Abstract
Objective
Failure to resolve inflammation is a novel feature of angiogenic-dependent diseases such as endometriosis and atherosclerosis.The correlation and causality between endometriosis and coronary heart disease is unclear. Here, we investigated the correlation and causality between endometriosis and coronary heart disease.
Methods
The relevant data was extracted from the NHANES database (Year range: 1999–2006) and analyzed to determine the correlation between endometriosis and coronary heart disease using weighted multivariable logistic regression models. Mendelian randomization analysis was used to assess the causal relationship between endometriosis and coronary heart disease risk.
Results
We evaluated the data obtained from 3943 participants and did not observe any association between endometriosis and coronary heart disease in weighted multivariable logistic regression models (Model 1, OR: 1.85, 95% CI: 0.66–5.15, P: 0.23; Model 2, OR: 0.89, 95% CI: 0.30–2.62, P: 0.82; Model 3, OR: 0.61, 95% CI: 0.19–1.95, P: 0.38). Two-sample Mendelian randomization analysis did not reveal a causal relationship between endometriosis and coronary heart disease (OR: 1.00, 95% CI: 0.94–1.07, P: 0.882).
Conclusion
Our findings did not indicate any association or causal relationship between endometriosis and coronary heart disease.
Keywords: endometriosis, coronary heart disease, cross-sectional study, NHANES, Mendelian randomization
Graphical Abstract
Introduction
Cardiovascular disease is the leading cause of mortality worldwide and coronary heart disease is one of the well-known and widely studied diseases.1 The full name for coronary heart disease(CHD) is coronary atherosclerotic heart disease.2 Atherosclerosis is a chronic inflammatory disease that is the main cause of heart attacks.2 Atherosclerosis accounts for more than 15% of deaths worldwide.3 Inflammation is an important factor in coronary artery disease and atherosclerosis.4 Chronic inflammation of the coronary artery wall driven by innate and adaptive immune system activation can lead to plaque rupture and thrombus formation.5 Cytokines, such as IL-1, IL-18, and IL-6, are involved in the formation of atherosclerosis by stimulating the movement of cells to the site of inflammation.6 Diseases, such as diabetes, obesity, and hypertension, significantly impact the risk of developing cardiovascular disease in women.7–9
Endometriosis is a common gynecological disease with hereditary and heterogeneous characteristics.2 Endometriosis is estimated to affect 10% of women of reproductive age, and its diagnosis is often delayed because it is difficult to detect early.10 Clinical manifestations vary from person to person and often include gastrointestinal symptoms, urogenital tract symptoms, pelvic pain, and infertility.10 Endometriosis is closely associated with inflammation and pathological angiogenesis.2 Cardiovascular disease is also associated with inflammation, pathological angiogenesis, and atherosclerosis.2 Failure to resolve inflammation is a novel feature of angiogenic-dependent diseases such as endometriosis and atherosclerosis.11 A case-control study in Japan involving 28 patients with endometriosis revealed that ≥30-year-old patients with endometriosis had significantly higher brachial-ankle pulse wave velocity (baPWV) than ≥30-year-old women without the disease (P < 0.05).12 The result suggested that endometriosis might cause hardening of the arteries in ≥30-year-old women.12 Therefore, women with endometriosis may be at high risk of cardiovascular disease.2 A cross-sectional study involving hospitalized women <35 years old revealed that the probability of being diagnosed with atherosclerosis was 42% higher in patients with endometriosis (OR = 1.421; 95% CI: 1.058–1.910).13 This suggested that endometriosis was associated with an increased risk of atherosclerosis in young patients; however, other information, such as disease severity, laboratory test results, or medical treatment provided, could not be obtained.13 A study on 21 patients with endometriosis and 24 healthy controls showed that endothelial dysfunction in patients with endometriosis was a preclinical manifestation of increased cardiovascular risk.14 However, the sample size was small and lacked long-term follow-up.14 The biochemical changes in cytokines and chemokines in patients with endometriosis were also recapitulated in a mouse model.15 Azari et al reported that both endometriosis and atherosclerosis were characterized by elevated levels of inflammation and cytokines, but the underlying causal relationship was unknown.16
The National Health and Nutrition Examination Survey (NHANES) combines interviews and physical examinations and is used to determine the risk factors for several diseases.17 Mendelian randomization(MR) uses genetic variation as an instrumental variable to study the causal relationship between exposure and outcome. MR analysis can reduce the bias caused by confounding factors and reverse causality.18
Here, we explored the correlation between endometriosis and CHD using the data obtained from the NHANES database from 1999 to 2006 and explored the causal impact of endometriosis on CHD through two-sample MR analysis, providing the corresponding guidance for the prevention of CHD.
Methods
Data Source
NHANES is a survey that combines interviews and physical examinations to assess the health and nutritional status of Americans.17 After 1999, it became a continuity plan, with a two-year cycle and an inclusion sample of approximately 5,000 participants per year.17 Survey data from the NHANES database is used in epidemiological studies and health science research to help develop sound public health policy.17
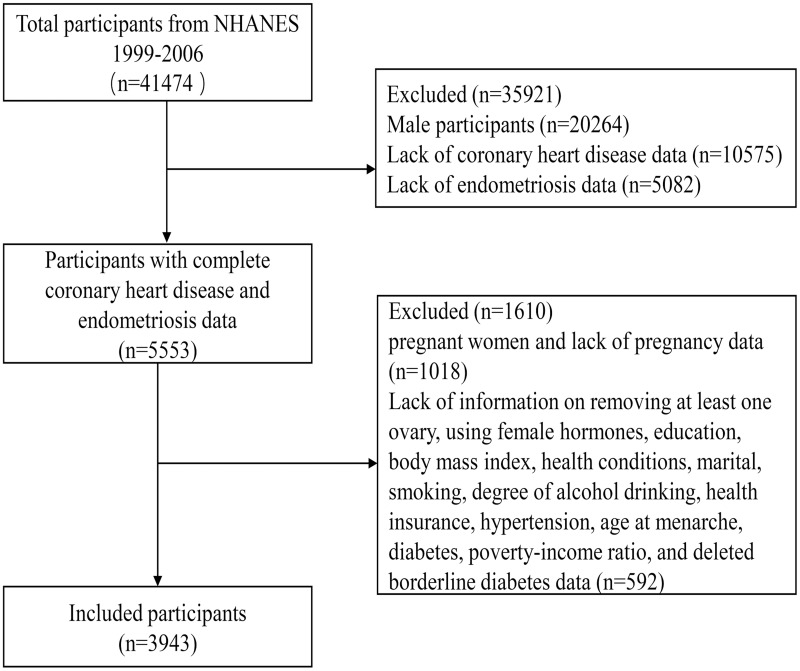
This cross-sectional study used data obtained in four two-year cycles from 1999 to 2006. The inclusion criteria were: 1) Age ≥20 years; 2) Relevant variables: coronary heart disease, endometriosis, age, race, marital status, education, body mass index, poverty–income ratio, age at menarche, hysterectomy, removing at least one ovary, using female hormones, cancer, diabetes, hypertension, stroke, using antidiabetic drugs, using antihypertensive drugs, using antihyperlipidemic drugs, smoking, degree of alcohol drinking, health conditions, a routine place to go for healthcare, and health insurance. We finally included 3943 participants in our analysis after removing the missing values (Figure 1).
Figure 1.
Flow chart for the selection of participants from the NHANES.
Diagnosis of Coronary Heart Disease
Participants were asked whether they had CHD through the MCQ160C questionnaire. The participants responded either yes or no to “Has a doctor or other health professional ever told you that you had coronary heart disease?”. The answer was either yes or no. When participants answered “Don’t know” or “Refused”, the response was classified as missing and deleted.
Diagnosis of Endometriosis
The RHQ360 questionnaire was used to determine whether the patient had endometriosis. The participants responded either yes or no to “Has a doctor or other health professional ever told you that you had endometriosis?” When participants answered “Do not know” or “Refused”, the response was classified as missing and deleted.
Mendelian Randomization
MR uses genetic variation as an instrumental variable to study the causal relationship between the exposure and outcome. Instrumental variables (IVs) must meet three conditions simultaneously: 1) strong association with the exposure factors; 2) no relation to the confounding factors; and 3) affecting outcomes only through exposure factors.19
Data on endometriosis were obtained from the FinnGen database (http://finngen.fi/) and included 15,088 cases and 107,564 controls from European populations. The single nucleotide polymorphisms (SNPs) (n = 16380466) associated with CHD were derived from genome-wide association studies (GWAS) data, including 21,012 cases and 197,780 controls from European populations. SNPs strongly associated with endometriosis at the genome-wide significance level (P < 5e-08) and without linkage disequilibrium (r2 < 0.001; kb = 10000) were selected for further analysis.20 The SNPs with F statistic ≥10 were screened from the strongly correlated SNPs.21 The F statistic is calculated using the formula: F statistic = R2× (N−2)/(1−R2), where N is the sample size of the exposure factor and R2 is the degree to which instrumental variables explain the exposure.22
Statistical Analysis
The included data were collected from the NHANES database (1999–2006) over four cycles, and all analyses were weighted. The 4-year mec weight (wtmec4yr) was used from 1999 to 2002, and the 2-year mec weight (wtmec2yr) was used from 2003 to 2006. Continuous variables were expressed by weighted average (weighted standard error), and categorical variables were expressed by weighted percentage. Weighted univariable and weighted multivariable logistic regression were used to analyze the relationship between endometriosis and CHD. Results were expressed as OR (95% CI). We established three logistic regression models. Model 1 was not adjusted. Model 2 was adjusted for age, marital status, education, body mass index (BMI), age at menarche, hysterectomy, removing at least one ovary, using female hormones, diabetes, hypertension, stroke, using antidiabetic drugs, using antihypertensive drugs, using antihyperlipidemic drugs, smoking, and degree of alcohol drinking. Model 3 was adjusted for age, race, marital status, education, BMI, poverty–income ratio, age at menarche, hysterectomy, removing at least one ovary, using female hormones, cancer, diabetes, hypertension, stroke, using antidiabetic drugs, using antihypertensive drugs, using antihyperlipidemic drugs, smoking, degree of alcohol drinking, a routine place to go for healthcare, health conditions, and health insurance.
MR analysis of the causal relationship between endometriosis and CHD risk was performed using SNPs strongly associated with endometriosis. Inverse-variance weighted (IVW), MR Egger, weighted median, weighted model, and simple model methods were used to determine whether IVs influenced CHD through their separate effect on endometriosis. The IVW method was the main analysis method to estimate the incidence of CHD OR and 95% CI. The outliers were detected using Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO). The egger_intercept of MR-Egger was used to evaluate the horizontal pleiotropy of IVs. In addition, the heterogeneity of IVs was estimated using the Q test. The reliability of causal estimates was assessed using the leave-one-out analysis.
All analyses were performed using R 4.3.1. P < 0.05 was considered statistically significant.
Results
Baseline Data of Included Individuals (Weighted)
We included 3943 participants in the study over four two-year cycles from 1999 to 2006 (Figure 1). A 4-year mec weight (wtmec4yr) was used from 1999 to 2002, and a 2-year mec weight (wtmec2yr) was used from 2003 to 2006. In women with CHD, BMI ≥30.0 accounted for 55.56% (weighted), hysterectomy accounted for 31.63% (weighted), and age ≥ 50 accounted for 58.57% (weighted). Endometriosis accounted for 16.71% (weighted), and antihypertensive drugs accounted for 75.28% (weighted). The variables, including age, education, BMI, hysterectomy, removing at least one ovary, using female hormones, diabetes, hypertension, stroke, using antidiabetic drugs, using antihypertensive drugs, and using antihyperlipidemic drugs, were significantly different between women with and without CHD (P value < 0.05). However, no significant differences were observed in race, marital status, poverty–income ratio, age at menarche, cancer, smoking, endometriosis, degree of alcohol drinking, health conditions, a routine place to go for healthcare, and health insurance between women with and without CHD (P value > 0.05; Table 1).
Table 1.
Comparison of Characteristics in Groups with and without CHD(Weighted)
| Variables | Total | Without CHD (%) | With CHD (%) | P value |
|---|---|---|---|---|
| Age(continuous) | 37.99(0.21) | 37.92(0.21) | 47.26(1.54) | < 0.0001 |
| Age(Grouped) | < 0.0001 | |||
| ≥50 | 15.06 | 14.73 | 58.57 | |
| 40–49 | 33.12 | 33.18 | 25.26 | |
| 30–39 | 27.71 | 27.86 | 8.90 | |
| 20–29 | 24.10 | 24.23 | 7.27 | |
| Race | 0.39 | |||
| Mexican American | 7.23 | 7.23 | 7.11 | |
| Non-Hispanic Black | 11.91 | 11.89 | 14.01 | |
| Non-Hispanic White | 70.61 | 70.56 | 76.44 | |
| Other Hispanic | 5.46 | 5.49 | 1.79 | |
| Other Race - Including Multi-Racial | 4.80 | 4.83 | 0.65 | |
| Marital | 0.23 | |||
| Divorced | 10.86 | 10.76 | 24.83 | |
| Living with partner | 7.68 | 7.67 | 9.01 | |
| Married | 57.28 | 57.32 | 51.60 | |
| Never married | 18.88 | 18.95 | 9.10 | |
| Separated | 3.87 | 3.86 | 5.46 | |
| Widowed | 1.42 | 1.43 | 0.00 | |
| Education | 0.00 | |||
| 9–11th Grade (Includes 12th grade with no diploma) | 10.74 | 10.58 | 32.22 | |
| College Graduate or above | 26.99 | 27.12 | 8.85 | |
| High School Grad/GED or Equivalent | 23.44 | 23.36 | 34.41 | |
| Less Than 9th Grade | 3.54 | 3.52 | 6.77 | |
| Some College or AA degree | 35.29 | 35.43 | 17.74 | |
| Poverty income ratio | 0.40 | |||
| <5 | 75.59 | 75.53 | 83.53 | |
| ≥5 | 24.41 | 24.47 | 16.47 | |
| Body mass index | 0.01 | |||
| <18.5 | 3.14 | 3.17 | 0.00 | |
| ≥30.0 | 33.28 | 33.11 | 55.56 | |
| 18.5–25.0 | 38.54 | 38.79 | 5.76 | |
| 25.0–30.0 | 25.04 | 24.93 | 38.68 | |
| Age at menarche | 0.23 | |||
| <11 | 8.79 | 8.71 | 18.78 | |
| >16 | 1.74 | 1.75 | 0.00 | |
| 11–16 | 89.47 | 89.53 | 81.22 | |
| Hysterectomy | 0.02 | |||
| Missing | 64.78 | 64.96 | 41.04 | |
| No | 22.18 | 22.14 | 27.32 | |
| Yes | 13.04 | 12.90 | 31.63 | |
| Removed at least one ovary | 0.00 | |||
| No | 89.33 | 89.49 | 68.29 | |
| Yes | 10.67 | 10.51 | 31.71 | |
| Use female hormones | < 0.0001 | |||
| No | 82.93 | 83.14 | 54.86 | |
| Yes | 17.07 | 16.86 | 45.14 | |
| Endometriosis | 0.23 | |||
| No | 90.15 | 90.21 | 83.29 | |
| Yes | 9.85 | 9.79 | 16.71 | |
| Cancer | 0.40 | |||
| No | 93.61 | 93.77 | 97.18 | |
| Yes | 6.19 | 6.23 | 2.82 | |
| Diabetes | 0.01 | |||
| No | 96.12 | 96.20 | 85.22 | |
| Yes | 3.88 | 3.80 | 14.78 | |
| Hypertension | < 0.0001 | |||
| No | 81.20 | 81.47 | 45.81 | |
| Yes | 18.80 | 18.53 | 54.19 | |
| Stroke | < 0.0001 | |||
| No | 98.62 | 98.74 | 89.01 | |
| Yes | 1.33 | 1.26 | 10.99 | |
| Antidiabetic drugs | 0.01 | |||
| No | 96.83 | 96.93 | 86.90 | |
| Yes | 3.14 | 3.07 | 13.10 | |
| Antihypertensive drugs | < 0.0001 | |||
| No | 87.27 | 87.77 | 24.72 | |
| Yes | 12.70 | 12.23 | 75.28 | |
| Antihyperlipidemic drugs | < 0.0001 | |||
| No | 96.13 | 96.49 | 52.62 | |
| Yes | 3.84 | 3.51 | 47.38 | |
| Smoke | 0.07 | |||
| Former | 17.05 | 16.92 | 34.40 | |
| Never | 57.01 | 57.13 | 40.62 | |
| Now | 25.94 | 25.95 | 24.98 | |
| Degree of alcohol drinking | 0.13 | |||
| Former | 13.47 | 13.37 | 26.43 | |
| Heavy | 22.21 | 22.26 | 15.13 | |
| Mild | 27.40 | 27.38 | 30.13 | |
| Moderate | 23.28 | 23.42 | 4.73 | |
| Never | 13.65 | 13.57 | 23.58 | |
| Health condition | 0.25 | |||
| Good | 97.61 | 97.63 | 95.05 | |
| Poor | 2.39 | 2.37 | 4.95 | |
| Routine place to go for healthcare | 0.60 | |||
| No | 10.84 | 10.86 | 7.36 | |
| Yes | 89.16 | 89.14 | 92.64 | |
| Health insurance | 0.11 | |||
| No | 18.20 | 18.11 | 29.38 | |
| Yes | 81.80 | 81.89 | 70.62 |
Notes: Continuous variables were shown as weighted mean (weighted standard error); Categorical variables were shown as the weighted percentage (%).
Abbreviation: CHD, coronary heart disease.
Univariable Analysis for CHD (Weighted)
Weighted univariable logistic regression analysis showed that age, marital status, education, BMI, age at menarche, hysterectomy, removing at least one ovary, using female hormones, diabetes, hypertension, stroke, using antidiabetic drugs, using antihypertensive drugs, using antihyperlipidemic drugs, smoking, and degree of alcohol drinking were associated with CHD (P value < 0.05; Table 2).
Table 2.
Univariable Analysis for CHD (Weighted)
| Variables | OR (95% CI) | P value |
|---|---|---|
| Age(continuous) | 1.14(1.06,1.23) | <0.001 |
| Age(grouped) | ||
| ≥50 | Ref | Ref |
| 40–49 | 0.19(0.07,0.50) | 0.001 |
| 30–39 | 0.08(0.02,0.34) | 0.001 |
| 20–29 | 0.08(0.02,0.29) | <0.001 |
| Race | ||
| Mexican American | Ref | Ref |
| Non-Hispanic Black | 1.20(0.33,4.28) | 0.78 |
| Non-Hispanic White | 1.10(0.38,3.16) | 0.86 |
| Other Hispanic | 0.33(0.04,3.07) | 0.32 |
| Other Race - Including Multi-Racial | 0.14(0.01,1.33) | 0.09 |
| Marital | ||
| Divorced | Ref | Ref |
| Living with partner | 0.51(0.09,2.86) | 0.44 |
| Married | 0.39(0.16,0.98) | 0.04 |
| Never married | 0.21(0.05,0.81) | 0.02 |
| Separated | 0.61(0.12,3.18) | 0.55 |
| Widowed | 0.00(0.00,0.00) | <0.0001 |
| Education | ||
| 9–11th Grade (Includes 12th grade with no diploma) | Ref | Ref |
| College Graduate or above | 0.11(0.02,0.55) | 0.01 |
| High School Grad/GED or Equivalent | 0.48(0.19,1.24) | 0.13 |
| Less Than 9th Grade | 0.63(0.25,1.62) | 0.33 |
| Some College or AA degree | 0.16(0.04,0.65) | 0.01 |
| Poverty income ratio | ||
| <5 | Ref | Ref |
| ≥5 | 0.61(0.19,2.00) | 0.41 |
| Body mass index | ||
| <18.5 | Ref | Ref |
| ≥30.0 | 1663259.86(843439.29,3279943.68) | <0.0001 |
| 18.5–25.0 | 147052.75(43963.09,491878.76) | <0.0001 |
| 25.0–30.0 | 1537449.35(796993.49,2965834.15) | <0.0001 |
| Age at menarche | ||
| <11 | Ref | Ref |
| >16 | 0.00(0.00,0.00) | <0.0001 |
| 11–16 | 0.42(0.15,1.15) | 0.09 |
| Hysterectomy | ||
| Missing | Ref | Ref |
| No | 1.95(0.65,5.83) | 0.23 |
| Yes | 3.88(1.63,9.26) | 0.003 |
| Removed at least one ovary | ||
| No | Ref | Ref |
| Yes | 3.95(1.66,9.43) | 0.002 |
| Used female hormones | ||
| No | Ref | Ref |
| Yes | 4.06(1.97,8.38) | <0.001 |
| Endometriosis | ||
| No | Ref | Ref |
| Yes | 1.85(0.66,5.15) | 0.23 |
| Cancer | ||
| No | Ref | Ref |
| Yes | 0.44(0.06,3.32) | 0.42 |
| Diabetes | ||
| No | Ref | Ref |
| Yes | 4.40(1.32,14.67) | 0.02 |
| Hypertension | ||
| No | Ref | Ref |
| Yes | 5.20(2.18,12.43) | <0.001 |
| Stroke | ||
| No | Ref | Ref |
| Yes | 9.70(2.64,35.68) | <0.001 |
| Antidiabetic drugs | ||
| No | Ref | Ref |
| Yes | 4.77(1.45,15.68) | 0.01 |
| Antihypertensive drugs | ||
| No | Ref | Ref |
| Yes | 21.85(8.77,54.44) | <0.0001 |
| Antihyperlipidemic drugs | ||
| No | Ref | Ref |
| Yes | 24.76(10.39,58.98) | <0.0001 |
| Smoke | ||
| Former | Ref | Ref |
| Never | 0.35(0.13,0.92) | 0.03 |
| Now | 0.47(0.16,1.40) | 0.17 |
| Degree of alcohol drinking | ||
| Former | Ref | Ref |
| Heavy | 0.34(0.05,2.20) | 0.25 |
| Mild | 0.56(0.18,1.73) | 0.31 |
| Moderate | 0.10(0.02,0.49) | 0.01 |
| Never | 0.88(0.27,2.86) | 0.83 |
| Health condition | ||
| Good | Ref | Ref |
| Poor | 2.15(0.55,8.32) | 0.26 |
| Routine place to go for healthcare | ||
| No | Ref | Ref |
| Yes | 1.53(0.29,8.03) | 0.61 |
| Health insurance | ||
| No | Ref | Ref |
| Yes | 0.53(0.24,1.18) | 0.12 |
Abbreviations: OR, odds ratio; CI, confidence interval; CHD, coronary heart disease.
Multivariable Logistic Regression
Table 3 presented weighted multivariable logistic regression models for the association between endometriosis and CHD. Model 1 was a crude model, and the result revealed no significant correlation between endometriosis and CHD (OR: 1.85, 95% CI: 0.66–5.15, P = 0.23, P for trend: 0.23). Model 2 did not reveal any significant correlation between endometriosis and CHD (OR: 0.89, 95% CI: 0.30–2.62, P = 0.82, P for trend: 0.82). Further, model 3 also revealed no significant association between endometriosis and CHD (OR: 0.61, 95% CI: 0.19–1.95, P = 0.38, P for trend: 0.38).
Table 3.
Multivariable Logistic Regression Models of the Association Between Endometriosis and CHD (Weighted)
| CHD | Endometriosis | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| Character | 95% CI | P | 95% CI | P | 95% CI | P |
| No | Ref | Ref | Ref | |||
| Yes | 1.85(0.66,5.15) | 0.23 | 0.89(0.30,2.62) | 0.82 | 0.61(0.19,1.95) | 0.38 |
| p for trend | 0.23 | 0.82 | 0.38 | |||
Notes: Model 1 was a crude model; Model 2 was adjusted for age, marital, education, BMI, age at menarche, hysterectomy, removing at least one ovary, using female hormones, diabetes, hypertension, stroke, using antidiabetic drugs, using antihypertensive drugs, using antihyperlipidemic drugs, smoking, degree of alcohol drinking; Model 3 was adjusted for age, race, marital, education, BMI, poverty–income ratio, age at menarche, hysterectomy, removing at least one ovary, using female hormones, cancer, diabetes, hypertension, stroke, using antidiabetic drugs, using antihypertensive drugs, using antihyperlipidemic drugs, smoking, degree of alcohol drinking, health conditions, a routine place to go for healthcare, health insurance.
Abbreviations: CI, confidence interval; CHD, coronary heart disease.
MR Analysis
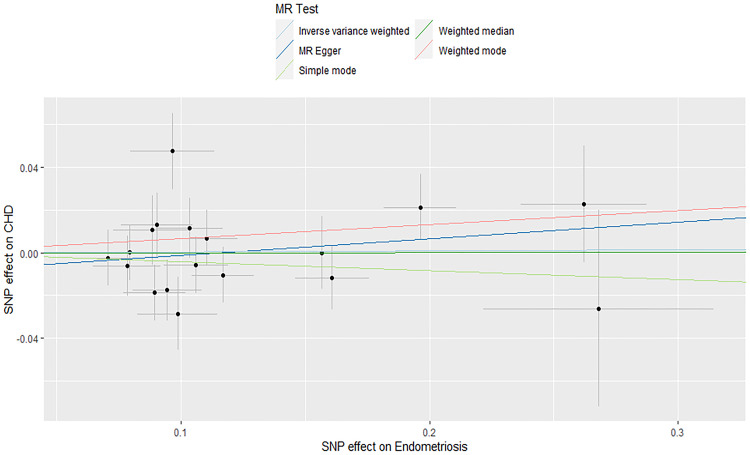
Cross-sectional studies were unable to explore causality. MR analysis was performed using 18 SNPs strongly associated with endometriosis to assess causality between endometriosis and CHD risk. IVW, MR Egger, weighted median, weighted model, and simple model did not find a causal relationship between endometriosis and CHD risk (Table 4). The forest plot (Supplementary Figure S1) showed the relationship between each instrumental variable and CHD. Q test and MR Egger intercept showed no heterogeneity and horizontal pleiotropy (Supplementary Tables S1 and S2). Figure 2 presented the scatter diagram of the two-sample MR analysis. The funnel plot was roughly symmetrical, indicating no significant heterogeneity in the results (Supplementary Figure S2). The leave-one-out analysis showed that the result remained stable when any SNP was deleted, indicating that the MR analysis was reliable (Supplementary Figure S3).
Table 4.
Results of Five MR Analysis Methods
| Method | nSNP | OR (95% CI) | P value |
|---|---|---|---|
| MR Egger | 18 | 1.08 (0.89~1.31) | 0.448 |
| Weighted median | 18 | 1.00 (0.92~1.09) | 0.993 |
| Inverse variance weighted | 18 | 1.00 (0.94~1.07) | 0.882 |
| Simple mode | 18 | 0.96(0.83~1.11) | 0.573 |
| Weighted mode | 18 | 1.07 (0.94~1.21) | 0.310 |
Abbreviations: OR, odds ratio; CI, confidence interval.
Figure 2.
Scatter plot of two-sample MR analysis.
Discussion
We collected data from the NHANES database (1999 to 2006) to investigate the association between endometriosis and CHD. The causal relationship between endometriosis and CHD risk was evaluated using two-sample MR analysis. Cross-sectional studies did not find an association between endometriosis and CHD. In addition, MR analysis did not find a causal relationship between endometriosis and CHD risk. Sensitivity analysis showed that the results of this study were reliable and robust.
Some of the existing studies are inconsistent with our findings. Mamillapalli et al reported that an animal study showed that mice with endometriosis had significantly higher serum levels of inflammatory factors, increased atherosclerosis, and significantly reduced aortic luminal area compared with mice with sham surgery, suggesting that elevated levels of inflammatory factors in endometriosis lesions might be associated with cardiovascular risk.23 A study involving 81 patients with endometriosis (stages I–II) showed elevated levels of CRP, homocysteine, fibrinogen, IL-17, and IL-33 in these patients.24 These inflammatory markers were associated with cardiovascular disease, suggesting an increased risk of cardiovascular disease in patients with endometriosis.24 The findings of a cross-sectional study in Europe involving 37 patients with endometriosis indicated that young women with endometriosis who had no structural vascular changes but had impaired endothelial function and subclinical atherosclerosis were at higher risk for future cardiovascular disease.25 However, the study also suggested that endometriosis might have no effect on vascular smooth muscle function.25 The findings of a large prospective cohort study (116430 patients with endometriosis) revealed an association of laparoscopically confirmed endometriosis with an increased risk of CHD; however, the extent of removal of endometriosis lesions during surgery was not covered.26 Li et al conducted a retrospective cohort study involving 19,454 patients with endometriosis and suggested that women with the disease were at higher risk of coronary artery disease (CAD), and women diagnosed with endometriosis should be aware of the risk of CAD.27 However, cigarette smoking and age at menarche were not recorded in the database.27 The results of a cohort study in Taiwan (13988 patients with endometriosis patients and 358 patients with CAD) showed an elevated risk of CAD in symptomatic endometriosis patients (aHR, 1.60; 95% CI: 1.29–1.98; P < 0.001), where the association was most significant in women aged 20–39 years.28 however, some potential confounding factors of CAD, such as obesity and smoking, were not covered in this study.28 Santoro et al assessed endothelial function in 22 patients with endometriosis who had undergone surgery and found that endothelial function improved in patients who underwent laparoscopic surgery and did not develop structural atherosclerosis.29 Endometriosis is a potential risk factor for CHD.28 Gynecologists should increase their awareness of endometriosis risk and screening for CAD in women with endometriosis aged 20–39 years.28 Gynecologists and cardiologists should work together to reduce the incidence of CAD.28 Notably, these studies reported on the association between endometriosis and CHD were mostly cohort studies, lacking large sample randomized controlled trials. In particular, clinical trials on the relationship between the severity of endometriosis and cardiovascular risk were limited.
The strength of our study is its large sample size. We investigated the correlation between endometriosis and CHD through a large sample cross-sectional study and used the FinnGen database and GWAS data to explore their causal relationship. The number of participants with CHD was 411,872 after weighted, and the results were more representative by the weighted analysis. MR analysis can reduce the bias caused by confounding factors and reverse causality.18 The results of MR analysis showed no pleiotropy and heterogeneity, and the conclusion was reliable. However, our study had some limitations. We did not investigate the types of endometriosis, and the diagnosis of endometriosis and CHD was based on a self-reported questionnaire with possible recall bias. In addition, the limited time frame (endometriosis occurs early in life while CAD occurs late) and the lack of accurate diagnosis of endometriosis were also limitations. In addition, MR analysis included all European populations, eliminating the demographic stratification bias. However, whether these findings could be generalized to other populations remains to be discussed.
Conclusion
Although several cohort studies have suggested a possible association between endometriosis and CHD, our cross-sectional study of a large NHANES database showed no association between these diseases. Two-sample MR analysis did not find a causal relationship between endometriosis and CHD risk. In the future, large randomized controlled trials and long-term follow-up based on the severity of endometriosis are needed to validate our findings.
Funding Statement
This study was supported by the Gusu Health Talent Plan Research Project (No.GSWS2023016); the Science and Technology Development Plan of Suzhou (No.SLT2022012, SKYD2022010); School-Land Collaborative Innovation Research Project of Jiangsu Pharmaceutical Vocational College (No.20239602); and the Youth Natural Science Foundation of Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine (No. ZZYQ2206).
Data Sharing Statement
The data of this cross-sectional study was publicly available on the NHANES website (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). MR analysis data could be publicly accessible from the finngen database (https://r9.finngen.fi/) and GWAS database (https://gwas.mrcieu.ac.uk/).
Ethics Statement
The National Health and Nutrition Examination Survey was approved by the National Center for Health Statistics Research Ethics Review Board (approval number: 98-12, 2005-06) (https://www.cdc.gov/nchs/nhanes/irba98.htm). The patients/participants provided their written informed consent to participate in this study. The study received ethical approval from the Ethics Committee of Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine (approval number: KY2024SZK0123).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Wu H, Chiou J. Potential benefits of probiotics and prebiotics for coronary heart disease and stroke. Nutrients. 2021;13(8):2878. doi: 10.3390/nu13082878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vazgiourakis VM, Zervou MI, Papageorgiou L, et al. Association of endometriosis with cardiovascular disease: genetic aspects (Review). Int J Mol Med. 2023;51(3):29. doi: 10.3892/ijmm.2023.5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemenway G, Frishman WH. Therapeutic implications of NLRP3-mediated inflammation in coronary artery disease. Cardiol Rev. 2022;30(2):90–99. doi: 10.1097/CRD.0000000000000391 [DOI] [PubMed] [Google Scholar]
- 4.Attiq A, Afzal S, Ahmad W, Kandeel M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur J Pharmacol. 2024;966:176338. doi: 10.1016/j.ejphar.2024.176338 [DOI] [PubMed] [Google Scholar]
- 5.Montarello NJ, Nguyen MT, Wong DTL, Nicholls SJ, Psaltis PJ. Inflammation in coronary atherosclerosis and its therapeutic implications. Cardiovasc Drugs Ther. 2022;36(2):347–362. doi: 10.1007/s10557-020-07106-6 [DOI] [PubMed] [Google Scholar]
- 6.Boland J, Long C. Update on the inflammatory hypothesis of coronary artery disease. Curr Cardiol Rep. 2021;23(2):6. doi: 10.1007/s11886-020-01439-2 [DOI] [PubMed] [Google Scholar]
- 7.Dei Cas A, Aldigeri R, Mantovani A, et al. Sex differences in cardiovascular disease and cardiovascular risk estimation in patients with type 1 diabetes. J Clin Endocrinol Metab. 2023;108(9):e789–e798. doi: 10.1210/clinem/dgad127 [DOI] [PubMed] [Google Scholar]
- 8.Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14(3):185–201. doi: 10.1038/nrneph.2017.189 [DOI] [PubMed] [Google Scholar]
- 9.Wang MC, Lloyd-Jones DM. Cardiovascular risk assessment in hypertensive patients. Am J Hypertens. 2021;34(6):569–577. doi: 10.1093/ajh/hpab021 [DOI] [PubMed] [Google Scholar]
- 10.Tan J, Taskin O, Iews M, et al. Atherosclerotic cardiovascular disease in women with endometriosis: a systematic review of risk factors and prospects for early surveillance. Reprod Biomed Online. 2019;39(6):1007–1016. doi: 10.1016/j.rbmo.2019.05.021 [DOI] [PubMed] [Google Scholar]
- 11.Kelly AG, Panigrahy D. Targeting angiogenesis via resolution of inflammation. Cold Spring Harb Perspect Med. 2023;13(3):a041172. doi: 10.1101/cshperspect.a041172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tani A, Yamamoto S, Maegawa M, et al. Arterial stiffness is increased in young women with endometriosis. J Obstet Gynaecol. 2015;35(7):711–715. doi: 10.3109/01443615.2014.992871 [DOI] [PubMed] [Google Scholar]
- 13.Nassiri Kigloo H, Suarthana E, Montreuil T, Tulandi T. Endometriosis, anxiety, and atherosclerosis: a study of eight million young hospitalized women in United States. Gynecol Obstet Invest. 2024;1:1–15. doi: 10.1159/000542049 [DOI] [PubMed] [Google Scholar]
- 14.Smyk JM, Danielecka Z, Kotowska M, et al. Cardiovascular risks and endothelial dysfunction in reproductive-age women with endometriosis. Sci Rep. 2024;14(1):24127. doi: 10.1038/s41598-024-73841-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fattori V, Zaninelli TH, Rasquel-Oliveira FS, et al. Nociceptor-to-macrophage communication through CGRP/RAMP1 signaling drives endometriosis-associated pain and lesion growth in mice. Sci Transl Med. 2024;16(772):eadk8230. doi: 10.1126/scitranslmed.adk8230 [DOI] [PubMed] [Google Scholar]
- 16.Azari ZD, Aljubran F, Nothnick WB. Inflammatory MicroRNAs and the pathophysiology of endometriosis and atherosclerosis: common pathways and future directions towards elucidating the relationship. Reprod Sci. 2022;29(8):2089–2104. doi: 10.1007/s43032-022-00955-6 [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed May 16, 2024.
- 18.Chen TX, Zhang ZL, Zhu YQ, Yang SP. The causal relationship between cancer and bone mineral density: a bi-directional two-sample Mendelian randomization analysis. Mod Preventive Med. 2023;18:3276–3280+3287. [Google Scholar]
- 19.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98. doi: 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai B, Jiang H, Gao R, Zhou X. Association between alcohol intake and bone mineral density: results from the NHANES 2005-2020 and two-sample Mendelian randomization. Arch Osteoporos. 2024;19(1):21. doi: 10.1007/s11657-024-01382-7 [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Lei S, Li D, Li Z, Zhang Y, Guo Y. Relationship of Klotho with cognition and dementia: results from the NHANES 2011-2014 and Mendelian randomization study. Transl Psychiatry. 2023;13(1):337. doi: 10.1038/s41398-023-02632-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Han H, Jin J, Zhou G, Li Z. Osteoarthritis and sarcopenia-related traits: the cross-sectional study from NHANES 2011-2014 and Mendelian randomization study. J Orthop Surg Res. 2023;18(1):502. doi: 10.1186/s13018-023-03960-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamillapalli R, Toffoloni N, Habata S, et al. Endometriosis promotes atherosclerosis in a murine model. Am J Obstet Gynecol. 2022;227(2):248.e1–248.e8. doi: 10.1016/j.ajog.2022.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafi U, Ahmad S, Bokhari SS, et al. Association of inflammatory markers/cytokines with cardiovascular risk manifestation in patients with endometriosis. Mediators Inflamm. 2021;2021:3425560. doi: 10.1155/2021/3425560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santoro L, D’Onofrio F, Campo S, et al. Endothelial dysfunction but not increased carotid intima-media thickness in young European women with endometriosis. Hum Reprod. 2012;27(5):1320–1326. doi: 10.1093/humrep/des062 [DOI] [PubMed] [Google Scholar]
- 26.Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9(3):257–264. doi: 10.1161/CIRCOUTCOMES.115.002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li PC, Yang YC, Wang JH, Lin SZ, Ding DC. Endometriosis is associated with an increased risk of coronary artery disease in Asian Women. J Clin Med. 2021;10(18):4173. doi: 10.3390/jcm10184173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei CH, Chang R, Wan YH, Hung YM, Wei JC. Endometriosis and new-onset coronary artery disease in Taiwan: a nationwide population-based study. Front Med. 2021;8:619664. doi: 10.3389/fmed.2021.619664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santoro L, D’Onofrio F, Campo S, et al. Regression of endothelial dysfunction in patients with endometriosis after surgical treatment: a 2-year follow-up study. Hum Reprod. 2014;29(6):1205–1210. doi: 10.1093/humrep/deu074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this cross-sectional study was publicly available on the NHANES website (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). MR analysis data could be publicly accessible from the finngen database (https://r9.finngen.fi/) and GWAS database (https://gwas.mrcieu.ac.uk/).