Abstract
With improving living standards, functional and healthy foods are accounting for an increased share in human food. The development of dairy products that are rich in virgin omega-3 polyunsaturated fatty acids (n-3 PUFAs) has become a topic of interest. Virgin n-3 PUFA milk can provide high-quality protein and calcium, as well as provide n-3 PUFAs to improve human health. This review aims to investigate the effect of virgin n-3 PUFAs in milk on human health and discuss the content of virgin n-3 PUFAs in milk regulated by dairy animal diet and the effect of food processing on the content of virgin n-3 PUFAs in dairy production. The interaction between n-3 PUFAs and proteins in milk is the key to improving the nutritional value of n-3 PUFAs in milk. n-3 PUFA supplementation in the diet of dairy animals is the key method to improve n-3 PUFAs in raw milk, as well as to adjust the types of virgin n-3 PUFAs. Compared with a common source, virgin n-3 PUFAs in milk show higher antioxidant activity, but elevated temperatures and long-term thermal processing should be avoided.
Keywords: Omega-3 polyunsaturated fatty acids, Milk, Functional food, Lactoferrin–n-3 PUFAs complex, Heat treatment
1. Introduction
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) are essential fatty acids (mainly including α-linolenic acid: ALA, C18:3, cis-9,cis-12,cis-15-octadecatrienoic acid; C20:3, cis-11,cis-14,cis-17-eicosatrienoic acid, ETE; C20:5, cis-5,cis-8,cis-11,cis-14,cis-17-eicosapentaenoic acid, EPA; C22:5, cis-7,cis-10,cis-13,cis-16,cis-19-docosapentaenoic acid, DPA; and C22:6, cis-4,cis-7,cis-10,cis-13,cis-16,cis-19-docosahexaenoic acid, DHA) that play critical roles in most vital organs. The n-3 PUFAs are important substances for brain and muscle structure [1,2]. n-3 PUFAs are also beneficial for cardiovascular health and other conditions [3,4]. Some n-3 PUFA every day is good for health. Studies found that 1 g/day of ALA intake can reduce cardiovascular disease [3], and the optimal intake of DHA + EPA is 0.25 g/day [5]. Even in the context of the Coronavirus disease 2019 (COVID-19) outbreak, n-3 PUFAs have been shown to reduce the risk of death [6].
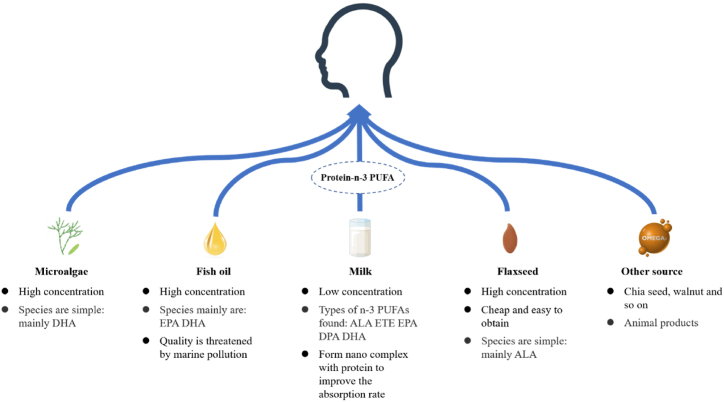
The main n-3 PUFA foods for humans are fish, oil seeds (flax, chia, perilla, etc.), and microalgae [[7], [8], [9]] as shown in Fig. 1, and other n-3 PUFA products such as eggs that are specifically rich in n-3 PUFAs [10]. The n-3 PUFAs in microalgae are mainly DHA and in flaxseed are mainly ALA. Fish oil is a good source of n-3 PUFAs, but its quality is affected by marine pollutants [11]. Milk contains multiple virgin n-3 PUFAs (such as ALA, ETE, EPA, DPA, and DHA) which are potential dietary sources. In addition, n-3 PUFAs in milk play a special role, and studies have found that virgin n-3 PUFA milk could reduce the risk of asthma in children [12].
Fig. 1.
Characteristics of n-3 PUFAs from various sources. n-3 PUFAs: omega-3 polyunsaturated fatty acids, ALA: α-linolenic acid, ETE: eicosatrienoic acid, EPA: eicosapentaenoic acid, DPA: docosapentaenoic acid, DHA: docosahexaenoic acid.
Milk is considered a complete food source because of its extraordinary nutritional value [13]. Milk fat is the most important constituent of milk and is easily digested and absorbed compared with other fats. Thus, unlike other n-3 PUFA sources, n-3 PUFAs in milk are easily absorbed by the human body. Similar to calcium, n-3 PUFAs can also combine with proteins to form nanocomplexes [14], and this nanocomplex shows many benefits for fatty acids: 1) increases fatty acid absorption, 2) protects fatty acids from oxidation or other stresses, and 3) changes the bioavailability of fatty acids [15]. In milk, there are numerous kinds of proteins, such as β-lactoglobulin (β-LG), α-lactalbumin (α-LA), and lactoferrin (LF). The n-3 PUFAs exert multiple functions by binding to different proteins. For example, compared with other complexes, only the LF–ALA complex showed a higher inhibitory effect on cancer cells. Therefore, increasing the n-3 PUFA content in dairy production is a good way to improve human health [16].
The concentration of virgin n-3 PUFAs in milk can be adjusted via the diet of the mammal [17,18]. Supplementation of flaxseed in the diet can increase the content of ALA, ETE, EPA, DHA, and total n-3 PUFAs in raw milk [19]. Fish oil and microalgae can increase the concentration of virgin n-3 PUFAs in milk, but the type varies [17]. The processing of dairy products, such as heat treatment, also affects the concentration of virgin n-3 PUFAs in milk [20].
To our knowledge, there is no detailed information about the nutritional characteristics, production, and processing of virgin n-3 PUFAs in dairy products. Therefore, this study aimed to highlight the nutritional characteristics and key factors affecting the concentration and type of virgin n-3 PUFAs in raw milk and dairy products.
2. Methodology
The scholarly articles used in this review were searched from the web using Google Scholar, Science Direct, PubMed, and subject-specific professional websites, and the data from 1999 to 2022. The keywords “omega-3,” “α-linolenic acid,” “eicosatrienoic acid,” “docosapentaenoic acid,” “docosahexaenoic acid,” “microalgae,” “fish oil,” “flaxseed,” “dairy,” and “cheese” were used in the search. The articles chosen in this review should show the nutrition, production, or processing of virgin n-3 PUFAs in dairy.
2.1. Virgin n-3 PUFAs in milk
There are many kinds of n-3 PUFAs in milk, such as ALA, ETE, EPA, DPA, and DHA, but their contents are low, as shown in Table 1. There are three main forms of n-3 PUFAs in milk: phospholipids (PLs) [21,22], triacylglycerol (TAG) type [23], and free fatty acids [24]. Different n-3 PUFAs have different nutritional and functional roles in human health [25].
Table 1.
Mean values of n-3 PUFAs in Holstein and Jersey cows, buffalo, yak, human, goat, donkey, camel [26], moose [27], and sheep [28] milk, and flaxseed oil [29], fish oil [10], and microalgae [30] (g/100 g fatty acid).
| Fatty acid | Holstein | Jersey | Buffalo | Yak | Human | Goat | Donkey | Camel | Flaxseed oil | Fish oil | Microalgae oil |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALA | 0.40 | 0.30 | 0.14 | 1.12 | 0.88 | 0.30 | 2.57 | 0.99 | 50.8 | 1.11 | 0.03 |
| DPA | 0.01 | 0.01 | 0.01 | 0.02 | 0.04 | 0.01 | 0.01 | 0.03 | 0.00 | – | – |
| EPA | 0.03 | 0.04 | 0.03 | 0.06 | 0.04 | 0.04 | – | 0.06 | 0.00 | 7.06 | 0.41 |
| ETE | 0.07 | 0.07 | 0.07 | 0.07 | 0.15 | 0.11 | 0.08 | 0.18 | 0.00 | 1.34 | – |
| DHA | 0.01 | 0.01 | 0.01 | 0.03 | 0.38 | 0.03 | – | 0.03 | 0.00 | 6.76 | 29.98 |
| Σ n-3 | 0.52 | 0.44 | 0.25 | 1.43 | 1.49 | 0.49 | 2.75 | 1.28 | 50.8 | 17.62 | 30.50 |
“-”: unreported, n-3 PUFAs: omega-3 polyunsaturated fatty acids, ALA: α-linolenic acid, ETE: eicosatrienoic acid, EPA: eicosapentaenoic acid, DPA: docosapentaenoic acid, DHA: docosahexaenoic acid. Σ n-3 = ALA + ETE + EPA + DPA + DHA.
2.1.1. n-3 PUFAs in phospholipids
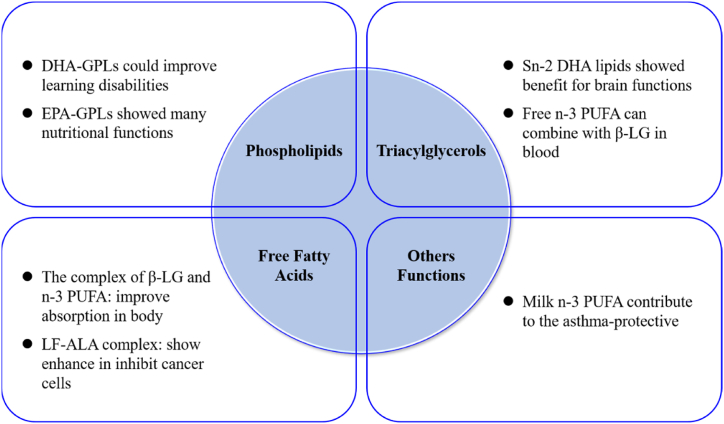
Lipids distributed in milk fat globules mainly exist in the form of PLs. PLs play an important role in the human body and infant development [31,32]. In general, PLs have a lower content of saturated fatty acids as well as short-chain fatty acids (C4–C10), but a higher content of unsaturated fatty acids in milk [33,34]. PLs containing PUFAs play an important role in the body, for example, DHA contained in PLs could improve the function of mitochondria [35]. Previous studies in animals and humans showed that n-3 PUFAs in the form of PL could have superior biological and nutritional functions [36]. Compared with the traditional n-3 PUFAs, the PL forms showed higher efficacy in the body [37], such as EPA-PLs and DHA-PLs. EPA-PLs and DHA-PLs can reduce neuroinflammation and anti-oxidation in the brain through changes in the brain cell structure and neurotransmitters, which are beneficial for brain function [38,39]. PLs can be classified into three types according to their molecular structure: glycerophospholipids (GPLs), sphingomyelins (SMs), and phospholipids (PLs) [40]. Recently, n-3 PUFAs connected with PLs, such as EPA-GPLs and DHA-GPLs, have received increasing attention. A previous study reported that DHA-GPLs could improve learning disabilities by altering brain lipid composition [41]. EPA-GPLs have many nutritional functions in humans, improving brain function, favoring visual and nervous system development, and regulating lipids in the blood [42].
2.1.2. n-3 PUFAs in triacylglycerols
The main form of milk fatty acids is TAGs, which account for more than 98 % of milk fat. n-3 PUFAs are located at three positions (sn-1, sn-2, and sn-3) in TAGs [20,43], and different positions in fatty acids play different roles in human health [44]. n-3 PUFAs in sn-1 and sn-3 are hydrolyzed by pancreatic lipase to form free n-3 PUFAs and sn-2 monoacylglycerols [45]. Free n-3 PUFAs can bind to β-LG because of their higher affinity for β-LG than for other blood components [14]. The n-3 PUFAs located at the sn-2 position showed higher absorption efficiency in the body compared with other positions [46,47]. Owing to their high absorption efficiency, sn-2 n-3 PUFA lipids play important roles in the body. A previous study reported that sn-2 DHA lipids are beneficial for brain functions, such as anxiety, cognitive decline, stress, and stroke [48]. Dietary supplementation with n-3 PUFAs in dairy animals can increase the concentration of n-3 PUFAs at the sn-2 position in milk [49]. Thus, regulating the dietary fatty acid composition can increase the content of virgin n-3 PUFAs in milk, which has many benefits for human health.
2.1.3. n-3 PUFAs in free fatty acids
The combination of fatty acids can alter the function of proteins [50]. The combination of β-LG and n-3 PUFAs can promote the absorption of n-3 PUFAs, and LF and n-3 PUFAs can enhance the inhibitory effect on cancer cell growth [50].
2.1.3.1. Functions of β-LG–n-3 PUFAs and α-LA–n-3 PUFAs complexes
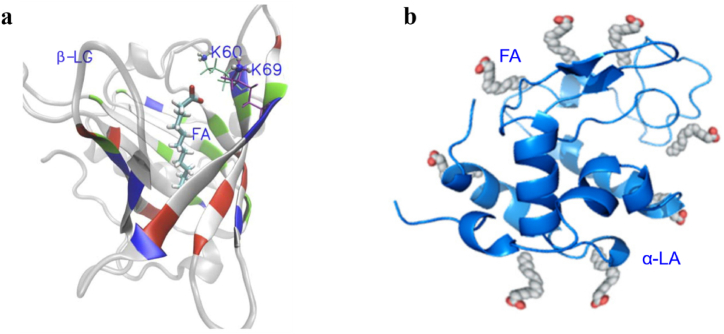
β-LG and α-LA are milk-specific proteins synthesized by breast epithelial cells, accounting for about 50 % and 36 % of the total whey protein in milk [51]. As members of the lipid carrier protein family, β-LG and α-LA have multiple ligand binding sites, which can bind with fatty acids, such as oleic acid, linoleic acid, and conjugated linoleic acid (CLA) to form complexes as showed in Fig. 2a [52] and Fig. 2b [53,54]. These complexes can increase their conformational stability to tryptic degradation [55], and can effectively embed, transfer, and protect bioactive components from oxidation and degradation [56]. β-LG and CLA can synthesize β-LG–CLA complexes. β-LG–CLA showed good stability in the gastrointestinal environment; thus, the complex can increase the absorption of CLA compared with CLA monomer (85.9 vs 45.8 μmol/L) [57]. Oleic acid (OA) and linoleic acid (LA) can bind to β-LG to form β-LG–OA and β-LG–LA, respectively. The two complexes, β-LG–OA and β-LG–LA, have cytotoxic activity on tumor cells and can induce cell decay [58]. In addition, β-LG–LA exhibited better thermostability than β-LG–OA [58]. β-LG and n-3 PUFAs can spontaneously combine to form nanoscale complexes [54], and these nanocomplexes have a good protective effect on n-3 PUFAs [14]. β-LG–n-3 PUFAs can delay the oxidative degradation of n-3 PUFAs during storage. β-LG can also effectively inhibit the degradation of n-3 PUFAs in the intestine and improve their absorption in the body. Most studies on α-LA have focused on the complexes of α-LA and OA. Although some studies have reported that α-LA and ALA can combine to form the complex α-LA–ALA [54], few studies have reported on the function of α-LA–n-3 PUFAs.
Fig. 2.
Protein structure representation of bovine β-lactoglobulin [52] (a) and α-lactalbumin [53,54] (b) bound to fatty acids. β-LG: β-lactoglobulin, FA: fatty acid, α-LA: α-lactalbumin.
2.1.3.2. Functions of lactoferrin–n-3 PUFAs complex
LF is a natural, harmless, bioactive iron-binding glycoprotein with a molecular weight of approximately 80 kDa. LF is produced by mammalian glandular epithelial cells and neutrophils and is widely expressed in various body fluids [59], such as milk. Previous studies found that LF plays a positive role in the human body [[60], [61], [62]]. The combination of LF and n-3 PUFAs may be similar to the complex of LF and OA, and the LF–OA complex induces apoptosis in tumor cells [63]. LF combined with n-3 PUFAs can enhance the inhibitory effect on cancer cells, especially in combination with ALA [51]. Thus, LF and n-3 PUFAs may also synthesize the LF–n-3 PUFA complex (LF–ALA complex), which may play a role in the body. Nowadays, the Coronavirus disease 2019 (COVID-19), which is caused by the SARS-CoV-2 virus, is rapidly spreading globally. LF may play an important role in the prevention of SARS-CoV-2 virus infection [64], and it can prevent SARS-CoV-2 virus infection by blocking the virus’s attachment to cellular heparin sulfate [65]. The LF–n-3 PUFA complex may have an inhibitory effect on SARS-CoV-2 infection.
Overall, as shown in Fig. 3, protein combined with n-3 PUFAs can increase the absorption of n-3 PUFAs and exert stronger physiological functions. β-LG–n-3 PUFAs may increase the absorption of n-3 PUFAs and avoid oxidation of n-3 PUFAs in milk. LF combined with n-3 PUFAs can enhance the inhibitory effect on cancer cells. Currently, there is limited research on the functionality of protein–n-3 PUFAs.
Fig. 3.
Potential benefits of different kinds of n-3 PUFAs to human health. n-3 PUFAs: omega-3 polyunsaturated fatty acids, ALA: α-linolenic acid, ETE: eicosatrienoic acid, EPA: eicosapentaenoic acid, DHA: docosahexaenoic acid, β-LG: β-lactoglobulin, LF: lactoferrin, GPL: glycerophospholipids.
2.2. Regulation of virgin n-3 PUFAs in milk by feeding
Although n-3 PUFAs are similar in structure, different types of n-3 PUFAs play different functions in the body. ALA is an essential fatty acid that cannot be synthesized in the body and must be obtained from food [66]. Longer n-3 PUFAs can be synthesized using ALA. DHA has the highest concentration of n-3 PUFAs in the brain and plays an important role in maintaining normal brain development and function [67]. DHA is beneficial for cognitive behavior and can improve the health of children [68]. EPA mainly affects blood physiology and EPA intake can decrease serum and hepatic lipid levels; however, DHA is ineffective [69]. Therefore, the development of milk rich in different types of n-3 PUFAs may become a trend.
The kinds of n-3 PUFAs in milk, including free fatty acid, PLs and TAG, can be regulated by the diet of dairy animals [70]. The main n-3 PUFA feeds include flaxseed, fish oil, microalgae, and algae. These feeds can be used to produce milk that is rich in different types of n-3 PUFAs.
2.2.1. Regulation of virgin ALA in milk
Flaxseed has high ALA content [71], which can effectively increase the content of ALA in raw milk as dairy cow feed [72]. As shown in Table 2, there are many forms of flaxseed (including whole flaxseed, extruded flaxseed, and flaxseed oil) in the diet of different dairy animals (buffalo, goat, and sheep). All studies found that supplementation with flaxseed in the diet can increase the content of ALA in milk. However, different studies have reported different results for other longer-chain n-3 PUFAs. Studies have reported that flaxseed supplementation in the diet can cause an increase in virgin EPA [[73], [74], [75]] or DPA [28] in milk. However, some studies have shown no influence on the concentration of EPA [28,[76], [77], [78], [79]] or DPA [80,81] in milk. Several studies have reported that flaxseed supplementation can increase the concentration of virgin DHA in milk [82]. A study on buffalo milk found that the DHA content in milk can be increased with flaxseed supplementation in the diet [81,82]. ALA can be converted to longer-chain n-3 PUFAs (ALA → ETE → EPA → DPA → DHA) via enzyme action in vitro [83]. In general, flaxseed is used to produce virgin ALA dairy products.
Table 2.
Summary of the content of virgin n-3 PUFA in milk with dietary flaxseed supplementation.
| Core material | Dosage | Breed | n-3 PUFA in milk (g/100 g fatty acid) |
Results | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| ALA | ETE | EPA | DPA | DHA | |||||
| Whole flaxseed | 0.00 % | Holstein cows | 0.52 | – | 0.01 | 0.01 | – | –The concentration in milk Increased: ALA No influence: EPA and DPA |
[78] |
| 5.00 % | 0.66 | – | 0.01 | 0 | – | ||||
| 10.00 % | 0.82 | – | 0.02 | 0.21 | – | ||||
| 15.00 % | 0.96 | – | 0.01 | 0 | – | ||||
| Extruded flaxseed | 0.00 % | Holstein cows | 0.44 | 0 | 0.03 | 0.008 | 0.003 | –The concentration in milk Increased: ALA and EPA No influence: DPA and DHA |
[74] |
| 5.00 % | 0.8 | 0.001 | 0.05 | 0.009 | 0 | ||||
| Flaxseed oil | 0.00 % | Holsteins cows | 0.31 | <0.01 | 0.03 | – | <0.01 | –The concentration in milk Increased: ALA No influence: ETE, EPA and DHA |
[79] |
| 2.50 % | 0.99 | <0.01 | 0.04 | – | <0.01 | ||||
| Flaxseed oil | 0 mL | Holstein cows | 0.21 | 0 | 0.01 | 0.05 | – | –The concentration in milk Increased: ALA and DPA No influence: ETE and EPA |
[28] |
| 110 mL | 1.89 | 0 | 0.02 | 0.08 | – | ||||
| 220 mL | 3.3 | 0.01 | 0.02 | 0.08 | – | ||||
| Whole flaxseed | 0.00 % | Friesian cows | 0.26 | 0.29 | 0.015 | 0.05 | 0.02 | –The concentration in milk Increased: ALA No influence: ETE, EPA, DPA and DHA |
[84] |
| 1.93 % | 0.31 | 0.27 | 0.015 | 0.05 | 0.02 | ||||
| 0.00 % | Jersey cows | 0.22 | 0.23 | 0.011 | 0.03 | 0.02 | –The concentration in milk Increased: ALA No influence: ETE, EPA, DPA and DHA |
[84] | |
| 1.93 % | 0.28 | 0.23 | 0.013 | 0.04 | 0.02 | ||||
| Whole flaxseed | 0.00 % | Sarda goats | 1.05 | 0.01 | 0.005 | – | 0.012 | –The concentration in milk Increased: ALA and EPA No influence: ETE and DHA |
[73] |
| 21.00 % | 1.65 | 0.02 | 0.037 | – | 0.013 | ||||
| Flaxseed oil | 0 g/d | Malagueña goats | 0.35 | – | 0.03 | 0.09 | 0.02 | –The concentration in milk Increased: ALA and EPA No influence: DPA and DHA |
[75] |
| 30 g/d | 0.66 | – | 0.04 | 0.09 | 0.02 | ||||
| Extruded flaxseed | 0.00 % | Alpine goats | 0.62 | – | 0.085 | 0.121 | 0.049 | –The concentration in milk Increased: ALA No influence: EPA, DPA and DHA |
[77] |
| 21.60 % | 2.19 | – | 0.101 | 0.107 | 0.031 | ||||
| Extruded flaxseed | 0.00 % | ewes | 0.36 | 0.012 | 0.02 | 0.06 | 0.03 | –The concentration in milk Increased: ALA, EPA and DPA No influence: DHA |
[85] |
| 6.00 % | 1.27 | 0.01 | 0.04 | 0.08 | 0.03 | ||||
| 12.00 % | 1.91 | 0.01 | 0.04 | 0.08 | 0.03 | ||||
| Flaxseed | 0.00 kg | sheep | 0.74 | 0.01 | 0.07 | 0.07 | 0.02 | –The concentration in milk Increased: ALA No influence: ETE, EPA, DPA and DHA |
[80] |
| 0.22 kg | 1.87 | 0.02 | 0.07 | 0.08 | 0.02 | ||||
| Whole flaxseed | 0.00 % | Comisana ewes | 0.57 | 0.02 | 0.07 | 0.08 | 0.05 | –The concentration in milk Increased: ALA, ETE and EPA No influence: DPA and DHA |
[81] |
| 9.06 % | 1.53 | 0.03 | 0.08 | 0.09 | 0.05 | ||||
| Flaxseed oil | 0.00 % | Water buffaloes | 0.383 | – | 0.024 | – | 0.016 | –The concentration in milk Increased: ALA and DHA No influence: EPA |
[82] |
| 2.50 % | 0.768 | – | 0.021 | – | 0.069 | ||||
“-”: means not detected, n-3 PUFAs: omega-3 polyunsaturated fatty acids, ALA: α-linolenic acid, ETE: eicosatrienoic acid, EPA: eicosapentaenoic acid, DPA: docosapentaenoic acid, DHA: docosahexaenoic acid.
2.2.2. Regulation of virgin EPA and DHA in milk
Fish oil and microalgae contain high concentrations of n-3 PUFAs [17] (mainly EPA and DHA) and can be used as feed sources to increase the content of virgin milk EPA and DHA. However, as shown in Table 3, the EPA and DHA contents in fish oil and algae are different, and the EPA concentration in fish oil is higher than that in microalgae. The main n-3 PUFAs in microalgae are DPA and DHA.
Table 3.
Summary of the content of n-3 PUFAs in fish oil and microalgae.
| Core material | n-3 PUFAs g/100 g fatty acid |
Reference | ||||
|---|---|---|---|---|---|---|
| ALA | ETE | EPA | DPA | DHA | ||
| Fish oil | 2.84 | 0.2 | 8.19 | 1.62 | 13.83 | [86] |
| Fish oil | – | – | 8.261 | – | 37.374 | [87] |
| Fish oil | 1.95 | – | 35.81 | – | 28.43 | [88] |
| Microalgae | 0.1 | – | 1.0 | 13.2 | 35.3 | [89] |
| Microalgae oil | 0 | – | 1.4 | 15.4 | 39.5 | [89] |
| Microalgae | 0.03 | – | 0.41 | 6.31 | 29.89 | [30] |
“-”: means not detected, n-3 PUFAs: omega-3 polyunsaturated fatty acids, ALA: α-linolenic acid, ETE: eicosatrienoic acid, EPA: eicosapentaenoic acid, DPA: docosapentaenoic acid, DHA: docosahexaenoic acid.
The main n-3 PUFAs in fish oil are EPA and DHA [80], and dietary supplementation of dairy animals can increase the concentration of EPA and DHA in raw milk [88,91], as shown in Table 4. In addition, the addition of fish oil to the diet of dairy animals can also increase the levels of other kinds of n-3 PUFAs such as ETE and DPA [[92], [93], [94]]. Furthermore, fish oil supplementation in the diet of dairy animals does not influence the concentration of ALA in raw milk [91,95,96]. However, some research reported that fish oil addition to the diet can increase the concentration of ALA in raw milk. This may be due to the composition of n-3 PUFAs in fish oil being different. Some fish oils contain ALA [86], so some researchers found that the content of ALA in raw milk can be increased with ALA supplementation in the diet. However, the content of ALA in fish oil is very low [87]. Thus, fish oil can be used to produce virgin EPA and DHA dairy products.
Table 4.
Summary of the content of virgin n-3 PUFAs in milk with fish oil supplement in the diet.
| Fish oil Dosage | Breed | n-3 PUFAs in milk g/100 g fatty acid |
Results | References | ||||
|---|---|---|---|---|---|---|---|---|
| ALA | ETE | EPA | DPA | DHA | ||||
| 0 g/d | cow | 0.41 | 0.013 | 0.06 | 0.09 | 0.03 | –The concentration in milk Increased: ETE, EPA, DPA, and DHA No influence: ALA |
[93] |
| 75 g/d | 0.38 | 0.017 | 0.06 | 0.08 | 0.03 | |||
| 150 g/d | 0.39 | 0.056 | 0.07 | 0.1 | 0.05 | |||
| 300 g/d | 0.48 | 0.323 | 0.17 | 0.18 | 0.10 | |||
| 0 % | Holstein cows | 0.51 | – | 0.23 | – | 0.59 | –The concentration in milk Increased: EPA and DHA No influence: ALA |
[88] |
| 1.80 % | 0.44 | – | 1.66 | – | 3.11 | |||
| 0 % | Holstein cows | 0.75 | 0.001 | 0.003 | – | 0.001 | –The concentration in milk Increased: ALA, ETE, EPA, DPA, and DHA |
[77] |
| 1.10 % | 0.84 | 0.019 | 0.060 | – | 0.117 | |||
| 0 | Holstein cows | 0.4 | 0 | 0.03 | 0.06 | 0.02 | –The concentration in milk Increased: ETE, EPA, DPA, and DHA No influence: ALA |
[92] |
| 0.80 % | 0.41 | 0.04 | 0.05 | 0.08 | 0.03 | |||
| 0 % | Brown Swiss cows | 1.04 | – | 0.14 | 0.2 | 0.06 | –The concentration in milk Increased: EPA, DPA, and DHA No influence: ALA |
[90] |
| 2.00 % | 1.35 | – | 0.39 | 0.3 | 0.15 | |||
| 0 % | Assaf ewes | 0.33 | 0.02 | 0.03 | 0.06 | 0.02 | –The concentration in milk Increased: ETE, EPA, DPA, and DHA No influence: ALA |
[94] |
| 1 % | 0.34 | 0.05 | 0.15 | 0.18 | 0.38 | |||
| 0 % | Assaf ewes | 0.56 | – | 0.06 | 0.09 | 0.16 | –The concentration in milk Increased: EPA, DPA, and DHA No influence: ALA |
[96] |
| 1.70 % | 0.4 | – | 0.42 | 0.44 | 1.18 | |||
| 0 % | Assaf ewes | 0.58 | – | 0.07 | 0.1 | 0.03 | –The concentration in milk Increased: EPA, DPA, and DHA No influence: ALA |
[95] |
| 1.50 % | 0.46 | – | 0.35 | 0.35 | 0.99 | |||
n-3 PUFAs: omega-3 polyunsaturated fatty acids, ALA: α-linolenic acid, ETE: eicosatrienoic acid, EPA: eicosapentaenoic acid, DPA: docosapentaenoic acid, DHA: docosahexaenoic acid.
2.2.3. Regulation of virgin DHA in milk
As high-quality DHA feed [97], microalgae can be used to produce virgin DHA dairy products (shown in Table 5) [98]. In addition to DHA, microalgae can also increase the content of ETE [99], EPA [29], and DPA [100] in raw milk. One study reported that microalgae supplementation can increase the concentration of ETE (0.018–0.051 g/100 g fatty acid; 283 times), EPA (0.072–0.360 g/100 g fatty acid; 500 times), DPA (0.110–0.290 g/100 g fatty acid; 264 times), and ETE (0.074–1.150 g/100 g fatty acid; 1554 times) [101]. In addition, some studies reported that microalgae supplementation did not affect the concentration of ETE [98] and EPA [99] in milk and may have even decreased the ETE concentration [102] in milk. Most studies have reported that microalgae supplementation does not influence or decrease the concentration of ALA in milk [29,101]. Thus, microalgae can be used to produce virgin DHA in dairy products, as a feed for cows.
Table 5.
Summary of the content of virgin n-3 polyunsaturated fatty acids (PUFAs) in milk with microalgae supplement in the diet.
| Microalgae Dosage | Breed | n-3 PUFAs in milk g/100 g fatty acid |
Results | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| ALA | ETE | EPA | DPA | DHA | ||||
| 0 g/d | Holstein cows | 0.273 | – | 0.022 | – | 0.002 | –The concentration in milk Increased: ALA, EPA, and DHA |
[29] |
| 100 g/d | 0.318 | – | 0.028 | – | 0.242 | |||
| 0 g/d | Holstein cows | 0.45 | 0.13 | 0.07 | – | 0.08 | –The concentration in milk Increased: ALA, ETE, and DHA No influence: EPA |
[99] |
| 50 g/d | 0.46 | 0.14 | 0.07 | – | 0.15 | |||
| 100 g/d | 0.49 | 0.14 | 0.06 | – | 0.25 | |||
| 150 g/d | 0.05 | 0.16 | 0.07 | – | 0.37 | |||
| 0 g/d | Holstein cows | 0.48 | 0.18 | 0.08 | – | 0.04 | –The concentration in milk Increased: DHA No influence: ALA, ETE, and EPA |
[98] |
| 100 g/d | 0.47 | 0.17 | 0.09 | – | 0.22 | |||
| 0 g/d | cows | 0.66 | 0.018 | 0.072 | 0.11 | 0.074 | –The concentration in milk Increased: ETE, EPA. DPA, and DHA Decreased: ALA |
[101] |
| 310 g/d | 0.53 | 0.051 | 0.36 | 0.29 | 1.15 | |||
| 0 g/d | Alpine goats | 1.18 | – | 0.09 | 0.22 | 0.04 | –The concentration in milk Increased: ETE, EPA. DPA, and DHA Decreased: ALA |
[103] |
| 10 g/d | 0.95 | – | 0.07 | 0.18 | 0.32 | |||
| 0 g/d | goats | 0.62 | 0.018 | 0.052 | 0.1 | 0.078 | –The concentration in milk Increased: EPA. DPA, and DHA Decreased: ALA No influence: ETE |
[102] |
| 40 g/d | 0.51 | 0.019 | 0.15 | 0.18 | 0.77 | |||
| 0 g/d | ewes | 3.33 | 0.18 | 0.00 | 0.00 | 0.00 | –The concentration in milk Increased: EPA. DPA, and DHA Decreased: ALA and ETE |
[100] |
| 23.5 g/d | 0.31 | 0.19 | 0.04 | 0.21 | 0.43 | |||
| 47 g/d | 0.33 | 0.09 | 0.12 | 0.28 | 0.69 | |||
| 94 g/d | 0.25 | 0.05 | 0.21 | 0.31 | 1.24 | |||
| 0 g/d | Assaf sheep | 0.49 | – | 0.04 | 0.10 | 0.05 | –The concentration in milk Increased: EPA. DPA, and DHA No influence: ALA |
[102] |
| 0.70 % | 0.48 | – | 0.06 | 0.12 | 0.38 | |||
n-3 PUFA: omega-3 polyunsaturated fatty acid, ALA: α-linolenic acid, ETE: eicosatrienoic acid, EPA: eicosapentaenoic acid, DPA: docosapentaenoic acid, DHA: docosahexaenoic acid.
2.3. Virgin n-3 PUFAs changes in various dairy products
Raw milk can be used to produce various dairy products. In particular, milk and cheese are the two main dairy foods in our daily lives.
2.3.1. Dairy products
2.3.1.1. Liquid milk
Sterilization is the main process for liquid milk, which is an important means of killing pathogens and destroying bacteria in raw milk. The sterilization methods include thermal technology (pasteurization, ultrahigh temperature processing and high-pressure processing) and nonthermal techniques (ultraviolet radiation, high-power ultrasound, and bactofugation).
Thermal technology is a commonly used sterilization method in commercial production. However, because of the high unsaturation, the n-3 PUFAs are easily oxidized by heat treatment [104]. The temperature and processing time of thermal technologies are the two main factors that influence the content of n-3 PUFAs in dairy products. As shown in Table 6, when the temperature was not higher than 120 °C, short-term (15 s) heat treatment did not affect the n-3 PUFA content in milk [105]. However, the concentration of n-3 PUFAs (ALA, EPA, and DHA) in milk decreases [19] after treatment at 135 °C for 15 s. In addition, milk heated at 62.5 °C for 30 min also showed no influence on the concentration of n-3 PUFAs in milk [106,107]. However, treatment at 65 °C for 30 min or boiling for 1 min decreased the ALA content in milk. In general, controlling the temperature and duration of heat treatment can provide healthy and safe dairy products for humans.
Table 6.
Summary of the content of virgin n-3 PUFAs in milk with heat treatments.
| Milk | Heat treatments | n-3 PUFAs in milk g/100 g fatty acid |
Reference | |||
|---|---|---|---|---|---|---|
| ALA | EPA | DPA | DHA | |||
| Cow | 65 °C for 30 min | decrease 29.63 % |
– | – | – | [111] |
| Boiling for 1 min | decrease 52.85 % |
– | – | – | ||
| Buffalo | 65 °C for 30 min | decrease 15.38 % |
– | – | – | |
| Boiling for 1 min | decrease 40.38 % |
– | – | – | ||
| Cow | 85 °C for 15 s | no | no | – | – | [19] |
| 135 °C for 15 s | decrease 8.50 % |
decrease 21.11 % |
– | – | ||
| Cow | 72 °C for 15 s | no | no | – | – | [105] |
| 75 °C for 15 s | no | no | – | – | ||
| 80 °C for 15 s | no | no | – | – | ||
| 85 °C for 15 s | no | no | – | – | ||
| 90 °C for 15 s | no | no | – | – | ||
| 95 °C for 15 s | no | no | – | – | ||
| 100 °C for 15 s | no | no | – | – | ||
| 105 °C for 15 s | no | no | – | – | ||
| 110 °C for 15 s | no | no | – | – | ||
| 115 °C for 15 s | no | no | – | – | ||
| 120 °C for 15 s | no | no | – | – | ||
| Human | 62.5 °C for 30 min | no | no | no | no | [106] |
| 62.5 °C for 30 min and pressurized for 5 min at 400 MPa | no | no | no | no | ||
| 62.5 °C for 30 min and pressurized for 5 min at 500 MPa | no | no | no | no | ||
| 62.5 °C for 30 min and pressurized for 5 min at 600 MPa | no | no | no | no | ||
| Human | 62.5 °C for 30 min | – | – | – | no | [107] |
Decrease = (raw milk fatty acid – dairy products fatty acid) × 100/raw milk fatty acid, no: no influence.
n-3 PUFA: omega-3 polyunsaturated fatty acid, ALA: α-linolenic acid, EPA: eicosapentaenoic acid, DPA: docosapentaenoic acid, DHA: docosahexaenoic acid.
The main nonthermal technologies include ultraviolet radiation, high-power ultrasound, and bactofugation. Ultraviolet radiation, as one of the emerging food processing technologies [108], has a huge potential in milk processing. Ultraviolet radiation kills microorganisms in milk without producing heat. Thus, studies have found that ultraviolet radiation does not affect n-3 PUFAs in milk [109]. High-power ultrasound and bactofugation are two sterilization methods used in milk production. High-power ultrasound and bactofugation could reduce the loss of nutrients in milk while sterilizing [110]. Therefore, the development of nonthermal technologies is conducive to the production of n-3 PUFA-rich dairy products.
2.3.1.2. Cheese
Cheese is a nutritious and versatile food whose nutritional value is affected by the type of milk used [112]. Thus, increasing the concentration of virgin n-3 PUFAs in raw milk can increase the n-3 PUFA content in cheese [113,114]. Because the fat content in cheese is influenced by several factors, such as the method of manufacture, studies have reported that higher concentrations of n-3 PUFAs were observed in cheese than in raw milk (cheese vs raw milk: 4.05 vs 3.71) [115].
2.3.2. Oxidation of virgin n-3 PUFAs in dairy products
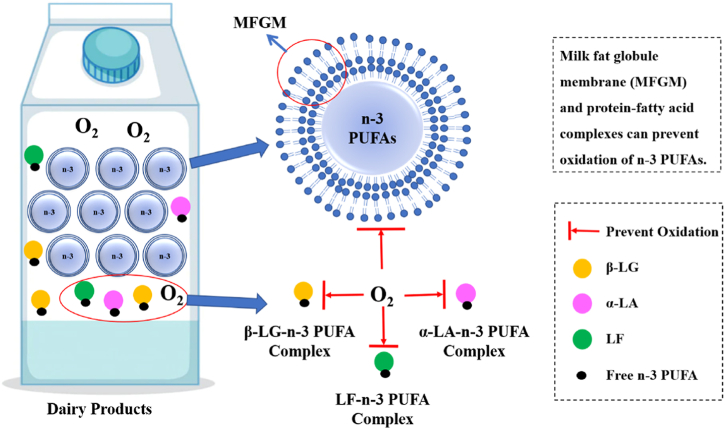
The n-3 PUFAs have high oxidation sensitivity [116], thus the concentration of n-3 PUFAs in milk decreases with storage time by direct incorporation of n-3 PUFA sources [117,118]. Fatty acid oxidation can cause off-flavor in milk [119]. However, virgin n-3 PUFAs are hardly oxidized during storage time [91], and compared with added n-3 PUFAs in milk there is no odor due to the oxidation of virgin n-3 PUFAs during storage [91,120]. A previous study found that the concentration of virgin n-3 PUFAs in cheese was not influenced by storage [115]. This may be due to approximately 99 % of fatty acid being compartmented in milk fat globules, and most n-3 PUFAs are surrounded by the milk fat globule membrane (MFGM). The MFGM can protect n-3 PUFAs against lipolysis and dispersion [121] and reduce the oxidation of virgin n-3 PUFAs in milk [112], as shown in Fig. 4. In addition, nanocomplexes of n-3 PUFA–β-LG could also protect n-3 PUFAs against lipolysis and dispersal.
Fig. 4.
Virgin n-3 PUFAs on lipid oxidation in dairy foods. BLG: β-lactoglobulin, α-LA: α-lactalbumin, LF: lactoferrin, n-3 PUFAs: omega-3 polyunsaturated fatty acids, MFGM: milk fat globule membrane.
In conclusion, the main factor influencing the concentration of virgin n-3 PUFAs in dairy products is heat treatment. Owing to the protection provided by the MFGM and milk protein, the content of virgin n-3 PUFAs remained constant during storage.
3. Conclusion
Dairy products with virgin n-3 PUFAs are potentially healthy functional foods. However, the development of virgin n-3 PUFA dairy products is still at the primary stage. To date, most studies only focused on how to obtain a stable content of virgin n-3 PUFA raw milk. However, studies on the nutrition of virgin n-3 PUFA dairy products are still insufficient, such as the relationship between the production and preservation of virgin n-3 PUFA dairy. Thus, further studies can focus on two aspects: 1) comprehensive functional evaluation of virgin n-3 PUFA milk and 2) the relationship between the concentration of virgin n-3 PUFAs in milk and heat treatment, and the development of emerging nonthermal technologies.
CRediT authorship contribution statement
Guoxin Huang: Writing – original draft, Formal analysis, Data curation, Conceptualization. Ning Li: Investigation. Xufang Wu: Software. Nan Zheng: Writing – review & editing. Shengguo Zhao: Validation. Yangdong Zhang: Supervision. Jiaqi Wang: Supervision, Formal analysis.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests.
Acknowledgments
This study was financially supported by the National Key R&D Program of China (No. 2022YFD1301004), the Agricultural Science and Technology Innovation Program (ASTIP-IAS12), the Modern Agro-Industry Technology Research System of the PR China, and the Scientific Research Project for Major Achievements of the Agricultural Science and Technology Innovation Program (CAAS-ZDXT2019004).
Contributor Information
Yangdong Zhang, Email: zhangyangdong@caas.cn.
Jiaqi Wang, Email: jiaqiwang@vip.163.com.
References
- 1.Chen C., Xun P., Kaufman J.D., Hayden K.M., Espeland M.A., Whitsel E.A.M.L., Serre, Vizuete W., Orchard T., Harris W.S., Wang X., Chui H.C., Chen J., He K. Erythrocyte omega-3 index, ambient fine particle exposure, and. brain aging. Neurology. 2020;95:e995–e1007. doi: 10.1212/WNL.0000000000010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanchais K., Capel F., Tournadre A. Could omega 3 fatty acids preserve muscle health in rheumatoid arthritis? Nutrients. 2020;12:223. doi: 10.3390/nu12010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naghshi S., Aune D., Beyene J., Mobarak S., Asadi M., Sadeghi O. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of cohort studies. BMJ. 2021;375:n2213. doi: 10.1136/bmj.n2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lázaro I., Rueda F., Cediel G., Ortega E., García-García C., Sala-Vila A., Bayés-Genís A. Circulating omega-3 fatty acids and incident adverse events in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2020;76:2089–2097. doi: 10.1016/j.jacc.2020.08.073. [DOI] [PubMed] [Google Scholar]
- 5.Afshin A., Sur P.J., Fay K.A., Cornaby L., Ferrara G., Salama J., Mullany E.C., Abate K.H., Abbafati C., Abebe Z., Afarideh M., Aggarwal A., Agrawal S., Akinyemiju T., Alahdab F., Bacha U., Bachman V.F., Badali H., Badawi A., Bensenor I.M., Bernabe E., Biadgilign S., Biryukov S., Cahill L.E., Carrero J.J., Cercy K., Dandona L., Dandona R., Dang A.K., Degefa M.G., Zaki M.E.S., Esteghamati A., Esteghamati S., Fanzo J., Farinha C.S.E.S., Farvid M.S., Farzadfar F., Feigin V.L., Fernandes J.C., Flor L.S., Foigt N., Forouzanfar M.H., Ganji M., Geleijnse J.M., Gillum R.F., Goulart A.C., Grosso G., Guessous I., Hamidi S., Hankey G.J., Harikrishnan S., Hassen H.Y., Hay S.I., Hoang C.L., Horino M., Islami F., Jackson M.D., James S.L., Johansson L., B Jonas J., Kasaeian A., Khader Y., Khalil I.A., Khang Y., Kimokoti R.W., Kokubo Y., Kumar G.A., Lallukka T., Lopez A., Lorkowski S., Lotufo P.A., Lozano R., Malekzadeh R., Marz W., Meier T., Melaku Y.A., Mendoza W., Mensink G.B.M., Micha R., Miller T.R., Mirarefin M., Mohan V., Mokdad A.H., Mozaffarian D., Nagel G., Naghavi M., Nguyen C.T., Nixon M.R., Ong K.L., Pereira D.M., Poustchi H., Rai M. Qorbani R.K., Razogarcia C., Rehm C.D., Rivera J.A., Rodriguezramirez S., Roshandel G., Roth G.A., Sanabria J.R., Sanchezpimienta T.G., Sartorius B., Schmidhuber J., Schutte A.E., Sepanlou S.G., Shin M., Sorensen R.J.D., Springmann M., Szponar L., Thornelyman A.L., Thrift A.G., Touvier M., Tran B.X., Tyrovolas S., Ukwaja K.N., Ullah I., Uthman O.A., Vaezghasemi M., Vasankari T., Vollset S.E., Vos T., Vu G.T., Vu L.G., Weiderpass E.V., Werdecker A., Wijeratne T., Willett W.C., Wu J.H.Y., Xu G., Yonemoto N., Yu C., Murray C.J.L. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asher A., Tintle N.L., Myers M., Lockshon L., Bacareza H., Harris W.S. Blood omega-3 fatty acids and death from COVID-19: a pilot study. Prostagl. Leukot. Essent. Fat. Acids. 2021;166 doi: 10.1016/j.plefa.2021.102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saini R.K., Keum Y. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance-A review. Life Sci. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 8.Yu X., Wang Q., Lu W., Zhang M., Chen K., Xue J., Zhao Q., Wang P., Luo P., Shen Q. Fast and specific screening of EPA/DHA-Enriched phospholipids in fish oil extracted from different species by HILIC–MS. J. Agric. Food Chem. 2021;69:7997–8007. doi: 10.1021/acs.jafc.1c01709. [DOI] [PubMed] [Google Scholar]
- 9.Perdana B.A., Chaidir Z., Kusnanda A.J., Dharma A., Zakaria I.J., Bayu A., Putra M.Y. Omega-3 fatty acids of microalgae as a food supplement: a review of exogenous factors for production enhancement. Algal Res. 2021;60 doi: 10.1016/j.algal.2021.102542. [DOI] [Google Scholar]
- 10.Lemahieu C., Bruneel C., Ryckebosch E., Muylaert K., Buyse J., Foubert I. Impact of different omega-3 polyunsaturated fatty acid (n-3 PUFA) sources (flaxseed, Isochrysis galbana, fish oil and DHA Gold) on n-3 LC-PUFA enrichment (efficiency) in the egg yolk. J. Funct.Foods. 2015;19:821–827. doi: 10.1016/j.jff.2015.04.021. [DOI] [Google Scholar]
- 11.Brick T., Schober Y., Böcking C., Pekkanen J., Genuneit J., Loss G., Dalphin J.C., Riedler J., Lauener R., Nockher W.A., Renz H., Vaarala O., Braun-Fahrländer C., von Mutius E., Ege M.J., Pfefferle P.I. PASTURE study group. ω-3 fatty acids contribute to the asthma-protective effect of unprocessed cow's milk. J. Allergy Clin. Immunol. 2016;137(6):1699–1706. doi: 10.1016/j.jaci.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Sun S., Hua X., Deng Y., Zhang Y., Li J., Wu Z., Limbu S.M., Lu D., Yin H., Wang G., Waagbø R., Frøyland L., Zhang M., Du Z. Tracking pollutants in dietary fish oil: from ocean to table. Environmental Pollution. 2018;240:733–744. doi: 10.1016/j.envpol.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Ishaq Z., Nawaz M.A. Analysis of contaminated milk with organochlorine pesticide residues using gas chromatography. Int. J. Food Prop. 2018;21:879–891. doi: 10.1080/10942912.2018.1460607. [DOI] [Google Scholar]
- 14.Zimet P., Livney Y.D. Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocolloids. 2009;23:1120–1126. doi: 10.1016/j.foodhyd.2008.10.008. [DOI] [Google Scholar]
- 15.Honest K.N., Zhang H.W., Zhang L. Lycopene: isomerization effects on bioavailability and bioactivity properties. Food Rev. Int. 2011;27:248–258. doi: 10.1080/87559129.2011.563392. [DOI] [Google Scholar]
- 16.Gumus C.E., Gharibzahedi S.M.T. Yogurts supplemented with lipid emulsions rich in omega-3 fatty acids: new insights into the fortification, microencapsulation, quality properties, and health-promoting effects. Trends Food Sci. Technol. 2021;110:267–279. doi: 10.1016/j.tifs.2021.02.016. [DOI] [Google Scholar]
- 17.Huang G.X., Zhang Y.D., Xu Q.B., Zheng N., Zhao S.G., Liu K.Z., Qu X.Y., Yu J., Wang J.Q. DHA content in milk and biohydrogenation pathway in rumen: a review. PeerJ. 2020;8 doi: 10.7717/peerj.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamed S., Soliman T., Hassan L., Abo-Elwafa G. Preparation of functional yogurt fortified with fish oil-in-water nanoemulsion. Egypt. J. Chem. 2019;62:6–7. doi: 10.21608/ejchem.2019.18621.2149. [DOI] [Google Scholar]
- 19.Huang G.X., Wang J., Liu K.Z., Wang F.E., Zheng N., Zhao S.G., Qu X.Y., Yu J., Zhang Y.D., Wang J.Q. Effect of flaxseed supplementation on milk and plasma fatty acid composition and plasma parameters of Holstein dairy cows. Animals. 2022;15:1898. doi: 10.3390/ani12151898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q.B., Zhang Y.D., Zheng N., Wang Q., Li S., Zhao S.G., Wen F., Meng L., Wang J.Q. Short communication: decrease of lipid profiles in cow milk by ultra-high-temperature treatment but not by pasteurization. J. Dairy Sci. 2020;103:1900–1907. doi: 10.3168/jds.2019-17329. [DOI] [PubMed] [Google Scholar]
- 21.Zou X., Huang J., Jin Q., Guo Z., Liu Y., Cheong L., Xu X., Wang X. Lipid composition analysis of milk fats from different mammalian species: potential for use as human milk fat substitutes. J. Agric. Food Chem. 2013;61(29):7070–7080. doi: 10.1021/jf401452y. [DOI] [PubMed] [Google Scholar]
- 22.Yao Y., Zhao G., Xiang J., Zou X., Jin Q., Wang X. Lipid composition and structural characteristics of bovine, caprine and human milk fat globules. Int. Dairy J. 2016;56:64–73. doi: 10.1016/j.idairyj.2015.12.013. [DOI] [Google Scholar]
- 23.Wang X., Zhu H., Zhang W., Zhang Y., Zhao P., Zhang S., Pang X., Vervoort J., Lu J., Lv J. Triglyceride and fatty acid composition of ruminants milk, human milk, and infant formulae. J. Food Compos. Anal. 2022;106 doi: 10.1016/j.jfca.2021.104327. [DOI] [Google Scholar]
- 24.Garcia C., Antona C., Robert B., Lopez C., Armand M. The size and interfacial composition of milk fat globules are key factors controlling triglycerides bioavailability in simulated human gastro-duodenal digestion. Food Hydrocolloids. 2014;35:494–504. doi: 10.1016/j.foodhyd.2013.07.005. [DOI] [Google Scholar]
- 25.Howe P., Buckley J. Metabolic health benefits of long-chain omega-3 polyunsaturated fatty acids. Mil. Med. 2014 Nov;179(11 Suppl):138–143. doi: 10.7205/MILMED-D-14-00154. [DOI] [PubMed] [Google Scholar]
- 26.Wang F.E., Chen M.Q., Luo R.B., Huang G.X., Wu X.F., Zheng N., Zhang Y.D., Wang J.Q. Fatty acid profiles of milk from Holstein cows, Jersey cows, buffalos, yaks, humans, goats, camels, and donkeys based on gas chromatography–mass spectrometry. J. Dairy Sci. 2021;105:1687–1700. doi: 10.3168/jds.2021-20750. [DOI] [PubMed] [Google Scholar]
- 27.Dreiucker J., Vetter W. Fatty acids patterns in camel, moose, cow and human milk as determined with GC/MS after silver ion solid phase extraction. Food Chem. 2011;126:762–771. doi: 10.1016/j.foodchem.2010.11.061. [DOI] [Google Scholar]
- 28.Basdagianni Z., Papaloukas L., Kyriakou G., Karaiskou C., Parissi Z., Sinapis E., Kasapidou E. A comparative study of the fatty acid and terpene profiles of ovine and caprine milk from Greek mountain sheep breeds and a local goat breed raised under a semi-extensive production system. Food Chem. 2019;278:625–629. doi: 10.1016/j.foodchem.2018.11.093. [DOI] [PubMed] [Google Scholar]
- 29.Moallem U., Vyas D., Teter B.B., Delmonte P., Zachut M., Erdman R.A. Transfer rate of α-linolenic acid from abomasally infused flaxseed oil into milk fat and the effects on milk fatty acid composition in dairy cows. J. Dairy Sci. 2012;95:5276–5284. doi: 10.3168/jds.2012-5415. [DOI] [PubMed] [Google Scholar]
- 30.Sinedino L., Honda P.M., Souza L., Lock A.L., Santos J. Effects of supplementation with docosahexaenoic acid on reproduction of dairy cows. Reproduction. 2017;153:707–723. doi: 10.1530/REP-16-0642. [DOI] [PubMed] [Google Scholar]
- 31.Ahmmed M.K., Ahmmed F., Tian H.S., Carne A., Bekhit A.E.D. Marine omega‐3 (n‐3) phospholipids: a comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2019;19:64–123. doi: 10.1111/1541-4337.12510. [DOI] [PubMed] [Google Scholar]
- 32.McPherson A.V., Kitchen B.J. Reviews of the progress of Dairy Science: the bovine milk fat globule membrane–its formation, composition, structure and behaviour in milk and dairy products. J. Dairy Res. 1983;50:107–133. doi: 10.1017/S0022029900032581. [DOI] [Google Scholar]
- 33.Castañeda-Gutiérrez E., Benefield B.C., de Veth M.J., Santos N.R., Gilbert R.O., Butler W.R., Bauman D.E. Evaluation of the mechanism of action of conjugated linoleic acid isomers on reproduction in dairy cows. J. Dairy Sci. 2007;90:4253–4264. doi: 10.3168/jds.2007-0117. [DOI] [PubMed] [Google Scholar]
- 34.Ting H., Chao Y., Hsu Y.H. Polyunsaturated fatty acids incorporation into cardiolipin in H9c2 cardiac myoblast. J. Nutr. Biochem. 2015;26:769–775. doi: 10.1016/j.jnutbio.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Liu X., Cui J., Li Z., Xu J., Wang J., Xue C., Wang Y. Research Article Comparative study of DHA‐enriched phospholipids and EPA‐enriched phospholipids on metabolic disorders in diet ‐ induced ‐ obese C57BL/6J mice. Eur. J. Lipid Sci. Technol. 2014;116:255–265. doi: 10.1002/ejlt.201300407. [DOI] [Google Scholar]
- 36.Li Q., Wu F., Wen M., Yanagita T., Xue C., Zhang T., Wang Y. The protective effect of antarctic krill oil on cognitive function by inhibiting oxidative stress in the brain of senescence-accelerated prone mouse strain 8 (SAMP8) mice. J. Food Sci. 2018;83:543–551. doi: 10.1111/1750-3841.14044. [DOI] [PubMed] [Google Scholar]
- 37.Che H., Zhang T., Ding L., Zhang L., Shi H., Yanagita T., Xue C., Chang Y., Wang Y. A comparative study of EPA-enriched ethanolamine plasmalogen and EPA-enriched phosphatidylethanolamine on Aβ42 induced cognitive deficiency in a rat model of Alzheimer's disease. Food Funct. 2018;9:3008–3017. doi: 10.1039/c8fo00643a. [DOI] [PubMed] [Google Scholar]
- 38.Wang C., Wang D., Xu J., Yanagita T., Xue C., Zhang T., Wang Y. DHA enriched phospholipids with different polar groups (PC and PS) had different improvements on MPTP-induced mice with Parkinson's disease. J. Funct.Foods. 2018;45:417–426. doi: 10.1016/j.jff.2018.04.017. [DOI] [Google Scholar]
- 39.Fahy E., Subramaniam S., Brown H.A., Glass C.K., Merrill A.H., Murphy R.C., Raetz C.R.H., Russell D.W., Seyama Y., Shaw W., Shimizu T., Spener F., van Meer G., VanNieuwenhze M.S., White S.H., Witztum J.L., Dennis E.A. A comprehensive classification system for lipids. JLR (J. Lipid Res.) 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita S., Hashimoto M., Haque A.M., Nakagawa K., Kinoshita M., Shido O., Miyazawa T. Oral administration of ethanolamine glycerophospholipid containing a high level of plasmalogen improves memory impairment in amyloid β-infused rats. Lipids. 2017;52:575–585. doi: 10.1007/s11745-017-4260-3. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z., Zhao J., Wang Y., Zhang T., Liu R., Chang M., Wang X. Advances in EPA-GPLs: structural features, mechanisms of nutritional functions and sources. Trends Food Sci. Technol. 2021;114:521–529. doi: 10.1016/j.tifs.2021.06.019. [DOI] [Google Scholar]
- 42.Zhang X., Wei W., Tao G., Jin Q., Wang X. Identification and quantification of triacylglycerols using ultraperformance supercritical fluid chromatography and quadrupole time-of-flight mass spectrometry: comparison of human milk, infant formula, other mammalian milk, and plant oil. J. Agric. Food Chem. 2021;69:8991–9003. doi: 10.1021/acs.jafc.0c07312. [DOI] [PubMed] [Google Scholar]
- 43.He C., Cao J., Jiang X., Wen C., Bai X., Li C. Fatty acid profiles of triacylglycerols and phospholipids of sea-cage cultured Trachinotus blochii: a comparative study of head, viscera, skin, bone, and muscle. J. Food Sci. 2019;84:650–658. doi: 10.1111/1750-3841.14458. [DOI] [PubMed] [Google Scholar]
- 44.Carlier H., Bernard A., Caselli C. Digestion and absorption of polyunsaturated fatty acids. Reprod. Nutr. Dev. 1991;31:475–500. doi: 10.1051/rnd:19910501. [DOI] [PubMed] [Google Scholar]
- 45.Christensen M., Høy C., Becker C., Redgrave T. Intestinal absorption and lymphatic transport of eicosapentaenoic (EPA), docosahexaenoic (DHA), and decanoic acids: dependence on intramolecular triacylglycerol structure. Am. J. Clin. Nutr. 1995;61:56–61. doi: 10.1093/ajcn/61.1.56. [DOI] [PubMed] [Google Scholar]
- 46.Couëdelo L., Vaysse C., Vaique E., Guy A., Gosse I., Durand T., Pinet S., Cansell M., Combe N. The fraction of α-linolenic acid present in the sn-2 position of structured triacylglycerols decreases in lymph chylomicrons and plasma triacylglycerols during the course of lipid absorption in rats. J. Nutr. 2012;142:70–75. doi: 10.3945/jn.111.146290. [DOI] [PubMed] [Google Scholar]
- 47.Jin J., Jin Q., Wang X., Akoh C.C. High Sn-2 docosahexaenoic acid lipids for brain benefits, and their enzymatic syntheses: a review. Engineering. 2020;6:424–431. doi: 10.1016/j.eng.2020.02.009. [DOI] [Google Scholar]
- 48.Serra A., Conte G., Ciucci F., Bulleri E., Corrales-Retana L., Cappucci A., Buccioni A., Mele M. Dietary linseed supplementation affects the fatty acid composition of the sn-2 position of triglycerides in sheep milk. J. Dairy Sci. 2018;101:6742–6751. doi: 10.3168/jds.2017-14188. [DOI] [PubMed] [Google Scholar]
- 49.Chen B., Yang S., Niu J., Jarugumilli G.K., Xu W. Protein lipidation in cell signaling and diseases-function, regulation, and therapeutic opportunities. Cell Chem. Biol. 2018;25:817–831. doi: 10.1016/j.chembiol.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao Q., Li H., Fan L., Huang S., Wang J., Zheng N. The combination of lactoferrin and linolenic acid inhibits colorectal tumor growth through activating AMPK/JNK-related apoptosis pathway. PeerJ. 2021;9 doi: 10.7717/peerj.11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boitz L.I., Fiechter G., Seifried R.K., Mayer H.K. A novel ultra-high performance liquid chromatography method for the rapid determination of β-lactoglobulin as heat load indicator in commercial milk samples. Journal of Chromatography a. 2015;1386:98–102. doi: 10.1016/j.chroma.2015.01.081. [DOI] [PubMed] [Google Scholar]
- 52.Yi C., Wambo T.O. Factors affecting the interactions between beta-lactoglobulin and fatty acids as revealed in molecular dynamics simulations. Phys. Chem. Chem. Phys. 2015;35:23074–23080. doi: 10.1039/c5cp02312b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delgado Y., Morales-Cruz M., Figueroa C.M., Hernández-Román J., Hernández G., Griebenow K. The cytotoxicity of BAMLET complexes is due to oleic acid and independent of the α‐lactalbumin component. FEBS Open Bio. 2015;5:397–404. doi: 10.1016/j.fob.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng X., Zeng Z., Gao J., Tong P., Wu Y., Li X., Chen H. Conformational changes in bovine α-lactalbumin and β-lactoglobulin evoked by interaction with C18 unsaturated fatty acid give insights into increased allergic potentia. Food Funct. 2020;11:9240–9251. doi: 10.1039/d0fo02028a. [DOI] [PubMed] [Google Scholar]
- 55.Puyol P., Perez M.D., Mata L., Ena J., Calvo M. Effect of retinol and fatty acid binding by bovine β-lactoglobulin on its resistance to trypsin digestion. Int. Dairy J. 1993;7:589–597. doi: 10.1016/0958-6946(93)90102-6. [DOI] [Google Scholar]
- 56.Wu S., Zhang Y., Ren F., Qin Y., Liu J., Liu J., Wang Q., Zhang H. Structure–affinity relationship of the interaction between phenolic acids and their derivatives and β-lactoglobulin and effect on antioxidant activity. Food Chem. 2018;245:613–619. doi: 10.1016/j.foodchem.2017.10.122. [DOI] [PubMed] [Google Scholar]
- 57.Jiang H.R., Liu N. Self-assembled β-lactoglobulin–conjugated linoleic acid complex for colon cancer-targeted substance. J. Dairy Sci. 2010;93:3931–3939. doi: 10.3168/jds.2010-3071. [DOI] [PubMed] [Google Scholar]
- 58.Fang B., Zhang M., Tian M., Ren F.Z. Self-assembled β-lactoglobulin–oleic acid and β-lactoglobulin–linoleic acid complexes with antitumor activities. J. Dairy Sci. 2015;98:2898–2907. doi: 10.3168/jds.2014-8993. [DOI] [PubMed] [Google Scholar]
- 59.Puddu P., Valenti P., Gessani S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie. 2009;91:11–18. doi: 10.1016/j.biochi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Sriramoju B., Kanwar R.K., Kanwar J.R. Lactoferrin induced neuronal differentiation: a boon for brain tumours. Int. J. Dev. Neurosci. 2015;41:28–36. doi: 10.1016/j.ijdevneu.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Morishita S., Kawaguchi H., Ono T., Miura N., Murakoshi M., Sugiyama K., Kato H., Tanimoto A., Nishino H. Enteric lactoferrin attenuates the development of high-fat and high-cholesterol diet-induced hypercholesterolemia and atherosclerosis in Microminipigs. Bioscience, biotechnology, and biochemistry. 2016;80:295–303. doi: 10.1080/09168451.2015.1091713. [DOI] [PubMed] [Google Scholar]
- 62.Gibbons J.A., Kanwar R.K., Kanwar J.R. Lactoferrin and cancer in different cancer models. Front. Biosci. 2011;S3:1080–1088. doi: 10.2741/212. [DOI] [PubMed] [Google Scholar]
- 63.Fang B., Zhang M., Tian M., Jiang L., Guo H.Y., Ren F.Z. Bovine lactoferrin binds oleic acid to form an anti-tumor complex similar to HAMLET. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2014;1841:535–543. doi: 10.1016/j.bbalip.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Ren G., Cheng G., Wang J. Understanding the role of milk in regulating human homeostasis in the context of the COVID-19 global pandemic. Trends Food Sci. Technol. 2021;107:157–160. doi: 10.1016/j.tifs.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mirabelli C., Wotring J.W., Zhang C.J., McCarty S.M., Fursmidt R., Pretto C.D., Qiao Y., Zhang Y., Frum T., Kadambi N.S., Amin A.T., O Meara T.R., Spence J.R., Huang J., Alysandratos K.D., Kotton D.N., Handelman S.K., Wobus C.E., Weatherwax K.J., Mashour G.A., O Meara M.J., Chinnaiyan A.M., Sexton J.Z. vol. 118. Proceedings of the National Academy of Sciences; 2021. (Morphological Cell Profiling of SARS-CoV-2 Infection Identifies Drug Repurposing Candidates for COVID-19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan Q., Xie F., Huang W., Hu M., Yan Q., Chen Z., Zheng Y., Liu L. The review of alpha‐linolenic acid: sources, metabolism, and pharmacology. Phytother Res. 2022;36:164–188. doi: 10.1002/ptr.7295. [DOI] [PubMed] [Google Scholar]
- 67.Zhang T.T., Xu J., Wang Y.M., Xue C.H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019;75 doi: 10.1016/j.plipres.2019.100997. [DOI] [PubMed] [Google Scholar]
- 68.Al-Ghannami S.S., Al-Adawi S., Ghebremeskel K., Hussein I.S., Min Y., Jeyaseelan L., Al-Shammakhi S.M., Mabry R.M., Al-Oufi H.S. Randomised open-label trial of docosahexaenoic acid -enriched fish oil and fish meal on cognitive and behavioural functioning in Omani children. Nutrition. 2019;57:167–172. doi: 10.1016/j.nut.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 69.Ding L., Zhang L., Wen M., Che H., Du L., Wang J. Eicosapentaenoic acid-enriched phospholipids improve atherosclerosis by mediating cholesterol metabolism. J. Funct.Foods. 2017;32:90–97. doi: 10.1016/j.jff.2017.02.020. [DOI] [Google Scholar]
- 70.Guerra E., Verardo V., Caboni M.F. Determination of bioactive compounds in cream obtained as a by-product during cheese-making: influence of cows' diet on lipid quality. Int. Dairy J. 2015;42:16–25. doi: 10.1016/j.idairyj.2014.11.004. [DOI] [Google Scholar]
- 71.Vargas-Bello-Pérez E., García Montes De Oca C.A., Pescador Salas N., Estrada Flores J.G., Romero Bernal J., Robles-Jimenez L.E., Gonzalez-Ronquillo M. Productive performance, milk composition and milk fatty acids of goats supplemented with sunflower and linseed whole seeds in grass silage-based diets. Animals. 2020;10:1143. doi: 10.3390/ani10071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leduc M., Létourneau-Montminy M.P., Gervais R., Chouinard P.Y. Effect of dietary flax seed and oil on milk yield, gross composition, and fatty acid profile in dairy cows: a meta-analysis and meta-regression. J. Dairy Sci. 2017;100:8906–8927. doi: 10.3168/jds.2017-12637. [DOI] [PubMed] [Google Scholar]
- 73.Caroprese M., Albenzio M., Bruno A., Fedele V., Santillo A., Sevi A. Effect of solar radiation and flaxseed supplementation on milk production and fatty acid profile of lactating ewes under high ambient temperature. J. Dairy Sci. 2011;94:3856–3867. doi: 10.3168/jds.2010-4067. [DOI] [PubMed] [Google Scholar]
- 74.Livingstone K.M., Humphries D.J., Kirton P., Kliem K.E., Givens D.I., Reynolds C.K. Effects of forage type and extruded linseed supplementation on methane production and milk fatty acid composition of lactating dairy cows. J. Dairy Sci. 2015;98:4000–4011. doi: 10.3168/jds.2014-8987. [DOI] [PubMed] [Google Scholar]
- 75.Gómez-Cortés P., Cívico A., de la Fuente M.A., Núñez Sánchez N., Peña Blanco F., Martínez Marín A.L. Effects of dietary concentrate composition and linseed oil supplementation on the milk fatty acid profile of goats. Animal. 2018;12:2310–2317. doi: 10.1017/S1751731118000381. [DOI] [PubMed] [Google Scholar]
- 76.Bernard L., Leroux C., Rouel J., Delavaud C., Shingfield K.J., Chilliard Y. Effect of extruded linseeds alone or in combination with fish oil on intake, milk production, plasma metabolite concentrations and milk fatty acid composition in lactating goats. Animal. 2015;9:810–821. doi: 10.1017/S1751731114003048. [DOI] [PubMed] [Google Scholar]
- 77.Caroprese M., Marzano A., Marino R., Gliatta G., Muscio A., Sevi A. Flaxseed supplementation improves fatty acid profile of cow milk. J. Dairy Sci. 2010;93:2580–2588. doi: 10.3168/jds.2008-2003. [DOI] [PubMed] [Google Scholar]
- 78.Petit H.V., Gagnon N. Concentration of the mammalian lignans enterolactone and enterodiol in milk of cows fed diets containing different concentrations of whole flaxseed. Animal. 2009;10:1428–1435. doi: 10.1017/S1751731109990346. [DOI] [PubMed] [Google Scholar]
- 79.Oliveira M.X.S., Palma A.S.V., Reis B.R., Franco C.S.R., Marconi A.P.S., Shiozaki F.A., Reis L.G., Salles M.S.V., Netto A.S. Inclusion of soybean and linseed oils in the diet of lactating dairy cows makes the milk fatty acid profile nutritionally healthier for the human diet. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Correddu F., Gaspa G., Pulina G., Nudda A. Grape seed and linseed, alone and in combination, enhance unsaturated fatty acids in the milk of Sarda dairy sheep. J. Dairy Sci. 2016;99:1725–1735. doi: 10.3168/jds.2015-10108. [DOI] [PubMed] [Google Scholar]
- 81.Caroprese M., Ciliberti M.G., Marino R., Santillo A., Sevi A., Albenzio M. Polyunsaturated fatty acid supplementation: effects of seaweed Ascophyllum nodosum and flaxseed on milk production and fatty acid profile of lactating ewes during summer. J. Dairy Res. 2016;83:289–297. doi: 10.1017/S0022029916000431. [DOI] [PubMed] [Google Scholar]
- 82.Agustinho B.C., Zeoula L.M., Santos N.W., Machado E., Yoshimura E.H., Ribas J.C.R., Bragatto J.M., Stemposki M.R., Santos V.J.D., Faciola A.P. Effects of flaxseed oil and vitamin E supplementation on digestibility and milk fatty composition and antioxidant capacity in water buffaloes. Animals. 2020;10:1294. doi: 10.3390/ani10081294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kabeya N., Fonseca M.M., Ferrier D.E.K., Navarro J.C., Bay L.K., Francis D.S., Tocher D.R., Castro L.F.C., Monroig Ó. Genes for de novo biosynthesis of omega-3 polyunsaturated fatty acids are widespread in animals. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aar6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caroprese M., Mancino R., Ciliberti M.G., Di Luccia A., La Gatta B., Albenzio M. Fatty acid profile and coagulating ability of milk from Jersey and Friesian cows fed whole flaxseed. J. Dairy Res. 2017;84:14–22. doi: 10.1017/S002202991600073X. [DOI] [PubMed] [Google Scholar]
- 85.Gómez-Cortés P., Bach A., Luna P., Juárez M., de la Fuente M.A. Effects of extruded linseed supplementation on n-3 fatty acids and conjugated linoleic acid in milk and cheese from ewes. J. Dairy Sci. 2009;92:4122–4134. doi: 10.3168/jds.2008-1909. [DOI] [PubMed] [Google Scholar]
- 86.Kupczyński R., Szołtysik M., Janeczek W., Chrzanowska J., Kinal S., Króliczewska B. Effect of dietary fish oil on milk yield, fatty acids content and serum metabolic profile in dairy cows. J. Anim. Physiol. Anim. Nutr. 2011;95:512–522. doi: 10.1111/j.1439-0396.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 87.Thanh L.P., Phakachoed N., Suksombat W., Loor J.J., Hang T.T.T. Partial substitution of fish oil for linseed oil enhances beneficial fatty acids from rumen biohydrogenation but reduces ruminal fermentation and digestibility in growing goats. Translational Animal Science. 2021;5 doi: 10.1093/tas/txab116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mattos R., Staples C.R., Arteche A., Wiltbank M.C., Diaz F.J., Jenkins T.C., Thatcher W.W. The effects of feeding fish oil on uterine secretion of PGF2α, milk composition, and metabolic status of periparturient Holstein cows. J. Dairy Sci. 2004;87:921–932. doi: 10.3168/jds.S0022-0302(04)73236-1. [DOI] [PubMed] [Google Scholar]
- 89.Stamey J.A., Shepherd D.M., de Veth M.J., Corl B.A. Use of algae or algal oil rich in n-3 fatty acids as a feed supplement for dairy cattle. J. Dairy Sci. 2012;95:5269–5275. doi: 10.3168/jds.2012-5412. [DOI] [PubMed] [Google Scholar]
- 90.Li Z., Zhong W., Liu S., Kraus V.B., Zhang Y., Gao X., Lv Y., Shen D., Zhang X., Zhang P., Huang Q., Chen Q., Wu X., Shi X., Wang D., Mao C. Associations of habitual fish oil supplementation with cardiovascular outcomes and all cause mortality: evidence from a large population based cohort study. BMJ. 2020;368:m456. doi: 10.1136/bmj.m456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramaswamy N., Baer R.J., Schingoethe D.J., Hippen A.R., Kasperson K.M., Whitlock L.A. Composition and flavor of milk and butter from cows fed fish oil, extruded soybeans, or their combination. J. Dairy Sci. 2001;84:2144–2151. doi: 10.3168/jds.S0022-0302(01)74659-0. [DOI] [PubMed] [Google Scholar]
- 92.Pirondini M., Colombini S., Mele M., Malagutti L., Rapetti L., Galassi G., Crovetto G.M. Effect of dietary starch concentration and fish oil supplementation on milk yield and composition, diet digestibility, and methane emissions in lactating dairy cows. J. Dairy Sci. 2015;98:357–372. doi: 10.3168/jds.2014-8092. [DOI] [PubMed] [Google Scholar]
- 93.Kairenius P., Ärölä A., Leskinen H., Toivonen V., Ahvenjärvi S., Vanhatalo A., Huhtanen P., Hurme T., Griinari J.M., Shingfield K.J. Dietary fish oil supplements depress milk fat yield and alter milk fatty acid composition in lactating cows fed grass silage-based diets. J. Dairy Sci. 2015;98:5653–5671. doi: 10.3168/jds.2015-9548. [DOI] [PubMed] [Google Scholar]
- 94.Toral P.G., Frutos P., Hervás G., Gómez-Cortés P., Juárez M., de la Fuente M.A. Changes in milk fatty acid profile and animal performance in response to fish oil supplementation, alone or in combination with sunflower oil, in dairy ewes. J. Dairy Sci. 2010;93:1604–1615. doi: 10.3168/jds.2009-2530. [DOI] [PubMed] [Google Scholar]
- 95.Carreno D., Hervas G., Toral P.G., Castro-Carrera T., Frutos P. Fish oil-induced milk fat depression and associated downregulation of mammary lipogenic genes in dairy ewes. J. Dairy Sci. 2016;99:7971–7981. doi: 10.3168/jds.2016-11019. [DOI] [PubMed] [Google Scholar]
- 96.Toral P.G., Hervás G., Carreño D., Frutos P. Does supplemental 18:0 alleviate fish oil-induced milk fat depression in dairy ewes? J. Dairy Sci. 2016;99:1133–1144. doi: 10.3168/jds.2015-10304. [DOI] [PubMed] [Google Scholar]
- 97.Rodríguez-España M., Mendoza-Sánchez L.G., Magallón-Servín P., Salgado-Cervantes M.A., Acosta-Osorio A.A., García H.S. Supercritical fluid extraction of lipids rich in DHA from Schizochytrium sp. J. Supercrit. Fluids. 2022;179 doi: 10.1016/j.supflu.2021.105391. [DOI] [Google Scholar]
- 98.Till B.E., Huntington J.A., Kliem K.E., Taylor-Pickard J., Sinclair L.A. Long term dietary supplementation with microalgae increases plasma docosahexaenoic acid in milk and plasma but does not affect plasma 13,14-dihydro-15-keto PGF2α concentration in dairy cows. J. Dairy Res. 2020;87:14–22. doi: 10.1017/S002202991900102X. [DOI] [PubMed] [Google Scholar]
- 99.Till B.E., Huntington J.A., Posri W., Early R., Taylor-Pickard J., Sinclair L.A. Influence of rate of inclusion of microalgae on the sensory characteristics and fatty acid composition of cheese and performance of dairy cows. J. Dairy Sci. 2019;102:10934–10946. doi: 10.3168/jds.2019-16391. [DOI] [PubMed] [Google Scholar]
- 100.Bichi E., Hervás G., Toral P.G., Loor J.J., Frutos P. Milk fat depression induced by dietary marine algae in dairy ewes: persistency of milk fatty acid composition and animal performance responses. J. Dairy Sci. 2013;96:524–532. doi: 10.3168/jds.2012-5875. [DOI] [PubMed] [Google Scholar]
- 101.Fougère H., Delavaud C., Bernard L. Diets supplemented with starch and corn oil, marine algae, or hydrogenated palm oil differentially modulate milk fat secretion and composition in cows and goats: a comparative study. J. Dairy Sci. 2018;101:8429–8445. doi: 10.3168/jds.2018-14483. [DOI] [PubMed] [Google Scholar]
- 102.Papadopoulos G., Goulas C., Apostolaki E., Abril R. Effects of dietary supplements of algae, containing polyunsaturated fatty acids, on milk yield and the composition of milk products in dairy ewes. J. Dairy Res. 2002;69:357–365. doi: 10.1017/s0022029902005599. [DOI] [PubMed] [Google Scholar]
- 103.Pajor F., Egerszegi I., Szűcs Á., Póti P., Bodnár Á. Effect of marine algae supplementation on somatic cell count, prevalence of udder pathogens, and fatty acid profile of dairy goats' milk. Animals (Basel) 2021;11:1097. doi: 10.3390/ani11041097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li A., Ha Y., Wang F., Li W., Li Q. Determination of thermally induced trans-fatty acids in soybean oil by attenuated total reflectance fourier transform infrared spectroscopy and gas chromatography analysis. J. Agric. Food Chem. 2012;60:10709–10713. doi: 10.1021/jf3033599. [DOI] [PubMed] [Google Scholar]
- 105.Wu H., Wang Y., Hao X., Meng L., Li H., Cheng M., Zheng N., Wang J. Effect of TBC of raw milk and thermal treatment intensity on endotoxin contents of milk products. Food Res. Int. 2022;152 doi: 10.1016/j.foodres.2021.110816. [DOI] [PubMed] [Google Scholar]
- 106.Moltó-Puigmartí C., Permanyer M., Castellote A.I., López-Sabater M.C. Effects of pasteurisation and high-pressure processing on vitamin C, tocopherols and fatty acids in mature human milk. Food Chem. 2011;124:697–702. doi: 10.1016/j.foodchem.2010.05.079. [DOI] [Google Scholar]
- 107.Henderson T.R., Fay T.N., Hamosh M. Effect of pasteurization on long chain polyunsaturated fatty acid levels and enzyme activities of human milk. J. Pediatr. 1998;132:876–878. doi: 10.1016/S0022-3476(98)70323-3. [DOI] [PubMed] [Google Scholar]
- 108.Delorme M.M., Guimarães J.T., Coutinho N.M., Balthazar C.F., Rocha R.S., Silva R., Margalho L.P., Pimentel T.C., Silva M.C., Freitas M.Q., Granato D., Sant'Ana A.S., Duart M.C., Cruz A.G. Ultraviolet radiation: an interesting technology to preserve quality and safety of milk and dairy foods. Trends in food science & technology. 2020;102:146–154. doi: 10.1016/j.tifs.2020.06.001. [DOI] [Google Scholar]
- 109.Matak K.E., Sumner S.S., Duncan S.E., Hovingh E., Worobo R.W., Hackney C.R., Pierson M.D. Effects of ultraviolet irradiation on chemical and sensory properties of goat milk. J. Dairy Sci. 2007;90:3178–3186. doi: 10.3168/jds.2006-642. [DOI] [PubMed] [Google Scholar]
- 110.Juraga E., Vukušić Pavičić T., Gajdoš Kljusurić J., Brnčić M., Juraga T., Herceg Z. Properties of milk treated with high-power ultrasound and bactofugation. Food Technol. Biotechnol. 2021 Mar;59(1):92–102. doi: 10.17113/ftb.59.01.21.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khan I.T., Nadeem M., Imran M., Ayaz M., Ajmal M., Ellahi M.Y., Khalique A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids Health Dis. 2017;16:163. doi: 10.1186/s12944-017-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fox P.F., O'Connor T.P., Mcsweeney P.L.H., Guinee T.P., O'Brien N.M. Cheese: physical, biochemical, and nutritional aspects. Adv. Food Nutr. Res. 1996;39:163–328. doi: 10.1016/s1043-4526(08)60075-3. [DOI] [PubMed] [Google Scholar]
- 113.Zhang R.H., Mustafa A.F., Zhao X. Effects of feeding oilseeds rich in linoleic and linolenic fatty acids to lactating ewes on cheese yield and on fatty acid composition of milk and cheese. Anim. Feed Sci. Technol. 2006;127:220–233. doi: 10.1016/j.anifeedsci.2005.09.001. [DOI] [Google Scholar]
- 114.Vargas-Bello-Pérez E., Vera R.R., Aguilar C., Lira R., Peña I., Tello F.A. Feeding extruded linseed to dairy ewes under extensive grazing conditions. Ciencia e investigación agraria. 2014;41:21–22. doi: 10.4067/S0718-16202014000100011. [DOI] [Google Scholar]
- 115.Santurino C., Calvo M.V., Gómez-Candela C., Fontecha J. Characterization of naturally goat cheese enriched in conjugated linoleic acid and omega-3 fatty acids for human clinical trial in overweight and obese subjects. PharmaNutrition. 2017;5:8–17. doi: 10.1016/j.phanu.2016.12.001. [DOI] [Google Scholar]
- 116.Let M.B., Jacobsen C., Pham K.A., Meyer A.S. Protection against oxidation of fish-oil-enriched milk emulsions through addition of rapeseed oil or antioxidants. J. Agric. Food Chem. 2005;53:5429–5437. doi: 10.1021/jf047960f. [DOI] [PubMed] [Google Scholar]
- 117.Dal Bello B., Torri L., Piochi M., Zeppa G. Healthy yogurt fortified with n-3 fatty acids from vegetable sources. J. Dairy Sci. 2015;98:8375–8385. doi: 10.3168/jds.2015-9688. [DOI] [PubMed] [Google Scholar]
- 118.Rasti B., Erfanian A., Selamat J. Novel nanoliposomal encapsulated omega-3 fatty acids and their applications in food. Food Chem. 2017;230:690–696. doi: 10.1016/j.foodchem.2017.03.089. [DOI] [PubMed] [Google Scholar]
- 119.Salles M., D Abreu L., Júnior L., César M., Guimarães J., Segura J., Rodrigues C., Zanetti M., Pfrimer K., Netto A. Inclusion of sunflower oil in the bovine diet improves milk nutritional profile. Nutrients. 2019;11:481. doi: 10.3390/nu11020481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nelson K.A.S., Martini S. Increasing omega fatty acid content in cow's milk through diet manipulation: effect on milk flavor. J. Dairy Sci. 2009;92:1378–1386. doi: 10.3168/jds.2008-1780. [DOI] [PubMed] [Google Scholar]
- 121.Kuchta A.M., Kelly P.M., Stanton C., Devery R.A. Milk fat globule membrane-a source of polar lipids for colon health? A review. Int. J. Dairy Technol. 2012;65:315–333. doi: 10.1111/j.1471-0307.2011.00759.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.