In this issue of Haematologica, Minhajuddin et al.1 provide a compelling analysis of the combinatorial therapy of venetoclax and tyrosine kinase inhibitors (TKI) for treating blast phase chronic myeloid leukemia (bpCML). Despite significant improvements in the outcomes of patients with chronic myeloid leukemia (CML) due to the development of TKI, a subset of patients still progresses to bpCML, which is associated with dismal prognosis and a median survival of less than 1 year.2 The treatment of bpCML remains a significant challenge due to drug resistance of leukemia stem cells (LSC),3 requiring the development of new therapeutic strategies that specifically target this resistant population. Preclinical studies have indicated that combining the selective BCL-2 inhibitor venetoclax with TKI holds promise for both chronic phase and bpCML.4 Minhajuddin et al. delve into the mechanistic underpinnings of this combinatorial therapy, identifying a key role for lysosomal acid lipase A (LIPA) in the adaptative response of bpCML LSC and highlighting a new vulnerability of these cells to the disruption of lysosomal function.1
BCL-2 family members are proteins involved in mitochondrial-related apoptosis and have been shown to be critical for the survival of leukemia cells. Indeed, selective inhibition of the anti-apoptotic protein BCL-2 by the Food and Drug Administration-approved compound venetoclax has emerged as a promising strategy for the treatment of various lymphoid and myeloid leukemias.5 In CML, BCL-2 levels are higher than in normal hematopoietic stem cells and are further increased in bpCML.6 Since TKI treatment alone often fails to eradicate CML LSC, combining venetoclax with TKI could enhance treatment efficacy, particularly in bpCML. Minhajuddin et al. demonstrate that the combination of venetoclax and dasatinib does indeed effectively target LSC in both the Bcr-Abl Nup98- Hoxa9 bpCML mouse model and in primary bpCML cells. This combination therapy led to complete elimination of bpCML cells and, importantly, LSC exposed to the drug combination were not able to engraft in secondary recipients, indicating successful eradication of the bpCML LSC compartment. Furthermore, they generated a novel bpCML mouse model harboring the T315I Bcr-Abl mutation in combination with Nup98-Hoxa9 translocation and showed enhanced efficacy of venetoclax in combination with the third-generation TKI ponatinib. This is of particular relevance since more than 40% of bpCML cases harbor tyrosine-kinase domain mutations in BCR-ABL, conferring resistance to earlier-generation TKI.7 It would be of special interest in future studies to assess the efficacy of venetoclax in combination with the next-generation TKI asciminib, which has been shown to be active in CML patients after the failure of ponatinib or other TKI.8
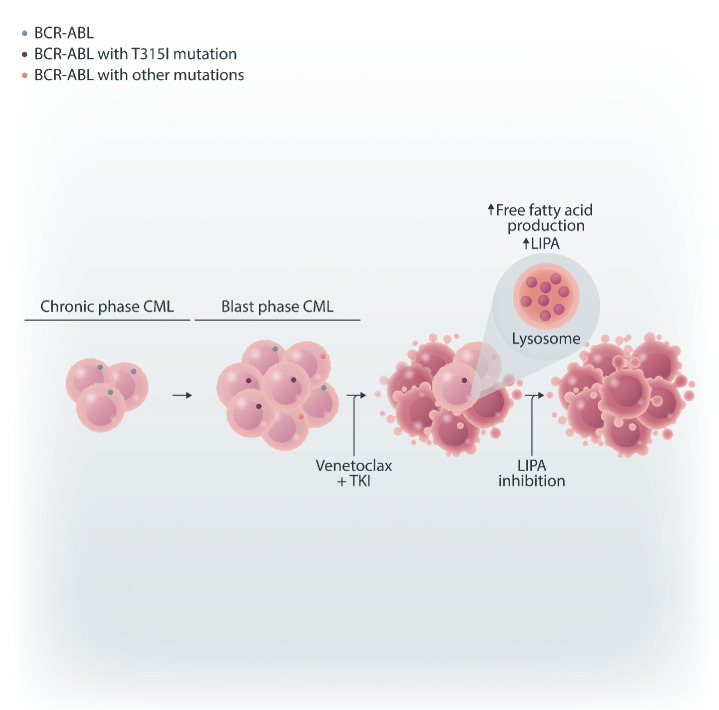
Understanding the molecular mechanisms underlying the bpCML LSC-targeting activity of venetoclax and TKI combination therapy is crucial for anticipating potential mechanisms of resistance and identifying new actionable pathways that could be leveraged to enhance therapeutic outcomes. Minhajuddin et al. performed RNA sequencing of bone marrow LSC-sorted populations treated with dasatinib alone or combined with venetoclax, revealing an upregulation of genes involved in lysosomal biology in cells exposed to the combination therapy. Notably, LIPA, an enzyme involved in free fatty acid regulation, was significantly elevated in LSC treated with both venetoclax and dasatinib, resulting in increased production of several fatty acids such as g-linolenic acid and dihomo-g-linolenic acid. LIPA overexpression in murine bpCML LSC or fatty acid media supplementation conferred partial resistance to the combination therapy. Conversely, LIPA knockout in murine bpCML LSC, LIPA knockdown in primary bpCML cells and inhibition of lysosomal function with bafilomycin increased sensitivity to the treatment. Collectively, these results indicate that LIPA-driven lysosomal and fatty acid pathways contribute to the protective response of bpCML LSC to venetoclax and TKI, laying the ground for further exploration of these pathways as potential targets for inhibition in the context of dual BCL-2 and tyrosine kinase inhibition.
Figure 1.
Lysosomal acid lipase A modulates the response of blast phase chronic myeloid leukemia stem cells to venetoclax and tyrosine kinase inhibition. In blast phase chronic myeloid leukemia (CML), the combination of venetoclax and tyrosine kinase inhibitors (TKI) upregulates lysosomal acid lipase A (LIPA) and other regulators of lysosomal biology, leading to increased free fatty acid production. Inhibiting LIPA or blocking free fatty acid upregulation sensitizes blast phase CML cells to venetoclax/TKI dual therapy.
The implications of fatty acid metabolism in the protective response of LSC to combined venetoclax and TKI therapy may extend to other BCR-ABL-driven malignancies. For instance, it would be critical to understand whether LIPA-dependent fatty acid generation is also involved in Philadelphia-positive B-cell acute lymphoblastic leukemia, in which venetoclax and TKI dual therapy has shown early preclinical synergy.9 If involved, blockade of free fatty acid production through LIPA inhibition would constitute a promising therapeutic strategy in combination with venetoclax/TKI in this subtype of acute leukemia, associated with dismal survival rates in children and adults. Similarly, targeting fatty acid production may be beneficial in lymphoid bpCML cases, in which current TKI and BCL-2 inhibitor combinations have been less successful.10 In summary, the work by Minhajuddin et al. advances our understanding of the preclinical efficacy of venetoclax and TKI combination therapy in bpCML with and without tyrosine kinase mutations, shedding light on the role of lysosomal function and fatty acid metabolism in treatment response. This research paves the way for exploring triple combinatorial therapies to enhance disease-free survival in bpCML patients, potentially transforming treatment approaches for this challenging condition in the future.
References
- 1.Minhajuddin M, Winters A, Ye H, et al. Lysosomal acid lipase A modulates leukemia stem cell response to venetoclax/tyrosine kinase inhibitor combination therapy in blast phase chronic myeloid leukemia. Haematologica. 2025;110(1):103-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107(11):4532-4539. [DOI] [PubMed] [Google Scholar]
- 4.Carter BZ, Mak PY, Mu H, et al. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci Transl Med. 2016;8(355):355ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts AW, Wei AH, Huang DCS. BCL2 and MCL1 inhibitors for hematologic malignancies. Blood. 2021;138(13):1120-1136. [DOI] [PubMed] [Google Scholar]
- 6.Goff DJ, Recart AC, Sadarangani A, et al. A pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell. 2013;12(3):316-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adnan-Awad S, Kankainen M, Mustjoki S. Mutational landscape of chronic myeloid leukemia: more than a single oncogene leukemia. Leuk Lymphoma. 2021;62(9):2064-2078. [DOI] [PubMed] [Google Scholar]
- 8.Hughes TP, Mauro MJ, Cortes JE, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381(24):2315-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moujalled DM, Hanna DT, Hediyeh-Zadeh S, et al. Cotargeting BCL-2 and MCL-1 in high-risk B-ALL. Blood Adv. 2020;4(12):2762-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parry N, Busch C, Aßmann V, et al. BH3 mimetics in combination with nilotinib or ponatinib represent a promising therapeutic strategy in blast phase chronic myeloid leukemia. Cell Death Discov. 2022;8(1):457. [DOI] [PMC free article] [PubMed] [Google Scholar]