Abstract
F-box and WD repeat domain-containing 7 (FBXW7), formerly known as hCdc4, hAGO Fbw7, or SEL10, plays a specific recognition function in SCF-type E3 ubiquitin ligases. FBXW7 is a well-established cancer suppressor gene that specifically controls proteasomal degradation and destruction of many key oncogenic substrates. The FBXW7 gene is frequently abnormal in human malignancies especially in gastrointestinal cancers. Accumulating evidence reveals that mutations and deletions of FBXW7 are participating in the occurrence, progression and treatment resistance of human gastrointestinal cancers. Considering the current therapeutic challenges faced by gastrointestinal cancers, elucidating the biological function and molecular mechanism of FBXW7 can provide new perspectives and references for future personalized treatment strategies. In this review, we elucidate the key molecular mechanisms by which FBXW7 and its substrates are involved in gastrointestinal cancers. Furthermore, we discuss the consequences of FBXW7 loss or dysfunction in tumor progression and underscore its potential as a prognostic and therapeutic biomarker. Lastly, we propose potential therapeutic strategies targeting FBXW7 to guide the precision treatment of gastrointestinal cancers.
Keywords: FBXW7, gastrointestinal cancers, molecular mechanism, therapeutic strategies, biomarker
Graphical Abstract
1 Background
Globally, gastrointestinal cancers have high morbidity and mortality rates, especially in recent years, with a trend toward affecting younger individuals (I et al., 2023; Huang et al., 2023; Ben-Aharon et al., 2023). Gastrointestinal malignancies are estimated to account for 17.7% and 28.5% of all expected newly diagnosed cancer cases and fatalities respectively in the United States in 2024 (Siegel et al., 2024). Gastrointestinal cancers refer to malignant tumors originating from the digestive tract and digestive organs, mainly including esophageal cancer, colorectal cancer, stomach cancer, pancreatic cancer, hepatocellular carcinoma and cholangiocarcinoma (Li et al., 2021; Li et al., 2023a). With the development of medical technology, certain progress has been made in the prognosis of patients with gastrointestinal cancer (Chong et al., 2024). However, gastrointestinal cancers are relatively heterogeneous, and different individuals usually face dramatic differences in outcomes. Molecularly stratified markers are used for patients with gastrointestinal cancers as shown in Figure 1. Precision therapy is the future direction for gastrointestinal cancers, but current therapeutic and prognostic molecular markers are limited. Therefore, to achieve precision management of gastrointestinal cancers, it is necessary to explore novel biomarkers that can improve diagnosis and treatment.
FIGURE 1.
Major molecular markers of gastrointestinal cancers. The key molecular markers for esophageal cancer include HER2 amplification or overexpression, PD-L1, EGFR, and VEGFR (Liu et al., 2020; Zhou and Hofstetter, 2020; Wang et al., 2025). For pancreatic cancer, the main molecular markers are NTRK fusion, BRCA1/2 mutations, KRAS mutations, BRAF mutations, and MSI (Collisson et al., 2019; Buscail et al., 2020; Wang S. et al., 2021; Schlick et al., 2021). Molecular markers for cholangiocarcinoma mainly include MSI, HER-2 amplification or overexpression KRAS mutations,IDH1/2 mutations, FGFR2 fusion and NTRK fusion (Rodrigues et al., 2021; Pavicevic et al., 2022; Kam et al., 2021). The major molecular markers for colorectal cancer include MMR/MSI, RAS/BRAF mutations, NTRK fusion, POLE/POLD1 mutations, PI3K mutations, and HER2 amplification or overexpression (Martelli et al., 2022; Taieb et al., 2019; Sepulveda et al., 2017). The main molecular markers for gastric cancer include MSI, PD-L1,HER-2 amplification or overexpression, Claudin18.2, TMB, and EBV (Guan et al., 2023; Joshi and Badgwell, 2021; Elimova et al., 2015). So far the molecular alterations in hepatocellular carcinoma have not led to effective treatments (Nault and Villanueva, 2021). In the figure we show several promising molecular markers such as ctDNA, ncRNA, TERT promoter mutation and MSI (Nault and Villanueva, 2021; Llovet et al., 2018; Chan et al., 2024a).
The ubiquitin-proteasome system (UPS) regulates many cellular processes such as cell division, cell differentiation, DNA damage repair, and apoptosis by controlling the degradation of a variety of proteins in eukaryotes (Park et al., 2020; Liu et al., 2021a). The protein degradation function of UPS mainly depends on the sequential activation of three enzymes: ubiquitin-activating enzyme E1, ubiquitin couplingase E2 and ubiquitin protein ligase E3 (Tekcham et al., 2020). FBXW7 (also known as Ago, hCdc4, Fbw7, and Sel10) is involved in the formation of the SCF-type E3 enzyme complex and performs specific substrate recognition (Yeh et al., 2018). FBXW7 has been identified as a cancer suppressor gene that frequently malfunctions in a variety of human cancers (Yumimoto and Nakayama, 2020). According to the COSMIC database, the overall mutation rate of FBXW7 in human tumors is 7.79%, with the highest prevalence observed in gynecologic tumors, hematologic malignancies, and gastrointestinal cancers (Fan et al., 2022). Extensive research has demonstrated that abnormal FBXW7 expression contributes to tumor initiation, progression, treatment resistance, and poor prognosis in cancer patients (Qi et al., 2024). While the molecular mechanisms and clinical significance of FBXW7 in gynecologic cancers, breast cancer, and hematologic malignancies have been thoroughly reviewed, a comprehensive analysis of its role in gastrointestinal cancers remains lacking (Di Fiore et al., 2023; Chen et al., 2023; N et al., 2015; Q et al., 2020). Therefore, further investigation into the role of FBXW7 in regulating various signaling pathways and key molecules in gastrointestinal cancers is warranted. It provides a reference for exploring biomarkers and molecular targets for the treatment of gastrointestinal cancers.
2 Structure and function of the FBXW7
The degradation of proteins in eukaryotes requires the coordinated activity of E1, E2, and E3 enzymes (Liu et al., 2017; Hershko and Ciechanover, 1998). First, the E1 enzyme activates and binds to the ubiquitin molecule (Ub) by consuming ATP (Bakos et al., 2018). Subsequently, the Ub bound to the E1 enzyme is transferred to the E2 enzyme (G and T, 2014). The SCF-type E3 enzyme transfers the Ub bound to the E2 enzyme to the specific substrate protein recruited (Pickart, 2001). Finally, the 26s proteasome degrades the target protein-Ub complex and releases Ub for recycling (Xu et al., 2015). F-box proteins are the recognition subunits of the SCF E3 ubiquitin ligase complex, and about 70 F-box proteins have been identified in humans (Ge et al., 2020). Based on their structure, F-box proteins are classified into three families: FBXW (WD40 repeat-containing domains), FBXL (leucine-rich repeats), and FBXO (other structural domains) (Ge et al., 2020; Skaar et al., 2013). FBXW7, due to its involvement in the destruction of many oncogenic proteins, is the most extensively studied F-box protein (Welcker and Clurman, 2008; Zhang et al., 2019). The structure and function of FBXW7 within the UPS are illustrated in Figure 2.
FIGURE 2.
Structure and function of FBXW7. The FBXW7 gene is located on 4q31.3 and encodes three FBXW7 isoforms: FBXW7α, FBXW7β, and FBXW7γ. Despite differences at their N-terminal regions, all three isoforms share dimerization domains, F-box domains, and WD40 repeat domains. FBXW7 primarily targets substrates such as TGF-β, c-Jun, Cyclin E, c-Myc, Notch1, MCL-1, YAP, and mTOR for degradation. Additionally, the expression of FBXW7 is regulated by p53, non-coding RNAs, LSD1, ERK1/2, USP28, HES5, and C/EBPδ.
The FBXW7 gene consists of 13 exons and 4 introns and is located on chromosome 4q31q.3 (Fan et al., 2022; Sailo et al., 2019). As shown in Figure 2, the proteins encoded by the FBXW7 gene contain three domains: F-box, dimerization (D-D), and WD40 (Roling et al., 2022). The F-box domain functions as a linker to the SCF E3 ligase complex by binding to the adaptor protein SKP1 (Welcker and Clurman, 2008; Thirimanne et al., 2022). The WD40 repeat domains fold into β-propeller-like structures, forming binding pockets that specifically recognize and bind phosphorylated substrates (Zhang et al., 2019; Schapira et al., 2017). The dimerization domain contributes to the specificity and stability of FBXW7 and substrate interactions (Welcker et al., 2013). The FBXW7 gene encodes three different mRNAs, which are translated into three isoforms: FBXW7α, FBXW7βand FBXW7γ, differing at the N-terminus (Kitade et al., 2016). These isoforms exhibit distinct subcellular localizations and functional roles.
FBXW7α is distributed in the nucleus and and mediates the degradation of the majority of FBXW7 substrates (Kar et al., 2021). FBXW7β isoforms and FBXW7γ are distributed in the endoplasmic reticulum and nucleolus, respectively (Lee et al., 2020). FBXW7β isoforms are involved in cellular lipid metabolism whereas the function of FBXW7γ is unclear (Wei et al., 2023). The range of substrate proteins degraded by FBXW7 is broad, including Notch1, c-Jun, c-Myc, mTOR, MCL-1, cyclin E, TGF-β, and YAP (Tekcham et al., 2020; Welcker and Clurman, 2008; Sailo et al., 2019; Mao et al., 2008; Bengoechea-Alonso and Ericsson, 2010; Tu et al., 2014). In addition to genetic alterations, the function of FBXW7 is regulated by the P53 , transcriptional (HES5 and C/EBPδ), translational (non-coding RNA), and post-translational regulators (LSD1, ERK1/2, USP28) (Chen et al., 2023). Given the carcinogenic effects of various regulatory proteins and molecules, FBXW7 has a broad and profound role in the occurrence and progression of human cancer (Fan et al., 2022).
3 Upstream regulatory mechanism and downstream key substrates of FBXW7
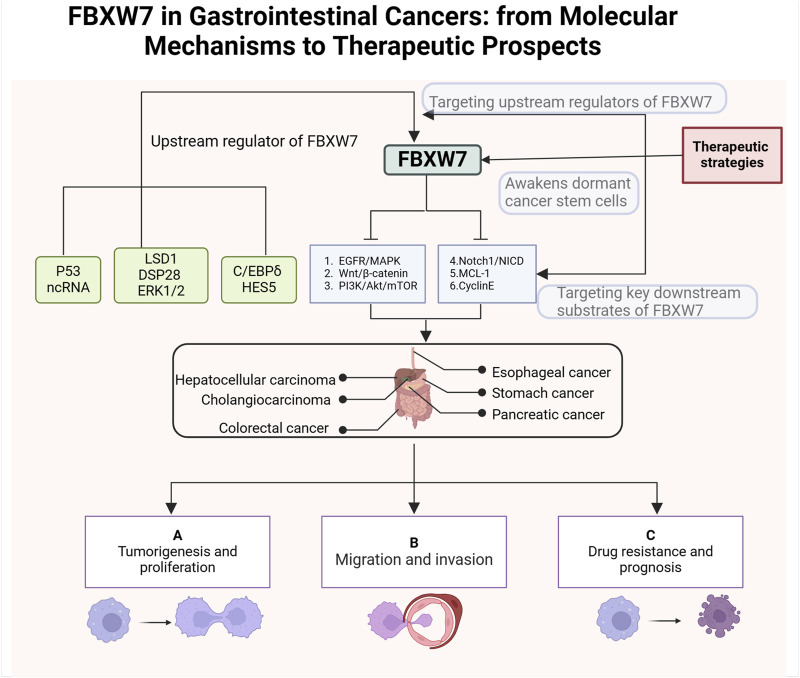
FBXW7 plays a pivotal role in various processes, including cancer cell proliferation, metastasis, invasion, apoptosis, and treatment resistance (Davis et al., 2014). Understanding the functional regulatory network of FBXW7 in greater depth is essential to elucidate its tumor-suppressive effects and to identify potential avenues for developing new cancer treatment strategies. The subsequent sections discuss the upstream regulatory mechanisms of FBXW7 and its downstream carcinogenic substrates.
3.1 Upstream regulation mechanism of FBXW7
3.1.1 Transcriptional regulation of FBXW7
The expression of the FBXW7 gene is regulated by various transcription factors, including TP53, C/EBPδ, PHF1, and Hes5. Mao et al. discovered that the exons of FBXW7 contain p53 binding sites and that heterozygous mutations in TP53 in mouse tumors are frequently accompanied by FBXW7 mutations (Mao et al., 2004). Subsequent studies have confirmed that FBXW7 gene expression depends on the TP53 gene status during tumor development (Perez-Losada et al., 2005; Grim et al., 2012). C/EBPδ directly binds to the FBXW7α promoter to inhibit FBXW7 expression, leading to reduced degradation of mTOR (Balamurugan et al., 2010). Elevated mTOR levels activate HIF-1 protein activity, thereby promoting metastatic tumorigenesis. Similarly, PHF1 regulates FBXW7 transcription by negatively affecting the expression of both the FBXW7 protein and E-cadherin, which promotes tumor proliferation and invasion (Liu et al., 2018a). HES5, a downstream effector of Notch signaling, directly suppresses the transcription of FBW7β, influencing the differentiation patterns of intestinal cells in mice (Sancho et al., 2013). Since Notch is negatively regulated by FBXW7, a significant feedback loop exists between HES5, FBXW7, and Notch. Additionally, the inactivation of the TGF-β signaling pathway is strongly associated with HES5-mediated inhibition of FBXW7 transcription (Chen et al., 2021).
3.1.2 Non-coding RNA regulation of FBXW7
Non-coding RNAs (ncRNAs), which are not translated into proteins, play critical roles in various physiological and pathological processes, primarily by regulating messenger RNA (mRNA) at the post-transcriptional level (Anastasiadou et al., 2018). Several types of ncRNAs, including microRNAs (MiRNAs), Circular RNAs (CircRNAs), and Long non-coding RNAs (LncRNAs), have been implicated in the post-transcriptional regulation of FBXW7 (Lin et al., 2019). In cancer, miRNAs can suppress FBXW7 expression, thereby disrupting its tumor-suppressive functions (Lin et al., 2019; Ling et al., 2016). For instance, MiR-27a-3p and MiR-92a-3p downregulate FBXW7 mRNA expression, promoting tumor cell proliferation and invasion (Wang et al., 2021a; Ben et al., 2020). Similarly, MiRNAs such as MiR-182, MiR-25, and MiR-32 inhibit FBXW7 expression in various cancers, facilitating tumor growth and migration (Chang et al., 2018; Xiang et al., 2015; Xia et al., 2017). LncRNAs often function as MiRNA sponges to regulate FBXW7 expression. For example, LncRNA-MIF acts as a competing endogenous RNA (ceRNA) for miR-586, counteracting miR-586-mediated inhibition of FBXW7, which in turn suppresses c-Myc activity and tumorigenesis (Zhang et al., 2016a). Additionally, circular RNA circPSD3 sponges MiR-25-3p, thereby restoring FBXW7 expression and inhibiting epithelial-mesenchymal transition (EMT) and tumor metastasis (Xie et al., 2022).
3.1.3 Post-translational modifications of FBXW7
3.1.3.1 Phosphorylation
The function and expression of FBXW7 are regulated by phosphorylation through several post-translational kinases, including ERK1/2,PI3K, Polo-like kinase-1 and -2 (PLK1/2), and cyclin-dependent kinase 5 (CDK5) (L et al., 2022; D et al., 2016; Cizmecioglu et al., 2012; Ko et al., 2019). ERK1/2 activation induces the phosphorylation of FBXW7 at Thr205, reducing its stability and leading to the accumulation of heat shock factor 1 (HSF1) (Gi et al., 2020). Similarly, PLK family members phosphorylate FBXW7, promoting its ubiquitin-mediated degradation and enhancing the oncogenic potential of Myc (D et al., 2016). The FBXW7α isoform is also phosphorylated in a PI3K-dependent manner at the S227 site (Schülein et al., 2011). Additionally, CDK5 phosphorylates FBXW7 at Ser349 and Ser372, resulting in its ubiquitination and degradation [22]. Phosphorylation by dual-specificity tyrosine-phosphorylation-regulated kinase 2 (DYRK2) has been reported to promote FBXW7 degradation via the proteasome, thereby impairing the turnover of oncogenic proteins (Jiménez-Izquierdo et al., 2023).
3.1.3.2 Auto-ubiquitination and deubiquitination
At the post-translational level, autoubiquitination and deubiquitinating enzymes (DUBs) collaboratively regulate the levels and functions of the FBXW7 protein (Xu et al., 2016). Sang’s colleagues demonstrated that Pin1 promotes FBXW7 autoubiquitination and degradation by disrupting its dimerization, thereby impairing its function (Min et al., 2012). Concurrently, Pin1 negatively regulates FBXW7-mediated substrate degradation, contributing to tumor development (Min et al., 2012). Additionally, tripartite motif-containing 25 (TRIM25) mediates the ubiquitination and degradation of FBXW7α, leading to increased stability and accumulation of Myc (Zhang et al., 2020). The HECT domain-based E3 ligase thyroid hormone receptor interactor 12 (TRIP12) promotes autoubiquitination and proteasomal degradation of FBXW7 by mediating K11-linked ubiquitination of lysine residues K404 and K412 (Khan et al., 2021a). Beyond genetic alterations, FBXW7 function is modulated by deubiquitinases. USP9X inhibits FBXW7 autoubiquitination, thereby reducing the activity of its downstream substrate c-Myc and suppressing tumor formation in mice (Khan et al., 2018). Similarly, USP28 prevents FBXW7 self-degradation in chronic lymphocytic leukemia (CLL), leading to elevated levels of Notch1 (Close et al., 2018).
3.1.3.3 Dimerization
FBXW7 forms dimers through its conserved D-domain to maintain stability and functional activity. This structural mechanism enhances the specificity and stability of FBXW7 binding to specific substrates (Welcker et al., 2013; Welcker and Clurman, 2007). Impaired dimerization of FBXW7 not only increases its autoubiquitination but also disrupts substrate ubiquitination and degradation. For instance, as previously discussed, Pin1 promotes FBXW7 autoubiquitination and degradation by interfering with its dimerization (Min et al., 2012). Similarly, LSD1 directly disrupts FBXW7 dimerization, leading to reduced stability and proteasomal degradation (Lan et al., 2019). These findings underscore the critical role of dimerization in regulating FBXW7 stability and its anti-tumor functions.
3.2 Downstream carcinogenic substrates of FBXW7
Cyclin E plays a critical role in the transition from the G1 phase to the S phase and regulates tumorigenesis, cell proliferation, and resistance to anticancer therapies (Chu et al., 2021; T et al., 2004). The normal progression of the cell cycle relies on the proper expression of cyclins and cyclin-dependent kinases (CDKs) (Mammalian cell cycle cyclins, 2024; Targeting cell, 2024). FBXW7 deficiency has been shown to enhance Cyclin E1/CDK activation, contributing to genomic instability and tumorigenesis (Minella et al., 2007; Grim et al., 2008; Takada et al., 2017). Cyclin E is a key downstream substrate of the miR-223/FBXW7 and miR-92a/FBXW7 regulatory pathways (Xu et al., 2010; M et al., 2011). Low levels of miR-223 or miR-27a result in FBXW7 upregulation, thereby inhibiting the ubiquitination and degradation of cyclin E (Xu et al., 2010; M et al., 2011). Consequently, FBXW7 plays a vital role in maintaining Cyclin E levels, and its dysfunction is sufficient to cause Cyclin E dysregulation (Ekholm-Reed et al., 2004).
Notch1, a member of the Notch family, plays a critical role in the initiation and progression of human malignancies (Notch1 in Cancer Therapy, 2024). The Notch signaling pathway is primarily involved in regulating embryonic development, normal cell growth, apoptosis, and differentiation (Notch signaling pathway in cancer, 2024). The intracellular domain of Notch1 (NICD) is a key target for FBXW7-mediated degradation (Kar et al., 2021). In mouse embryonic fibroblasts (MEFs), selective knockout of FBXW7 leads to aberrant activation of the Notch1/NICD signaling pathway, disrupting normal cell cycle regulation (Y et al., 2008). In various hematopoietic and solid tumors, impaired FBXW7-mediated degradation of Notch1 is associated with reduced drug response and poor patient prognosis (Close et al., 2019; Mori et al., 2018).
MCL-1, a member of the anti-apoptotic Bcl-2 protein family, is critically involved in tumor cell proliferation, apoptosis, and drug resistance (Wang et al., 2021b). The function and expression of MCL-1 are tightly regulated by various factors, including VEGF, IL-6, FBXW7, MULE, and MiRNAs (Mojsa et al., 2014; L et al., 2009; Shenoy et al., 2014; CXCR4, 2024; Inuzuka et al., 2011; Pervin et al., 2011). Specific alleles in the CPD domain of MCL-1—Ser159, Thr163, and Ser121—are phosphorylated by glycogen synthase kinase 3β (GSK3β) (Inuzuka et al., 2011; Morel et al., 2009; Senichkin et al., 2020). Subsequently, FBXW7 interacts with phosphorylated MCL-1, facilitating its degradation via the 26S proteasome (Yeh et al., 2018; Tang et al., 2023). Thus, FBXW7 influences multiple malignant processes in cancer, particularly drug resistance, by regulating MCL-1 activity or expression levels (Wu et al., 2024). In oral squamous cell carcinoma, FBXW7 has been reported to negatively regulate MCL-1 expression and autophagy (Sun et al., 2023). Furthermore, reduced FBXW7 expression decreases cisplatin sensitivity in oral squamous cell carcinoma (Yang et al., 2022). These findings suggest that targeting MCL-1 could enhance therapeutic sensitivity in patients with FBXW7-mutated gastrointestinal cancers (Wood, 2020; Mittal et al., 2021).
mTOR is a serine/threonine kinase belonging to the phosphoinositide 3-kinase-related kinase (PIKK) family, playing a pivotal role in tumor growth, metastasis, and drug resistance (Marques-Ramos and Cervantes, 2023; As, 2019). FBXW7 has been reported to recognize and degrade mTOR (Mao et al., 2008). Experiments have shown that the expression level of mTOR depends on the rhythmic change of FBXW7, which means that FBXW7 is a negative regulator of mTOR (Okazaki et al., 2014). This regulatory relationship has been well-demonstrated in breast cancer (Mao et al., 2008). Notably, FBXW7 knockout in tumor cells leads to increased epithelial-mesenchymal transition (EMT), enhanced stem cell properties, and greater migratory potential (Wang et al., 2013). Interestingly, mTOR inhibitors have been shown to suppress EMT and cancer stem cell traits induced by FBXW7 mutations (Wang et al., 2013). Furthermore, in murine tumor models undergoing radiotherapy, mTOR inhibitors delay tumor progression associated with FBXW7 mutations or deletions (Liu et al., 2013).
β-Catenin is a key effector of the canonical Wnt signaling pathway, which regulates cell proliferation, embryonic development, and homeostasis under physiological conditions (Liu et al., 2022; Song et al., 2024; Pai et al., 2017). Dysregulation of the Wnt/β-catenin pathway promotes malignant tumor invasion (Song et al., 2024; Zhang and Wang, 2020). Extensive research has demonstrated that this pathway is frequently overactivated in various cancers, particularly in gastrointestinal malignancies (Is et al., 2022; Bd et al., 2012; X C. et al., 2019a; Wu et al., 2016). FBXW7 plays a critical role in regulating the Wnt/β-catenin pathway by mediating the degradation of β-catenin and associated transcription factors (Yu et al., 2021). Additionally, chromatin domain-helicase-DNA-binding protein 4 (CHD4) promotes Wnt/β-catenin pathway activation, which FBXW7 counteracts by disrupting CHD4 (G et al., 2024). Overexpression of FBXW7 directly ubiquitinates and degrades β-catenin, thereby preventing pathway overactivation and inhibiting tumor growth and invasion (Jiang et al., 2016). Consequently, targeting the Wnt/β-catenin pathway is a promising strategy for treating FBXW7-mutated tumors. However, recent studies reveal resistance to Wnt/β-catenin inhibition therapy in FBXW7-mutant tumors, potentially due to increased activity of alternative FBXW7 substrates, such as Myc (Zhong and Virshup, 2024). This suggests that cancer proliferation and progression driven by FBXW7 mutations are not solely dependent on Wnt/β-catenin signaling and that targeting a single FBXW7 substrate may have limited therapeutic efficacy.
c-Myc is a proto-oncogene encoding a protein implicated in the development of numerous human cancers (Dhanasekaran et al., 2021). Aberrant activation of c-Myc drives hallmark features of cancer, including cell proliferation, growth, differentiation, metabolism, angiogenesis, and tumor microenvironment remodeling, through various genetic mechanisms (M et al., 2014). FBXW7 suppresses the oncogenic effects of c-Myc by ubiquitinating and degrading phosphorylated c-Myc at Thr58 and Ser62 (Yeh et al., 2018; M Y. et al., 2004). At the single-cell level, loss of FBXW7 expression results in the accumulation of several substrates, most notably c-Myc and cyclins (Meyer et al., 2020). This loss leads to the activation of c-Myc, contributing to the progression of various malignancies, particularly hematologic cancers (B et al., 2024; B et al., 2013). Findings by Markus et al. indicate that FBXW7 deletion disrupts control over c-Myc, resulting in rapid tumor cell proliferation (M W. et al., 2004).
4 FBXW7 is involved in the occurrence and development of various gastrointestinal cancers
FBXW7 mediates the degradation of numerous proteins, and alterations in its expression are strongly associated with cell proliferation, invasion, metastasis, and drug resistance in gastrointestinal cancers. Understanding the molecular mechanisms and clinical significance of FBXW7 in these cancers can aid in the development of targeted therapies and biomarkers. The following sections discuss the role of FBXW7 in the onset and progression of gastrointestinal cancers by tumor type, and summarize the associated molecular mechanisms and signaling pathways, as illustrated in Figure 3.
FIGURE 3.
Interaction of FBXW7 with signaling pathways and key molecules in gastrointestinal cancers. (1) FBXW7 directly targets EGFR and SHOC2, blocking the activation of the MAPK signaling pathway. ERK1/2 promotes FBXW7 phosphorylation, further activating the MAPK pathway. (2) FBXW7 directly targets β-catenin, inhibiting the activation of the Wnt/β-catenin signaling pathway. (3) FBXW7 directly targets Nox1, PTEN, and mTOR, inhibiting the activation of the Akt/mTOR signaling pathway. (4) FBXW7 degrades MCL-1, thereby promoting apoptosis. (5) FBXW7 targets Notch1/NICD, inhibiting MAPK signaling. The degradation of Notch1/NICD inhibits cell proliferation. (6) FBXW7 mediates Cyclin E degradation, maintaining a normal cell cycle. (7) MicroRNA-770, MiR-92a-3p, MiR-27a, and MiR-223 inhibit FBXW7 expression.
4.1 Colorectal cancer (CRC)
CRC is a highly heterogeneous disease, with different molecular alterations and genetic subtypes closely related to the prognosis of CRC (Schell et al., 2016; Linnekamp et al., 2018). Currently, the treatment and prognostic classification of CRC primarily depend on TNM staging, as well as molecular markers such as MMR/MSI, RAS, RAF, and PI3K. Mutations or deletions of FBXW7 are often found in patients with CRC, especially in younger patients (<45 years) (Kothari et al., 2016). A recent meta-analysis of 58 studies found that the overall mutation rate of FBXW7 in 13,974 CRC patients was 10.3% (Afolabi et al., 2024). Thus, investigating the possible role of the FBXW7 gene in CRC is essential. The role of FBXW7 in CRC is shown in Table 1.
TABLE 1.
The role of FBXW7 in colorectal cancer.
| Study | Source | FBXW7 status | Specific mechanisms | Significance |
|---|---|---|---|---|
| Babaei-Jadidi et al. (2011) | Mice | Deleted | FBXW7-Notch/c-Jun/β-catenin | Reduced life span and elevated risk of intestinal cancers in mice |
| Li et al. (2014) | HCT116 | Downregulated | MiR-182/MiR-503—FBXW7 | Contribute to the malignant progression of colon adenoma to adenocarcinoma |
| Wei et al. (2023) | CRC tissues | Downregulated | CSN6—FBXW7β | Reprogramming adipogenesis in CRC to increase tumour growth |
| Ou et al. (2016) | CRC tissues | Downregulated | Plk2—FBXW7—Cyclin E | Promotes tumor development by inhibiting CRC cell death |
| He et al. (2019) | CRC tissues | Downregulated | STYX -FBXW7 | Stimulates CRC cell migration, invasion, proliferation, and EMT while preventing apoptosis |
| Liu et al. (2021b) | HCT11 | Downregulated | MiR-223-FBXW7-Akt/mTOR/Notch1 | CRC proliferation increased, whereas apoptosis reduced |
| Gong et al. (2018) | HT29 HCT116 |
Downregulated | MiR-92b-3p -FBXW7 | Increased migration, invasion, and proliferation of CRC |
| Mu et al. (2017) | CRC cell lines CRC tissues |
Downregulated | FAM83D-FBXW7 -Notch1 | Encourages CRC cell growth, migration and invasion |
| Khan et al. (2018) | Mice model | Upregulated | Usp9x -FBXW7 -c-Myc | Inhibits tumor formation |
| Lin et al. (2020a) | Colon cancer cells | Downregulated | KDM5c- FBXW7 - c-Jun | Increase proliferation of colon cancer cells |
| Li et al. (2018) | CRC tissues | Low expression | FBXW7—HIF1α/CEACAM5 | Worse clinicopathologic features and poor patient outcome |
| Zhan et al. (2015) | HCT116 | Depletion | FBXW7—ENO1 | Lactate production, cell proliferation and migration |
| Kawashita et al. (2017) | CRC tissues | Low expression | MiR-223-FBXW7 | Worse disease-free survival and more susceptibility to recurrence |
| Honma et al. (2019) | CRC tissues CRC cell lines |
Downregulated | FBXW7 silence awakens CSCs | Enhanced susceptibility to anti-cancer medications both in vivo and in vitro |
| Izumi et al. (2017) | Colorectal CSCs | Upregulated | Elevated FBXW7 leads to cell cycle arrest of CSCs after chemotherapy | After chemotherapy, inhibiting FBXW7 overexpression in CSCs may improve their response to anticancer drugs |
| Huang and Long (2023); Wang et al. (2021c) | CRC cell lines HCT-116 |
Overexpression | FBXW7- Nox1- mTOR | CRC cells are sensitive to cisplatin and taxol |
| Li et al. (2019) | Mouse models | Depletion | FBXW7-ZEB2-EMT/CSCs | Causes chemoresistance and EMT. |
| J et al. (2017); Tong et al. (2017); Song et al. (2020); Lin et al. (2020b) | CRC cell lines | Inactivating mutations | FBXW7-MCL-1 | Less sensitive to regorafenib, sorafenib, Trametinib and Hsp90 inhibitors |
| Boretto et al. (2024); Nemecek et al. (2016) | CRC tissues Colon organoids |
Mutations | FBXW7-EGFR | Reduced effectiveness of anti-EGFR treatment |
| Liu et al. (2018b); Korphaisarn et al. (2017) | CRC tissues Clinical databases |
Downregulated Missense mutations |
Unclear | An independent unfavorable prognostic factor of CRC |
| Iwatsuki et al. (2010) | CRC tissues CRC cell lines |
Low expression | FBXW7-c-Myc/CyclinE | The prognosis was worse |
| Shang et al. (2021) | Database | Mutation or Low expression | N/A | Poorer OS of the CRC |
| Kawaguchi et al. (2021) | Genetic sequencing data | Low expression | N/A | Worse survival after CLM resection |
| Chang et al. (2015) | MassArray system | Mutation | N/A | FBXW7 mutations and patient prognosis did not significantly correlate |
| Y et al. (2023) | CRC tissues | Mutation | N/A | FBXW7 R465C hotspot mutation-afflicted CRC patients had worse OS |
4.1.1 FBXW7 is involved in regulating the growth and proliferation of CRC
FBXW7 influences the growth and proliferation of CRC by regulating the activity of various oncogenic substrates, including Notch, Akt/mTOR, Jun, and DEK (Babaei-Jadidi et al., 2011; Lu et al., 2019; Liu et al., 2021b). Mice with FBXW7 deficiency exhibit an increased likelihood of developing intestinal tumors (Babaei-Jadidi et al., 2011). For instance, the synergistic downregulation of FBXW7 expression by MiR-182 and MiR-503 has been shown to drive the progression of colon adenoma into adenocarcinoma (Li et al., 2014). Ou et al. reported that elevated expression of Polo-like kinase 2 (Plk2) in CRC samples stabilizes Cyclin E by suppressing FBXW7 expression, thereby promoting CRC growth (Ou et al., 2016). Similarly, the Rictor/FBXW7-mediated accumulation of c-Myc and Cyclin E plays a crucial role in CRC cell proliferation (Guo et al., 2012). Additionally, factors such as Serine/threonine/tyrosine interacting protein (STYX), MiR-223, MiR-92b-3p, FAM83D, USP9X, and KDM5c indirectly regulate various oncoproteins by modulating FBXW7 expression, contributing to CRC cell proliferation and apoptosis (Khan et al., 2018; Liu Z. et al., 2021; Gong et al., 2018; He et al., 2019; Mu et al., 2017; Lin H. et al., 2020). Fat metabolism, regulated by FBXW7, is also linked to tumor growth in CRC. The binding of COP9 signalosome subunit 6 (CSN6) to FBXW7β inhibits the degradation of fatty acid synthase (FASN), promoting lipogenic CRC and tumor progression (Wei et al., 2023).
4.1.2 FBXW7 is involved in regulating the invasion and metastasis of CRC
FBXW7 plays a significant role in the invasion and metastasis of CRC. It has been reported to suppress the migration of CRC cells by regulating the HIF1α/CEACAM5 axis (Li et al., 2018). Furthermore, the FBXW7 gene negatively regulates Enolase 1 (ENO1), thereby influencing the growth and metastasis of the CRC cell line HCT116 (163). SHOC2, a conserved protein that binds to RAS and RAF at its N-terminus, facilitates downstream signaling (Xie and Sun, 2019). FBXW7-mediated ubiquitination and degradation of SHOC2 block MAPK pathway activation, thereby inhibiting cancer growth signaling (Xie and Sun, 2019). In FBXW7 knockout CRC cells, increased epithelial-mesenchymal transition (EMT), enhanced stem cell properties, and elevated migration are observed (Wang et al., 2013). The use of mTOR inhibitors can mitigate EMT characteristics and cancer stem cell properties induced by FBXW7 mutations (Wang et al., 2013). Additionally, restoring FBXW7 expression partially suppresses CRC progression driven by the activation of the Wnt/β-catenin signaling pathway (Hu et al., 2019).
4.1.3 FBXW7 affects the therapeutic sensitivity of CRC
Loss of FBXW7 function reduces the sensitivity of CRC cells to anticancer drugs. In CRC cell models, FBXW7 downregulation increases the activity of NADPH oxidase 1 (Nox1) and mTOR, leading to resistance to paclitaxel and cisplatin (Huang and Long, 2023; Wang HP. et al., 2021). Additionally, FBXW7 deletion upregulates ZEB2 protein levels, inducing chemoresistance to 5-fluorouracil and oxaliplatin (Li et al., 2019). Conversely, high FBXW7 expression downregulates Cryptochrome 2 (CRY2), reducing resistance to oxaliplatin treatment (Fang et al., 2015). FBXW7 also plays a critical role in the effectiveness of molecularly targeted therapies for CRC. Sequencing data from CRC patients reveal that mutations in FBXW7 and SMAD4 are common in those resistant to cetuximab or panitumumab (Lupini et al., 2015). Recently, FBXW7 was shown to mediate the ubiquitination and degradation of EGFR, influencing the efficacy of EGFR inhibitors in human CRC-derived organoid lines (Boretto et al., 2024; Nemecek et al., 2016). Moreover, CRC cells harboring FBXW7 mutations exhibit resistance to regorafenib and sorafenib due to MCL-1 accumulation (Tong et al., 2017; Song et al., 2020). However, inhibiting MCL-1 restores the sensitivity of these cells to regorafenib (Tong et al., 2017; Song et al., 2020). Similar mechanisms underlie resistance to trametinib and Hsp90 inhibitors in FBXW7-mutant CRC (Lin L. et al., 2020; J et al., 2017). Interestingly, FBXW7 downregulation has been reported to enhance the therapeutic response of CRC stem cells to anticancer drugs (Honma et al., 2019). CRC stem cells may develop chemoresistance to CPT-11 by upregulating FBXW7, which degrades c-Myc and arrests the cell cycle (Izumi et al., 2017). Thus, further research on FBXW7 could aid in identifying novel predictive markers and treatment strategies for CRC.
4.1.4 FBXW7 affects the prognostic outcome of CRC
The functional status of FBXW7 may be linked to poorer prognosis in CRC patients (Liu H. et al., 2018; Korphaisarn et al., 2017). Specifically, reduced FBXW7 mRNA expression in tumor tissues is inversely associated with CRC prognosis (Iwatsuki et al., 2010). A meta-analysis further confirmed that FBXW7 mutations or low expression levels correlate with advanced T stage, shorter overall survival (OS), and lymph node metastases in CRC patients (Shang et al., 2021). Additionally, CRC patients with liver metastases who have FBXW7 mutations exhibit significantly lower 5-year OS rates compared to those with FBXW7 wild-type tumors (Kawaguchi et al., 2021; Kawashita et al., 2017). However, some studies indicate that the relationship between FBXW7 and patient prognosis may not be strong (Chang et al., 2015). For instance, Liu et al. found that while the R465C hotspot mutation was associated with worse OS, other FBXW7 mutations did not significantly affect survival outcomes (Y et al., 2023). Although FBXW7 appears to influence CRC prognosis, further research is required to establish more conclusive evidence. Nonetheless, FBXW7 shows significant potential as a biomarker for CRC treatment and prognosis.
4.2 Gastric cancer (GC)
According to recent statistics, gastric cancer (GC) ranks fifth in cancer-related mortality (Bray et al., 2024). Molecular biomarker-based classification, including markers such as PD-L1, MSI, and HER-2, has enabled some GC patients to benefit from immunotherapy or targeted therapies (Guan et al., 2023). However, there are few treatment options for patients with GC and the prognosis is still relatively poor (Sun et al., 2024). Thus, identifying more effective molecular markers is critical for improving the management of GC. Studies have reported mutations rate of FBXW7 in GC ranging from 9.2% to 18.5% (Li et al., 2016). More and more researchers have paid attention to the role of FBXW7 in GC (Table 2).
TABLE 2.
The role of FBXW7 in gastric cancer.
| Study | Source | FBXW7 status | Specific mechanisms | Significance |
|---|---|---|---|---|
| Jiang et al. (2017) | Gene Knockout mouse | Haploinsufficiency | FBXW7-c-Myc/DNA damage | Increased the risk of gastric carcinogenesis |
| Li et al. (2012); Gong et al. (2015) | GC cell lines GC tissues |
Downregulated | MiR-223/MiR-25-FBXW7 | FBXW7 regulate cellular apoptosis, proliferation, and invasion in GC |
| Y et al. (2021); Li et al. (2020) | Xenograft tumor model GC cell lines |
Upregulated | FBXW-c-Myc FBXW-MCL-1 |
Dem and Lycorine hydrochloride inhibits the proliferation, apoptosis, and invasion of GC cells |
| X et al. (2019b) | GC tissue | N/A | FBXW7/GSK3β-GFI1-GKN2 | Gastric cancer cell proliferation and tumorigenesis |
| Li et al. (2016) | GC tissue | Downregulated | FBXW7-Snail 1/ZEB 1-EMT FBXW7- RhoA |
FBXW7 induces apoptosis and growth arrest and inhibits the EMT in GC |
| Hou et al. (2022) | GC cell lines | Downregulated | ZC3H15- FBXW-c-Myc | Promote GC cell proliferation, migration and invasion |
| Huang et al. (2018) | GC tissue | N/A | FBXW7- Brg1 | Brg1 degradation by FBXW7 inhibits GC metastasis |
| Huang et al. (2022) | GC tissue | Downregulated | BDNF-AS-WDR5-FBXW7-VDAC3- Ferroptosis | Promotes progression of GC and peritoneal metastases |
| Zhou et al. (2015); Eto et al. (2015) | GC cell lines | Downregulated | MiR-223-FBXW7 | FBXW7 is involved in cisplatin and trastuzumab resistance in GC cells |
| Yokobori et al. (2009) | GC tissue | Downregulated | P53-FBXW7 | Disruption of both p53 and FBXW7 leads to poor GC prognosis |
| Li et al. (2017a) | GC tissue | Low expression | Unclear | Low FBXW7 expression is associated with poor differentiation and prognosis in GC |
4.2.1 FBXW7 is involved in regulating the occurrence and development of GC
Studies have shown that FBXW7-deficient mice exposed to the chemical carcinogen N-methyl-N-nitrosourea (MNU) are more prone to genetic damage and c-Myc accumulation, increasing the risk of gastric cancer (GC) (Jiang et al., 2017). In addition, MiR-223 and MiR-25 can indirectly regulate GC cell proliferation, apoptosis, and invasiveness by controlling the post-transcriptional expression of FBXW7 (191,192). Certain compounds, such as Demethylzeylasteral (a naturally occurring monomer from Tripterygium wilfordii) and Lycorine hydrochloride (LH), can restore FBXW7 expression, thereby reducing the growth and invasiveness of GC cells (Y et al., 2021; Li et al., 2020). Growth factor independent 1 (GFI1) encourages the growth of GC cells and disease progression by inhibiting the transcription of gastric factor-2 (GKN2) (X K. et al., 2019). In GC patients with elevated GFI1 protein levels, FBXW7 function were reduced, leading to rapid GC cell proliferation and disease progression (X K. et al., 2019). Li et al. demonstrated that low FBXW7 expression activates the RhoA pathway in GC, promoting EMT and disease progression (Li et al., 2016). ZC3H15 inhibits FBXW7 expression at the transcriptional level, hindering c-Myc degradation and thereby contributing to GC development and progression (Hou et al., 2022). Higher Brg1 (also known as SMARCA4) expression has been linked to distant and lymph node metastasis in GC patients (Sentani et al., 2001). Huang et al. analyzed human GC tissue samples and found that increased Brg1, due to low FBXW7 expression, is a key mechanism driving GC metastasis (Huang et al., 2018). Moreover, FBXW7 is negatively regulated by lncRNA BDNF-AS and is involved in ferroptosis and peritoneal metastasis of GC in animal models (Huang et al., 2022).
4.2.2 FBXW7 affects the treatment and prognosis of GC
The functional status of FBXW7 also profoundly affects the treatment resistance and prognosis of GC patients. It has been reported that MiR-223 modulates the sensitivity of GC to chemotherapy and molecularly targeted therapy (Zhou et al., 2015; Eto et al., 2015). Mechanistic studies have shown that MiR-223 induces resistance to cisplatin and trastuzumab in GC cells by downregulating FBXW7 expression (Zhou et al., 2015; Eto et al., 2015). The restoration of FBXW7 function by consuming MiR-223 inhibits the resistance of GC cells to cisplatin trastuzumab (Zhou et al., 2015; Eto et al., 2015). Furthermore, in patients with GC, dysregulated FBXW7 is related to more severe clinical characteristics and a worse prognosis (Yokobori et al., 2009). For Chinese patients, the results also suggest that GC patients with low expression of FBXW7 have poor tumor differentiation and worse prognosis (Li MR. et al., 2017). In summary, the function of FBXW7 acts on the whole process of the occurrence and development of GC, which offers an innovative approach for the static treatment of patients.
4.3 Esophageal squamous cell carcinoma (ESCC)
In ESCC, low FBXW7 expression is related to high aggressiveness, while FBXW7 overexpression significantly inhibits tumor growth and invasion (Gong et al., 2016; Bi et al., 2023). FBXW7 deficiency in ESCC can overactivate the ANXA2-ERK pathway, worsening the tumor’s biological behavior (Li Z. et al., 2023). In addition, FBXW7-MAP4-ERK axis is implicated in ESCC(207). MAP4 overexpression and ERK activation caused by FBXW7 inactivation can promote the growth, invasion and migration of ESCC cells (Pan et al., 2023). Upregulation of MiR-27a-3p in ESCC has been shown to reduce FBXW7 expression, leading to accelerated tumor proliferation and disease progression (Wu et al., 2015). Moreover, the functional status of FBXW7 can also affect the prognosis of ESCC patients (Yu et al., 2015; Y et al., 2010). In ESCC, both FBXW7 mutations and copy number loss affect the prognosis of patients (Yokobori et al., 2012). When the expression of FBXW7 in ESCC is inhibited by MiR-223, the prognosis of patients becomes worse (Kurashige et al., 2012). A retrospective study found that high FBXW7 expression was correlated with a favorable response to chemoradiotherapy (CRT) in advanced ESCC(213). Therefore, the status of FBXW7 is also an important indicator for predicting the efficacy of ESCC treatment (Table 3).
TABLE 3.
The role of FBXW7 in esophageal squamous cell carcinoma.
| Study | Source | FBXW7 status | Specific mechanisms | Significance |
|---|---|---|---|---|
| Bi et al. (2023) | TCGA database | Overexpression | ESCC stem cell formation | Inhibit ESCC cell proliferation, migration, invasion and angiogenesis |
| Li et al. (2023b) | Xenograft tumors | Loss of function | FBXW7- ANXA2-ERK | Promoting esophageal carcinogenesis through ANXA2 overexpression |
| Pan et al. (2023) | ESCC tissues | Inactivation | FBXW7-MAP4/ERK | Promote ESCC proliferation |
| Wu et al. (2015) | ESCC tissues and cell lines | Downregulated | MiR-27a-3p-FBXW7 | Promoted ESCC cell proliferation |
| Yu et al. (2015) | ESCC tissues | Downregulated | N/A | Poor prognosis of ESCC. |
| Y et al. (2010) | ESCC tissues | Downregulated | N/A | Progression and local invasiveness |
| Yokobori et al. (2012) | ESCC tissues | Downregulated | FBXW7-cMyc | ECSS proliferation, worse prognosis |
| Kurashige et al. (2012) | ESCC tissues | Downregulated | MiR-223-FBXW7 | Low FBXW7 expression adversely affects survival in ESCC patients |
| Gombodorj et al. (2018) | ESCC tissues and cell lines | Overexpression | FBXW7-MCL-1 | ESCC patients have a favorable response to CRT. |
4.4 Hepatocellular carcinoma (HCC)
Studies from small samples have reported that the frequency of FBXW7 mutations in hepatocellular carcinoma (HCC) tissues is approximately 7.7% (1/13) (Dl et al., 2014). Patients with HCC have a worse prognosis and a more aggressive tumor when FBXW7 expression is lost (Table 4) (Wang et al., 2015; Zhou et al., 2012; Feng et al., 2022). The functional impairment of FBXW7 in HCC is connected to accumulation of the Notch1 and poorer clinicopathological features (Wang et al., 2015). The Assembly Factor for Spindle Microtubules (ASPM) promotes tumorigenesis in HCC by preventing the binding and degradation of Notch1 by FBXW7 (218). Except for Notch1, FBXW7 can control the invasion ability and prognosis of HCC by regulating the stability of YAP protein and Myc protein (Tu et al., 2014; Zhang et al., 2020). Moreover, the level of FBXW7 in HCC cells was found to be controlled by a variety of regulators. For instance, by controlling FBXW7 expression, FAM83D, MiR-27b, MiR-92a, and MiR-155-3p affect the development and prognosis of HCC (219–222).
TABLE 4.
The Role of FBXW7 in hepatocellular carcinoma.
| Study | Source | FBXW7 status | Specific mechanisms | Significance |
|---|---|---|---|---|
| Wang et al. (2015) | HCC tissues | Downregulated | FBXW7-Notch1 | Promote HCC cell invasion and worse prognosis |
| Zhou et al. (2012) | HCC tissues | Downregulated | N/A | Adverse clinicopathologic features and cell proliferation |
| Feng et al. (2022) | HCC tissues | Downregulated | MiR-25-FBXW7-Autophagy | Sorafenib resistance and autophagy |
| Chan et al. (2024b) | HCC cell lines | Loss of function | ASPM-i1-FBXW7- Notch1 | Leading to the occurrence of HCC |
| Zhang et al (2020) | HCC tissues | Downregulated | MAP3K13-TRIM25-FBXW7- Myc | Treatment resistance and poor prognosis |
| Nie et al. (2022) | TCGA database GEO database |
Downregulated | FAM83D-FBXW7-MCL-1 | Promote the proliferation and migration of HCC cells |
| Sun et al. (2016) | HCC tissues HCC cell lines |
Downregulated | MiR-27b-FBXW7 | Poor prognostic features and reduced survival |
| Yang et al. (2015) | HCC tissues | Downregulated | MiR-92a-FBXW7 | Independent risk factors for tumor growth and prognosis |
| Tang et al. (2016) | HCC tissues HCC cell lines |
Downregulated | MiR-155-3p-FBXW7 | HCC cell proliferation |
| Tu et al. (2014) | HCC tissues | Downregulated | FBXW7-YAP | Poor clinicopathologic features and prognosis |
| Yu et al. (2014) | HCC cell lines | Downregulated | FBXW7-EMT | Diminished efficacy of adriamycin and increased HCC aggressiveness |
| Tang et al. (2019) | HCC cell lines | Downregulated | MiR-223-FBXW7 | HCC cells develop resistance to sorafenib |
| S et al. (2014) | HCC tissues | Low expression | N/A | Low expression of FBXW7 is an independent risk factor for HCC recurrence |
| El-Mezayen et al. (2021) | HCC tissues | Downregulated | MiR-25-FBXW7 | HCC tumor progression |
4.5 Pancreatic cancer (PC)
Pancreatic cancer (PC) is a high-threat gastrointestinal cancer characterized by its insidious onset and resistance to chemotherapy (Lin et al., 2022). Due to the significant heterogeneity and limited treatment options for PC, the survival rate for patients with advanced disease remains extremely low (Pancreatic Cancer). Therefore, identifying new therapeutic targets and biomarkers is crucial. The mutation rate of FBXW7 in PC has been reported to be approximately 2%–3% (Heestand and Kurzrock, 2015).
FBXW7 plays a crucial role in the differentiation of pancreatic ductal epithelial cells (Sancho et al., 2014). Elevated levels of Ngn3 after FBXW7 inactivation can induce the differentiation potential of pancreatic ductal cells, which may lead to the development of PC (Sancho et al., 2014; Ba and Y, 2014). Zhang et al. showed that deletion of FBXW7 accelerates KRAS G12D-driven pancreatic tumorigenesis (Zhang Q. et al., 2016). Mechanistic studies have shown that FBXW7 loss in mice depends on Yap accumulation to drive pancreatic tumor formation (Zhang Q. et al., 2016). The activation of the KRAS-ERK pathway in PC further suppresses FBXW7 expression, facilitating cancer progression (Ji et al., 2015). This occurs because the ERK pathway degrades FBXW7 in a phosphorylation-dependent manner, and pancreatic cancer cells with defective FBXW7 phosphorylation sites exhibit oncogenic resistance to ERK pathway activation (Ji et al., 2015). FBXW7 has been reported to hinder PC cell growth and invasion by inhibiting Wnt/β-catenin signaling (Jiang et al., 2016; Liu et al., 2019). Protein database analysis suggests that FBXW7 also inhibits the downstream TORC2/AKT signaling pathway by degrading salt-inducible kinase 2 (SIK2), thereby reducing pancreatic cancer cell proliferation (Mx et al., 2020). Moreover, FBXW7 suppresses tumor aggressiveness by degrading other substrates, such as Enhancer of zeste homolog 2 (EZH2) (Jin et al., 2017). The epigenetic regulator protein arginine methyltransferase 5 (PRMT5) stabilizes c-Myc levels in pancreatic cancer cells by downregulating FBXW7, promoting tumor cell proliferation (Qin et al., 2019).
FBXW7 can predict the response of PC to chemotherapy drugs. On one hand, FBXW7 can enhance the therapeutic response to gemcitabine by inhibiting the expression of proteins like stearoyl-CoA desaturase (SCD) or increasing the expression of equilibrative nucleoside transporter 1 (ENT1) (Ye et al., 2020; Q et al., 2017). And on the other hand, the low expression of FBXW7 can promote resistance to gemcitabine and paclitaxel in pancreatic cancer (PC) cells by leading to the accumulation of MCL-1 (240). Consequently, it is anticipated that FBXW7 function restoration will enhance the therapeutic efficacy and prognosis of PC patients (Table 5).
TABLE 5.
The role of FBXW7 in pancreatic cancer.
| Study | Source | FBXW7 status | Specific mechanisms | Significance |
|---|---|---|---|---|
| Zhang et al. (2016b) | Mice | Deletion | FBXW7-Yap | Induced pancreatic tumorigenesis |
| Ji et al. (2015) | PC tissues | Downregulated | KRAS-ERK-FBXW7 | Promote PC progression |
| Jiang et al. (2016) | PC tissues | Inactivation | FBXW7-Wnt/β-catenin | PC cell growth and invasion |
| Mx et al. (2020) | Human protein database | N/A | FBXW7- SIK2/TORC2/AKT | Suppress PC cell division and cell cycle progression |
| Jin et al. (2017) | PC cell lines | N/A | FBXW7-EZH2 | Suppress PC cell invasion |
| Qin et al. (2019) | TCGA Mouse model |
Downregulated | PRMT5 - FBW7-cMyc | Improvement in PC cell growth and aerobic glycolysis |
| Ye et al. (2020) | Xenograft tumors | Normal | FBXW7-NRA41-SCD1 | Activating ferroptosis and apoptosis |
| Q et al. (2017) | PC cell lines | Overexpression | FBXW7-ENT1 | Improving the therapeutic efficacy of gemcitabine |
| Ishii et al. (2017) | PC tissues PC cell lines |
Downregulated | FBXW7-MCL-1 | Improving gemcitabine and paclitaxel efficacy in PC cells |
The invasiveness and sensitivity of HCC cells to doxorubicin are also affected by FBXW7 levels. Restoring FBXW7 expression in HCC cells reduces their invasiveness and enhances the efficacy of doxorubicin (Yu et al., 2014). Sorafenib, commonly used in patients with advanced liver cancer, faces significant challenges due to drug resistance. FBXW7 has been implicated in sorafenib resistance in HCC cells (Feng et al., 2022; Tang et al., 2019). This resistance is linked to the downregulation of FBXW7 by MiR-223 and MiR-25, and can be reversed by restoring FBXW7 levels (Feng et al., 2022; Tang et al., 2019). For prognosis, HCC patients with high FBXW7 mRNA expression exhibit better DFS compared to those with low expression (S et al., 2014). Survival analysis indicates that low FBXW7 expression is an independent risk factor for predicting HCC recurrence (S et al., 2014). Therefore, FBXW7 may be a molecular marker in the treatment and prognosis assessment of HCC (Tu et al., 2014; El-Mezayen et al., 2021).
4.6 Cholangiocarcinoma and other gastrointestinal cancers
The mutation rate of FBXW7 in extrahepatic cholangiocarcinoma (CCA) has been reported to be 15% (n = 20) (Churi et al., 2014). Wang et al. demonstrated that FBXW7 deletion and AKT pathway activation synergistically upregulated the expression of c-Myc in animal experiment, contributing to the occurrence of intrahepatic cholangiocarcinoma (iCCA) (Wang et al., 2019). Overexpression of FBXW7 enables CCA cells to restore normal c-Myc expression levels, which inhibits cell proliferation and progression (Li M. et al., 2017). Consequently, they proposed a novel therapeutic strategy for iCCA that targets the FBXW7-c-Myc pathway (Wang et al., 2019; Li M. et al., 2017). Besides c-Myc, FBXW7 regulates the progression of CCA cells and their resistance to cisplatin by targeting other substrates, including Notch1 and MCL-1 (Mori et al., 2018). The loss of FBXW7 expression can promote EMT and CSC development in CCA, resulting in tumors with increased malignant potential (H et al., 2015). Studies indicate that FBXW7-deficient CCA exhibits poor differentiation, a propensity for regional lymph node metastasis, and a later tumor stage (H et al., 2015; Enkhbold et al., 2014). Furthermore, FBXW7 expression is closely linked to the prognosis of CCA patients. Patients with low FBXW7 expression have significantly poorer outcomes, indicating that FBXW7 levels are a critical prognostic factor for CCA (Mori et al., 2018; Enkhbold et al., 2014). Recent research has revealed that numerous cytokines and chemokines are upregulated in CCA with FBXW7 mutations, showing favorable responses to immunotherapy (Feng et al., 2024). These findings suggest that FBXW7-mutant CCA may possess a distinct tumor immune microenvironment, making FBXW7 expression status a potential indicator of immunotherapy efficacy.
Approximately 75% of patients with gastrointestinal stromal tumors (GIST) harbor functional mutations in KIT (Shima et al., 2024). Imatinib, a KIT inhibitor, is the primary treatment for inoperable or metastatic GIST. However, the occurrence of drug resistance remains inevitable (Blanke et al., 2008). Current research has emphasized the role of FBXW7 in predicting GIST treatment resistance and prognosis. Both in vivo and in vitro experiments demonstrate that FBXW7 expression enhances sensitivity to imatinib treatment in GIST by downregulating MCL-1 (Wu et al., 2024). Following radical resection, GIST patients with high FBXW7 expression exhibit better prognoses and lower recurrence rates (Koga et al., 2019). The incidence of FBXW7 mutations in rectal neuroendocrine tumors (NETs) is reported to be 25% (Venizelos et al., 2021). In rectal NETs, the levels of FBXW7 mutations and non-coding RNAs may correlate with the Ki67 index, though further research is required to confirm this relationship (Kang et al., 2023). Table 6 illustrates the role of FBXW7 in CCA and other gastrointestinal cancers.
TABLE 6.
The role of FBXW7 in cholangiocarcinoma and other gastrointestinal cancers.
| Study | Source | FBXW7 status | Specific mechanisms | Significance |
|---|---|---|---|---|
| Wang et al. (2019) | iCCA tissues Mouse model |
Downregulated | FBXW7/AKT-c-Myc | Leading to tumorigenesis in mice |
| Li et al. (2017b) | CCA cell lines | Overexpression | FBXW7 -c-Myc | Restrained cell multiplication in vitro and CCA xenograft tumor growth |
| A et al. (2018) | CCA cell lines CCA tissues |
Normal expression | FBXW7-MCL-1/Notch1 | Inhibited CCA progression and CCA cells are sensitive to cisplatin |
| H et al. (2015) | CCA cell lines CCA tissues |
Depletion | FBXW7-EMT/CSCS | Resulting in the metastasis of CCA cells |
| Enkhbold et al. (2014) | CCA tissues | Low expression | N/A | Tumor progression and poor prognosis |
| Feng et al. (2024) | CCA cell lines Mouse |
Downregulated | FBXW7- Stat | Accelerating tumor formation and growth |
| Wu et al. (2024) | GIST tissues | N/A | FBXW7-MCL-1 | Regulates the sensitivity of imatinib |
| Koga et al. (2019) | GIST tissues | Low expression | FBXW7-c-Myc/Notch 1 | Potential predictive marker of recurrence |
| Kang et al. (2023) | Rectal NET tissues | Mutation | N/A | Playing critical roles in tumor behaviors |
5 Strategies to target FBXW7 for the treatment of cancers
As previously noted, FBXW7 is pivotal in the pathogenesis, progression, treatment resistance, and poor prognosis of gastrointestinal cancers, thereby presenting opportunities for targeted therapy. We propose several promising strategies for the treatment of cancer by FBXW7 as shown in Figure 4. At the same time, we also propose the current challenges of FBXW7 in the treatment of cancer.
FIGURE 4.
Strategies for targeting FBXW7 in the treatment of cancers (A). Inhibition of regulators such as miRNA and ERK can upregulate the expression of FBXW7. Additionally, inhibiting FBXW7 downstream substrates, such as MCL-1 and mTOR, may reduce cancer cell proliferation and drug resistance (B). Inhibiting FBXW7 expression in cancer stem cells (CSCs) can awaken cell cycle-arrested CSCs, making them more sensitive to treatment.
5.1 Targeting upstream regulators to increase FBXW7 expression levels
Aberrant FBXW7 expression is a key factor contributing to poor cancer prognosis, drug resistance, and treatment challenges (Khan AQ. et al., 2021). Restoring or increasing FBXW7 expression has been identified as a viable strategy to halt cancer progression and improve treatment outcomes (Figure 4). Several studies have shown that modulating MiR-223 levels can indirectly upregulate FBXW7 expression (Liu Z. et al., 2021; Kurashige et al., 2012). As previously reported, MiR-223-mediated inhibition of FBXW7 leads to GC resistance to trastuzumab and cisplatin, whereas reducing MiR-223 levels reverses this resistance (Zhou et al., 2015; Eto et al., 2015). Notably, genistein has been found to downregulate MiR-223 and upregulate FBXW7 expression (Ma et al., 2013). Elevated FBXW7 expression subsequently inhibits the growth and aggressiveness of PC cells (Ma et al., 2013). Recent findings also suggest that inhibiting ERK, another upstream regulator of FBXW7, improves the sensitivity of CRC cells to chemotherapeutic agents (Ruan et al., 2024). Mechanistically, this is attributed to Clitocine, an adenosine analog, which promotes FBXW7 expression and MCL-1 degradation by preventing the A2B/cAMP/ERK signaling axis (Ruan et al., 2024). Thus, targeting upstream regulators of FBXW7 presents a feasible approach to enhance drug sensitivity in gastrointestinal cancers.
5.2 Targeting key downstream substrates for FBXW7
FBXW7 exerts anti-tumor effects by regulating the degradation of various downstream oncoproteins. Thus, developing drugs that target key carcinogenic substrates may counteract the carcinogenic effects resulting from FBXW7 inactivation. For example, selective MCL-1 inhibitors restore the sensitivity of CRC cells with FBXW7 mutations to regorafenib (Tong et al., 2017). It has been found that deguelin can promote the destruction of MCL-1 by FBXW7, which is beneficial to the apoptosis of cancer cells (Gao et al., 2020). Furthermore, FBXW7 deficiency-induced resistance of CRC to Hsp90 inhibitors can be overcome by selective MCL-1 inhibitors (J et al., 2017). Research on MCL-1 inhibitors has garnered significant attention and may be applied in clinical treatment in the near future (Wang H. et al., 2021; Chen and Fletcher, 2017). Alongside MCL-1 inhibitors, mTOR inhibitors also exhibit significant anti-cancer effects on tumors with FBXW7 mutations (Mao et al., 2008). FBXW7 deletion leads to increased EMT and aggressiveness in CRC cell lines, which is mediated by overactivation of the mTOR pathway (Wang et al., 2013). Subsequently, mTOR inhibitors were applied to CRC cells with FBXW7 deficiency, resulting in significant inhibition of EMT and the aggressiveness of cancer cells (Wang et al., 2013). Therefore, inhibiting downstream oncoproteins to treat gastrointestinal cancers with FBXW7 deletions or mutations has the potential to enhance the efficacy of anticancer therapy.
5.3 Awakens dormant cancer stem cells (CSCs)
FBXW7 plays a critical role in regulating the differentiation and malignant transformation of various stem/progenitor cells, including pluripotent stem cells, hematopoietic stem cells, intestinal stem cells, and neural stem cells (N et al., 2015; Takeishi and Nakayama, 2014). Additionally, FBXW7 is involved in the characterization of CSCs by modulating key oncoproteins, such as c-Myc and Notch1, in human malignancies (G et al., 2024; Li et al., 2019). For instance, FBXW7 contributes to the self-renewal and stem-like properties of hepatocellular carcinoma (HCC) cells by promoting the degradation of Actin-like 6A (ACTL6A) (X et al., 2022). CSCs, a small subset of tumor cells with the capacity for self-renewal and differentiation, play a pivotal role in tumor recurrence, progression, and resistance to treatment. While cytotoxic drugs target proliferating tumor cells, CSCs in a quiescent state are often resistant to such treatments (Takeishi and Nakayama, 2016). In non-small cell lung cancer (NSCLC), FBXW7 maintains gefitinib-resistant CSCs in a quiescent state by targeting the degradation of c-Myc (Hidayat et al., 2019). Moreover, downregulation of FBXW7 gene expression has been shown to significantly decrease the number of cells in the G0/G1 phase (Hidayat et al., 2019). In chronic myeloid leukemia, FBXW7 preserves dormant leukemia-initiating cells (LICs) through a similar mechanism, and its removal can awaken quiescent LICs, restoring sensitivity to imatinib (Takeishi et al., 2013). Elevated FBXW7 expression has also been detected in drug-resistant colorectal cancer (CRC) stem cells, with FBXW7 silencing enhancing sensitivity to anticancer drugs (Honma et al., 2019). These findings suggest that silencing FBXW7 may help convert CSCs from a quiescent to a proliferative state, potentially improving treatment resistance. However, this effect is primarily directed at CSCs or tumor cells with stem-like properties, which represent only a small portion of the overall tumor.
5.4 Other approaches to target FBXW7 for cancer treatment
FBXW7 is frequently inactivated in cancer through mutations, deletions, or promoter hypermethylation (Fan et al., 2022). Therefore, restoring the function of the FBXW7 cancer suppressor gene in tumor cells lacking expression at the molecular and genetic levels represents a potential therapeutic strategy. Evidence from mouse lung cancer models indicates that decitabine promotes FBXW7 expression, leading to MCL-1 degradation and enhanced sensitivity to anticancer drugs (DiNardo et al., 2018). Decitabine, as a DNMT1 inhibitor, induces a shift from hypermethylation to unmethylation of the FBXW7 promoter, thereby facilitating FBXW7 transcription (DiNardo et al., 2018). As discussed in Section 3, FBXW7 protein levels are regulated by autoubiquitination and deubiquitinases (DUBs). USP9X prevents the ubiquitination and degradation of FBXW7 in mouse models and inhibits intestinal tumor development (Khan et al., 2018). In tumors with downregulated FBXW7 expression, it may be possible to counteract FBXW7 self-ubiquitination and degradation by developing deubiquitinase-targeting chimeric (DUBTAC) technology to sustain FBXW7 expression and function (Henning et al., 2022; Willson, 2022). Furthermore, the development of proteolysis-targeting chimeric (PROTAC) technology, where two covalently linked PROTAC molecules recruit specific substrates and FBXW7, could enhance the degradation efficiency of FBXW7 (Li et al., 2022). Although research in this area is limited, the potential significance of DUBTACs and PROTACs will become clearer as the functional mechanisms of FBXW7 are further explored.
5.5 Current challenges in targeting FBXW7 in the treatment of cancer
The FBXW7 gene is frequently mutated or deleted in human tumors, leading to impaired tumor suppression. Currently, restoring the tumor-suppressive function of FBXW7 in vivo through gene editing or gene transfer therapies remains challenging (Fan et al., 2022). No drug or therapeutic technology currently available targets the FBXW7 gene directly for cancer treatment. While PROTAC and DUBTAC approaches may prove useful in tumors with low FBXW7 activity, they are ineffective in tumors with FBXW7 mutations or deletions. A large network of regulators and oncogenic substrates surrounds FBXW7, offering multiple potential pathways for cancer treatment. However, the tumor-suppressive effects of FBXW7 arise from the integrated function of this regulatory network, making it difficult to predict whether targeting a single pathway will produce the desired therapeutic effect. As noted earlier, there is resistance to Wnt/β-catenin pathway inhibition in FBXW7-mutant tumors (Zhong and Virshup, 2024), which is linked to the activation of other carcinogenic substrates regulated by FBXW7. However, simultaneously targeting multiple oncogenic substrates or regulators may lead to unforeseen toxic side effects. Furthermore, the knockdown of FBXW7 to enhance the sensitivity of dormant cancer stem cells (CSCs) to therapeutic agents presents a dual challenge. CSCs, while a small fraction of the overall tumor, tend to increase as the disease progresses. Whether the enhanced drug sensitivity resulting from FBXW7 knockdown can counterbalance the tumor-suppressive dysfunction caused by FBXW7 deletion requires further investigation.
6 Discussion and conclusions
FBXW7 is a substrate recognition component of the SCF-type E3 ubiquitin ligase complex, frequently inactivated or mutated in human cancers. Given the wide range of FBXW7 functions in cancer, this review focuses on its role in gastrointestinal cancers. As a recognized tumor suppressor gene, the inactivation of FBXW7 promotes tumorigenesis, proliferation, differentiation, invasion, and apoptosis in gastrointestinal tumor cells by interacting with the EGFR-MAPK, Wnt/β-catenin, and PI3K/Akt/mTOR signaling pathways (Wu et al., 2016; Liu Z. et al., 2021; Xie and Sun, 2019; Hu et al., 2019; Huang and Long, 2023; Pan et al., 2023; Ji et al., 2015; Escobar et al., 2022; R et al., 2023). In addition, FBXW7 is involved in the aggressiveness and drug resistance of gastrointestinal cancers by mediating the degradation of downstream substrates such as Cyclin E, MCL-1, and Notch1 (Ou et al., 2016; Guo et al., 2012; Lin et al., 2020b; J et al., 2017; Wang et al., 2015). Therefore, FBXW7 forms a complex and extensive regulatory network in gastrointestinal cancers by modulating various signaling pathways and substrate proteins. Both in vitro and in vivo studies have shown that the loss of FBXW7 expression leads to the accumulation of oncogenic proteins, which is strongly connected to the initiation, development, metastasis, and drug resistance of various gastrointestinal cancers, including CRC, GC, ESCC, HCC, CCA, and PC (Wei et al., 2023; Khan et al., 2018; Mori et al., 2018; Ou et al., 2016; Gong et al., 2018; He et al., 2019; Mu et al., 2017; Lin H. et al., 2020; Li et al., 2018; Zhan et al., 2015; Huang and Long, 2023; Wang et al., 2021c; Kawashita et al., 2017; Jiang et al., 2017; Li et al., 2012; Gong et al., 2015; Y et al., 2021; Li et al., 2020; X et al., 2019b; Hou et al., 2022; Zhou et al., 2015; Eto et al., 2015; Bi et al., 2023; Li et al., 2023b; Wu et al., 2015; Y et al., 2010; Zhou et al., 2012; Feng et al., 2022; Chan et al., 2024b; Yang et al., 2015; Yu et al., 2014; Zhang Q. et al., 2016; Mx et al., 2020; Ye et al., 2020; Wang et al., 2019). Impaired FBXW7 function in gastrointestinal cancers is generally associated with poor patient outcomes (Liu H. et al., 2018; Iwatsuki et al., 2010; Kawaguchi et al., 2021; Yokobori et al., 2009; Li MR. et al., 2017; Gombodorj et al., 2018; S et al., 2014; Feng et al., 2024; Koga et al., 2019). Therefore, strategies aimed at targeting FBXW7 may offer potential therapeutic approaches for cancer treatment. Although no drugs currently directly target FBXW7, promising therapeutic strategies have been proposed based on existing research (Zeng et al., 2024). Future treatments may focus on restoring or replacing FBXW7 expression by targeting its upstream regulators, such as MiRNA and ERK, or by inhibiting downstream oncoproteins such as MCL-1, mTOR, and c-Myc (Zhou et al., 2015; Eto et al., 2015; Ma et al., 2013; Ruan et al., 2024; Gao et al., 2020). The numerous regulators and substrates of FBXW7 provide a wide range of targets for treating gastrointestinal cancers. However, these treatment strategies are often difficult to implement, with uncertain efficacy and significant side effects. To address the key challenges currently faced, the following approaches may be considered: i) Identifying and inhibiting the most critical carcinogenic substrates or upstream regulators of FBXW7 to maximize its tumor-suppressive function. ii) Activating or enhancing the activity of other tumor suppressor genes in the presence of FBXW7 mutations or deletions. iii) Tailoring strategies based on tumor stage and the number of CSCs present to minimize the adverse effects of FBXW7 knockdown.
In conclusion, FBXW7 has the potential to be an important molecular marker for the treatment and prognosis of gastrointestinal cancers. Continued research into the tumor biology and molecular mechanisms of FBXW7 will offer valuable insights for improving the precision treatment of gastrointestinal cancer patients.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Jilin Provincial Education Department (grant number: 3D5196778428), and Jilin Provincial Finance Department (grant number: 3D5214495428).
Author contributions
WW: Investigation, Project administration, Software, Visualization, Writing–original draft. XL: Investigation, Software, Writing–original draft. LZ: Supervision, Writing–original draft. KJ: Software, Writing–original draft. ZY: Software, Writing–original draft. RY: Investigation, Writing–original draft. WZ: Investigation, Writing–original draft. JC: Conceptualization, Supervision, Writing–review and editing. TL: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We acknowledge Biorender (https://www.biorender.com/) since Graphical Abstract, Figures 1–4 of this manuscript were made using this software.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- C/EBPδCCAAT
enhancer-binding protein-delta
- CHD4
Chromodomain helicase DNA-binding protein 4
- CLM
Colorectal cancer liver metastases
- CoA
Coactivator
- CPD
Cdc4 phosphodegron
- CRC
Colorectal cancer
- CSCs
Cancer stem cells
- CSL
CBF-1/suppressor of hairless/Lag1
- CSN6
COP9 signalosome subunit 6
- ctDNA
Circulating tumor DNA
- E1
Ubiquitin-activating enzymes
- E2
Ubiquitin-conjugating enzymes
- E3
Ubiquitin ligase
- EBV
Epstein-Barr virus
- EGFR
Epidermal Growth Factor Receptor
- EMT
Epithelial-mesenchymal transition
- EMT
Epithelial-mesenchymal transition
- ENO1
Enolase 1
- ERK1/2
Extracellular signal-regulated kinases 1 and 2
- ESCC
Esophageal squamous cell carcinoma
- FASN
Fatty acid synthase
- FBXW7
F-Box and WD Repeat Domain Containing 7
- FGFR
Fibroblast growth factor receptor 2
- GBM
Glioblastoma
- GC
Gastric cancer
- GIST
Gastrointestinal stromal tumors
- GSK3β
Glycogen synthase kinase-3β
- GSK3β
Glycogen synthase kinase-3β
- HCC
Hepatocellular carcinoma
- HER-2
Human epidermal growth factor receptor 2
- HES5
HES family bHLH transcription factor 5
- Hsp90
Heat shock protein 90
- iCCA
Intrahepatic Cholangiocarcinoma
- IDH1/2
Isocitrate dehydrogenase 1 or 2
- KRAS
Kirsten rat sarcoma viral oncogene
- KRAS
Kirsten rat sarcoma viral oncogene
- LEF
lymphocyte enhancer factor-1
- LSD1
Lysine-specific demethylase 1
- MAMLs
Mastermind-like proteins
- MAP4
Microtubule-associated proteins 4
- MAPK
Mitogen-activated protein kinase
- MCL-1
Myeloid leukemia 1
- MiRNA
micro-RNA
- MMR
Mismatch Repair
- MSI
Microsatellite instability
- mTOR
Mammalian target of rapamycin
- N/A
Not assessable
- ncRNA
Noncoding RNAs
- NICD
NOTCH intracellular domain
- Nox1
NADPH oxidase 1
- NTRK
Neurotrophic tyrosine receptor kinase
- NTRK
Neurotrophic tyrosine receptor kinase
- PC
Pancratic cancer
- PD-L1
Programmed cell death ligand 1
- PI3K
Phosphoinositide 3-kinase
- Plk2
Polo-like kinase 2
- PTEN
Phosphatase and tensin homolog
- SCF
Skp1-Cullin1-F-box
- SKP1
S-phase kinase-associated protein 1
- STYX
Serine/threonine/tyrosine interacting protein
- TCF
T cell factor
- TERT
Telomerase reverse transcriptase
- TGF-β
Transforming growth factor beta
- TMB
Tumor mutation burden
- TNBC
Triple negative breast cancer
- UPS
Ubiquitin-proteasome system
- USP28
Ubiquitin-specific proteases 28
- Usp9x
Ubiquitin-specific peptidase 9X
- VEGFR
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor
- YAP
Yes-associated protein
References
- Afolabi H. A., Salleh S. M., Zakaria Z., Seng C. E., Nafi N. M., Bin AbdulAziz A. A., et al. (2024). Targeted variant prevalence of FBXW7 gene mutation in colorectal carcinoma propagation. The first systematic review and meta-analysis. Heliyon 10 (11), e31471. 10.1016/j.heliyon.2024.e31471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadou E., Jacob L. S., Slack F. J. (2018). Non-coding RNA networks in cancer. Nat. Rev. Cancer 18 (1), 5–18. 10.1038/nrc.2017.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- As A. (2019). PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin. Cancer Biol. 59, 125–132. 10.1016/j.semcancer.2019.07.009 [DOI] [PubMed] [Google Scholar]
- B K., T T., L R., L X., J M., P N., et al. (2013). The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell 153 (7), 1552–1566. 10.1016/j.cell.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- B F., Pa C., Bj V. F., El R., V R., B I., et al. (2024). A germline point mutation in the MYC-FBW7 phosphodegron initiates hematopoietic malignancies. Genes Dev. 38 (5–6), 253–272. 10.1101/gad.351292.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba S., Y X. (2014). Out of the F-box: reawakening the pancreas. Cell Stem Cell 15 (2), 111–112. 10.1016/j.stem.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei-Jadidi R., Li N., Saadeddin A., Spencer-Dene B., Jandke A., Muhammad B., et al. (2011). FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation. J. Exp. Med. 208 (2), 295–312. 10.1084/jem.20100830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakos G., Yu L., Gak I. A., Roumeliotis T. I., Liakopoulos D., Choudhary J. S., et al. (2018). An E2-ubiquitin thioester-driven approach to identify substrates modified with ubiquitin and ubiquitin-like molecules. Nat. Commun. 9, 4776. 10.1038/s41467-018-07251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan K., Wang J. M., Tsai H. H., Sharan S., Anver M., Leighty R., et al. (2010). The tumour suppressor C/EBPδ inhibits FBXW7 expression and promotes mammary tumour metastasis. EMBO J. 29 (24), 4106–4117. 10.1038/emboj.2010.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bd W., Aj C., Dw D. (2012). Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology 142 (2), 219–232. 10.1053/j.gastro.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben W., Zhang G., Huang Y., Sun Y. (2020). MiR-27a-3p regulated the aggressive phenotypes of cervical cancer by targeting FBXW7. Cancer Manag. Res. 12, 2925–2935. 10.2147/CMAR.S234897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Aharon I., van Laarhoven H. W. M., Fontana E., Obermannova R., Nilsson M., Lordick F. (2023). Early-onset cancer in the gastrointestinal tract is on the rise-evidence and implications. Cancer Discov. 13 (3), 538–551. 10.1158/2159-8290.CD-22-1038 [DOI] [PubMed] [Google Scholar]
- Bengoechea-Alonso M. T., Ericsson J. (2010). Tumor suppressor Fbxw7 regulates TGFβ signaling by targeting TGIF1 for degradation. Oncogene 29 (38), 5322–5328. 10.1038/onc.2010.278 [DOI] [PubMed] [Google Scholar]
- Bi Y., Yang Y., Zhang Y., Cheng C., Tang P., Xiao H., et al. (2023). FBXW7 inhibits the progression of ESCC by directly inhibiting the stemness of tumor cells. Neoplasma 70 (6), 733–746. 10.4149/neo_2023_230104N8 [DOI] [PubMed] [Google Scholar]
- Blanke C. D., Rankin C., Demetri G. D., Ryan C. W., von Mehren M., Benjamin R. S., et al. (2008). Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 26 (4), 626–632. 10.1200/JCO.2007.13.4452 [DOI] [PubMed] [Google Scholar]
- Boretto M., Geurts M. H., Gandhi S., Ma Z., Staliarova N., Celotti M., et al. (2024). Epidermal growth factor receptor (EGFR) is a target of the tumor-suppressor E3 ligase FBXW7. Proc. Natl. Acad. Sci. U. S. A. 121 (12), e2309902121. 10.1073/pnas.2309902121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R. L., Soerjomataram I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- Buscail L., Bournet B., Cordelier P. (2020). Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 17 (3), 153–168. 10.1038/s41575-019-0245-4 [DOI] [PubMed] [Google Scholar]
- Chan Y. T., Zhang C., Wu J., Lu P., Xu L., Yuan H., et al. (2024a). Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol. Cancer 23 (1), 189. 10.1186/s12943-024-02101-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. S., Cheng L. H., Hsu C. C., Yang P. M., Liao T. Y., Hsieh H. Y., et al. (2024b). ASPM stabilizes the NOTCH intracellular domain 1 and promotes oncogenesis by blocking FBXW7 binding in hepatocellular carcinoma cells. Mol. Oncol. 18 (3), 562–579. 10.1002/1878-0261.13589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Lin H. H., Lin J. K., Lin C. C., Lan Y. T., Wang H. S., et al. (2015). FBXW7 mutation analysis and its correlation with clinicopathological features and prognosis in colorectal cancer patients. Int. J. Biol. Markers 30 (1), e88–e95. 10.5301/jbm.5000125 [DOI] [PubMed] [Google Scholar]
- Chang H., Liu Y. H., Wang L. L., Wang J., Zhao Z. H., Qu J. F., et al. (2018). MiR-182 promotes cell proliferation by suppressing FBXW7 and FBXW11 in non-small cell lung cancer. Am. J. Transl. Res. 10 (4), 1131–1142. [PMC free article] [PubMed] [Google Scholar]
- Chen L., Fletcher S. (2017). Mcl-1 inhibitors: a patent review. Expert Opin. Ther. Pat. 27 (2), 163–178. 10.1080/13543776.2017.1249848 [DOI] [PubMed] [Google Scholar]
- Chen L. J., Hu B., Han Z. Q., Liu W., Zhu J. H., Chen X. X., et al. (2021). Repression of FBXW7 by HES5 contributes to inactivation of the TGF-β signaling pathway and alleviation of endometriosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 35 (2), e20938. 10.1096/fj.202000438RRR [DOI] [PubMed] [Google Scholar]
- Chen S., Leng P., Guo J., Zhou H. (2023). FBXW7 in breast cancer: mechanism of action and therapeutic potential. J. Exp. Clin. Cancer Res. CR 42 (1), 226. 10.1186/s13046-023-02767-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong X., Madeti Y., Cai J., Li W., Cong L., Lu J., et al. (2024). Recent developments in immunotherapy for gastrointestinal tract cancers. J. Hematol. OncolJ Hematol. Oncol. 17 (1), 65. 10.1186/s13045-024-01578-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Geng Y., Zhou Y., Sicinski P. (2021). Cyclin E in normal physiology and disease states. Trends Cell Biol. 31 (9), 732–746. 10.1016/j.tcb.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churi C. R., Shroff R., Wang Y., Rashid A., Kang H. C., Weatherly J., et al. (2014). Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PloS One 9 (12), e115383. 10.1371/journal.pone.0115383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizmecioglu O., Krause A., Bahtz R., Ehret L., Malek N., Hoffmann I. (2012). Plk2 regulates centriole duplication through phosphorylation-mediated degradation of Fbxw7 (human Cdc4). J. Cell Sci. 125 (4), 981–992. 10.1242/jcs.095075 [DOI] [PubMed] [Google Scholar]
- Close V., Close W., Kugler S. J., Reichenzeller M., Yosifov D. Y., Bloehdorn J., et al. (2018). NOTCH1 signaling is activated in CLL by mutations of FBXW7 and low expression of USP28 at 11q23. Blood 132, 946. 10.1182/blood-2018-99-114491 [DOI] [Google Scholar]
- Close V., Close W., Kugler S. J., Reichenzeller M., Yosifov D. Y., Bloehdorn J., et al. (2019). FBXW7 mutations reduce binding of NOTCH1, leading to cleaved NOTCH1 accumulation and target gene activation in CLL. Blood 133 (8), 830–839. 10.1182/blood-2018-09-874529 [DOI] [PubMed] [Google Scholar]
- Collisson E. A., Bailey P., Chang D. K., Biankin A. V. (2019). Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 16 (4), 207–220. 10.1038/s41575-019-0109-y [DOI] [PubMed] [Google Scholar]
- CXCR4. CXCR4 inhibitor BL-8040 induces AML blast apoptosis by altering miR-15a/16-1 expression and downregulating ERK, BCL-2, MCL-1, and cyclin-D1 | Leukemia. (2024). Available at: https://www.nature.com/articles/leu201782 [DOI] [PubMed]
- D X., M Y., H S., P R., J J., F L., et al. (2016). Polo-like kinase-1 regulates myc stabilization and activates a feedforward circuit promoting tumor cell survival. Mol. Cell 64 (3), 493–506. 10.1016/j.molcel.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Davis R. J., Welcker M., Clurman B. E. (2014). Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell 26 (4), 455–464. 10.1016/j.ccell.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran R., Deutzmann A., Mahauad-Fernandez W. D., Hansen A. S., Gouw A. M., Felsher D. W. (2021). The MYC oncogene — the grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 19 (1), 23–36. 10.1038/s41571-021-00549-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore R., Suleiman S., Drago-Ferrante R., Subbannayya Y., Suleiman S., Vasileva-Slaveva M., et al. (2023). The role of FBXW7 in gynecologic malignancies. Cells 12 (10), 1415. 10.3390/cells12101415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo C. D., Pratz K. W., Letai A., Jonas B. A., Wei A. H., Thirman M., et al. (2018). Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 19 (2), 216–228. 10.1016/S1470-2045(18)30010-X [DOI] [PubMed] [Google Scholar]
- Dl J., Jj W., K H., Am T., R Z., J F., et al. (2014). FBXW7 mutations in patients with advanced cancers: clinical and molecular characteristics and outcomes with mTOR inhibitors. PloS One 9 (2), e89388. 10.1371/journal.pone.0089388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm-Reed S., Spruck C. H., Sangfelt O., van Drogen F., Mueller-Holzner E., Widschwendter M., et al. (2004). Mutation of hCDC4 leads to cell cycle deregulation of cyclin E in cancer. Cancer Res. 64 (3), 795–800. 10.1158/0008-5472.can-03-3417 [DOI] [PubMed] [Google Scholar]
- Elimova E., Wadhwa R., Shiozaki H., Sudo K., Estrella J. S., Badgwell B. D., et al. (2015). Molecular biomarkers in gastric cancer. J. Natl. Compr. Cancer Netw. JNCCN 13 (4), e19–e29. 10.6004/jnccn.2015.0064 [DOI] [PubMed] [Google Scholar]
- El-Mezayen H., Yamamura K., Yusa T., Nakao Y., Uemura N., Kitamura F., et al. (2021). MicroRNA-25 exerts an oncogenic function by regulating the ubiquitin ligase Fbxw7 in hepatocellular carcinoma. Ann. Surg. Oncol. 28 (12), 7973–7982. 10.1245/s10434-021-09778-2 [DOI] [PubMed] [Google Scholar]
- Enkhbold C., Utsunomiya T., Morine Y., Imura S., Ikemoto T., Arakawa Y., et al. (2014). Loss of FBXW7 expression is associated with poor prognosis in intrahepatic cholangiocarcinoma. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 44 (14), E346–E352. 10.1111/hepr.12314 [DOI] [PubMed] [Google Scholar]
- Escobar D., Bushara O., Sun L., Liao J., Yang G. Y. (2022). Clinicopathologic characteristics of FBXW7-mutated colorectal adenocarcinoma and association with aberrant beta-catenin localization. Hum. Pathol. 119, 51–58. 10.1016/j.humpath.2021.10.003 [DOI] [PubMed] [Google Scholar]
- Eto K., Iwatsuki M., Watanabe M., Ishimoto T., Ida S., Imamura Y., et al. (2015). The sensitivity of gastric cancer to trastuzumab is regulated by the miR-223/FBXW7 pathway. Int. J. Cancer 136 (7), 1537–1545. 10.1002/ijc.29168 [DOI] [PubMed] [Google Scholar]
- Fan J., Bellon M., Ju M., Zhao L., Wei M., Fu L., et al. (2022). Clinical significance of FBXW7 loss of function in human cancers. Mol. Cancer 21 (1), 87. 10.1186/s12943-022-01548-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Yang Z., Zhou J., Tung J. Y., Hsiao C. D., Wang L., et al. (2015). Circadian clock gene CRY2 degradation is involved in chemoresistance of colorectal cancer. Mol. Cancer Ther. 14 (6), 1476–1487. 10.1158/1535-7163.MCT-15-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Zou B., Nan T., Zheng X., Zheng L., Lan J., et al. (2022). MiR-25 enhances autophagy and promotes sorafenib resistance of hepatocellular carcinoma via targeting FBXW7. Int. J. Med. Sci. 19 (2), 257–266. 10.7150/ijms.67352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Zhao M., Wang L., Li L., Lei J. H., Zhou J., et al. (2024). The heterogeneity of signaling pathways and drug responses in intrahepatic cholangiocarcinoma with distinct genetic mutations. Cell Death Dis. 15 (1), 34. 10.1038/s41419-023-06406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- G K., T M. (2014). Perilous journey: a tour of the ubiquitin-proteasome system. Trends Cell Biol. 24 (6), 352–359. 10.1016/j.tcb.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- G X., W L., J Y., Z L., P W., H F. (2024). Fbxw7 suppresses carcinogenesis and stemness in triple-negative breast cancer through CHD4 degradation and Wnt/β-catenin pathway inhibition. J. Transl. Med. 22 (1), 99. 10.1186/s12967-024-04897-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Yu X., Li M., Zhou L., Liu W., Li W., et al. (2020). Deguelin suppresses non-small cell lung cancer by inhibiting EGFR signaling and promoting GSK3β/FBW7-mediated Mcl-1 destabilization. Cell Death Dis. 11 (2), 143. 10.1038/s41419-020-2344-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge M. K., Zhang N., Xia L., Zhang C., Dong S. S., Li Z. M., et al. (2020). FBXO22 degrades nuclear PTEN to promote tumorigenesis. Nat. Commun. 11, 1720. 10.1038/s41467-020-15578-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gi M., E C., Y L., Ys L. (2020). Decreased expression of FBXW7 by ERK1/2 activation in drug-resistant cancer cells confers transcriptional activation of MDR1 by suppression of ubiquitin degradation of HSF1. Cell Death Dis. 11 (5), 395. 10.1038/s41419-020-2600-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombodorj N., Yokobori T., Tanaka N., Suzuki S., Kuriyama K., Kumakura Y., et al. (2018). Correlation between high FBXW7 expression in pretreatment biopsy specimens and good response to chemoradiation therapy in patients with locally advanced esophageal cancer: a retrospective study. J. Surg. Oncol. 118 (1), 101–108. 10.1002/jso.25127 [DOI] [PubMed] [Google Scholar]
- Gong J., Cui Z., Li L., Ma Q., Wang Q., Gao Y., et al. (2015). MicroRNA-25 promotes gastric cancer proliferation, invasion, and migration by directly targeting F-box and WD-40 Domain Protein 7, FBXW7. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 36 (10), 7831–7840. 10.1007/s13277-015-3510-3 [DOI] [PubMed] [Google Scholar]
- Gong J., Huang Z., Huo J. R. (2016). Involvement of F-box proteins in esophageal cancer (Review). Int. J. Oncol. 48 (3), 886–894. 10.3892/ijo.2016.3325 [DOI] [PubMed] [Google Scholar]
- Gong L., Ren M., Lv Z., Yang Y., Wang Z. (2018). miR-92b-3p promotes colorectal carcinoma cell proliferation, invasion, and migration by inhibiting FBXW7 in vitro and in vivo . DNA Cell Biol. 37 (5), 501–511. 10.1089/dna.2017.4080 [DOI] [PubMed] [Google Scholar]
- Grim J. E., Gustafson M. P., Hirata R. K., Hagar A. C., Swanger J., Welcker M., et al. (2008). Isoform- and cell cycle–dependent substrate degradation by the Fbw7 ubiquitin ligase. J. Cell Biol. 181 (6), 913–920. 10.1083/jcb.200802076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim J. E., Knoblaugh S. E., Guthrie K. A., Hagar A., Swanger J., Hespelt J., et al. (2012). Fbw7 and p53 cooperatively suppress advanced and chromosomally unstable intestinal cancer. Mol. Cell Biol. 32 (11), 2160–2167. 10.1128/MCB.00305-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W. L., He Y., Xu R. H. (2023). Gastric cancer treatment: recent progress and future perspectives. J. Hematol. OncolJ Hematol. Oncol. 16 (1), 57. 10.1186/s13045-023-01451-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Zhou Y., Evers B. M., Wang Q. (2012). Rictor regulates FBXW7-dependent c-Myc and cyclin E degradation in colorectal cancer cells. Biochem. Biophys. Res. Commun. 418 (2), 426–432. 10.1016/j.bbrc.2012.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- H Y., X L., Z L., L C., Y X., Y W., et al. (2015). FBXW7 suppresses epithelial-mesenchymal transition, stemness and metastatic potential of cholangiocarcinoma cells. Oncotarget 6 (8), 6310–6325. 10.18632/oncotarget.3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D., Ma Z., Fang C., Ding J., Yang W., Chen P., et al. (2019). Pseudophosphatase STYX promotes tumor growth and metastasis by inhibiting FBXW7 function in colorectal cancer. Cancer Lett. 454, 53–65. 10.1016/j.canlet.2019.04.014 [DOI] [PubMed] [Google Scholar]
- Heestand G. M., Kurzrock R. (2015). Molecular landscape of pancreatic cancer: implications for current clinical trials. Oncotarget 6 (7), 4553–4561. 10.18632/oncotarget.2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning N. J., Boike L., Spradlin J. N., Ward C. C., Liu G., Zhang E., et al. (2022). Deubiquitinase-targeting chimeras for targeted protein stabilization. Nat. Chem. Biol. 18 (4), 412–421. 10.1038/s41589-022-00971-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. (1998). The ubiquitin system. Annu. Rev. Biochem. 67, 425–479. 10.1146/annurev.biochem.67.1.425 [DOI] [PubMed] [Google Scholar]
- Hidayat M., Mitsuishi Y., Takahashi F., Tajima K., Yae T., Miyahara K., et al. (2019). Role of FBXW7 in the quiescence of gefitinib-resistant lung cancer stem cells in EGFR-mutant non-small cell lung cancer. Bosn. J. Basic Med. Sci. 19 (4), 355–367. 10.17305/bjbms.2019.4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S., Hisamori S., Nishiuchi A., Itatani Y., Obama K., Shimono Y., et al. (2019). F-Box/WD repeat domain-containing 7 induces chemotherapy resistance in colorectal cancer stem cells. Cancers 11 (5), E635. 10.3390/cancers11050635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Huang P., Lan C., Geng S., Xu M., Liu Y., et al. (2022). ZC3H15 promotes gastric cancer progression by targeting the FBXW7/c-Myc pathway. Cell Death Discov. 8 (1), 32. 10.1038/s41420-022-00815-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. L., Wang W., Lan X. L., Zeng Z. C., Liang Y. S., Yan Y. R., et al. (2019). CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 18 (1), 91. 10.1186/s12943-019-1019-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Long K. (2023). Sensitization of colon cancer cells to cisplatin by Fbxw7 via negative regulation of the Nox1-mTOR pathway. Pathol. Res. Pract. 247, 154479. 10.1016/j.prp.2023.154479 [DOI] [PubMed] [Google Scholar]
- Huang L. Y., Zhao J., Chen H., Wan L., Inuzuka H., Guo J., et al. (2018). SCFFBW7-mediated degradation of Brg1 suppresses gastric cancer metastasis. Nat. Commun. 9, 3569. 10.1038/s41467-018-06038-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Xiang Z., Wu H., He Q., Dou R., Lin Z., et al. (2022). The lncRNA BDNF-AS/WDR5/FBXW7 axis mediates ferroptosis in gastric cancer peritoneal metastasis by regulating VDAC3 ubiquitination. Int. J. Biol. Sci. 18 (4), 1415–1433. 10.7150/ijbs.69454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Lucero-Prisno D. E., Zhang L., Xu W., Wong S. H., Ng S. C., et al. (2023). Updated epidemiology of gastrointestinal cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 20 (5), 271–287. 10.1038/s41575-022-00726-3 [DOI] [PubMed] [Google Scholar]
- I B. A., Hwm van L., E F., R O., M N., F L. (2023). Early-onset cancer in the gastrointestinal tract is on the rise-evidence and implications. Cancer Discov. 13 (3), 538–551. 10.1158/2159-8290.CD-22-1038 [DOI] [PubMed] [Google Scholar]
- Inuzuka H., Shaik S., Onoyama I., Gao D., Tseng A., Maser R. S., et al. (2011). SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471 (7336), 104–109. 10.1038/nature09732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Is H., A G., W S., Ap K., G S. M. G., Garg M. (2022). The multidimensional role of the Wnt/β‐catenin signaling pathway in human malignancies. J. Cell Physiol. 237 (1), 199–238. 10.1002/jcp.30561 [DOI] [PubMed] [Google Scholar]
- Ishii N., Araki K., Yokobori T., Gantumur D., Yamanaka T., Altan B., et al. (2017). Reduced FBXW7 expression in pancreatic cancer correlates with poor prognosis and chemotherapeutic resistance via accumulation of MCL1. Oncotarget 8 (68), 112636–112646. 10.18632/oncotarget.22634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki M., Mimori K., Ishii H., Yokobori T., Takatsuno Y., Sato T., et al. (2010). Loss of FBXW7, a cell cycle regulating gene, in colorectal cancer: clinical significance. Int. J. Cancer 126 (8), 1828–1837. 10.1002/ijc.24879 [DOI] [PubMed] [Google Scholar]
- Izumi D., Ishimoto T., Miyake K., Eto T., Arima K., Kiyozumi Y., et al. (2017). Colorectal cancer stem cells acquire chemoresistance through the upregulation of F-box/WD repeat-containing protein 7 and the consequent degradation of c-myc. Stem Cells Dayt Ohio 35 (9), 2027–2036. 10.1002/stem.2668 [DOI] [PubMed] [Google Scholar]
- J T., S T., Z N. C., J Y., F Z., L Z. (2017). FBW7-Dependent mcl-1 degradation mediates the anticancer effect of Hsp90 inhibitors. Mol. Cancer Ther. 16 (9), 1979–1988. 10.1158/1535-7163.MCT-17-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S., Qin Y., Shi S., Liu X., Hu H., Zhou H., et al. (2015). ERK kinase phosphorylates and destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell Res. 25 (5), 561–573. 10.1038/cr.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. X., Sun C. Y., Tian S., Yu C., Chen M. Y., Zhang H. (2016). Tumor suppressor Fbxw7 antagonizes WNT signaling by targeting β-catenin for degradation in pancreatic cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 37 (10), 13893–13902. 10.1007/s13277-016-5217-5 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Qi X., Liu X., Zhang J., Ji J., Zhu Z., et al. (2017). Fbxw7 haploinsufficiency loses its protection against DNA damage and accelerates MNU-induced gastric carcinogenesis. Oncotarget 8 (20), 33444–33456. 10.18632/oncotarget.16800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Izquierdo R., Morrugares R., Suanes-Cobos L., Correa-Sáez A., Garrido-Rodríguez M., Cerero-Tejero L., et al. (2023). FBXW7 tumor suppressor regulation by dualspecificity tyrosine-regulated kinase 2. Cell Death Dis. 14 (3), 202. 10.1038/s41419-023-05724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Yang C., Fan P., Xiao J., Zhang W., Zhan S., et al. (2017). CDK5/FBW7-dependent ubiquitination and degradation of EZH2 inhibits pancreatic cancer cell migration and invasion. J. Biol. Chem. 292 (15), 6269–6280. 10.1074/jbc.M116.764407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S. S., Badgwell B. D. (2021). Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 71 (3), 264–279. 10.3322/caac.21657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam A. E., Masood A., Shroff R. T. (2021). Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol. Hepatol. 6 (11), 956–969. 10.1016/S2468-1253(21)00171-0 [DOI] [PubMed] [Google Scholar]
- Kang H. S., Park H. Y., Lim H., Son I. T., Kim M. J., Kim N. Y., et al. (2023). Different miRNAs related to FBXW7 mutations or high mitotic indices contribute to rectal neuroendocrine tumors: a pilot study. Int. J. Mol. Sci. 24 (7), 6329. 10.3390/ijms24076329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar R., Jha S. K., Ojha S., Sharma A., Dholpuria S., Raju V. S. R., et al. (2021). The FBXW7-NOTCH interactome: a ubiquitin proteasomal system-induced crosstalk modulating oncogenic transformation in human tissues. Cancer Rep. 4 (4), e1369. 10.1002/cnr2.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Newhook T. E., Tran Cao H. S., Tzeng C. W. D., Chun Y. S., Aloia T. A., et al. (2021). Alteration of FBXW7 is associated with worse survival in patients undergoing resection of colorectal liver metastases. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 25 (1), 186–194. 10.1007/s11605-020-04866-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashita Y., Morine Y., Ikemoto T., Saito Y., Iwahashi S., Yamada S., et al. (2017). Loss of Fbxw7 expression is a predictor of recurrence in colorectal liver metastasis. J. Hepato-Biliary-Pancreat Sci. 24 (10), 576–583. 10.1002/jhbp.500 [DOI] [PubMed] [Google Scholar]
- Khan O. M., Carvalho J., Spencer-Dene B., Mitter R., Frith D., Snijders A. P., et al. (2018). The deubiquitinase USP9X regulates FBW7 stability and suppresses colorectal cancer. J. Clin. Invest 128 (4), 1326–1337. 10.1172/JCI97325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan O. M., Almagro J., Nelson J. K., Horswell S., Encheva V., Keyan K. S., et al. (2021a). Proteasomal degradation of the tumour suppressor FBW7 requires branched ubiquitylation by TRIP12. Nat. Commun. 12 (1), 2043. 10.1038/s41467-021-22319-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. Q., Al-Tamimi M., Uddin S., Steinhoff M. (2021b). F-box proteins in cancer stemness: an emerging prognostic and therapeutic target. Drug Discov. Today 26 (12), 2905–2914. 10.1016/j.drudis.2021.07.006 [DOI] [PubMed] [Google Scholar]
- Kitade S., Onoyama I., Kobayashi H., Yagi H., Yoshida S., Kato M., et al. (2016). FBXW7 is involved in the acquisition of the malignant phenotype in epithelial ovarian tumors. Cancer Sci. 107 (10), 1399–1405. 10.1111/cas.13026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y. U., Kim C., Lee J., Kim D., Kim Y., Yun N., et al. (2019). Site-specific phosphorylation of Fbxw7 by Cdk5/p25 and its resulting decreased stability are linked to glutamate-induced excitotoxicity. Cell Death Dis. 10 (8), 579. 10.1038/s41419-019-1818-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga Y., Iwatsuki M., Yamashita K., Kiyozumi Y., Kurashige J., Masuda T., et al. (2019). The role of FBXW7, a cell-cycle regulator, as a predictive marker of recurrence of gastrointestinal stromal tumors. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 22 (6), 1100–1108. 10.1007/s10120-019-00950-y [DOI] [PubMed] [Google Scholar]
- Korphaisarn K., Morris V. K., Overman M. J., Fogelman D. R., Kee B. K., Raghav K. P. S., et al. (2017). FBXW7 missense mutation: a novel negative prognostic factor in metastatic colorectal adenocarcinoma. Oncotarget 8 (24), 39268–39279. 10.18632/oncotarget.16848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari N., Teer J. K., Abbott A. M., Srikumar T., Zhang Y., Yoder S. J., et al. (2016). Increased incidence of FBXW7 and POLE proofreading domain mutations in young adult colorectal cancers. Cancer 122 (18), 2828–2835. 10.1002/cncr.30082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashige J., Watanabe M., Iwatsuki M., Kinoshita K., Saito S., Hiyoshi Y., et al. (2012). Overexpression of microRNA-223 regulates the ubiquitin ligase FBXW7 in oesophageal squamous cell carcinoma. Br. J. Cancer 106 (1), 182–188. 10.1038/bjc.2011.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L V., O T., P V., F D., G D., E C., et al. (2009). Strong correlation between VEGF and MCL-1 mRNA expression levels in B-cell chronic lymphocytic leukemia. Leuk. Res. 33 (12), 1623–1626. 10.1016/j.leukres.2009.05.003 [DOI] [PubMed] [Google Scholar]
- L S., A C., L L., L Y., Y H., J X., et al. (2022). NRG1 regulates Fra-1 transcription and metastasis of triple-negative breast cancer cells via the c-Myc ubiquitination as manipulated by ERK1/2-mediated Fbxw7 phosphorylation. Oncogene 41 (6), 907–919. 10.1038/s41388-021-02142-4 [DOI] [PubMed] [Google Scholar]
- Lan H., Tan M., Zhang Q., Yang F., Wang S., Li H., et al. (2019). LSD1 destabilizes FBXW7 and abrogates FBXW7 functions independent of its demethylase activity. Proc. Natl. Acad. Sci. U. S. A. 116 (25), 12311–12320. 10.1073/pnas.1902012116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. J., An H. J., Kim S. M., Yoo S. M., Park J., Lee G. E., et al. (2020). FBXW7-mediated stability regulation of signal transducer and activator of transcription 2 in melanoma formation. Proc. Natl. Acad. Sci. U. S. A. 117 (1), 584–594. 10.1073/pnas.1909879116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Guo Y., Liang X., Sun M., Wang G., De W., et al. (2012). MicroRNA-223 functions as an oncogene in human gastric cancer by targeting FBXW7/hCdc4. J. Cancer Res. Clin. Oncol. 138 (5), 763–774. 10.1007/s00432-012-1154-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Sarver A. L., Khatri R., Hajeri P. B., Kamenev I., French A. J., et al. (2014). Sequential expression of miR-182 and miR-503 cooperatively targets FBXW7, contributing to the malignant transformation of colon adenoma to adenocarcinoma. J. Pathol. 234 (4), 488–501. 10.1002/path.4407 [DOI] [PubMed] [Google Scholar]
- Li H., Wang Z., Zhang W., Qian K., Xu W., Zhang S. (2016). Fbxw7 regulates tumor apoptosis, growth arrest and the epithelial-to-mesenchymal transition in part through the RhoA signaling pathway in gastric cancer. Cancer Lett. 370 (1), 39–55. 10.1016/j.canlet.2015.10.006 [DOI] [PubMed] [Google Scholar]
- Li M. R., Zhu C. C., Ling T. L., Zhang Y. Q., Xu J., Zhao E. H., et al. (2017a). FBXW7 expression is associated with prognosis and chemotherapeutic outcome in Chinese patients with gastric adenocarcinoma. BMC Gastroenterol. 17 (1), 60. 10.1186/s12876-017-0616-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Ouyang L., Zheng Z., Xiang D., Ti A., Li L., et al. (2017b). E3 ubiquitin ligase FBW7α inhibits cholangiocarcinoma cell proliferation by downregulating c-Myc and cyclin E. Oncol. Rep. 37 (3), 1627–1636. 10.3892/or.2017.5432 [DOI] [PubMed] [Google Scholar]
- Li Q., Li Y., Li J., Ma Y., Dai W., Mo S., et al. (2018). FBW7 suppresses metastasis of colorectal cancer by inhibiting HIF1α/CEACAM5 functional axis. Int. J. Biol. Sci. 14 (7), 726–735. 10.7150/ijbs.24505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Babaei-Jadidi R., Lorenzi F., Spencer-Dene B., Clarke P., Domingo E., et al. (2019). An FBXW7-ZEB2 axis links EMT and tumour microenvironment to promote colorectal cancer stem cells and chemoresistance. Oncogenesis 8 (3), 13. 10.1038/s41389-019-0125-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Deng C., Pan G., Wang X., Zhang K., Dong Z., et al. (2020). Lycorine hydrochloride inhibits cell proliferation and induces apoptosis through promoting FBXW7-MCL1 axis in gastric cancer. J. Exp. Clin. Cancer Res. CR 39, 230. 10.1186/s13046-020-01743-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xu Q., Huang Z. jian, Mao N., Lin Z. tao, Cheng L., et al. (2021). CircRNAs: a new target for the diagnosis and treatment of digestive system neoplasms. Cell Death Dis. 12 (2), 205–213. 10.1038/s41419-021-03495-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Pu W., Zheng Q., Ai M., Chen S., Peng Y. (2022). Proteolysis-targeting chimeras (PROTACs) in cancer therapy. Mol. Cancer 21 (1), 99. 10.1186/s12943-021-01434-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. P., Qu J., Teng X. Q., Zhuang H. H., Dai Y. H., Yang Z., et al. (2023a). The emerging role of super-enhancers as therapeutic targets in the digestive system tumors. Int. J. Biol. Sci. 19 (4), 1036–1048. 10.7150/ijbs.78535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Pan Y., Yao J., Gao Y., Qian Y., Zheng M., et al. (2023b). ANXA2 as a novel substrate of FBXW7 promoting esophageal squamous cell carcinoma via ERK phosphorylation. Biochem. Biophys. Res. Commun. 649, 93–100. 10.1016/j.bbrc.2023.01.082 [DOI] [PubMed] [Google Scholar]
- Lin M., Xu Y., Gao Y., Pan C., Zhu X., Wang Z. W. (2019). Regulation of F-box proteins by noncoding RNAs in human cancers. Cancer Lett. 466, 61–70. 10.1016/j.canlet.2019.09.008 [DOI] [PubMed] [Google Scholar]
- Lin H., Ma N., Zhao L., Yang G., Cao B. (2020a). KDM5c promotes colon cancer cell proliferation through the FBXW7-c-jun regulatory Axis. Front. Oncol. 10, 535449. 10.3389/fonc.2020.535449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Ding D., Xiao X., Li B., Cao P., Li S. (2020b). Trametinib potentiates TRAIL-induced apoptosis via FBW7-dependent Mcl-1 degradation in colorectal cancer cells. J. Cell Mol. Med. 24 (12), 6822–6832. 10.1111/jcmm.15336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Wang X., Zhai S., Shi M., Peng C., Deng X., et al. (2022). Hypoxia-induced exosomal circPDK1 promotes pancreatic cancer glycolysis via c-myc activation by modulating miR-628-3p/BPTF axis and degrading BIN1. J. Hematol. OncolJ Hematol. Oncol. 15 (1), 128. 10.1186/s13045-022-01348-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H., Pickard K., Ivan C., Isella C., Ikuo M., Mitter R., et al. (2016). The clinical and biological significance of mir-224 expression in colorectal cancer metastasis. Gut 65 (6), 977–989. 10.1136/gutjnl-2015-309372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnekamp J. F., Hooff S. R. van, Prasetyanti P. R., Kandimalla R., Buikhuisen J. Y., Fessler E., et al. (2018). Consensus molecular subtypes of colorectal cancer are recapitulated in in vitro and in vivo models. Cell Death Differ. 25 (3), 616–633. 10.1038/s41418-017-0011-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Huang Y., Wang Z., Huang Y., Li X., Louie A., et al. (2013). Temporal mTOR inhibition protects Fbxw7-deficient mice from radiation-induced tumor development. Aging 5 (2), 111–119. 10.18632/aging.100535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhao B., Sun L., Bhuripanyo K., Wang Y., Bi Y., et al. (2017). Orthogonal ubiquitin transfer identifies ubiquitination substrates under differential control by the two ubiquitin activating enzymes. Nat. Commun. 8, 14286. 10.1038/ncomms14286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Gao J., Yang Y., Qiu R., Zheng Y., Huang W., et al. (2018a). PHD finger protein 1 (PHF1) is a novel reader for histone H4R3 symmetric dimethylation and coordinates with PRMT5–WDR77/CRL4B complex to promote tumorigenesis. Nucleic Acids Res. 46 (13), 6608–6626. 10.1093/nar/gky461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang K., Fu H., Song J. (2018b). Low expression of the ubiquitin ligase FBXW7 correlates with poor prognosis of patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 11 (1), 413–419. [PMC free article] [PubMed] [Google Scholar]
- Liu F., Xia Z., Zhang M., Ding J., Feng Y., Wu J., et al. (2019). SMARCAD1 promotes pancreatic cancer cell growth and metastasis through wnt/β-catenin-mediated EMT. Int. J. Biol. Sci. 15 (3), 636–646. 10.7150/ijbs.29562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhao L., Xue L., Hou Y. (2020). Selected updates in molecular and genomic pathology of esophageal cancer. Ann. N. Y. Acad. Sci. 1482 (1), 225–235. 10.1111/nyas.14527 [DOI] [PubMed] [Google Scholar]
- Liu Q., Aminu B., Roscow O., Zhang W. (2021a). Targeting the ubiquitin signaling cascade in tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 22 (2), 791. 10.3390/ijms22020791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Ma T., Duan J., Liu X., Liu L. (2021b). MicroRNA-223-induced inhibition of the FBXW7 gene affects the proliferation and apoptosis of colorectal cancer cells via the Notch and Akt/mTOR pathways. Mol. Med. Rep. 23 (2), 154. 10.3892/mmr.2020.11793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Xiao Q., Xiao J., Niu C., Li Y., Zhang X., et al. (2022). Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target Ther. 7, 3. 10.1038/s41392-021-00762-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet J. M., Montal R., Sia D., Finn R. S. (2018). Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 15 (10), 599–616. 10.1038/s41571-018-0073-4 [DOI] [PubMed] [Google Scholar]
- Lu H., Yao B., Wen X., Jia B. (2019). FBXW7 circular RNA regulates proliferation, migration and invasion of colorectal carcinoma through NEK2, mTOR, and PTEN signaling pathways in vitro and in vivo . BMC Cancer 19 (1), 918. 10.1186/s12885-019-6028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupini L., Bassi C., Mlcochova J., Musa G., Russo M., Vychytilova-Faltejskova P., et al. (2015). Prediction of response to anti-EGFR antibody-based therapies by multigene sequencing in colorectal cancer patients. BMC Cancer 15, 808. 10.1186/s12885-015-1752-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- M Y., S H., T K., M N., R T., H I., et al. (2004a). Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23 (10), 2116–2125. 10.1038/sj.emboj.7600217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- M W., A O., J J., Je G., Jw H., Rn E., et al. (2004b). The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. U. S. A. 101 (24), 9085–9090. 10.1073/pnas.0402770101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- M L., J L., S A., A J., Hf N., F A. M., et al. (2011). MiRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell Cycle Georget Tex 10 (13), 2172–2183. 10.4161/cc.10.13.16248 [DOI] [PubMed] [Google Scholar]
- M G., Y L., Dw F. (2014). MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect. Med. 4 (6), a014241. 10.1101/cshperspect.a014241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Cheng L., Liu H., Zhang J., Shi Y., Zeng F., et al. (2013). Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr. Drug Targets 14 (10), 1150–1156. 10.2174/13894501113149990187 [DOI] [PubMed] [Google Scholar]
- Mammalian cell cycle cyclins (2024). Available at: http://pubmed6.0089.lunwenlib.com/32334991/ [DOI] [PubMed]
- Mao J. H., Perez-Losada J., Wu D., Delrosario R., Tsunematsu R., Nakayama K. I., et al. (2004). Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature 432 (7018), 775–779. 10.1038/nature03155 [DOI] [PubMed] [Google Scholar]
- Mao J. H., Kim I. J., Wu D., Climent J., Kang H. C., DelRosario R., et al. (2008). FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 321 (5895), 1499–1502. 10.1126/science.1162981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Ramos A., Cervantes R. (2023). Expression of mTOR in normal and pathological conditions. Mol. Cancer 22 (1), 112. 10.1186/s12943-023-01820-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli V., Pastorino A., Sobrero A. F. (2022). Prognostic and predictive molecular biomarkers in advanced colorectal cancer. Pharmacol. Ther. 236, 108239. 10.1016/j.pharmthera.2022.108239 [DOI] [PubMed] [Google Scholar]
- Meyer A. E., Furumo Q., Stelloh C., Minella A. C., Rao S. (2020). Loss of Fbxw7 triggers mammary tumorigenesis associated with E2F/c-Myc activation and Trp53 mutation. Neoplasia N. Y. N. 22 (11), 644–658. 10.1016/j.neo.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S. H., Lau A. W., Lee T. H., Inuzuka H., Wei S., Huang P., et al. (2012). Negative regulation of the stability and tumor suppressor function of Fbw7 by the Pin1 prolyl isomerase. Mol. Cell 46 (6), 771–783. 10.1016/j.molcel.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minella A. C., Grim J. E., Welcker M., Clurman B. E. (2007). p53 and SCFFbw7 cooperatively restrain cyclin E-associated genome instability. Oncogene 26 (48), 6948–6953. 10.1038/sj.onc.1210518 [DOI] [PubMed] [Google Scholar]
- Mittal P., Singh S., Sinha R., Shrivastava A., Singh A., Singh I. K. (2021). Myeloid cell leukemia 1 (MCL-1): structural characteristics and application in cancer therapy. Int. J. Biol. Macromol. 187, 999–1018. 10.1016/j.ijbiomac.2021.07.166 [DOI] [PubMed] [Google Scholar]
- Mojsa B., Lassot I., Desagher S. (2014). Mcl-1 ubiquitination: unique regulation of an essential survival protein. Cells 3 (2), 418–437. 10.3390/cells3020418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel C., Carlson S. M., White F. M., Davis R. J. (2009). Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol. Cell Biol. 29 (14), 3845–3852. 10.1128/MCB.00279-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A., Masuda K., Ohtsuka H., Shijo M., Ariake K., Fukase K., et al. (2018). FBXW7 modulates malignant potential and cisplatin-induced apoptosis in cholangiocarcinoma through NOTCH1 and MCL1. Cancer Sci. 109 (12), 3883–3895. 10.1111/cas.13829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y., Zou H., Chen B., Fan Y., Luo S. (2017). FAM83D knockdown regulates proliferation, migration and invasion of colorectal cancer through inhibiting FBXW7/Notch-1 signalling pathway. Biomed. Pharmacother. Biomedecine Pharmacother. 90, 548–554. 10.1016/j.biopha.2017.03.073 [DOI] [PubMed] [Google Scholar]
- Mx Z., H W., Gp S. (2020). Tumor-suppressor Fbxw7 targets SIK2 for degradation to interfere with TORC2-AKT signaling in pancreatic cancer. Cell Biol. Int. 44 (9), 1900–1910. 10.1002/cbin.11396 [DOI] [PubMed] [Google Scholar]
- N K., A S., I A. (2015). Emerging roles for the FBXW7 ubiquitin ligase in leukemia and beyond. Curr. Opin. Cell Biol. 37, 28–34. 10.1016/j.ceb.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault J. C., Villanueva A. (2021). Biomarkers for hepatobiliary cancers. Hepatol. Balt. Md 73 (Suppl. 1), 115–127. 10.1002/hep.31175 [DOI] [PubMed] [Google Scholar]
- Nemecek R., Berkovcova J., Radova L., Kazda T., Mlcochova J., Vychytilova-Faltejskova P., et al. (2016). Mutational analysis of primary and metastatic colorectal cancer samples underlying the resistance to cetuximab-based therapy. OncoTargets Ther. 9, 4695–4703. 10.2147/OTT.S102891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Lu L., Du C., Gao X. (2022). FAM83D promotes the proliferation and migration of hepatocellular carcinoma cells by inhibiting the FBXW7/MCL1 pathway. Transl. Cancer Res. 11 (10), 3790–3802. 10.21037/tcr-22-2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notch signaling pathway in cancer: from mechanistic insights to targeted therapies. 2024. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11128457/ [DOI] [PMC free article] [PubMed]
- Notch1 in Cancer Therapy (2024). Possible clinical implications and challenges. Available at: https://pubmed.ncbi.nlm.nih.gov/32913140/. [DOI] [PubMed]
- Okazaki H., Matsunaga N., Fujioka T., Okazaki F., Akagawa Y., Tsurudome Y., et al. (2014). Circadian regulation of mTOR by the ubiquitin pathway in renal cell carcinoma. Cancer Res. 74 (2), 543–551. 10.1158/0008-5472.CAN-12-3241 [DOI] [PubMed] [Google Scholar]
- Ou B., Zhao J., Guan S., Wangpu X., Zhu C., Zong Y., et al. (2016). Plk2 promotes tumor growth and inhibits apoptosis by targeting Fbxw7/Cyclin E in colorectal cancer. Cancer Lett. 380 (2), 457–466. 10.1016/j.canlet.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Pai S. G., Carneiro B. A., Mota J. M., Costa R., Leite C. A., Barroso-Sousa R., et al. (2017). Wnt/beta-catenin pathway: modulating anticancer immune response. J. Hematol. OncolJ Hematol. Oncol. 10, 101. 10.1186/s13045-017-0471-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Liu J., Gao Y., Guo Y., Wang C., Liang Z., et al. (2023). FBXW7 loss of function promotes esophageal squamous cell carcinoma progression via elevating MAP4 and ERK phosphorylation. J. Exp. Clin. Cancer Res. CR 42 (1), 75. 10.1186/s13046-023-02630-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancreatic Cancer Pancreatic cancer: changing epidemiology and new approaches to risk assessment, early detection, and prevention - PubMed Available at: https://pubmed.ncbi.nlm.nih.gov/36804602/ . 10.1053/j.gastro.2023.02.012 [DOI] [PMC free article] [PubMed]
- Park J., Cho J., Song E. J. (2020). Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch. Pharm. Res. 43 (11), 1144–1161. 10.1007/s12272-020-01281-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavicevic S., Reichelt S., Uluk D., Lurje I., Engelmann C., Modest D. P., et al. (2022). Prognostic and predictive molecular markers in cholangiocarcinoma. Cancers 14 (4), 1026. 10.3390/cancers14041026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Losada J., Mao J. H., Balmain A. (2005). Control of genomic instability and epithelial tumor development by the p53-Fbxw7/Cdc4 pathway. Cancer Res. 65 (15), 6488–6492. 10.1158/0008-5472.CAN-05-1294 [DOI] [PubMed] [Google Scholar]
- Pervin S., Tran A., Tran L., Urman R., Braga M., Chaudhuri G., et al. (2011). Reduced association of anti-apoptotic protein Mcl-1 with E3 ligase Mule increases the stability of Mcl-1 in breast cancer cells. Br. J. Cancer 105 (3), 428–437. 10.1038/bjc.2011.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart C. M. (2001). Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533. 10.1146/annurev.biochem.70.1.503 [DOI] [PubMed] [Google Scholar]
- Q H., Y Q., B Z., C L., S J., S S., et al. (2017). FBW7 increases the chemosensitivity of pancreatic cancer cells to gemcitabine through upregulation of ENT1. Oncol. Rep. 38 (4), 2069–2077. 10.3892/or.2017.5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Q Z., L H., Y G., Z X., Q X., X T. (2020). FBW7 in hematological tumors. Oncol. Lett. 19 (3), 1657–1664. 10.3892/ol.2020.11264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Rezaeian A. H., Wang J., Huang D., Chen H., Inuzuka H., et al. (2024). Molecular insights and clinical implications for the tumor suppressor role of SCFFBXW7 E3 ubiquitin ligase. Biochim. Biophys. Acta Rev. Cancer 1879 (5), 189140. 10.1016/j.bbcan.2024.189140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Hu Q., Xu J., Ji S., Dai W., Liu W., et al. (2019). PRMT5 enhances tumorigenicity and glycolysis in pancreatic cancer via the FBW7/cMyc axis. Cell Commun. Signal CCS 17 (1), 30. 10.1186/s12964-019-0344-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R S., S M., J X., X R., P G., H L., et al. (2023). A novel polypeptide encoded by the circular RNA ZKSCAN1 suppresses HCC via degradation of mTOR. Mol. Cancer 22 (1), 16. 10.1186/s12943-023-01719-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues P. M., Vogel A., Arrese M., Balderramo D. C., Valle J. W., Banales J. M. (2021). Next-generation biomarkers for cholangiocarcinoma. Cancers 13 (13), 3222. 10.3390/cancers13133222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roling L., Flammersfeld A., Pradel G., Bennink S. (2022). The WD40-protein PfWLP1 ensures stability of the PfCCp-based adhesion protein complex in plasmodium falciparum gametocytes. Front. Cell Infect. Microbiol. 12, 942364. 10.3389/fcimb.2022.942364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan F., Ruan Y., Gu H., Sun J., Chen Q. (2024). Clitocine enhances the drug sensitivity of colon cancer cells by promoting FBXW7-mediated MCL-1 degradation via inhibiting the A2B/cAMP/ERK axis. Am. J. Physiol-Cell Physiol. 327, C884–C900. 10.1152/ajpcell.00310.2024 [DOI] [PubMed] [Google Scholar]
- S I., Lo T., T U., Y M., T I., Y A., et al. (2014). Role of Fbxw7 expression in hepatocellular carcinoma and adjacent non-tumor liver tissue. J. Gastroenterol. Hepatol. 29 (10), 1822–1829. 10.1111/jgh.12623 [DOI] [PubMed] [Google Scholar]
- Sailo B. L., Banik K., Girisa S., Bordoloi D., Fan L., Halim C. E., et al. (2019). FBXW7 in cancer: what has been unraveled thus far? Cancers 11 (2), 246. 10.3390/cancers11020246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R., Blake S. M., Tendeng C., Clurman B. E., Lewis J., Behrens A. (2013). Fbw7 repression by hes5 creates a feedback loop that modulates Notch-mediated intestinal and neural stem cell fate decisions. PLoS Biol. 11 (6), e1001586. 10.1371/journal.pbio.1001586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R., Gruber R., Gu G., Behrens A. (2014). Loss of Fbw7 reprograms adult pancreatic ductal cells into α, δ, and β cells. Cell Stem Cell 15 (2), 139–153. 10.1016/j.stem.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Tyers M., Torrent M., Arrowsmith C. H. (2017). WD-repeat domain proteins: a novel target class? Nat. Rev. Drug Discov. 16 (11), 773–786. 10.1038/nrd.2017.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. J., Yang M., Teer J. K., Lo F. Y., Madan A., Coppola D., et al. (2016). A multigene mutation classification of 468 colorectal cancers reveals a prognostic role for APC. Nat. Commun. 7, 11743. 10.1038/ncomms11743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlick K., Kiem D., Greil R. (2021). Recent advances in pancreatic cancer: novel prognostic biomarkers and targeted therapy—a review of the literature. Biomolecules 11 (10), 1469. 10.3390/biom11101469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schülein C., Eilers M., Popov N. (2011). PI3K-dependent phosphorylation of Fbw7 modulates substrate degradation and activity. FEBS Lett. 585 (14), 2151–2157. 10.1016/j.febslet.2011.05.036 [DOI] [PubMed] [Google Scholar]
- Senichkin V. V., Streletskaia A. Y., Gorbunova A. S., Zhivotovsky B., Kopeina G. S. (2020). Saga of Mcl-1: regulation from transcription to degradation. Cell Death Differ. 27 (2), 405–419. 10.1038/s41418-019-0486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentani K., Oue N., Kondo H., Kuraoka K., Motoshita J., Ito R., et al. (2001). Increased expression but not genetic alteration of BRG1, a component of the SWI/SNF complex, is associated with the advanced stage of human gastric carcinomas. Pathobiol. J. Immunopathol. Mol. Cell Biol. 69 (6), 315–320. 10.1159/000064638 [DOI] [PubMed] [Google Scholar]
- Sepulveda A. R., Hamilton S. R., Allegra C. J., Grody W., Cushman-Vokoun A. M., Funkhouser W. K., et al. (2017). Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American society for clinical pathology, college of American pathologists, association for molecular pathology, and the American society of clinical oncology. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 35 (13), 1453–1486. 10.1200/JCO.2016.71.9807 [DOI] [PubMed] [Google Scholar]
- Shang W., Yan C., Liu R., Chen L., Cheng D., Hao L., et al. (2021). Clinical significance of FBXW7 tumor suppressor gene mutations and expression in human colorectal cancer: a systemic review and meta-analysis. BMC Cancer 21 (1), 770. 10.1186/s12885-021-08535-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy A. R., Kirschnek S., Häcker G. (2014). IL-15 regulates Bcl-2 family members Bim and Mcl-1 through JAK/STAT and PI3K/AKT pathways in T cells. Eur. J. Immunol. 44 (8), 2500–2507. 10.1002/eji.201344238 [DOI] [PubMed] [Google Scholar]
- Shima T., Taniguchi K., Inomata Y., Arima J., Lee S. W. (2024). Glycolysis in gastrointestinal stromal tumor: a brief overview. Neoplasia N. Y. 55, 101022. 10.1016/j.neo.2024.101022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Giaquinto A. N., Jemal A. (2024). Cancer statistics, 2024. CA Cancer J. Clin. 74 (1), 12–49. 10.3322/caac.21820 [DOI] [PubMed] [Google Scholar]
- Skaar J. R., Pagan J. K., Pagano M. (2013). Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell Biol. 14 (6), 369–381. 10.1038/nrm3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Shen L., Tong J., Kuang C., Zeng S., Schoen R. E., et al. (2020). Mcl-1 inhibition overcomes intrinsic and acquired regorafenib resistance in colorectal cancer. Theranostics 10 (18), 8098–8110. 10.7150/thno.45363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P., Gao Z., Bao Y., Chen L., Huang Y., Liu Y., et al. (2024). Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. OncolJ Hematol. Oncol. 17 (1), 46. 10.1186/s13045-024-01563-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. F., Sun J. P., Hou H. T., Li K., Liu X., Ge Q. X. (2016). MicroRNA-27b exerts an oncogenic function by targeting Fbxw7 in human hepatocellular carcinoma. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 37 (11), 15325–15332. 10.1007/s13277-016-5444-9 [DOI] [PubMed] [Google Scholar]
- Sun Y., Nie W., Qiu B., Yang Q., Zhao H. (2023). FBXW7 affects autophagy through MCL1 in oral squamous cell carcinoma. Oral Dis. 29, 3259–3267. 10.1111/odi.14325 [DOI] [PubMed] [Google Scholar]
- Sun D., Mülder D. T., Li Y., Nieboer D., Park J. Y., Suh M., et al. (2024). The effect of nationwide organized cancer screening programs on gastric cancer mortality: a synthetic control study. Gastroenterology 166 (3), 503–514. 10.1053/j.gastro.2023.11.286 [DOI] [PubMed] [Google Scholar]
- T M., C G., Cyclin E. (2004). Int. J. Biochem. Cell Biol. 36 (8). 10.1016/j.biocel.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Taieb J., Jung A., Sartore-Bianchi A., Peeters M., Seligmann J., Zaanan A., et al. (2019). The evolving biomarker landscape for treatment selection in metastatic colorectal cancer. Drugs 79 (13), 1375–1394. 10.1007/s40265-019-01165-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M., Zhang W., Suzuki A., Kuroda T. S., Yu Z., Inuzuka H., et al. (2017). FBW7 loss promotes chromosomal instability and tumorigenesis via cyclin E1/CDK2-mediated phosphorylation of CENP-A. Cancer Res. 77 (18), 4881–4893. 10.1158/0008-5472.CAN-17-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi S., Nakayama K. I. (2014). Role of Fbxw7 in the maintenance of normal stem cells and cancer-initiating cells. Br. J. Cancer 111 (6), 1054–1059. 10.1038/bjc.2014.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi S., Nakayama K. I. (2016). To wake up cancer stem cells, or to let them sleep, that is the question. Cancer Sci. 107 (7), 875–881. 10.1111/cas.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi S., Matsumoto A., Onoyama I., Naka K., Hirao A., Nakayama K. I. (2013). Ablation of Fbxw7 eliminates leukemia-initiating cells by preventing quiescence. Cancer Cell 23 (3), 347–361. 10.1016/j.ccr.2013.01.026 [DOI] [PubMed] [Google Scholar]
- Tang B., Lei B., Qi G., Liang X., Tang F., Yuan S., et al. (2016). MicroRNA-155-3p promotes hepatocellular carcinoma formation by suppressing FBXW7 expression. J. Exp. Clin. Cancer Res. CR 35 (1), 93. 10.1186/s13046-016-0371-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Yang W., Shu Z., Shen X., Zhang W., Cen C., et al. (2019). MicroRNA-223 promotes hepatocellular carcinoma cell resistance to sorafenib by targeting FBW7. Oncol. Rep. 41 (2), 1231–1237. 10.3892/or.2018.6908 [DOI] [PubMed] [Google Scholar]
- Tang G. L. Q., Lai J. X. H., Pervaiz S. (2023). Ubiquitin-proteasome pathway-mediated regulation of the Bcl-2 family: effects and therapeutic approaches. Haematologica 109 (1), 33–43. 10.3324/haematol.2023.283730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targeting cell-cycle machinery in cancer - PubMed (2024). Available at: http://pubmed6.0089.lunwenlib.com/33891890/
- Tekcham D. S., Chen D., Liu Y., Ling T., Zhang Y., Chen H., et al. (2020). F-box proteins and cancer: an update from functional and regulatory mechanism to therapeutic clinical prospects. Theranostics 10 (9), 4150–4167. 10.7150/thno.42735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirimanne H. N., Wu F., Janssens D. H., Swanger J., Diab A., Feldman H. M., et al. (2022). Global and context-specific transcriptional consequences of oncogenic Fbw7 mutations. eLife 11, e74338. 10.7554/eLife.74338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J., Tan S., Zou F., Yu J., Zhang L. (2017). FBW7 mutations mediate resistance of colorectal cancer to targeted therapies by blocking Mcl-1 degradation. Oncogene 36 (6), 787–796. 10.1038/onc.2016.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu K., Yang W., Li C., Zheng X., Lu Z., Guo C., et al. (2014). Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma. Mol. Cancer 13 (1), 110. 10.1186/1476-4598-13-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venizelos A., Elvebakken H., Perren A., Nikolaienko O., Deng W., Lothe I. M. B., et al. (2021). The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 29 (1), 1–14. 10.1530/ERC-21-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu Y., Lu J., Zhang P., Wang Y., Xu Y., et al. (2013). Rapamycin inhibits FBXW7 loss-induced epithelial-mesenchymal transition and cancer stem cell-like characteristics in colorectal cancer cells. Biochem. Biophys. Res. Commun. 434 (2), 352–356. 10.1016/j.bbrc.2013.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang J., Zhou L., Sun W., Zheng Z. G., Lu P., et al. (2015). Fbxw7 regulates hepatocellular carcinoma migration and invasion via Notch1 signaling pathway. Int. J. Oncol. 47 (1), 231–243. 10.3892/ijo.2015.2981 [DOI] [PubMed] [Google Scholar]
- Wang J., Wang H., Peters M., Ding N., Ribback S., Utpatel K., et al. (2019). Loss of Fbxw7 synergizes with activated Akt signaling to promote c-Myc dependent cholangiocarcinogenesis. J. Hepatol. 71 (4), 742–752. 10.1016/j.jhep.2019.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zheng Y., Yang F., Zhu L., Zhu X. Q., Wang Z. F., et al. (2021a). The molecular biology of pancreatic adenocarcinoma: translational challenges and clinical perspectives. Signal Transduct. Target Ther. 6 (1), 249. 10.1038/s41392-021-00659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Guo M., Wei H., Chen Y. (2021b). Targeting MCL-1 in cancer: current status and perspectives. J. Hematol. OncolJ Hematol. Oncol. 14, 67. 10.1186/s13045-021-01079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. P., Chen W. J., Shen J. M., Ye T., Xie H. W. (2021c). Attenuating glucose metabolism by Fbxw7 promotes Taxol sensitivity of colon cancer cells through downregulating NADPH oxidase 1 (Nox1). Ann. Transl. Med. 9 (10), 886. 10.21037/atm-21-2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Ye L., Li H., Chen W., Hong W., Mao W., et al. (2025). A narrative review on advances in neoadjuvant immunotherapy for esophageal cancer: molecular biomarkers and future directions. Int. J. Cancer 156, 20–33. 10.1002/ijc.35153 [DOI] [PubMed] [Google Scholar]
- Wei W., Qin B., Wen W., Zhang B., Luo H., Wang Y., et al. (2023). FBXW7β loss-of-function enhances FASN-mediated lipogenesis and promotes colorectal cancer growth. Signal Transduct. Target Ther. 8 (1), 187. 10.1038/s41392-023-01405-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker M., Clurman B. E. (2007). Fbw7/hCDC4 dimerization regulates its substrate interactions. Cell Div. 2, 7. 10.1186/1747-1028-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker M., Clurman B. E. (2008). FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer 8 (2), 83–93. 10.1038/nrc2290 [DOI] [PubMed] [Google Scholar]
- Welcker M., Larimore E. A., Swanger J., Bengoechea-Alonso M. T., Grim J. E., Ericsson J., et al. (2013). Fbw7 dimerization determines the specificity and robustness of substrate degradation. Genes Dev. 27 (23), 2531–2536. 10.1101/gad.229195.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson J. (2022). DUBTACs for targeted protein stabilization. Nat. Rev. Drug Discov. 21 (4), 258. 10.1038/d41573-022-00039-9 [DOI] [PubMed] [Google Scholar]
- Wood K. C. (2020). Overcoming MCL-1-driven adaptive resistance to targeted therapies. Nat. Commun. 11 (1), 531. 10.1038/s41467-020-14392-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. Z., Wang K. P., Song H. J., Xia J. H., Jiang Y., Wang Y. L. (2015). MiR-27a-3p promotes esophageal cancer cell proliferation via F-box and WD repeat domain-containing 7 (FBXW7) suppression. Int. J. Clin. Exp. Med. 8 (9), 15556–15562. [PMC free article] [PubMed] [Google Scholar]
- Wu W. J., Shi J., Hu G., Yu X., Lu H., Yang M. L., et al. (2016). Wnt/β-catenin signaling inhibits FBXW7 expression by upregulation of microRNA-770 in hepatocellular carcinoma. Tumor Biol. 37 (5), 6045–6051. 10.1007/s13277-015-4452-5 [DOI] [PubMed] [Google Scholar]
- Wu X., Iwatsuki M., Takaki M., Saito T., Hayashi T., Kondo M., et al. (2024). FBXW7 regulates the sensitivity of imatinib in gastrointestinal stromal tumors by targeting MCL1. Gastric Cancer 27 (2), 235–247. 10.1007/s10120-023-01454-6 [DOI] [PubMed] [Google Scholar]
- X C., X X., D C., F Z., W W. (2019a). Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. Biomedecine Pharmacother. 110, 473–481. 10.1016/j.biopha.2018.11.082 [DOI] [PubMed] [Google Scholar]
- X K., L L., R C., K W., M C., B C., et al. (2019b). SCFFBXW7/GSK3β-Mediated GFI1 degradation suppresses proliferation of gastric cancer cells. Cancer Res. 79 (17), 4387–4398. 10.1158/0008-5472.CAN-18-4032 [DOI] [PubMed] [Google Scholar]
- X W., Y L., P L., S L., Y P., Pan Y. (2022). FBXW7 reduces the cancer stem cell-like properties of hepatocellular carcinoma by regulating the ubiquitination and degradation of ACTL6A. Stem Cells Int. 2022, 3242482. 10.1155/2022/3242482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Zhou J., Luo H., Liu Y., Peng C., Zheng W., et al. (2017). MicroRNA-32 promotes cell proliferation, migration and suppresses apoptosis in breast cancer cells by targeting FBXW7. Cancer Cell Int. 17, 14. 10.1186/s12935-017-0383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J., Hang J. B., Che J. M., Li H. C. (2015). miR-25 is up-regulated in non-small cell lung cancer and promotes cell proliferation and motility by targeting FBXW7. Int. J. Clin. Exp. Pathol. 8 (8), 9147–9153. [PMC free article] [PubMed] [Google Scholar]
- Xie C. M., Sun Y. (2019). The MTORC1-mediated autophagy is regulated by the FBXW7-SHOC2-RPTOR axis. Autophagy 15 (8), 1470–1472. 10.1080/15548627.2019.1609864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Li H., Gao C., Lai Y., Liang J., Chen Z., et al. (2022). Downregulation of circular RNA circPSD3 promotes metastasis by modulating FBXW7 expression in clear cell renal cell carcinoma. J. Oncol. 2022 (1), 5084631. 10.1155/2022/5084631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Sengupta T., Kukreja L., Minella A. C. (2010). MicroRNA-223 regulates cyclin E activity by modulating expression of F-box and WD-40 domain protein 7. J. Biol. Chem. 285 (45), 34439–34446. 10.1074/jbc.M110.152306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Shan B., Lee B. H., Zhu K., Zhang T., Sun H., et al. (2015). Phosphorylation and activation of ubiquitin-specific protease-14 by Akt regulates the ubiquitin-proteasome system. eLife 4, e10510. 10.7554/eLife.10510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Taranets L., Popov N. (2016). Regulating Fbw7 on the road to cancer. Semin. Cancer Biol. 36, 62–70. 10.1016/j.semcancer.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Y I., I O., Ki N., K N. (2008). Notch-dependent cell cycle arrest and apoptosis in mouse embryonic fibroblasts lacking Fbxw7. Oncogene 27 (47), 6164–6174. 10.1038/onc.2008.216 [DOI] [PubMed] [Google Scholar]
- Y N., H I., Y K., M K., A M., T K., et al. (2010). Decreased expression of FBXW7 is correlated with poor prognosis in patients with esophageal squamous cell carcinoma. Exp. Ther. Med. 1 (5), 841–846. 10.3892/etm.2010.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Y L., Y S., Y Z., X H., G Z., J H., et al. (2021). Demethylzeylasteral inhibits proliferation, migration, and invasion through FBXW7/c-Myc axis in gastric cancer. MedComm 2 (3), 467–480. 10.1002/mco2.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Y L., H C., H B., J Z., R W., L Z. (2023). Comprehensive characterization of FBXW7 mutational and clinicopathological profiles in human colorectal cancers. Front. Oncol. 13, 1154432. 10.3389/fonc.2023.1154432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Dou C., Wang Y., Jia Y., Li C., Zheng X., et al. (2015). MicroRNA-92a contributes to tumor growth of human hepatocellular carcinoma by targeting FBXW7. Oncol. Rep. 34 (5), 2576–2584. 10.3892/or.2015.4210 [DOI] [PubMed] [Google Scholar]
- Yang Q., Sun Y., Qiu B., Zhao H. (2022). FBXW7 enhances cisplatin-induced apoptosis in oral cancer cell lines. Int. Dent. J. 73 (5), 620–627. 10.1016/j.identj.2022.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Zhuo Q., Hu Q., Xu X., liu M., Zhang Z., et al. (2020). FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox Biol. 38, 101807. 10.1016/j.redox.2020.101807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. H., Bellon M., Nicot C. (2018). FBXW7: a critical tumor suppressor of human cancers. Mol. Cancer 17 (1), 115. 10.1186/s12943-018-0857-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokobori T., Mimori K., Iwatsuki M., Ishii H., Onoyama I., Fukagawa T., et al. (2009). p53-Altered FBXW7 expression determines poor prognosis in gastric cancer cases. Cancer Res. 69 (9), 3788–3794. 10.1158/0008-5472.CAN-08-2846 [DOI] [PubMed] [Google Scholar]
- Yokobori T., Mimori K., Iwatsuki M., Ishii H., Tanaka F., Sato T., et al. (2012). Copy number loss of FBXW7 is related to gene expression and poor prognosis in esophageal squamous cell carcinoma. Int. J. Oncol. 41 (1), 253–259. 10.3892/ijo.2012.1436 [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang W., Gao F., Liu Y. X., Chen Z. Y., Cheng L. Y., et al. (2014). FBW7 increases chemosensitivity in hepatocellular carcinoma cells through suppression of epithelial-mesenchymal transition. Hepatobiliary Pancreat. Dis. Int. HBPD Int. 13 (2), 184–191. 10.1016/s1499-3872(14)60029-1 [DOI] [PubMed] [Google Scholar]
- Yu H., Ling T., Shi R., Shu Q., Li Y., Tan Z. (2015). Expression of FBXW7 in esophageal squamous cell carcinoma and its clinical significance. Zhonghua Zhong Liu Za Zhi 37 (5), 347–351. 10.3760/cma.j.issn.0253-3766.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Yu F., Yu C., Li F., Zuo Y., Wang Y., Yao L., et al. (2021). Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target Ther. 6, 307. 10.1038/s41392-021-00701-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumimoto K., Nakayama K. I. (2020). Recent insight into the role of FBXW7 as a tumor suppressor. Semin. Cancer Biol. 67 (Pt 2), 1–15. 10.1016/j.semcancer.2020.02.017 [DOI] [PubMed] [Google Scholar]
- Zeng J., Chen Z., He Y., Jiang Z., Zhang Y., Dong Q., et al. (2024). A patent review of SCF E3 ligases inhibitors for cancer: structural design, pharmacological activities and structure–activity relationship. Eur. J. Med. Chem. 278, 116821. 10.1016/j.ejmech.2024.116821 [DOI] [PubMed] [Google Scholar]
- Zhan P., Wang Y., Zhao S., Liu C., Wang Y., Wen M., et al. (2015). FBXW7 negatively regulates ENO1 expression and function in colorectal cancer. Lab. Investig. J. Tech. Methods Pathol. 95 (9), 995–1004. 10.1038/labinvest.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang X. (2020). Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. OncolJ Hematol. Oncol. 13 (1), 165. 10.1186/s13045-020-00990-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Cao L., Fan P., Mei Y., Wu M. (2016a). LncRNA‐MIF, a c‐Myc‐activated long non‐coding RNA, suppresses glycolysis by promoting Fbxw7‐mediated c‐Myc degradation. EMBO Rep. 17 (8), 1204–1220. 10.15252/embr.201642067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhang Y., Parsels J. D., Lohse I., Lawrence T. S., Pasca di Magliano M., et al. (2016b). Fbxw7 deletion accelerates KrasG12D-driven pancreatic tumorigenesis via Yap accumulation. Neoplasia N. Y. N. 18 (11), 666–673. 10.1016/j.neo.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Mady A. S. A., Ma Y., Ryan C., Lawrence T. S., Nikolovska-Coleska Z., et al. (2019). The WD40 domain of FBXW7 is a poly(ADP-ribose)-binding domain that mediates the early DNA damage response. Nucleic Acids Res. 47 (8), 4039–4053. 10.1093/nar/gkz058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Li X., Cui K., Liu C., Wu M., Prochownik E. V., et al. (2020). The MAP3K13-TRIM25-FBXW7α axis affects c-Myc protein stability and tumor development. Cell Death Differ. 27 (2), 420–433. 10.1038/s41418-019-0363-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Hofstetter W. L. (2020). Prognostic and therapeutic molecular markers in the clinical management of esophageal cancer. Expert Rev. Mol. Diagn 20 (4), 401–411. 10.1080/14737159.2020.1731307 [DOI] [PubMed] [Google Scholar]
- Zhou Z. yu, sheng Tu K., Zhang J., Zheng X., Gao J., Yao Y. min, et al. (2012). [Expression of Fbxw7 and its correlation with cell proliferation in human hepatocellular carcinoma]. Xi bao yu fen zi mian yi xue za zhi. Chin. J. Cell Mol. Immunol. 28 (12), 1303–1306. [PubMed] [Google Scholar]
- Zhou X., Jin W., Jia H., Yan J., Zhang G. (2015). MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J. Exp. Clin. Cancer Res. CR 34 (1), 28. 10.1186/s13046-015-0145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Virshup D. M. (2024). Recurrent mutations in tumor suppressor FBXW7 bypass Wnt/β-catenin addiction in cancer. Sci. Adv. 10 (14), eadk1031. 10.1126/sciadv.adk1031 [DOI] [PMC free article] [PubMed] [Google Scholar]