Abstract
Purpose
To assess the effectiveness of Amide Proton Transfer (APT) imaging in predicting the histopathological characteristics of cervical cancer.
Methods
A comprehensive literature search was conducted across multiple databases, covering studies until December 27, 2023. The meta-analysis was performed using Stata 15 and Review Manager 5.4 software. Key metrics analyzed included pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio (DOR), and summary receiver operating characteristic curves. The analysis focused on differentiating cervical cancer types, squamous carcinoma differentiation, and lymph node involvement. Meta-regression was employed to investigate heterogeneity.
Results
Thirteen studies involving 868 patients were included in the meta-analysis. For differentiating adenocarcinoma from squamous carcinoma, the pooled sensitivity was 0.82 (95%CI: 0.71–0.90), specificity was 0.65 (95%CI: 0.48–0.79), and DOR was 9 (95%CI: 1.6–3.5). When distinguishing poorly differentiated from moderately/well-differentiated squamous carcinoma, the sensitivity was 0.74 (95%CI: 0.66–0.81), specificity was 0.83 (95%CI: 0.75–0.89), and DOR was 14 (95%CI: 8–23). For identifying lymph node involvement, the sensitivity was 0.87 (95%CI: 0.78–0.92), specificity was 0.66 (95%CI: 0.59–0.73), and DOR was 13 (95%CI: 7–26). No publication bias was detected.
Conclusions
APT imaging demonstrates high sensitivity and specificity in distinguishing between cervical cancer types, grading squamous carcinoma, and detecting lymph node involvement. It can be considered a reliable technique for predicting the pathological features of cervical cancer in clinical practice.
Keywords: Cervical cancer, Amide proton transfer, Systematic review
1. Introduction
Cervical cancer is among the most common malignant neoplasms affecting women globally. In 2020, an estimated 604,000 new cases were diagnosed, with 342,000 deaths attributed to the disease worldwide [1,2], making it a significant threat to women's health and lives across the globe. Prevention and early screening are crucial for reducing the incidence and mortality of cervical cancer [3]. However, once cervical cancer is diagnosed, a tailored treatment strategy is required. For early-stage cervical cancer, radical surgery is the preferred treatment. Studies have shown that laparoscopic radical hysterectomy (LRH) has similar overall survival (OS) rates to traditional radical open hysterectomy [4] and lower intraoperative estimated blood loss for LRH [5]. For locally advanced cervical cancer (IB2-IIB), a meta-analysis by Marchetti C. et al. [6] found that chemoradiotherapy (CRT) was associated with similar OS rates as neoadjuvant chemotherapy followed by surgery and lower severe acute toxicity of CRT. In addition, drug therapies such as immunotherapy and targeted therapy have demonstrated promise in treating cervical cancer [7]. A phase 3 clinical trial by Domenica Lorusso. et al. [8] showed that pembrolizumab in combination with CRT significantly improved the OS of patients with locally advanced cervical cancer. The treatment outcome of cervical cancer is not only related to the stage and treatment method, but also closely related to the pathological characteristics of cervical cancer (histological type, degree of differentiation and pelvic lymph node metastasis), because these characteristics reflecting the biological behavior of cancer affect tumor clinical stage and the choice of treatment [9]. Research indicates that patients with cervical adenocarcinoma have a 5-year survival rate that is roughly 10 %–20 % lower than those with cervical squamous cell carcinoma [10]. Poorly differentiated squamous cell carcinoma of the cervix is associated with a less favorable prognosis and reduced survival compared to well-differentiated cervical squamous cell carcinoma. Currently, pathological diagnosis remains the gold standard for diagnosing cervical cancer. However, both surgical resection of gross specimens and cervical biopsies carry the risk of sampling errors, influenced by factors such as lesion size, tumor heterogeneity, and the operator's expertise [11].
Magnetic Resonance Imaging (MRI) plays a crucial role in the diagnosis, differentiation, staging, and assessment of treatment response in cervical cancer [12], and it has become the preferred method for evaluating the disease [13]. Amide Proton Transfer (APT) imaging, a functional MRI technique based on Chemical Exchange Saturation Transfer (CEST), offers insight into the amide protons of proteins and peptides in human tissues. This allows APT imaging to reflect the distribution of mobile proteins and peptides within tumors at the cellular and molecular levels [14]. APT imaging has demonstrated its utility in assessing tumors across various anatomical regions, including the head and neck [15], liver [16], prostate [17], breast [18], and rectum [19]. Takayama et al. [20] pioneered the application of APT imaging for evaluating uterine lesions. Since this breakthrough, numerous studies have investigated the application of APT imaging in assessing cervical cancer. He et al. [21] demonstrated that APT imaging produces high-quality images in cervical cancer, with APT values of 2.745 ± 0.065 for cervical cancer tissue and 1.853 ± 0.059 for normal cervical stroma, showing a statistically significant difference between the two (P < 0.05). APT imaging demonstrated a sensitivity of 84.62 % and a specificity of 83.66 % in differentiating cervical cancer from normal cervical stroma, with an area under the ROC curve of 0.927. Meng et al. [22] found that Asymmetric magnetization transfer rate (MTRasym) values were higher in cervical adenocarcinoma compared to squamous cell carcinoma. Additionally, high-grade squamous cell carcinoma exhibited higher MTRasym values than low-grade squamous cell carcinoma. MTRasym demonstrated a sensitivity of 60.0 % and a specificity of 82.5 % for differentiating squamous cell carcinoma from adenocarcinoma, with an area under the receiver operating Characteristic (ROC) curve of 0.779. For distinguishing high-grade from low-grade squamous cell carcinoma, MTRasym showed a sensitivity of 68.8 % and a specificity of 83.3 %, with an area under the ROC curve of 0.756. These results suggest that APT imaging holds significant potential for the diagnosis and differential diagnosis of cervical cancer.
Current studies on APT imaging for assessing cervical cancer are predominantly single-center investigations with limited sample sizes. Consequently, there is substantial heterogeneity in the reported diagnostic performance parameters, such as sensitivity and specificity [23,24]. This systematic review aims to critically evaluate the reliability of APT imaging in predicting the histopathological characteristics of cervical cancer and to quantify its diagnostic value using meta-analytic techniques.
2. Methods
This assessment was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25]. The evidence-based analysis was prospectively registered in PROSPERO (CRD42024504903). Two researchers independently performed each step of the analysis. In case of disagreements, the researchers engaged in a discussion to reach a consensus.
2.1. Search strategy
Systematic searches were conducted in the PubMed, Web of Science, Embase, Wanfang, and CNKI databases up to December 27, 2023. The search terms were structured using the Patient/Intervention/Comparator/Outcome (PICO) framework as follows: (P) cervical cancer, cervical carcinoma, malignant cervical tumor; (I) amide proton transfer, APT, CEST. Categories within the PICO framework were combined using “AND,” while variations within each category were combined using “OR.” Additionally, the reference lists of included articles were reviewed to identify any further eligible studies.
2.2. Inclusion and exclusion criteria
The inclusion criteria covered peer-reviewed publications in Chinese and English, available through electronic databases, that met the following conditions: utilization of biopsy or surgical pathology results as the gold standard; application of APT imaging as the index test for evaluating the histopathological characteristics of cervical cancer tissues, including histological type, grade, and lymph node involvement; ensuring that radiologists and pathologists were blinded to the results; and the ability to calculate true positives, false positives, true negatives, and false negatives. Exclusion criteria included animal or laboratory studies, case reports, conference abstracts, comments, and responses.
2.3. Literature screening and data extraction
Duplicate publications were removed using EndNote 21 software. The titles and abstracts of the remaining articles were carefully reviewed to exclude reviews, conference abstracts, and individual case reports. A thorough evaluation of the screened articles was then performed to eliminate studies that did not focus on the diagnostic performance of APT or lacked extractable data. This rigorous selection process led to the final inclusion of eligible publications. Key information was meticulously extracted from each selected study, including the author's name, publication year, study location, study design, diagnostic method, histopathological type, degree of differentiation, lymph node involvement, number of cases, MRI field strength, scanner model, echo time, repetition time, pulse sequence, and diagnostic sensitivity and specificity. From these data, true positives, false positives, false negatives, and true negatives were calculated based on the number of cases, sensitivity, and specificity.
2.4. Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies −2 (QUADAS-2) tool was employed to evaluate the risk of bias and assess the quality of the study [26], and results were presented using Review Manager 5.4 software. The QUADAS-2 quality assessment tool was divided into four sections (case selection, index test, “gold standard”, flow and timing), encompassing 10 questions, and an evaluation of the clinical applicability of the first three sections.
2.5. Meta-analysis
Meta-analysis was performed using Stata 15 (Stata Corp, College Station, TX) and Review Manager 5.4. Drawing the summary receiver operating characteristic (SROC) curve was the optimal method for data merging; combined sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio (DOR) were calculated, and the SROC curve was plotted to calculate the area under curve (AUC). Heterogeneity among study results was analyzed using the chi-square test, and the extent of heterogeneity was quantitatively determined. Deck's funnel plot was used to detect the presence of publication bias. P < 0.05 was considered statistically significant.
3. Results
3.1. Literature screening
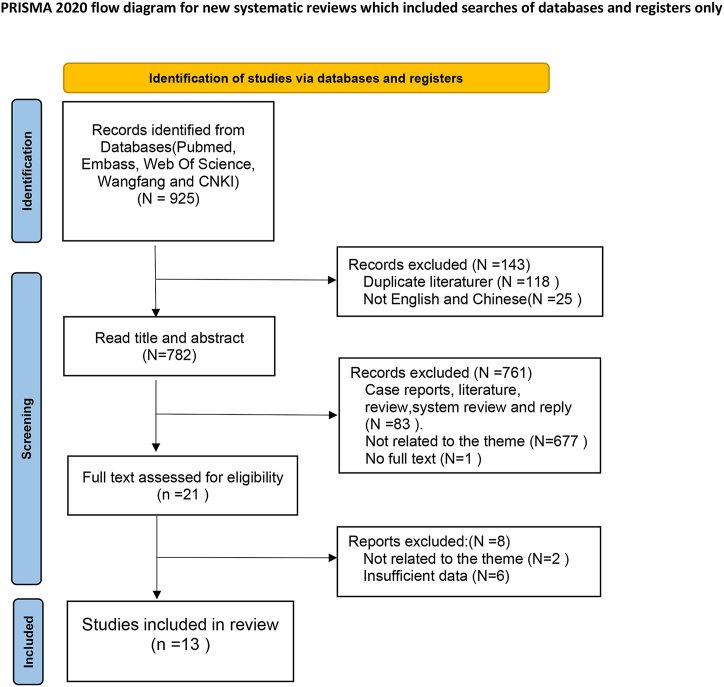
The database search identified a total of 925 articles, of which 118 were duplicates and subsequently removed. After screening the titles and abstracts, 21 full-text articles were assessed for eligibility. Ultimately, 13 studies were included in our quantitative analysis [[22], [23], [24],[27], [28], [29], [30], [31], [32], [33], [34], [35], [36]] (Fig. 1).
Fig. 1.
Flow diagram showing the process for selecting studies.
In all the studies, pathological examination served as the gold standard for confirming cervical cancer. Regarding the methods of obtaining pathological samples, six studies employed surgical procedures, one study used biopsy, and six studies did not specify the sampling method.
3.2. Basic characteristics of the included literature
Of the 13 studies, 2 were prospective and 11 were retrospective, with a total of 868 participants. All included studies utilized 3.0 T MRI scanners, with 8 studies using the Philips Ingenia CX, 4 studies using GE's MR750, and 1 study using Siemens' Magneton Skyra. Additionally, 3 studies employed 2D scanning, while 10 studies used 3D scanning. All study populations were Chinese. Five studies [22,23,27,28,32] aimed to differentiate between squamous carcinoma and adenocarcinoma of the cervix. Nine studies [[22], [23], [24],28,29,[31], [32], [33],36] focused on distinguishing low-grade from moderate-to-high-grade squamous cell carcinoma. Additionally, four studies [28,30,34,35] endeavored to identify lymph node invasion (Table 1).
Table 1.
The basic information of literatures.
| Author (years) | Type of research | MRI | D | TE (ms) | TR (ms) | Pulse Sequence | Power (μT) | Scan time (ms) | MIC | Totle number | CSCC | AC | Pathological Sampling Methods |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li Beibei (2018) | Retro | Philips Ingenia CX | 3D | 5.9 | 6294 | TSE | 2 | 5min46s | yes | 31 | 31 | NA | NA |
| Meng Nan (2019) | Retro | MR750, GE | 3D | 12 | 3000 | EPI | 2 | NA | yes | 76 | 60 | 16 | NA |
| Li Beibei (2019) | Pros | Philips Ingenia CX | 3D | 5.9 | 6294 | TSE | 2 | 5min46s | yes | 32 | 32 | NA | Surgery |
| Meng Nan (2020) | Retro | MR750, GE | 2D | 12 | 3000 | EPI | 2 | 2min36s | NA | 112 | 82 | 30 | NA |
| Li Shujian (2023) | Pros | Philips Ingenia CX | 3D | 1000 | 3000 | TSE | 2 | 6min0s | yes | 88 | 72 | 16 | NA |
| Meng Xing (2023) | Retro | Philips Ingenia CX | 3D | 7.8 | 5174 | FSE | NA | 4 min 45s | NA | 52 | 52 | NA | Surgery |
| Hou Mengyan (2021) | Retro | MR750, GE | 2D | NA | 3000 | EPI | NA | NA | NA | 52 | 52 | NA | Surgery |
| Meng nan (2019) | Retro | MR750, GE | 2D | NA | 3000 | EPI | NA | 2 min 36 s | NA | 38 | 28 | 10 | NA |
| Song Qingling (2022) | Retro | Philips Ingenia CX | 3D | 8 | 6400 | FSE | 2 | 5min3s | yes | 70 | 50 | 20 | biopsy |
| Huang Qiuhan (2023) | Retro | Magnetom Skyra, Siemens | 3D | 7.1 | 3100 | EPI | 2 | 3 min 20 s | yes | 105 | 88 | 17 | NA |
| Xu Qihao (2023) | Retro | Philips Ingenia CX | 3D | 8 | 6400 | FSE | 2 | 5min53s | yes | 69 | NA | NA | Surgery |
| Zhang Mengdi (2023) | Retro | Philips Ingenia CX | 3D | NA | 90 | FSE | 2 | 3min10s | yes | 75 | 43 | NA | Surgery |
| Meng Xing (2022) | Retro | Philips Ingenia CX | 3D | 7.8 | 5174 | FSE | 2 | 4min45s | yes | 87 | 64 | 23 | Surgery |
Retro: Retrospective; Pros: Prospective; TSE: turbo spin echo; FSE: fast spin-echo; EPI:echo planar imaging; D:Dimensionality; MIC: MTRasym at 3.5 ppm + B0 inhomogeneity correction; CSCC: cervical squamous cell carcinoma; AC: adenocarcinoma; NA:have no data.
3.3. Quality assessment
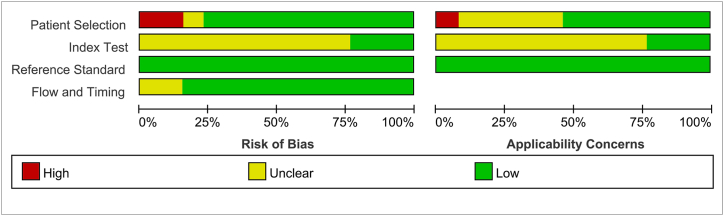
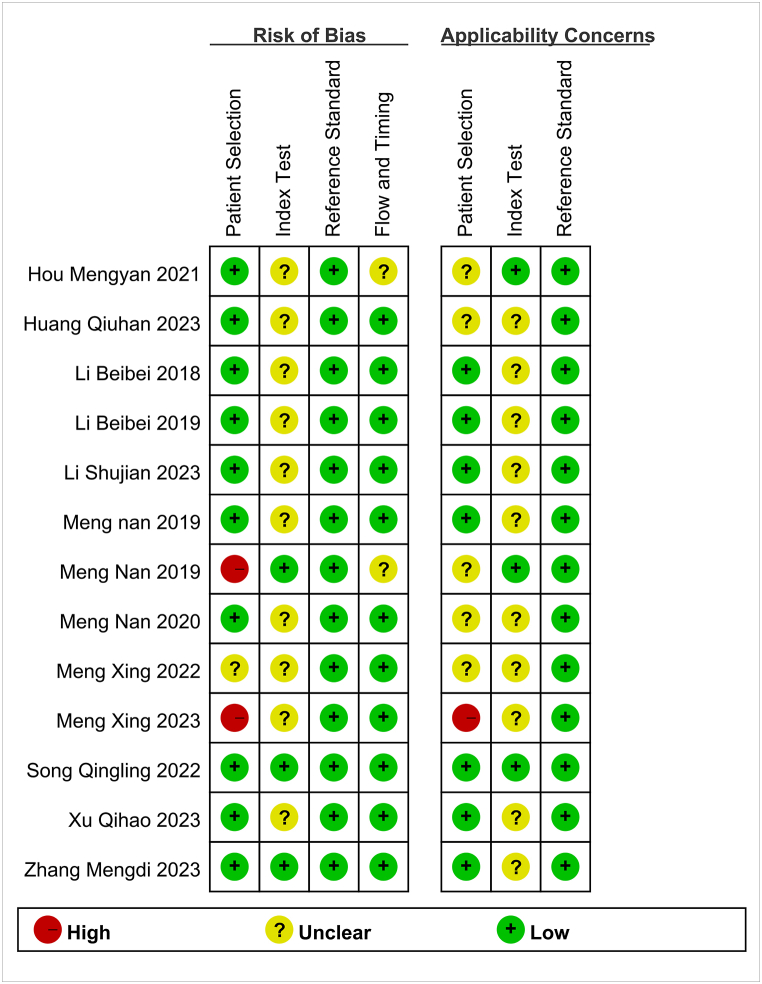
The overall methodological quality of the 13 studies assessing the pathological features of cervical cancer using APT was considered moderate. In terms of patient selection, 30.8 % (4/13) of the studies were rated as unclear due to a lack of information on whether patients were recruited consecutively or randomly. As for the reference standard, all studies (100 %) used pathological examination as the sole gold standard. The results of the risk of bias assessment are shown in Fig. 2, Fig. 3.
Fig. 2.
Bias risk assessment results of included studies.
Fig. 3.
Risk of bias and applicability concerns summary.
3.4. Differentiation diagnosis between cervical adenocarcinoma and squamous cell carcinoma
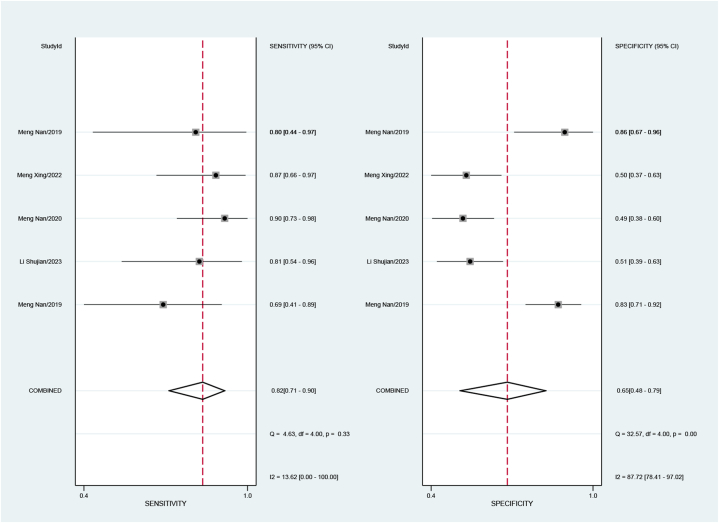
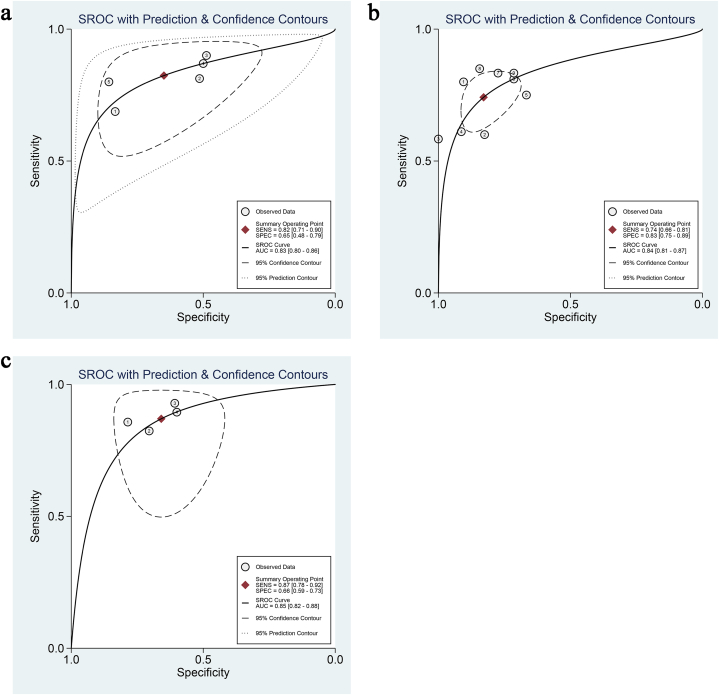
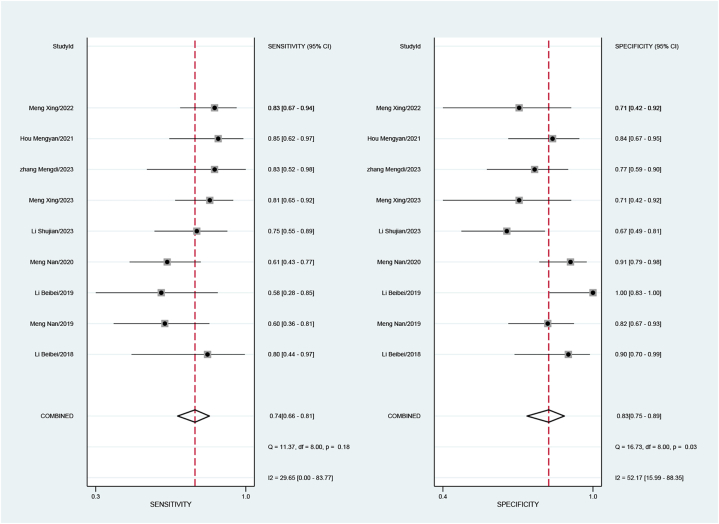
Five studies, involving a total of 401 patients, aimed to differentiate squamous cell carcinoma from adenocarcinoma of the cervix. The pooled sensitivity was 0.82 (95 % CI: 0.71–0.90), specificity was 0.65 (95 % CI: 0.48–0.79), the positive likelihood ratio was 2.3 (95 % CI: 1.6–3.5), the negative likelihood ratio was 0.27 (95 % CI: 0.17–0.43), and the DOR was 9 (95 % CI: 1.6–3.5). The sensitivity I2 for studies differentiating squamous cell carcinoma and adenocarcinoma was 13.62 %, and specificity I2 was 87.72 % (Fig. 4), with a combined AUC of 0.83 (95%CI: 0.80–0.86) (Fig. 5a).
Fig. 4.
Forest plot of sensitivity and specificity for assessment of Cervical squamous cell carcinoma and adenocarcinoma.
Fig. 5.
ROC curve. (a) ROC curve of APT distinguishing between cervical adenocarcinoma and squamous cell carcinoma; (b) ROC curve for APT to distinguish between low-differentiation and medium-differentiation of cervical squamous cell carcinoma; (c) ROC curve for APT to distinguish cervical cancer lymph node invasion.
3.5. Differentiation between low-grade and moderate-to-high-grade squamous cell carcinoma
Nine studies, encompassing 485 patients, endeavored to distinguish low-grade from moderate-to-high-grade squamous cell carcinoma. The pooled estimates were as follows: sensitivity of 0.74 (95 % CI: 0.66–0.81), specificity of 0.83 (95 % CI: 0.75–0.89), positive likelihood ratio of 4.3 (95 % CI: 2.9–6.4), negative likelihood ratio of 0.31 (95 % CI: 0.24–0.41), and DOR of 14 (95 % CI: 8–23). For studies differentiating low-grade and moderate-to-high-grade squamous cell carcinoma, the sensitivity I2 was 29.65 %, and the specificity I2 was 52.17 % (Fig. 6), with a combined AUC of 0.84 (95 % CI: 0.81–0.87) (Fig. 5b).
Fig. 6.
Forest plot of sensitivity and specificity for assessment of low and moder-high differentiation in cervical squamous cell carcinoma.
3.6. Identification of lymph node invasion
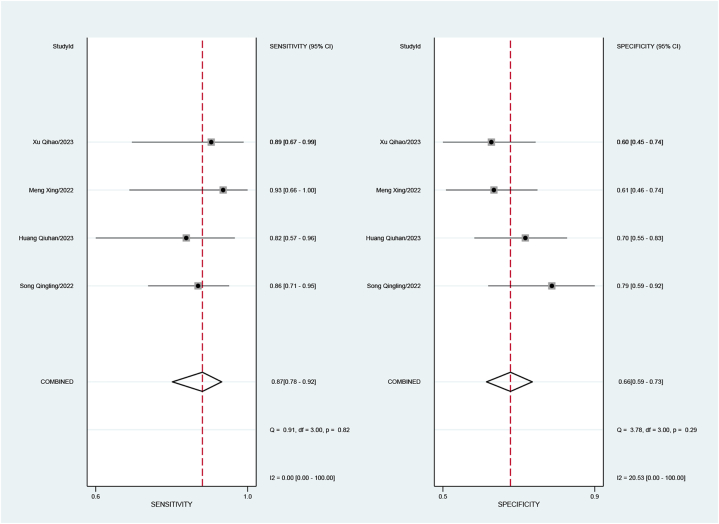
Four studies attempted to identify lymph node invasion, involving 308 patients. The combined sensitivity was 0.87 (95%CI: 0.78–0.92), specificity was 0.66 (95%CI: 0.59–0.73), positive likelihood ratio was 2.5 (95%CI: 2.0–3.2), negative likelihood ratio was 0.20 (95%CI: 0.12–0.34), and the DOR was 13 (95%CI: 7–26).
The sensitivity I2 for studies identifying lymph node invasion was 0.00 %, and specificity I2 was 20.53 % (Fig. 7), with a combined AUC of 0.85 (95%CI: 0.82–0.88) (Fig. 5 c).
Fig. 7.
Forest plot of sensitivity and specificity for assessment of Lymphatic invasion.
3.7. Publication bias
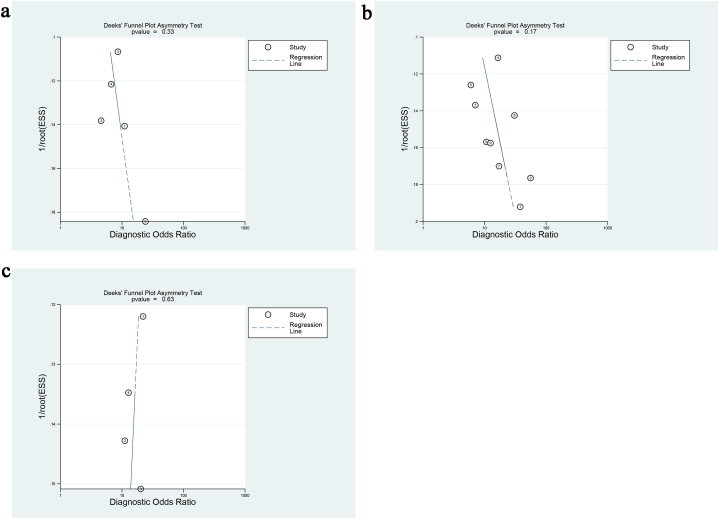
Funnel plots were generated for the included studies to assess potential publication bias. The results demonstrated that studies distinguishing cervical adenocarcinoma from squamous cell carcinoma, low-grade from moderate-to-high-grade squamous cell carcinoma, and cervical cancer lymph node invasion exhibited an approximately symmetrical distribution around the central axis (Fig. 8). The corresponding p-values were 0.33, 0.17, and 0.63, respectively, all exceeding the 0.05 threshold, suggesting no significant evidence of publication bias.
Fig. 8.
Deck's funnel diagram. (a) Funnel plot for APT to distinguish between cervical adenocarcinoma and squamous cell carcinoma; (b) funnel-shaped pattern of APT distinguishing between low-differentiation and medium-differentiation of cervical squamous cell carcinoma; (c) Funnel plot of APT to distinguish cervical cancer. lymph node invasion.
4. Discussion
APT imaging is a novel contrast-agent-free MRI technology developed in recent years, based on endogenous cytoplasmic protein and amide proton imaging [37]. Building on the outstanding clinical research outcomes of APT imaging in central nervous system tumors [38,39], researchers have also applied this technology to cervical cancer. Xu et al. [35] retrospectively analyzed data from 19 patients with lymph node metastasis (LNM)-positive and 50 patients with LNM negative cervical cancer, finding that the MTRasym (3.5 ppm) in the LNM positive group was higher than in the LNM negative group (3.260 ± 0.538 % vs 2.698 ± 0.597 %, P < 0.05). For predicting LNM status in cervical cancer, the MTRasym at 3.5 ppm exhibited a sensitivity of 89.47 % and a specificity of 60 %, with an AUC of 0.763. Furthermore, Deng et al. [40] also utilized APT imaging to evaluate the efficacy of concurrent chemoradiotherapy in locally advanced cervical cancer. Their results showed that APT values were lower in the complete remission group compared to the non-complete remission group. Additionally, APT imaging demonstrated a sensitivity of 82 % and a specificity of 67 % in predicting complete remission, with an AUC of 0.783.
Currently, APT imaging is used in various aspects of cervical cancer, including diagnosis and differential diagnosis, prediction of pathological type, degree of differentiation [41], LNM [35], and therapeutic effect assessment [40], with most studies focusing on the histological typing and grading diagnosis of cervical cancer.
This study employed a meta-analysis approach to summarize the research on APT imaging for predicting pathological features of cervical cancer. The results showed that the pooled sensitivity, specificity, and AUC for APT imaging in distinguishing cervical adenocarcinoma from squamous cell carcinoma were 0.82, 0.65, and 0.83, respectively. For differentiating poorly differentiated from moderately/highly differentiated cervical squamous carcinoma, the sensitivity, specificity, and AUC were 0.74, 0.83, and 0.84, respectively. Additionally, for detecting lymph node involvement, the sensitivity, specificity, and AUC were 0.87, 0.66, and 0.85, respectively.
Our findings suggest that APT imaging has exciting potential in differentiating pathological features such as cervical squamous carcinoma and adenocarcinoma, the degree of differentiation in cervical squamous carcinoma, and lymph node involvement. Similar meta-analyses have also demonstrated the capability of APT imaging in the pathological grading of gliomas [42]. The results of this study further clarify the value of APT imaging in the diagnosis of cervical cancer and provide a new and reliable method for accurately predicting pathological features of cervical cancer. In addition, the successful prediction of the pathological features of cervical cancer will help clinicians to personalize the treatment plan for cervical cancer patients and improve the survival prognosis of cervical cancer.
The impressive performance of APT imaging in predicting pathological features is closely tied to its underlying principles. APT imaging uses specific frequency pulses to saturate the amide protons on free proteins and peptides within cells. The chemical exchange between these amide protons and free water protons amplifies the signal intensity of the proteins and peptides, allowing for an indirect measurement of their content [37]. The APT signal intensity is closely related to the chemical exchange saturation transfer rate, amide proton concentration, and the pH of the microenvironment [37]. It may also be associated with factors such as cell density, nuclear atypia, tissue necrosis, microvascular density, and pH value [20,43]. Cervical adenocarcinoma, originating from endometrial cells, has a rich glandular structure and the ability to secrete mucin. In contrast, squamous carcinoma originates from cervical epithelial cells and has less mucin-secreting capability. Additionally, squamous carcinoma has a denser tissue structure than adenocarcinoma, which has a looser structure with more stromal components such as gland ducts, facilitating blood vessel growth and higher blood protein and peptide content in tumor vessels [43]. Higher tumor grades indicate higher malignancy, more mitotic and proliferative activity of cells, thus increasing cell density and the synthesis of proteins and peptides, which in turn leads to higher APT signals [44].
This meta-analysis included studies with certain heterogeneity (sensitivity I2 from 0.00 % to 29.65 %, specificity I2 from 20.53 % to 87.72 %), which could be related to different thresholds used in different studies. Additionally, there was considerable variability in APT imaging parameters across included studies, such as sequence types (gradient echo and spin echo), repetition times (90–6400 ms), echo times (5.9–100 m s), and acquisition times (3–6 min). Variations in APT imaging parameters contribute to differences in diagnostic performance. Therefore, standardized imaging parameters are essential. Currently, standardized parameters for cervical cancer APT imaging are unclear. Based on existing evidence, a standardized protocol should at least include: 3T MRI field strength, gradient echo pulse sequence, power of 2–3 μT, pulse duration of 2s, and 20–30 frequency offsets. However, further research is needed for exploration and validation. Moreover, although all studies used MTRasym3.5 ppm to calculate APT signal intensity, some studies used APT max [32] and APT mean [34] as evaluation measures, which could also affect diagnostic performance.
This study has several limitations. Firstly, the original studies included in this meta-analysis have small sample sizes, which may have a potential small sample size effect, and individual indicators are highly heterogeneous, leading to a decrease in the reliability of the results to some extent. Secondly, due to the limitation of the number of original studies and the overall sample size, this study only conducted group analysis based on pathological characteristics and could not conduct subgroup analysis on factors such as region, age and pathological stage. Finally, the study population included in this paper is all Chinese, which may lead to inevitable population selection bias in the results of this paper. Whether APT value in cervical cancer classification, grading, lymph node involvement and other aspects can be extended to populations in Europe, America, Africa and other regions needs further research for confirmation. Despite these limitations, this study represents the first meta-analysis worldwide to evaluate the value of APT in predicting cervical cancer histological type, grade, and lymph node involvement. The results support previous findings that APT is effective in diagnosing, typing, and grading cervical cancer, providing the latest and most comprehensive evidence-based support for the clinical use of APT in cervical cancer diagnosis.
5. Conclusion
APT imaging has demonstrated significant diagnostic value in distinguishing between cervical squamous cell carcinoma and adenocarcinoma, as well as between poorly differentiated and moderately/highly differentiated squamous cell carcinoma, and in assessing lymph node involvement. It shows promise as a reliable tool for predicting the pathological characteristics of cervical cancer in clinical practice. However, due to limitations such as small sample sizes, selection bias, and high heterogeneity among studies, the diagnostic accuracy of APT imaging for these purposes still requires further validation through larger-scale, prospective studies.
CRediT authorship contribution statement
Chongshuang Yang: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Hasyma Abu Hassan: Writing – review & editing, Writing – original draft, Software, Resources, Conceptualization. Nur Farhayu Omar: Writing – original draft, Supervision, Software, Resources. Tze Hui Soo: Writing – original draft, Resources, Investigation, Formal analysis. Ahmad Shuib bin Yahaya: Writing – original draft, Software, Investigation, Formal analysis. Tianliang Shi: Writing – original draft, Validation, Supervision, Software. Yinbin Luo: Writing – original draft, Software, Resources, Formal analysis. Min Wu: Writing – original draft, Resources, Investigation, Formal analysis.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The data used to support the findings of this study are included within the article.
Funding
Science and Technology Fund of Guizhou Provincial Health Commission (No. gzwkj2021-374).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Xia C., Dong X., Li H., Cao M., Sun D., He S., Yang F., Yan X., Zhang S., Li N., et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135(5):584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari F., Giannini A. Approaches to prevention of gynecological malignancies. BMC Wom. Health. 2024;24(1):254. doi: 10.1186/s12905-024-03100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrado G., Anchora L.P., Bruni S., Sperduti I., Certelli C., Chiofalo B., Giannini A., D'Oria O., Bizzarri N., Legge F., Cosentino F., Turco L.C., Vizza E., Scambia G., Ferrandina G. Patterns of recurrence in FIGO stage IB1-IB2 cervical cancer: comparison between minimally invasive and abdominal radical hysterectomy. Eur. J. Surg. Oncol.: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2023;49(11) doi: 10.1016/j.ejso.2023.107047. [DOI] [PubMed] [Google Scholar]
- 5.Pecorino B., D'Agate M.G., Scibilia G., Scollo P., Giannini A., Di Donna M.C., Chiantera V., Laganà A.S. Evaluation of surgical outcomes of abdominal radical hysterectomy and total laparoscopic radical hysterectomy for cervical cancer: a retrospective analysis of data collected before the LACC trial. Int. J. Environ. Res. Publ. Health. 2022;19(20) doi: 10.3390/ijerph192013176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchetti C., Fagotti A., Tombolini V., Scambia G., De Felice F. Survival and toxicity in neoadjuvant chemotherapy plus surgery versus definitive chemoradiotherapy for cervical cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2020;83 doi: 10.1016/j.ctrv.2019.101945. [DOI] [PubMed] [Google Scholar]
- 7.D'Oria O., Bogani G., Cuccu I., D'Auge T.G., Di Donato V., Caserta D., Giannini A. Pharmacotherapy for the treatment of recurrent cervical cancer: an update of the literature. Expet Opin. Pharmacother. 2023;25(1):55–65. doi: 10.1080/14656566.2023.2298329. [DOI] [PubMed] [Google Scholar]
- 8.Lorusso D., Xiang Y., Hasegawa K., Scambia G., Leiva M., Ramos-Elias P., Acevedo A., Cvek J., Randall L., Pereira de Santana Gomes A.J., Contreras Mejía F., Helpman L., Akıllı H., Lee J.Y., Saevets V., Zagouri F., Gilbert L., Sehouli J., Tharavichitkul E., Lindemann K. ENGOT-cx11/GOG-3047/KEYNOTE-A18 investigators. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 2024;404(10460):1321–1332. doi: 10.1016/S0140-6736(24)01808-7. [DOI] [PubMed] [Google Scholar]
- 9.Ni X., Ma X., Qiu J., Zhou S., Cheng W., Luo C. Development and validation of a novel nomogram to predict cancer-specific survival in patients with uterine cervical adenocarcinoma. Ann. Transl. Med. 2021;9(4):293. doi: 10.21037/atm-20-6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sindos M., Pisal N., Freeman-Wang T., Singer A. Cervical cancer: effect of glandular cell type on prognosis, treatment, and survival. Obstet. Gynecol. 2003;101(6):1354–1355. doi: 10.1016/s0029-7844(03)00347-8. author reply 1355. [DOI] [PubMed] [Google Scholar]
- 11.Moukarzel L.A., Angarita A.M., VandenBussche C., Rositch A., Thompson C.B., Fader A.N., Levinson K. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative pap result increases time to diagnosis. J. Low. Genit. Tract Dis. 2017;21(2):91–96. doi: 10.1097/LGT.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Re G.L., Cucinella G., Zaccaria G., Crapanzano A., Salerno S., Pinto A., Casto A.L., Chiantera V. Role of MRI in the assessment of cervical cancer. Semin. Ultrasound CT MR. 2023;44(3):228–237. doi: 10.1053/j.sult.2023.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Rustum N.R., Yashar C.M., Bean S., Bradley K., Campos S.M., Chon H.S., Chu C., Cohn D., Crispens M.A., Damast S., et al. NCCN guidelines insights: cervical cancer, version 1.2020. J Natl Compr Canc Netw. 2020;18(6):660–666. doi: 10.6004/jnccn.2020.0027. [DOI] [PubMed] [Google Scholar]
- 14.Jones C.K., Schlosser M.J., van Zijl P.C., Pomper M.G., Golay X., Zhou J. Amide proton transfer imaging of human brain tumors at 3T. Magn. Reson. Med. 2006;56(3):585–592. doi: 10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 15.Law B.K.H., King A.D., Ai Q.Y., Poon D.M.C., Chen W., Bhatia K.S., Ahuja A.T., Ma B.B., Ka-Wai Yeung D., Fai Mo F.K., et al. Head and neck tumors: amide proton transfer MRI. Radiology. 2018;288(3):782–790. doi: 10.1148/radiol.2018171528. [DOI] [PubMed] [Google Scholar]
- 16.Wu B., Jia F., Li X., Li L., Wang K., Han D. Comparative study of amide proton transfer imaging and intravoxel incoherent motion imaging for predicting histologic grade of hepatocellular carcinoma. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.562049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin H., Wang D., Yan R., Jin X., Hu Y., Zhai Z., Duan J., Zhang J., Wang K., Han D. Comparison of diffusion kurtosis imaging and amide proton transfer imaging in the diagnosis and risk assessment of prostate cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.640906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmermann F., Korzowski A., Breitling J., Meissner J.E., Schuenke P., Loi L., Zaiss M., Bickelhaupt S., Schott S., Schlemmer H.P., et al. A novel normalization for amide proton transfer CEST MRI to correct for fat signal-induced artifacts: application to human breast cancer imaging. Magn. Reson. Med. 2020;83(3):920–934. doi: 10.1002/mrm.27983. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Lin L., Gao X., Li S., Cheng J. Amide proton transfer weighted and intravoxel incoherent motion imaging in evaluation of prognostic factors for rectal adenocarcinoma. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.783544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takayama Y., Nishie A., Togao O., Asayama Y., Ishigami K., Ushijima Y., Okamoto D., Fujita N., Sonoda K., Hida T., et al. Amide proton transfer MR imaging of endometrioid endometrial adenocarcinoma: association with histologic grade. Radiology. 2018;286(3):909–917. doi: 10.1148/radiol.2017170349. [DOI] [PubMed] [Google Scholar]
- 21.He Y.L., Li Y., Lin C.Y., Qi Y.F., Wang X., Zhou H.L., Yang J.J., Xiang Y., Xue H.D., Jin Z.Y. Three-dimensional turbo-spin-echo amide proton transfer-weighted mri for cervical cancer: a preliminary study. J. Magn. Reson. Imag. 2019;50(4):1318–1325. doi: 10.1002/jmri.26710. [DOI] [PubMed] [Google Scholar]
- 22.Meng N., Wang J., Sun J., Liu W., Wang X., Yan M., Dwivedi A., Zheng D., Wang K., Han D. Using amide proton transfer to identify cervical squamous carcinoma/adenocarcinoma and evaluate its differentiation grade. Magn. Reson. Imaging. 2019;61:9–15. doi: 10.1016/j.mri.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Meng N., Wang X., Sun J., Han D., Ma X., Wang K., Wang M. Application of the amide proton transfer-weighted imaging and diffusion kurtosis imaging in the study of cervical cancer. Eur. Radiol. 2020;30(10):5758–5767. doi: 10.1007/s00330-020-06884-9. [DOI] [PubMed] [Google Scholar]
- 24.Li B., Sun H., Zhang S., Wang X., Guo Q. The utility of APT and IVIM in the diagnosis and differentiation of squamous cell carcinoma of the cervix: a pilot study. Magn. Reson. Imaging. 2019;63:105–113. doi: 10.1016/j.mri.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M., Sterne J.A., Bossuyt P.M., Group Q.- QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 27.Meng N., Yin H.J., Jin H.X., Wang H.X., Liu W.Y., Han D.M. Preliminary evaluation of histological characteristics of cervical cancer by proton amino transfer imaging. Journal of Clinical Radiology. 2019;38(2):290–293. doi: 10.13437/j.cnki.jcr.2019.02.027. [DOI] [Google Scholar]
- 28.Meng X. Dalian Medical University; 2022. The Value of Amide Proton Transfer Weighted Imaging in the Evaluation of Biological Behavior of Cervical Cancer [D] [DOI] [Google Scholar]
- 29.Hou M., Meng N., Zhang M., Liu W., Han D., Ren J. Amide proton transfer imaging and diffusion kurtosis imaging in differentiating histological grade of cervical squamous cell carcinoma. [Chinese] Chin. J. Med. Imaging Technol. 2020;36(4):564–568. [Google Scholar]
- 30.Huang Q., Wang Y., Meng X., Li J., Shen Y., Hu X., Feng C., Li Z., Kamel I. Amide proton transfer-weighted imaging combined with ZOOMit diffusion kurtosis imaging in predicting lymph node metastasis of cervical cancer. Bioengineering (Basel) 2023;10(3) doi: 10.3390/bioengineering10030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B., Sun H., Zhang S., Wang X., Guo Q. Amide proton transfer imaging to evaluate the grading of squamous cell carcinoma of the cervix: a comparative study using (18) F FDG PET. J. Magn. Reson. Imag. 2019;50(1):261–268. doi: 10.1002/jmri.26572. [DOI] [PubMed] [Google Scholar]
- 32.Li S., Liu J., Zhang Z., Wang W., Lu H., Lin L., Zhang Y., Cheng J. Added-value of 3D amide proton transfer MRI in assessing prognostic factors of cervical cancer: a comparative study with multiple model diffusion-weighted imaging. Quant. Imag. Med. Surg. 2023;13(12):8157–8172. doi: 10.21037/qims-23-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng X., Tian S., Ma C., Lin L., Zhang X., Wang J., Song Q., Liu A.L. APTw combined with mDixon-Quant imaging to distinguish the differentiation degree of cervical squamous carcinoma. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1105867. (no pagination) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Q., Tian S., Ma C., Meng X., Chen L., Wang N., Lin L., Wang J., Song Q., Liu A. Amide proton transfer weighted imaging combined with dynamic contrast-enhanced MRI in predicting lymphovascular space invasion and deep stromal invasion of IB1-IIA1 cervical cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.916846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Q., Song Q., Wang Y., Lin L., Tian S., Wang N., Wang J., Liu A. Amide proton transfer weighted combined with diffusion kurtosis imaging for predicting lymph node metastasis in cervical cancer. Magn. Reson. Imaging. 2023;106:85–90. doi: 10.1016/j.mri.2023.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M.D. Jilin University; 2023. Application of Amide Proton Transfer Imaging in the Diagnosis of Cervical Cancer Grading and Parauterine Invasion. MA thesis. [DOI] [Google Scholar]
- 37.Zhou J., Payen J.F., Wilson D.A., Traystman R.J., van Zijl P.C. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003;9(8):1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 38.Cheng D., Zhuo Z., Zhang P., Qu L., Duan Y., Xu X., Xie C., Liu X., Haller S., Barkhof F., et al. Amide proton transfer-weighted imaging of pediatric brainstem glioma and its predicted value for H3 K27 alteration. Acta Radiol. 2023;64(11):2922–2930. doi: 10.1177/02841851231197503. [DOI] [PubMed] [Google Scholar]
- 39.Chen L., Li T., Li Y., Zhang J., Li S., Zhu L., Qin J., Tang L., Zeng Z. Combining amide proton transfer-weighted and arterial spin labeling imaging to differentiate solitary brain metastases from glioblastomas. Magn. Reson. Imaging. 2023;102:96–102. doi: 10.1016/j.mri.2023.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Deng X., Liu M., Zhou Q., Zhao X., Li M., Zhang J., Shen H., Lan X., Zhang X., Zhang J. Predicting treatment response to concurrent chemoradiotherapy in squamous cell carcinoma of the cervix using amide proton transfer imaging and intravoxel incoherent motion imaging. Diagn Interv Imaging. 2022;103(12):618–624. doi: 10.1016/j.diii.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Hou M., Song K., Ren J., Wang K., Guo J., Niu Y., Li Z., Han D. Comparative analysis of the value of amide proton transfer-weighted imaging and diffusion kurtosis imaging in evaluating the histological grade of cervical squamous carcinoma. BMC Cancer. 2022;22(1):87. doi: 10.1186/s12885-022-09205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sotirios B., Demetriou E., Topriceanu C.C., Zakrzewska Z. The role of APT imaging in gliomas grading: a systematic review and meta-analysis. Eur. J. Radiol. 2020;133 doi: 10.1016/j.ejrad.2020.109353. [DOI] [PubMed] [Google Scholar]
- 43.Zheng S., van der Bom I.M., Zu Z., Lin G., Zhao Y., Gounis M.J. Chemical exchange saturation transfer effect in blood. Magn. Reson. Med. 2014;71(3):1082–1092. doi: 10.1002/mrm.24770. [DOI] [PubMed] [Google Scholar]
- 44.Tang Y., Dundamadappa S.K., Thangasamy S., Flood T., Moser R., Smith T., Cauley K., Takhtani D. Correlation of apparent diffusion coefficient with Ki-67 proliferation index in grading meningioma. AJR Am. J. Roentgenol. 2014;202(6):1303–1308. doi: 10.2214/AJR.13.11637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.