Abstract
Aim
This study examined the efficacy of AST‐001 for the core symptoms of autism spectrum disorder (ASD) in children.
Methods
This phase 2 clinical trial consisted of a 12‐week placebo‐controlled main study, a 12‐week extension, and a 12‐week follow‐up in children aged 2 to 11 years with ASD. The participants were randomized in a 1:1:1 ratio to a high‐dose, low‐dose, or placebo‐to‐high‐dose control group during the main study. The placebo‐to‐high‐dose control group received placebo during the main study and high‐dose AST‐001 during the extension. The a priori primary outcome was the mean change in the Adaptive Behavior Composite (ABC) score of the Korean Vineland Adaptive Behavior Scales II (K‐VABS‐II) from baseline to week 12.
Results
Among 151 enrolled participants, 144 completed the main study, 140 completed the extension, and 135 completed the follow‐up. The mean K‐VABS‐II ABC score at the 12th week compared with baseline was significantly increased in the high‐dose group (P = 0.042) compared with the placebo‐to‐high‐dose control group. The mean CGI‐S scores were significantly decreased at the 12th week in the high‐dose (P = 0.046) and low‐dose (P = 0.017) groups compared with the placebo‐to‐high‐dose control group. During the extension, the K‐VABS‐II ABC and CGI‐S scores of the placebo‐to‐high‐dose control group changed rapidly after administration of high‐dose AST‐001 and caught up with those of the high‐dose group at the 24th week. AST‐001 was well tolerated with no safety concern. The most common adverse drug reaction was diarrhea.
Conclusions
Our results provide preliminary evidence for the efficacy of AST‐001 for the core symptoms of ASD.
Keywords: autism, children, clinical trial, social communication
Autism spectrum disorder (ASD) is a lifelong neurodevelopmental disorder that manifests initially in childhood. The core symptoms of ASD include impaired social interactions and communication as well as the presence of restricted and repetitive behaviors. The current treatment options for the core symptoms of ASD are limited to psychosocial interventions, such as applied behavior analysis. Medications are reported to be effective in treating the combined behavioral symptoms, including irritability and aggression, 1 but there are no approved medications for the core symptoms of ASD, particularly deficits in social communication and interactions.
Novel medications, including cholinergic and glutamatergic agents, intranasal oxytocin, 2 and vasopressin 1a receptor antagonists, 3 have been of considerable interest. However, studies of these medications have failed to show significant improvement in the core symptoms of ASD compared with placebo. Several medications are currently undergoing clinical trials for the treatment of the core symptoms of ASD, but none have yet demonstrated effectiveness, especially for social communication and interactions.
AST‐001 is an L‐isomer of serine, which is a nonessential amino acid. AST‐001 plays a critical role in the biosynthesis of nucleotides, glycine, cysteine, phosphatidylserine, and sphingolipids, which are essential for maintaining the normal central nervous system. 4 , 5 AST‐001 acts as a modulator of the small conductance Ca2+‐activated K+ channel (SK channel) in dopamine neurons. 6 The roles of the SK channel in the developing brain are: (i) normal synapse formation, (ii) regulation of excitatory neurotransmitters, and (iii) generation of rhythmic and pacemaker activity of the brain. 7 , 8 In ASD animal models, AST‐001 improved social deficits by restoring synaptic imbalances in neural circuits, including dopamine pathways, through SK channel modulation. 6 It has also shown protective effects against oxidative damage in mitochondria and neuronal cells. 4 , 9 , 10
To date, L‐serine, the active ingredient in AST‐001 has also been studied for its beneficial effects and tolerability in hereditary sensory neuropathy type 1, 11 early‐stage Alzheimer disease, 12 amyotrophic lateral sclerosis, 13 3‐phosphoglycerate dehydrogenase deficiency, 14 , 15 and GRIN‐related disorders. 16 In these published reports, L‐serine was administered orally to adults and pediatric patients at doses ranging from 100 mg/kg per day to 850 mg/kg per day, for up to 2 years. The results showed improvement in clinical symptoms or slowing the progression of the disease, and no serious adverse events (AEs) were reported. In a phase I study that administered AST‐001, it was shown to be safe and well tolerated at a single dose of up to 30 g and multiple doses of 15 g bid for 7 days in healthy adults. 17 Also, in a previous open‐label study of AST‐001 (manuscript submitted), Vineland Adaptive Behavior Scales, 2nd Edition (VABS‐II) Adaptive Behavior Composite (ABC), Clinical Global Impression‐Severity (CGI‐S), and CGI‐Improvement (CGI‐I) scores at 12 weeks were significantly improved, providing preliminary evidence for AST‐001 in improving the core symptoms of ASD.
This phase 2 study was conducted to examine the efficacy of AST‐001 syrup for the core symptoms of ASD compared with placebo in a pediatric population with ASD. We also aimed to explore the optimal effective dose of AST‐001 syrup for ASD core symptom improvement.
Methods
This was a multicenter, randomized, double‐blind, placebo‐controlled, phase 2 study. Children with ASD were recruited from 10 hospitals in South Korea between 3 February 2021 and 12 May 2022. This study was approved by the institutional review boards of the participating institutions (Asan Medical Center, Hanyang University Medical Center, Inje University Ilsan Paik Hospital, Jeonbuk National University Hospital, Kangwon National University Hospital, Korea University Guro Hospital, Kyungpook National University Chilgok Hospital, Pusan National University Yangsan Hospital, Samsung Medical Center, Soon Chun Hyang University Cheonan Hospital) and conducted in accordance with the provisions of the Declaration of Helsinki. All of the parents/guardians provided written informed consent, and the participants provided written informed assent when possible. This study was registered at Clinical Research Information Service of Republic of Korea (KCT0007519).
Participants
All participants had to meet the following inclusion criteria: (i) age between 2 and 11 years, and (ii) a diagnosis of ASD defined by DSM‐5 and corroborated by the Autism Diagnostic Interview‐Revised (ADI‐R).
Participants were excluded from the study if they met one or more of the following exclusion criteria: (i) schizophrenia, other psychosis, or mood disorders including bipolar disorder and major depression according to DSM‐5 criteria; (ii) organic brain disease, neurological disorders, uncontrolled epilepsy, and history of seizures, except for simple febrile seizures; (iii) multisystem genetic disorder; (iv) sensory abnormalities such as congenital hearing loss; (v) severe self‐harm or injury to others requiring medical treatment; (vi) digestive disorders or surgical history that may influence the intestinal absorption of AST‐001; (vii) history of allergy to serine injections or serine supplements; and (viii) weight over 60 kg.
Study design
This clinical trial consisted of 12 weeks of the main study, 12 weeks of the extension study, and 12 weeks of the follow‐up study. All patients were treated with a body weight–based AST‐001 regimen that was already determined through modeling with AST‐001 pharmacokinetic data of healthy adults. 17 The high‐dose group took twice the dose compared with the low‐dose group. The appearance and taste of the placebo were identical to those of the investigational product. Participants were randomly assigned to high‐dose, low‐dose, or placebo‐to‐high‐dose control groups during the main study. The placebo‐to‐high‐dose control group received placebo during the main study and high‐dose AST‐001 during the extension study. For the high‐dose group, a low dose was administered for the first 2 weeks, then titrated to a high dose so that safety could be confirmed during the low‐dose period. The study design is displayed in Fig. S1.
During the screening period, all psychotropic medications (including antipsychotics, psychostimulants, antidepressants, anxiolytics, mood stabilizers, and neuroleptic agents) were washed out. Throughout the entire study period, antipsychotics and medications that may have influenced the symptoms of ASD, as well as natural/herbal medicines, were restricted from use. Only benzodiazepines and melatonin were permitted for managing temporary anxiety or sleep disturbances.
However, nonpharmacologic therapy for ASD (special education and other psychosocial treatment) was allowed under the discretion of the investigator if it had been continued for at least 3 months before participation in screening and if the therapy could be maintained throughout the study.
In this clinical trial, the randomization code was created using the sas software (SAS Institute Inc.) to assign a 1:1:1 ratio to the three groups (high‐dose, low‐dose, and placebo‐to‐high‐dose control groups) based on block randomization. Participants who had satisfied the inclusion/exclusion criteria were assigned to each group on a first‐come basis as they registered in the clinical trial using an interactive web response system in accordance with a randomization code.
Assessment
A diagnosis of ASD and/or comorbid psychiatric disorders was made based on the DSM‐5 criteria. The ADI‐R 18 was used to confirm the ASD diagnosis. Skilled interviewers, who were either previously trained and certified as ‘research reliable’ on the ADI‐R or who had completed a 2‐day rater training course by a certified ADI‐R trainer, conducted the ADI‐R.
Efficacy and safety assessments were performed at baseline, at the 12th, 24th, and 36th week, and, when applicable, at the time of early termination. The informant and rater for each child were not changed during the study. The primary end point was the mean change in the ABC score of the Korean VABS‐II (K‐VABS‐II) 19 , 20 from baseline to week 12. For those aged younger than 7 years, the ABC scores were the calculated scores (standard scores) from four major domains (Communication, Daily Living Skills, Socialization, Motor Skills); for those aged 7 years and older, the ABC score was calculated from three major domains excluding Motor Skills. 19 The secondary end points included the Ohio State University Autism CGI‐S and/or CGI‐I, 21 ABC, 22 , 23 Social Responsiveness Scale 2nd Edition (SRS‐2), and Korean‐Parenting Stress Index‐4th Edition‐Short Form (K‐PSI‐4‐SF). 24
Safety assessment measures, which included AE data, vital signs (blood pressure, pulse), physical measurements (body weight), and physical examinations, were collected at each visit. Electrocardiograms and laboratory tests (hematology, chemistry, and urinalysis) were performed at baseline, at the 12‐week visit, at the 24‐week end of treatment visit, and at the 36‐week end of study visit, if possible. At each visit, AEs were collected through open‐ended questions asked to parents/guardians and/or patients if possible. AE severity was classified based on the grade of the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI‐CTCAE) version 5.0. The causality criteria were assessed as ‘definitely related,’ ‘probably related,’ ‘possibly related,’ ‘unlikely related,’ and ‘unrelated’ and all cases except ‘unelated’ or ‘unlikely related’ were considered as if the causal relationship with AST‐001 could be ruled out.
Statistical analysis
For the efficacy evaluation, the main analysis was performed on the full analysis set using the intention‐to‐treat principle for those who received at least one dose of the investigational product after randomization and whose K‐VABS‐II ABC score was collected at least once after baseline. For the safety evaluation, all patients who received one or more doses of the investigational products were included.
Comparisons between groups at baseline were made using anova. Categorical variables are presented as frequencies and percentages and were compared using χ2 test or Fisher exact test, as appropriate. The comparison of the average change in scores at 12 weeks between the high‐dose and placebo‐to‐high‐dose control groups and between the low‐dose and placebo‐to‐high‐dose control groups was analyzed by ancova that included the baseline and age as covariates. For the difference between the groups, the least‐squares mean (LSM) difference, standard error, 90% confidence interval (CI), and P‐value of the change at 12 weeks are presented. A Statistical Analysis Plan (SAP) document was finalized before database lock and all statistical analyses were performed by preplanned SAP using the sas software version 9.4.
Results
Participants
A total of 151 participants were enrolled, and 145 children were included in the efficacy sample (Fig. 1). One‐hundred forty‐four children completed 12 weeks of the main study, 140 completed an additional 12 weeks of extended administration, and 135 completed an additional 12 weeks of the observational period. There were no significant differences in the number of participants who were withdrawn among the groups during the main study, the extended administration period, and the observational period (P = 0.405, P = 1.000, and P = 1.000, respectively). Sixteen children (10.6%) dropped out, and the reasons for dropout were withdrawal of consent (11), prohibited medication administered (2), difficult to continue study due to AEs (two), and unable to follow up (1). Discontinuation due to AEs was recorded in one case in the low‐dose group (osteomyelitis) and one case in the high‐dose group (enuresis), both of which were determined to have no causality with AST‐001.
Fig. 1.
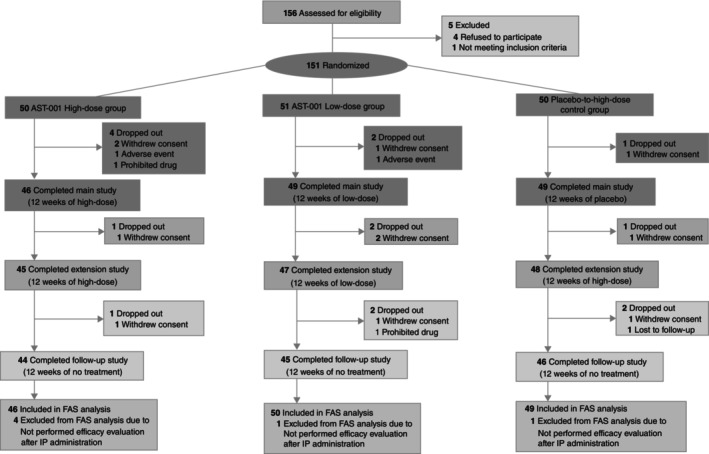
Consolidated Standards of Reporting Trials (CONSORT) flow diagram for this clinical trial.
The demographic and clinical characteristics of the participants at baseline are shown in Table 1. The mean age of the patients was 5.2 ± 2.2 years, and most were boys (n = 125, 82.8%). One‐hundred twenty‐two children (80.8%) were diagnosed with intellectual disability (intelligence quotient [IQ] < 70) based on standardized developmental assessments or intelligence tests, and 91 children (59.6%) were nonverbal by ADI‐R information. The mean weight and height at baseline were 23.3 ± 8.9 kg and 113.9 ± 14.0 cm, respectively. Twenty‐eight (18.5%) children had a history of use of psychotropic medications: aripiprazole, methylphenidate, risperidone, atomoxetine, quetiapine, escitalopram, and chlorpromazine for treatment of ASD or attention deficit/hyperactivity disorder. However, if these drugs were administered, a washout period of more than at least five times the half‐life before randomization was required.
Table 1.
Participant demographics and clinical characteristics at baseline
| High‐dose group | Low‐dose group | Placebo‐to‐high‐dose control group | |||
|---|---|---|---|---|---|
| Demographics and clinical characteristics | (n = 50) | (n = 51) | (n = 50) | F or x 2 | P‐value |
| Age, years, mean (SD) | 4.8 (1.7) | 5.3 (2.5) | 5.6 (2.2) | 1.92 | 0.150 |
| Age group, 2–6 years, n (%) | 43 (86.0) | 39 (37.3) | 34 (26.0) | 4.55 | 0.104 |
| Sex, male, n (%) | 45 (90.0) | 39 (76.5) | 41 (82.0) | 3.27 | 0.195 |
| Height, cm, mean (SD) | 111.8 (12.9) | 114.1 (15.8) | 116.0 (13.2) | 1.11 | 0.334 |
| Weight, kg, mean (SD) | 21.9 (7.5) | 23.2 (10.0) | 24.8 (9.1) | 1.30 | 0.279 |
| Comorbid psychiatric and genetic disorders | |||||
| Intellectual disability, n (%) | 40 (80.0) | 44 (86.3) | 38 (76.0) | 1.75 | 0.419 |
| ADHD, n (%) | 7 (14.0) | 8 (15.7) | 9 (18.0) | 0.30 | 0.890 |
| Tourette disorder, n (%) | 1 (2.0) | 0.662* | |||
| Fragile X syndrome, n (%) | 1 (2.0) | 0.662* | |||
| History of psychotropic medication use | 11 (22.0) | 8 (15.7) | 9 (18.0) | 0.702* | |
| History of psychosocial intervention | 50 (100.0) | 49 (96.1) | 49 (98.0) | 0.773* | |
| Baseline K‐VABS‐II ABC score | 49.9 (10.0) | 50.8 (11.6) | 48.5 (10.6) | 0.59 | 0.554 |
| Baseline K‐VABS‐II Communication score | 52.7 (12.5) | 54.2 (13.8) | 52.4 (11.8) | 0.29 | 0.750 |
| Baseline K‐VABS‐II Daily Living Skills score | 59.3 (13.8) | 59.2 (13.4) | 57.0 (14.2) | 0.42 | 0.659 |
| Baseline K‐VABS‐II Socialization score | 50.1 (9.5) | 50.5 (10.8) | 47.8 (10.5) | 0.97 | 0.382 |
| Baseline K‐VABS‐II Motor Skills score | 67.5 (9.8) | 69.2 (12.6) | 66.9 (10.5) | 0.45 | 0.638 |
| Baseline K‐VABS‐II Maladaptive Behavior score | 20.4 (2.0) | 19.9 (2.6) | 21.0 (2.4) | 2.56 | 0.081 |
| Baseline CGI‐S score | 5.1 (0.9) | 5.0 (0.9) | 5.0 (1.0) | 0.31 | 0.735 |
| Baseline SRS‐2 total T‐score | 76.2 (9.2) | 75.2 (10.2) | 80.0 (10.7) | 3.18 | 0.045 |
| Baseline Aberrant Behavior Checklist–Irritability | 11.7 (6.9) | 11.1 (8.0) | 13.7 (9.6) | 1.43 | 0.242 |
| Baseline K‐PSI‐4‐SF Parental Distress | 35.7 (8.5) | 35.3 (7.3) | 36.8 (8.3) | 0.44 | 0.643 |
Using Fisher exact test.
ABC, Adaptive Behavior Composite; ADHD, attention‐deficit/hyperactivity disorder; CGI‐S, Clinical Global Impression‐Severity; K‐PSI‐4‐SF, Korean‐Parenting Stress Index‐4th Edition‐Short Form; K‐VABS‐II, Korean Vineland Adaptive Behavior Scales, 2nd Edition; SRS‐2, Social Responsiveness Scale, 2nd Edition.
Efficacy outcomes
The changes in the K‐VABS‐II ABC and CGI‐S scores over 36 weeks are displayed in Figure 2a,b, respectively. When adjusted for age and baseline K‐VABS‐II ABC score, the mean K‐VABS‐II ABC score at the 12th week after administration compared with baseline was significantly increased in the high‐dose group (difference in adjusted LSM, 1.43 [90% CI, 0.28–2.58]; P = 0.042; Cohen d effect size, 0.43) compared with the placebo‐to‐high‐dose control group, but there was no significant difference in the mean change in the K‐VABS‐II ABC score between the low‐dose and placebo‐to‐high‐dose control groups (P = 0.128). The mean CGI‐S scores were significantly decreased at the 12th week in both the high‐dose (difference in adjusted LSM, −024 [90% CI, −0.45 to −0.04]; P = 0.046) and low‐dose (difference in adjusted LSM, −025 [90% CI, −0.42 to −0.08]; P = 0.017) groups compared with the placebo‐to‐high‐dose control group when adjusted for the baseline score and age. Figure 3 shows treatment differences in the VABS domain and K‐PSI‐4‐SF scores at the 12th week. Significant improvement in the Communication (difference in adjusted LSM, 2.56 [90% CI, 1.03–4.08]; P = 0.007) and Motor Skills (difference in adjusted LSM, 1.98 [90% CI, 0.33–3.63]; P = 0.049) domain scores of the VABS and in the Parental Distress subscale scores (difference in adjusted LSM, −1.99 [90% CI, −3.45 to −0.52]; P = 0.027) of the K‐PSI‐4‐SF were identified only in the high‐dose group compared with the placebo‐to‐high‐dose control group. Changes in the SRS‐2 and ABC at the 12th week were not significantly different among the groups.
Fig. 2.
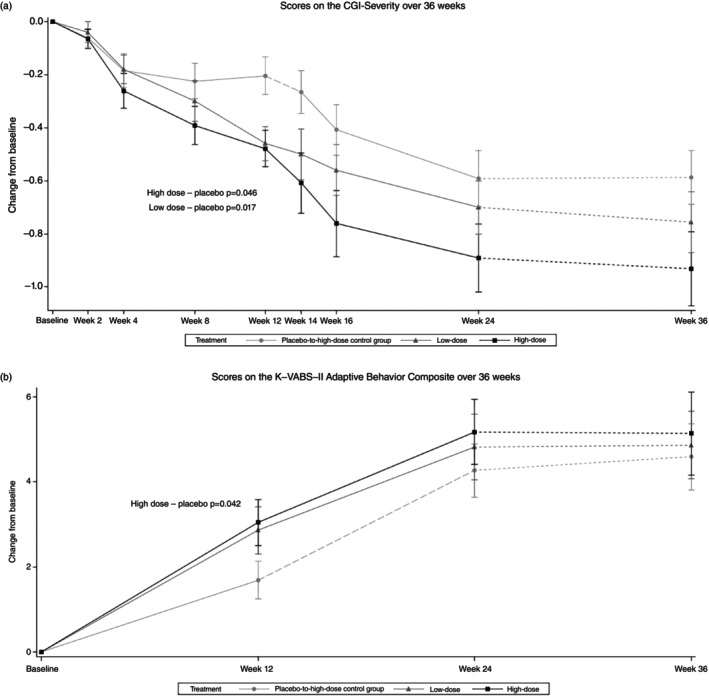
Mean Korean Vineland Adaptive Behavior Scales, 2nd Edition (K‐VABS‐II) Adaptive Behavior Composite (ABC) (a) and Clinical Global Impression‐Severity (CGI‐S) (b) scores over 36 weeks.
Fig. 3.
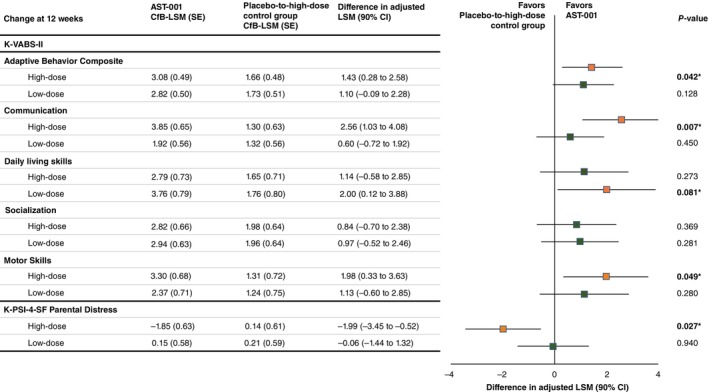
Treatment differences in Korean Vineland Adaptive Behavior Scales, 2nd Edition (K‐VABS‐II) domain and Korean‐Parenting Stress Index‐4th Edition‐Short Form (K‐PSI‐4‐SF) Parental Distress subscale scores at 12 weeks. cFB, confidence level for bounds; CI, confidence interval; LSM, least‐squares mean; SE, standard error.
The participants were divided into two groups according to chronological age (younger than 7 years vs 7 years or older), and efficacy analyses were performed again in each age group (Table S1). In children who were aged 2 to 6 years, the mean K‐VABS‐II ABC score at the 12th week compared with baseline was significantly increased both in the high‐dose (difference in adjusted LSM, 1.89 [90% CI, 0.59–3.18]; P = 0.018) and low‐dose (difference in adjusted LSM, 1.75 [90% CI, 0.31–3.19]; P = 0.047) groups compared with the placebo‐to‐high‐dose control group, but there was no significant difference in the mean change in the K‐VABS‐II ABC score between groups in children aged 7 years or older. In children who were aged 2 to 6 years, the mean CGI‐S scores were significantly decreased at the 12th week in the low‐dose group (difference in adjusted LSM, −0.25 [90% CI, −0.46 to −0.05]; P = 0.041) compared with the placebo‐to‐high‐dose control group, but there was no significant difference in the mean change in CGI‐S scores between groups in children aged 7 years or older.
During the 12‐week extended administration period, the K‐VABS‐II ABC and CGI‐S scores of the placebo‐to‐high‐dose control group changed rapidly after administration of high‐dose AST‐001 and caught up with those of the high‐dose group at the 24th week. After controlling for the baseline score and age, the change in the K‐VABS‐II ABC and CGI‐S scores at 24 weeks were not significantly different among the three groups (P = 0.555 and P = 0.441, respectively).
Although AST‐001 was not administered during the observation period, improvement in the K‐VABS‐II ABC and CGI‐S scores were maintained (Fig. 2), and there were no significant differences in the changes in the K‐VABS‐II ABC and CGI‐S scores at the 36th week among the three groups (P = 0.731 and P = 0.611, respectively).
Safety outcomes
Treatment‐emergent AEs (TEAEs) are listed in Table 2. Additionally, a summary of TEAEs showing data by period for the main study, extension study, and follow‐up study is provided in Table S2. During the 36 weeks of the study, 118 (78.2%) patients experienced at least one AE. The most common AE was cough, which was reported in 16.6% (25 of 151 patients, 46 events), followed by nasopharyngitis, pyrexia, and diarrhea. Most of the AEs were mild (263 of 316 events) in severity, and there were no significant differences among the three groups (P = 0.115). Discontinuation due to AEs without a causal relationship with the study medication occurred in two participants. A serious AE that was not related to the study medication occurred in five (3.3%) participants. Most AEs (163 of a total of 316; 51.6%) occurred during the main study period.
Table 2.
TEAEs occurring in ≥5% of patients
| Dose group | High‐dose group (n = 50) | Low‐dose group (n = 51) | Placebo‐to‐high‐dose control group* (n = 50) | |
|---|---|---|---|---|
| High dose for 24 weeks | Low dose for 24 weeks | Placebo for 12 weeks* | High‐dose for 12 weeks* | |
| TEAE | n (%) [case] | n (%) [case] | n (%) [case] | n (%) [case] |
| Cough | 9 (18.0) [11] | 9 (17.6) [17] | 6 (12.0) [9] | 7 (14.0) [10] |
| Nasopharyngitis | 6 (12.0) [10] | 10 (19.6) [17] | 2 (4.0) [2] | 8 (14.0) [9] |
| Pyrexia | 4 (8.0) [4] | 6 (11.8) [8] | 2 (4.0) [2] | 7 (14.0) [7] |
| Diarrhea | 4 (8.0) [4] | 6 (11.8) [6] | 6 (12.0) [6] | 4 (8.0) [4] |
| COVID‐19 | 3 (6.0) [3] | 5 (9.8) [5] | 0 | 8 (16.0) [8] |
| Vomiting | 1 (2.0) [1] | 5 (9.8) [6] | 3 (6.0) [3] | 3 (6.0) [5] |
| Decreased appetite | 5 (10.0) [6] | 4 (7.8) [4] | 0 | 1 (2.0) [1] |
| Constipation | 3 (6.0) [4] | 4 (7.8) [4] | 1 (2.00) [1] | 1 (2.0) [1] |
Adverse events were counted based on the onset date of the adverse event.
Percentages were calculated using the number of patients in each group as the denominator. Several cases of adverse events could be collected from one patient.
TEAE, treatment‐emergent adverse event.
During the study, adverse drug reactions (ADRs) related to the medication treatment were experienced in 12 children (24.0%) in the placebo‐to‐high‐dose control group, six (11.8%) in the low‐dose group, and seven (14.0%) in the high‐dose group; thus, there was no statistically significant difference among the groups (P = 0.209). Diarrhea was the most frequently reported ADR in all three groups.
There were no clinically significant results for changes in laboratory tests, vital signs, electrocardiograms, or physical examinations.
Discussion
This multicenter randomized controlled trial (RCT) showed significant improvements in ASD symptoms, especially in communication and motor skills, with AST‐001 compared with placebo in a pediatric population. After 12 weeks of extended administration of AST‐001, the placebo‐to‐high‐dose control group of patients who took high‐dose AST‐001 rapidly caught up with the development of patients in the other groups, and there were no significant differences in the K‐VABS‐II ABC and CGI‐S scores among the three groups. These results suggest that AST‐001 could improve the core symptoms of ASD and be a potential candidate for the treatment of ASD.
To the best of our knowledge, there are no previous larger‐scale RCTs that have shown the efficacy of a medication to improve social communications and/or interactions in ASD. Several compounds with initial promise, such as memantine, intranasal oxytocin, balovaptan, and bumetanide, have failed to demonstrate significant improvements in ASD symptoms when evaluated in large, multicenter RCTs. 2 , 3 , 25 , 26 However, this large, multicenter RCT showed the possibility of AST‐001 as a novel pharmacological agent that improves the core symptoms of ASD.
Although the mechanism of AST‐001 in ASD is not fully understood, previous studies have shown that AST‐001 improves sociability and social cognition in a mouse model of ASD by rescuing abnormal spontaneous firing of the ventral tegmental area dopamine neurons through normalization of SK channel activity. 6 In addition, oral administration of AST‐001 has been shown to pass through the blood–brain barrier and distribute to the brain. Moreover, a previous study showed that a high‐dose, but not a low‐dose, of AST‐001 administration improved social behaviors or neural excitability in an ASD model mouse, and AST‐001 dose‐dependently improved symptoms in ASD mouse models. 6 The results of our study are consistent with this nonclinical study and showed a significantly increased mean K‐VABS‐II ABC score at the 12th week only in the high‐dose group compared with the placebo‐to‐high‐dose control group.
There has been a robust placebo response reported for outcome measures used in ASD clinical trials. 2 , 3 , 25 , 26 Although different among studies, both caregiver‐reported measures, such as the SRS and ABC, and clinician‐rated measures, such as the VABS and CGI scales, are affected by placebo response. 27 Expectation bias can affect caregivers and clinician raters, increasing the treatment effect seen in placebo groups. 27 However, our study showed that the treatment effect of AST‐001 on the core symptoms of ASD was greater than the placebo effect.
In this study, the mean age of the children with ASD was 5.2 ± 2.2 years, and 77.2% (112 of 145 children) of the efficacy sample were 6 years or younger. Moreover, a treatment effect of AST‐001 on the K‐VABS‐II or CGI‐S scores was observed only in pediatric patients with ASD younger than 7 years in our results. In previous autism trials, buzmetanide 25 and intranasal oxytocin 28 showed marginal significance toward more improvement on the SRS, implying better efficacy for core symptoms in younger children with ASD. The effect of psychosocial interventions may also be enhanced at younger ages. 29 These potential age effects could be related to windows of plasticity in functional brain development, 30 suggesting a greater influence of interventions at early ages on brain circuitry. 31
In addition to age, our sample differed in intelligence profile from previous studies. Our study did not have inclusion criteria for IQ or adaptive functioning. Consequently, 80.8% of the children in our study had a medical history of intellectual disability, and the baseline mean K‐VABS‐II ABC score was around 50. This cognitive profile was lower than those in previous studies using balovaptan (inclusion criterion: IQ ≥70) 3 and bumetanide (inclusion criterion: IQ ≥55). 25
In the current study, a benefit of AST‐001 over placebo was also observed in the Parental Distress subscale of the K‐PSI‐4‐SF. Parents of children with ASD are more likely to experience higher levels of parenting stress than parents of typically developing children. 32 , 33 Previous studies about the impact of psychotropic medication on parenting stress among parents of children with ASD mostly focused on aripiprazole and risperidone. 34 Aripiprazole and risperidone could improve parenting stress through decreasing the behavioral symptoms of children with ASD, but our findings imply that improvement in social interactions and communication could also reduce parental stress among parents of children with ASD.
AST‐001 was safe and well‐tolerated in this study. The most common ADR was diarrhea, and the frequency was not different among the groups. Diarrhea seems to be more related to the drug additives such as sucrose used in syrup rather than to AST‐001 itself. Previous studies have suggested that high consumption of sugars, particularly sucrose, is associated with gastrointestinal disturbances, including diarrhea. 35 Placebo contains more sucrose than AST‐001 and diarrhea was more frequently reported in the placebo group. There were no treatment‐related serious AEs. Thus, our findings imply that AST‐001 could be safely administered to children with ASD.
Our current study findings should be interpreted in the context of some limitations. First, our sample may not have been representative of the whole ASD population because of the exclusion of patients with concomitant medication use and medical and psychiatric comorbidities as well as the weight limitation. However, the sex ratio and comorbid rate of intellectual disabilities in our sample were similar to those of children with ASD reported in previous studies. Second, there were few participants in the age range of 7 to 11 years in the current study. Although the treatment effect of AST‐001 seems to be increased in younger children, the study may not have had enough power to detect differences in older age groups owing to the small sample sizes. Third, although psychosocial treatment was allowed if the therapy could be maintained throughout the study, in some cases there were minor changes in psychosocial treatment according to COVID‐19 status.
Despite these caveats, our study suggests that AST‐001 could be a potential candidate to improve the core symptoms of ASD and could be safely used in children with ASD. Further phase 3 trials are needed.
Disclosure statement
Professor Su‐Kyeong Hwang is an employee of Astrogen and holds a patent for the study medication. Professor Hyo‐Won Kim was a medical consultant in this study and received a consulting fee of <$25,000 USD. No other authors have conflicts of interest.
Author contributions
Conception and design of the study: H.W.K. J.H.K, S.K.H, Y.S.J. Acquisition and analysis of data: H.W.K. J.H.K, U.S.C., J.I.K., S.S., T.W.P., MD, M.S.L, J.W.H., E.J.P., Y.S.J. Drafting the manuscript or figures: H.W.K. J.H.K, Y.S.J. All authors discussed the results and contributed to review the final article.
Supporting information
Figure S1. Design of the clinical trial.
Table S1. Changes from baseline to 12 weeks in the K‐VABS‐II ABC and CGI‐S scores by age subgroup: (A) comparison between the high‐dose group and the placebo group and (B) comparison between the low‐dose group and the placebo group.
Table S2. Summary of TEAEs.
Acknowledgment
This clinical trial was funded by Astrogen Inc. (Daegu, South Korea).
This study was presented as an abstract at the American Academy of Child and Adolescent Psychiatry's 70th Annual Meeting, New York City, NY, 23–28 October 2023.
References
- 1. Doyle CA, McDougle CJ. Pharmacotherapy to control behavioral symptoms in children with autism. Expert Opin. Pharmacother. 2012; 13: 1615–1629. [DOI] [PubMed] [Google Scholar]
- 2. Sikich L, Kolevzon A, King BH et al. Intranasal oxytocin in children and adolescents with autism spectrum disorder. N. Engl. J. Med. 2021; 385: 1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hollander E, Jacob S, Jou R et al. Balovaptan vs placebo for social communication in childhood autism spectrum disorder: A randomized clinical trial. JAMA Psychiatry 2022; 79: 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Koning TJ, Snell K, Duran M et al. L‐serine in disease and development. Biochem. J. 2003; 371: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattaini KR, Sullivan MR, Vander Heiden MG. The importance of serine metabolism in cancer. J. Cell Biol. 2016; 214: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Um KB, Kwak S, Choi M et al. AST‐001 improves social deficits and restores dopamine neuron activity in a mouse model of autism. Biomedicine 2023; 11: 3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat. Neurosci. 2005; 8: 635–641. [DOI] [PubMed] [Google Scholar]
- 8. Dwivedi D, Bhalla US. Physiology and therapeutic potential of SK, H, and M medium afterhyperpolarization ion channels. Front. Mol. Neurosci. 2021; 14: 658435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolpert L. Principles of Development. Oxford, United Kingdom. Oxford University Press, New York, 2019. [Google Scholar]
- 10. Kim KY, Hwang SK, Park SY, Kim MJ, Jun DY, Kim YH. L‐serine protects mouse hippocampal neuronal HT22 cells against oxidative stress‐mediated mitochondrial damage and apoptotic cell death. Free Radic. Biol. Med. 2019; 141: 447–460. [DOI] [PubMed] [Google Scholar]
- 11. Fridman V, Suriyanarayanan S, Novak P et al. Randomized trial of l‐serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology 2019; 92: e359–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yulug B, Altay O, Li X et al. Combined metabolic activators improve cognitive functions in Alzheimer's disease patients: A randomised, double‐blinded, placebo‐controlled phase‐II trial. Transl. Neurodegener. 2023; 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine TD, Miller RG, Bradley WG et al. Phase I clinical trial of safety of L‐serine for ALS patients. Amyotroph. Lateral Scler. Frontotemporal Degener. 2017; 18: 107–111. [DOI] [PubMed] [Google Scholar]
- 14. De Koning TJ, Jaeken J, Pineda M et al. Hypomyelination and reversible white matter attenuation in 3‐phosphoglycerate dehydrogenase deficiency. Neuropediatrics 2000; 31: 287–292. [DOI] [PubMed] [Google Scholar]
- 15. De Koning TJ, Duran M, Van Maldergem L et al. Congenital microcephaly and seizures due to 3‐phosphoglycerate dehydrogenase deficiency: Outcome of treatment with amino acids. J. Inherit. Metab. Dis. 2002; 25: 119–125. [DOI] [PubMed] [Google Scholar]
- 16. Krey I, von Spiczak S, Johannesen KM et al. L‐serine treatment is associated with improvements in behavior, EEG, and seizure frequency in individuals with GRIN‐related disorders due to null variants. Neurotherapeutics 2022; 19: 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee S, Hwang SK, Nam HS, Cho JS, Chung JY. Population pharmacokinetic model of AST‐001, L‐isomer of serine, combining endogenous production and exogenous Administration in Healthy Subjects. Front. Pharmacol. 2022; 13: 891227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lord C, Rutter M, Le Couteur A. Autism diagnostic interview‐revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994; 24: 659–685. [DOI] [PubMed] [Google Scholar]
- 19. Sparrow SS, Cicchetti VD, Balla AD. Vineland Adaptive Behavior Scales, 2nd edn. American Guidance Service, Circle Pines, MN, 2005. [Google Scholar]
- 20. Hwang S, Kim J, Hong S, Bae S, Cho S. Standardization study of the Korean Vineland Adaptive Behavior Scales‐II (K‐Vineland‐II). Kor. J. Clin. Psychol. 2015; 34: 851–876. [Google Scholar]
- 21. Holloway JA, Arnold LE, Aman MG. OSU Autism Rating Scale. DSM‐5 (OARS‐5). Ohio State University, Columbus, OH, 2017. [Google Scholar]
- 22. Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: A behavior rating scale for the assessment of treatment effects. Am. J. Ment. Defic. 1985; 89: 485–491. [PubMed] [Google Scholar]
- 23. Moon DS, Chung US, Jung SH, Cho AR, Bahn GH. A preliminary study for the rating of pharmacological effect with aberrant behavior checklist in children with autistic disorder. J. Korean Acad. Child Adolesc. Psychiatry 2013; 24: 164–169. [Google Scholar]
- 24. Abidin RR. Parenting Stress Index (Short Form). Psychological Assessment Resource, Odessa, TX, 1995. [Google Scholar]
- 25. Sprengers JJ, van Andel DM, Zuithoff NP et al. Bumetanide for core symptoms of autism spectrum disorder (BAMBI): A single center, double‐blinded, participant‐randomized, placebo‐controlled, phase‐2 superiority trial. J. Am. Acad. Child Adolesc. Psychiatry 2021; 60: 865–876. [DOI] [PubMed] [Google Scholar]
- 26. Brignell A, Marraffa C, Williams K, May T. Memantine for autism spectrum disorder. Cochrane Database Syst. Rev. 2022; 8: CD013845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacob S, Anagnostou E, Hollander E et al. Large multicenter randomized trials in autism: Key insights gained from the balovaptan clinical development program. Mol. Autism. 2022; 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guastella AJ, Boulton KA, Whitehouse AJO et al. The effect of oxytocin nasal spray on social interaction in young children with autism: A randomized clinical trial. Mol. Psychiatry 2023; 28: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris SL, Handleman JS. Age and IQ at intake as predictors of placement for young children with autism: A four‐ to six‐year follow‐up. J. Autism Dev. Disord. 2000; 30: 137–142. [DOI] [PubMed] [Google Scholar]
- 30. Hensch TK. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005; 6: 877–888. [DOI] [PubMed] [Google Scholar]
- 31. DeMayo MM, Young LJ, Hickie IB, Song YJC, Guastella AJ. Circuits for social learning: A unified model and application to autism spectrum disorder. Neurosci. Biobehav. Rev. 2019; 107: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baker BL, Blacher J, Crnic KA, Edelbrock C. Behavior problems and parenting stress in families of three‐year‐old children with and without developmental delays. Am. J. Ment. Retard. 2002; 107: 433–444. [DOI] [PubMed] [Google Scholar]
- 33. Emerson E, Einfeld S. Emotional and behavioural difficulties in young children with and without developmental delay: A bi‐national perspective. J. Child Psychol. Psychiatry 2010; 51: 583–593. [DOI] [PubMed] [Google Scholar]
- 34. Kim HW, Park EJ, Kim JH et al. Aripiprazole for irritability in Asian children and adolescents with autistic disorder: A 12‐week, multinational, multicenter, prospective open‐label study. J. Child Adolesc. Psychopharmacol. 2018; 28: 402–408. [DOI] [PubMed] [Google Scholar]
- 35. Arnone D, Chabot C, Heba AC et al. Sugars and gastrointestinal health. Clin. Gastroenterol. Hepatol. 2022; 20: 1912–1924.e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Design of the clinical trial.
Table S1. Changes from baseline to 12 weeks in the K‐VABS‐II ABC and CGI‐S scores by age subgroup: (A) comparison between the high‐dose group and the placebo group and (B) comparison between the low‐dose group and the placebo group.
Table S2. Summary of TEAEs.


