmou Li: Writing – original draft, Data curation. Bo Peng: Writing – review & editing. Junqiao Fan: Writing – review & editing. Dongqun Lin: Data curation. Kunyang He: Writing – review & editing. Rongjun Zou: Data curation. Xiaoping Fan: Writing – review & editing, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Rongjun Zou, Email: zourj3@mail2.sysu.edu.cn.
Xiaoping Fan, Email: fukui-hanson@hotmail.com.
References
- 1.Sattui S.E., Saag K.G. Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat Rev Endocrinol. 2014;10:592–602. doi: 10.1038/nrendo.2014.125. [DOI] [PubMed] [Google Scholar]
- 2.Cummings S.R., Melton L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 3.Lane N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3–S. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Yin J., Lu X., Qian Z., Xu W., Zhou X. New insights into the pathogenesis and treatment of sarcopenia in chronic heart failure. Theranostics. 2019;9:4019–4029. doi: 10.7150/thno.33000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai S.W., Liao K.F., Lai H.C., Tsai P.Y., Lin C.L., Chen P.C., Sung F.C. Risk of major osteoporotic fracture after cardiovascular disease: a population-based cohort study in Taiwan. J Epidemiol. 2013;23:109–114. doi: 10.2188/jea.JE20120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kado D.M., Browner W.S., Blackwell T., Gore R., Cummings S.R. Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res. 2000;15:1974–1980. doi: 10.1359/jbmr.2000.15.10.1974. [DOI] [PubMed] [Google Scholar]
- 7.Mundy G.R. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 8.Frangogiannis N.G. Pathophysiology of Myocardial Infarction. Compr Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 9.Prabhu S.D., Frangogiannis N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birney E. Mendelian Randomization. Cold Spring Harb Perspect Med. 2022;12 doi: 10.1101/cshperspect.a041302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess S., Labrecque J.A. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33:947–952. doi: 10.1007/s10654-018-0424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botelho J., Machado V., Mendes J.J., Mascarenhas P. Causal Association between Periodontitis and Parkinson's Disease: A Bidirectional Mendelian Randomization Study. Genes (basel) 2021;12 doi: 10.3390/genes12050772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z., Deng Y., Pan W. Combining the strengths of inverse-variance weighting and Egger regression in Mendelian randomization using a mixture of regressions model. PLoS Genet. 2021;17:e1009922. doi: 10.1371/journal.pgen.1009922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanazawa M., Matsumoto Y., Takahashi K., Suzuki H., Uzuka H., Nishimiya K., Shimokawa H. Treadmill exercise prevents reduction of bone mineral density after myocardial infarction in apolipoprotein E-deficient mice. European Journal of Preventive Cardiology. 2020;27:28–35. doi: 10.1177/2047487319834399. [DOI] [PubMed] [Google Scholar]
- 15.Tjandra P.M., Paralkar M.P., Osipov B., Chen Y.J., Zhao F., Ripplinger C.M., Christiansen B.A. Systemic bone loss following myocardial infarction in mice. Journal of Orthopaedic Research : Official Publication of the Orthopaedic Research Society. 2021;39:739–749. doi: 10.1002/jor.24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu S., Wang Y., Liu J. Tumor necrosis factor-α signaling in nonalcoholic steatohepatitis and targeted therapies. J Genet Genomics. 2022;49:269–278. doi: 10.1016/j.jgg.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Yao Z., Getting S.J., Locke I.C. Regulation of TNF-Induced Osteoclast Differentiation. Cells. 2021;11 doi: 10.3390/cells11010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilotić A., Nacka-Aleksić M., Pirković A., Bojić-Trbojević Ž., Dekanski D., Jovanović Krivokuća M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232314574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwan Tat S., Padrines M., Théoleyre S., Heymann D., Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Wei S., Kitaura H., Zhou P., Ross F.P., Teitelbaum S.L. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X., Sun Y., Xu W., Lin T., Zeng H. Expression of RANKL by peripheral neutrophils and its association with bone mineral density in COPD. Respirology. 2017;22:126–132. doi: 10.1111/resp.12878. [DOI] [PubMed] [Google Scholar]
- 22.Saraiva M., O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez-Rodriguez A., Abreu-Gonzalez P., de la Rosa A., Vargas M., Ferrer J., Garcia M. Role of endogenous interleukin-10 production and lipid peroxidation in patients with acute myocardial infarction treated with primary percutaneous transluminal coronary angioplasty, interleukin-10 and primary angioplasty. Int J Cardiol. 2005;99:77–81. doi: 10.1016/j.ijcard.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 24.Yi L., Li Z., Jiang H., Cao Z., Liu J., Zhang X. Gene Modification of Transforming Growth Factor β (TGF-β) and Interleukin 10 (IL-10) in Suppressing Mt Sonicate Induced Osteoclast Formation and Bone Absorption. Med Sci Monit. 2018;24:5200–5207. doi: 10.12659/MSM.909720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frangogiannis N.G. Targeting galectin-3 in myocardial infarction: a unique opportunity for biomarker-guided therapy. Cardiovascular Research. 2023;119:2495–2496. doi: 10.1093/cvr/cvad156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon D., Derer A., Andes F.T., Lezuo P., Bozec A., Schett G., Herrmann M., Harre U. Galectin-3 as a novel regulator of osteoblast-osteoclast interaction and bone homeostasis. Bone. 2017;105:35–41. doi: 10.1016/j.bone.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Chen W.T., Zhang F., Zhao X.Q., Yu B., Wang B.W. Galectin-3 and TRIM16 coregulate osteogenic differentiation of human bone marrow-derived mesenchymal stem cells at least partly via enhancing autophagy. Bone. 2020;131 doi: 10.1016/j.bone.2019.115059. [DOI] [PubMed] [Google Scholar]
- 28.Neumann J.T., Weimann J., Sörensen N.A., Hartikainen T.S., Haller P.M., Lehmacher J., Brocks C., Tenhaeff S., Karakas M., Renné T., Blankenberg S., Zeller T., Westermann D. A Biomarker Model to Distinguish Types of Myocardial Infarction and Injury. Journal of the American College of Cardiology. 2021;78:781–790. doi: 10.1016/j.jacc.2021.06.027. [DOI] [PubMed] [Google Scholar]
- 29.He B., Zhao J., Zhang M., Yin L., Quan Z., Ou Y., Huang W. Causal roles of circulating adiponectin in osteoporosis and cancers. Bone. 2022;155 doi: 10.1016/j.bone.2021.116266. [DOI] [PubMed] [Google Scholar]
- 30.Zhan J.K., Wang Y., He J.Y., Wang Y.J., Tan P., Tang Z.Y., Deng H.Q., Huang W., Liu Y.S. Artery calcification, osteoporosis, and plasma adiponectin levels in Chinese elderly. Heart & Lung : the Journal of Critical Care. 2015;44:539–543. doi: 10.1016/j.hrtlng.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Schneider U., Mukharyamov M., Beyersdorf F., Dewald O., Liebold A., Gaudino M., Fremes S., Doenst T. The value of perioperative biomarker release for the assessment of myocardial injury or infarction in cardiac surgery. European Journal of Cardio-Thoracic Surgery : Official Journal of the European Association for Cardio-Thoracic Surgery. 2022;61:735–741. doi: 10.1093/ejcts/ezab493. [DOI] [PubMed] [Google Scholar]
- 32.Abreu E.L., Vance A., Cheng A.L., Brotto M. Musculoskeletal Biomarkers Response to Exercise in Older Adults. Frontiers in Aging. 2022;3 doi: 10.3389/fragi.2022.867137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu P., Li H., Liu D., Zhang Y.F., Liu Y., Liang C. Association Between HDL-C and Bone Mineral Density: An Cross-Sectional Analysis. International Journal of General Medicine. 2021;14:8863–8872. doi: 10.2147/IJGM.S334972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G.H., Cheung C.L., Au P.C., Tan K.C., Wong I.C., Sham P.C. Positive effects of low LDL-C and statins on bone mineral density: an integrated epidemiological observation analysis and Mendelian randomization study. International Journal of Epidemiology. 2020;49:1221–1235. doi: 10.1093/ije/dyz145. [DOI] [PubMed] [Google Scholar]