Abstract
Clear cell renal cell carcinoma is the predominant subtype of kidney cancer. With distant metastasis, the overall survival rate for patients with renal cell carcinoma decreases significantly compared to localized disease. However, pembrolizumab plus axitinib combination is safe and improves long-term survival. Herein, we report a case of a pathological complete response from systemic pembrolizumab plus axitinib therapy in a 57-year-old male with locally advanced renal cell carcinoma with extensive kidney, inferior vena cava, and hepatic invasion, as well as metastatic disease to the retroperitoneal lymph nodes and lung. After 2-years of systemic treatment, there was absent radiographic evidence of renal cell carcinoma in the lung. The patient underwent right radical nephrectomy, adrenalectomy, partial hepatectomy, and inferior vena cava ligation. Pathology indicated no evidence of tumor consistent with a pathological complete response. This case highlights the possibility of a pathological complete response with pembrolizumab plus axitinib in metastatic renal cell carcinoma and potential disparate findings between radiological response and pathological response.
Keywords: Immunotherapy, inferior vena cava, ligations, urologic neoplasms
Introduction
Kidney cancer incidence has been on the rise over the past decade, largely attributed to increased abdominal imaging and incidental imaging. Advancements in medical diagnostic technology have contributed to heightened rates of early renal cell carcinoma (RCC) detection and favorable clinical prognoses. Among RCC cases, the clear cell histological subtype is the most common. 1 Approximately 30% of RCC cases with localized primary tumors will develop metastatic disease. The 5-year survival rate of metastatic RCC is 12% although improving steadily with the advent of combination immunotherapy-based treatments. 1
Sunitinib was previously considered the standard of care for advanced RCC. However, a recent randomized, open label, phase 3 KEYNOTE-426 trial investigating the combination of pembrolizumab and axitinib demonstrated an improvement in overall survival compared with sunitinib. 2 For patients with advanced clear cell RCC that is not suitable for immunotherapy, sunitinib and pazopanib as monotherapy can be considered. The STAR trial found that a drug-free interval treatment with tyrosine kinase inhibitor (TKI) monotherapy demonstrated noninferiority when compared to conventional continuation. 3 While no clinically significant reduction in life expectancy was observed, treatment breaks in patients receiving second-line TKI monotherapy should be approached prudently as these patients typically have shorter progression-free survival. In addition to this era of immunotherapy, a recent phase III trial examining belzutifan, a small-molecule inhibitor of hypoxia inducible factor 2α (HIF-2α) found increased progression-free survival and objective response compared to everolimus in patients with advanced clear-cell RCC previously receiving antiangiogenic and immune checkpoint therapies. 4
Currently, the combination of pembrolizumab and axitinib is approved by the U.S. Food and Drug Administration for the initial treatment of metastatic RCC. 5 To date, there is a paucity of cases documenting a pathological complete response (pCR) using this treatment approach, and there have been concerns regarding cytoreductive nephrectomy in the evolving landscape of immunotherapy.6,7
This case represents the first reported instance of a pCR in metastatic RCC following pembrolizumab and axitinib, with the patient also undergoing inferior vena cava (IVC) ligation during cytoreductive nephrectomy to manage persistent tumor thrombus. It demonstrates the need to optimize surgical and systemic treatment strategies in selected patients.
Case presentation
Case description
A 57-year-old male was referred for an evaluation of metastatic RCC to the lung after presenting with a cough. He has a past medical history of gastroesophageal reflux disease. His labs on presentation were significant for anemia (13.3 g/dL), but he had normal absolute neutrophil count (5.1 × 109/L), platelet count (252 × 109/L), and calcium. He was a prior heavy alcohol user (10–15 beers per day) with a 40-pack year smoking history. The patient had no family history of cancer. The patient’s Eastern Cooperative Oncology Group performance status was determined to be 0.
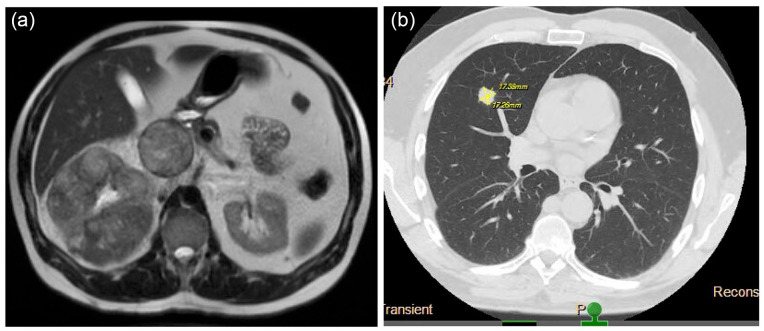
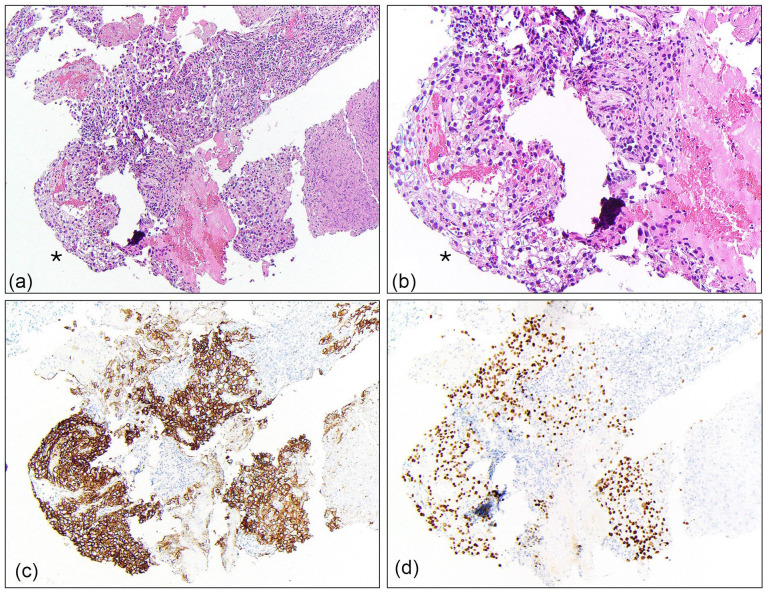
On presentation for cough, computed tomography (CT) of the chest revealed a 1.2 cm right middle lobe lung nodule and an incidental mass at the superior right kidney which prompted further evaluation of the abdomen and pelvis. CT of the abdomen and pelvis revealed a large right renal mass with IVC invasion and possible hepatic invasion, and multiple enlarged retroperitoneal lymph nodes. Subsequent magnetic resonance imaging (MRI) of the abdomen showed a heterogeneously enhancing 11.4 × 12.2 × 13 cm mass compatible with RCC in the upper pole of the right kidney with extensive venous invasion (Figure 1(a)). The tumor occluded the IVC and extended to the level of the hepatic IVC, indicative of a Level III thrombus according to the Mayo Clinic’s thrombus classification. Several subcentimeter para-aortic lymph nodes and three indeterminate subcentimeter arterially enhancing liver lesions were also observed (Figure 1(b)). A right middle lobe lung core biopsy showed malignant cells with clear cytoplasm morphologically consistent with carcinoma (Figure 2(a) and (b)). The malignant cells stained positive for PAX8 (Figure 2(c)), supporting renal origin, and carbonic anhydrase IX (CAIX) (Figure 2(d)) an immunophenotype which is most consistent with clear cell type RCC.
Figure 1.
(a) MRI of the right renal mass with IVC thrombus on presentation and (b) CT of the chest showing lung metastasis on presentation.
Figure 2.
Core biopsy of the lung right middle lobe. Cores contained focal malignant cells with clear cytoplasm (*) which showed cytologic architecture consistent with carcinoma (a, 4×; b, 20×). Immunohistochemical staining revealed the clear cells were positive for PAX8 (c) and carbonic anhydrase (CAIX) (d). This immunophenotype is characteristic of RCC.
The patient’s International Metastatic RCC Database Consortium risk score for metastatic RCC was determined to be two (low hemoglobin and time to systemic therapy of less than 1 year), which categorized his cancer as intermediate risk. The patient was treated with intravenous pembrolizumab 400 mg every 6 weeks plus oral axitinib 5 mg twice daily per KEYNOTE-426.
Clinical course
The patient experienced the typical side effects of VEGFR TKI therapy with grade 3 hypertension, grade 2 diarrhea, and grade 2 mucositis which required a dose reduction of axitinib to 3 mg twice daily and then 2 mg twice daily with subsequent improvement in his symptoms. Hypertension was well controlled on amlodipine alone. Diarrhea improved with dose reduction and supportive care with loperamide.
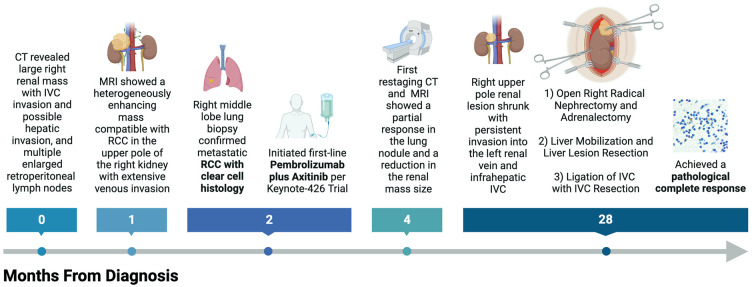
First restaging CT of the chest and MRI of the abdomen and pelvis after 8 weeks of treatment showed a partial response in his lung nodule and a reduction in the size of his renal mass. After 26 months of systemic treatment, CT of the right upper pole renal mass had shrunk to 6.2 cm × 4.5 cm with persistent invasion into the left renal vein and infrahepatic IVC (Figure 3). Additionally, his lung nodule was no longer visible. He therefore underwent open right radical nephrectomy including adrenalectomy, liver mobilization, and partial hepatectomy, along with IVC ligation and resection. The patient was discharged on postoperative day 4 with an uneventful inpatient hospital stay.
Figure 3.
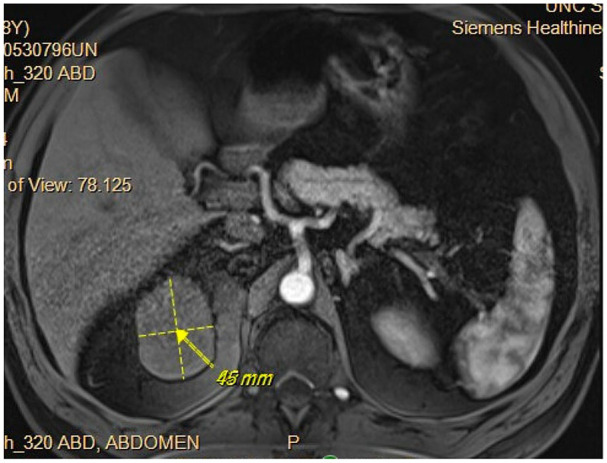
CT after pembrolizumab plus axitinib showing shrinking of right upper pole renal lesion with persistent invasion into the left renal vein and infrahepatic IVC.
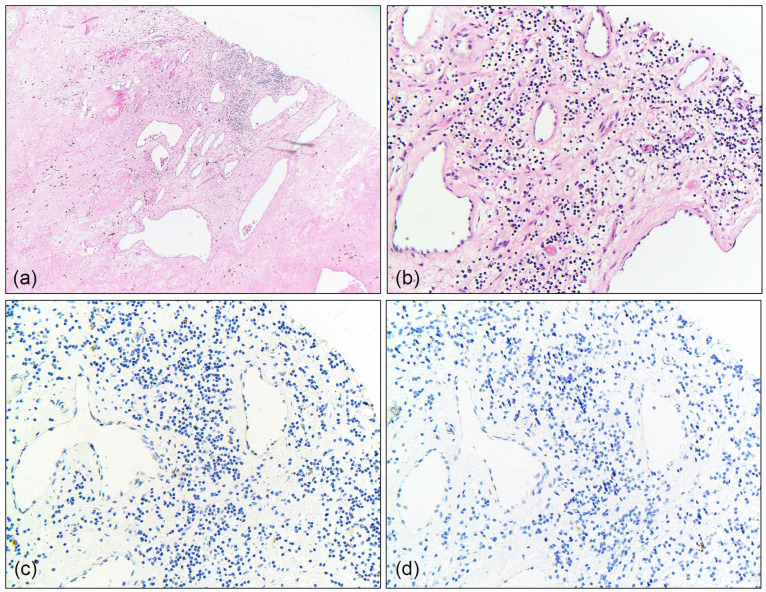
Pathologic evaluation of the nephrectomy, lymphadenectomy, IVC thrombectomy, and partial hepatectomy specimens revealed no viable carcinoma. The renal mass showed extensive treatment effect with abundant, dense fibrosis, hemorrhage, and hemosiderin deposition (ypT0) (Figure 4(a)). Within the resected segment of IVC, focal suspicious cells with clear cytoplasm were identified admixed with fibrosis (Figure 4(b)). Immunohistochemistry was used to further characterize these cells, which demonstrated negativity for pan-cytokeratin (Figure 4(c)) and PAX8 (Figure 4(d)), confirming they represented macrophages rather than residual tumor. The dissected interaortocaval lymph node involved on initial CT had no malignancy identified. Partial hepatectomy histology showed benign cuboidal epithelial lined cyst with focal fibrosis and hemosiderin deposition. Taken together, these findings were consistent with a pCR in the setting of pembrolizumab plus axitinib. The patient’s 1-month postoperative labs revealed normal calcium, elevated creatinine (1.65 mg/dL), decreased eGFR CKD-EPI (2021) Male (48 mL/min/1.73 m2), and decreased albumin (3.3 g/dL).
Figure 4.
Inferior vena cava with focal atypical cells with clear cytoplasm suspicious for residual clear cell RCC (a, 4×; b, 20×). The atypical cells showed negative staining for pan-cytokeratin (c, 20×) and PAX8 (d, 20×), consistent with macrophages rather than residual RCC.
Discussion
The patient herein presented with locally advanced and metastatic clear cell RCC. He initiated combination therapy with pembrolizumab plus axitinib as a standard of care for first-line treatment of metastatic RCC. 8 This case report reinforces the most up to date literature by underscoring the excellent outcomes associated with the combination therapy compared to the use of sunitinib monotherapy. Molecular studies have described drug resistance mechanisms in sunitinib that may explain its inferiority compared to combination therapies. Lysosome sequestration of sunitinib has been reported to contributes to reduced drug efficacy and resistance. 9 Despite ongoing challenges in metastatic RCC, drugs targeting specific pathological pathways have the potential to produce favorable outcomes. Lactotransferrin downregulation has been shown to promote metastasis in RCC. This attenuation renders the RCC tumor cells more responsive to mammalian target of rapamycin inhibitors, suggesting it may be a predictor for therapeutic effectiveness. However, clinical trial data indicated that pembrolizumab plus TKI therapy led to longer survival than sunitinib monotherapy, while everolimus plus TKI did not show this benefit. 10 Nevertheless, a more recent trial reports positive long-term follow-up outcomes with pembrolizumab plus axitninb. 8
After a 43-month follow-up of the Phase 3 KEYNOTE-426 randomized trial, the objective response rate for pembrolizumab plus axitinib was 60% with a 10% CR and 50% partial response. While this clinical trial demonstrates the efficacy of pembrolizumab plus axitinib in treating metastatic RCC, it is noteworthy that only four studies in the literature have documented a pCR of metastatic RCC following this specific regimen. A 48-year-old female achieved a pCR after 1 year of treatment for metastatic RCC involving lymph nodes and thyroid. 6 Similarly, a 69-year-old female experienced the same response after 3 months of treatment for RCC with lung metastasis. 11 In a 67-year-old male with RCC to the lymph node and IVC, a pCR was achieved after approximately 7 months of treatment. 7 In a more complex case, a 68-year-old female presented with metastases to lymph nodes, lungs, and liver. 12 After about 11 months of pembrolizumab plus axitinib therapy, pulmonary nodules and hepatic lesions were resolved and not observable on CT scan. A radical nephrectomy was subsequently performed.
What distinguishes our case from these other reports is that our patient received the longest duration of pembrolizumab plus axitinib to achieve a pCR for metastatic clear cell RCC (Figure 5). The length of his treatment was warranted given the extent of his pathology. Including our patient with other cases above, the median time from initiating immunotherapy to achieving a pCR was 11 months. Among all of these cases, there was a pCR, yet there was also an incomplete radiographic response. This further raises the questions about the necessity of cytoreductive nephrectomy in the time of immunotherapy, especially when pembrolizumab plus axitinib is able to accomplish such a robust outcome. A recent multicenter retrospective study found that systemic therapy before cytoreductive nephrectomy was associated with a 3-year overall survival rate of 70%. 13 Currently, there are two active clinical trials, PROBE, and NORDIC SUN, investigating surgical timing and patient selection for cytoreductive nephrectomy in the era of immune checkpoint inhibitors.14,15 The argument for cytoreductive nephrectomy after initial systemic therapy is the potential to eradicate immune resistant clones within the primary tumor. However, given the emerging evidence of a pCR in our patient and other cases following pembrolizumab plus axitinib, a similar clinical trial is warranted for examining whether cytoreductive nephrectomy provides any additional benefit.
Figure 5.
Schematic timeline of the patient’s clinical course from diagnosis to the achievement of a pathological complete response.
Additionally, our patient underwent concomitant IVC ligation alongside radical nephrectomy. RCC invasion into the IVC is a known risk factor for higher disease recurrence and poorer prognosis, 16 and radical resection of the invaded IVC wall improves the patient’s 5-year survival rate. 17 IVC ligation is very rarely performed during cytoreductive nephrectomy. IVC ligation is associated with a higher short-term postoperative complication rate compared to IVC thrombectomy (73% vs 39%, p = 0.004). 18 Given our patient’s postoperative pathology showing negative margins and a pCR, the necessity of the procedure is debatable. To our knowledge, this patient is the first to undergo such an extensive procedure following a robust systemic therapy course. Considering the complexity of his pathology, this may have optimized the outcome. However, the necessity of a more invasive surgical approach in our patient emphasizes the importance of careful risk–benefit assessment, especially considering the absence of viable carcinoma in postoperative specimens.
A limitation from our case is the lack of long-term follow-up data as this would be beneficial for elucidating the durability of the pCR and overall survival. However, we are hopeful that the patient will continue to maintain this response, given that the median duration of response from pembrolizumab plus axitinib is reported to be 24 months. Furthermore, the decision to discharge him on postoperative day 4 should be carefully interpreted, as it reflects a rare instance where no postoperative complications occurred despite the major operation. Patient safety remains the priority in all cases, with individualized discharge plans tailored to each situation. A strength from our case is highlighting the relevance of clinical trial findings in real-world patient management. Further, demonstration of a pCR herein suggests that this is an endpoint that future clinical trials should consider.
Conclusion
This case report describes a rare occurrence of a pCR of extensive metastatic RCC with the use of pembrolizumab plus axitinib. Despite the patient’s experience with gastrointestinal symptoms and VEGF-induced hypertension, his symptoms were effectively managed during the therapy and resulted in a significant response. This case suggests that further exploration may be warranted for pembrolizumab plus axitinib treatment in achieving a pCR of metastatic RCC to the IVC. This case also highlights the potential benefit of withholding cytoreductive nephrectomy before systemic treatment in patients having metastatic RCC and primary tumor invasion of the IVC.
Acknowledgments
None.
Footnotes
Author contributions: RW: Manuscript writing and editing, Review of literature; TLR: Treating physician, Manuscript writing and editing; H-JT: Treating physician, Manuscript writing and editing; CH: Manuscript writing and editing, Histology imaging; SEW: Manuscript writing and editing, Histology imaging; MAB: Treating physician, Data acquisition, Manuscript writing and editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series. This study was exempt from IRB Review.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Ryan Wong  https://orcid.org/0000-0002-1251-758X
https://orcid.org/0000-0002-1251-758X
References
- 1. Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol 2020; 11: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powles T, Tomczak P, Park SH, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022; 23: 1133–1144. [DOI] [PubMed] [Google Scholar]
- 3. Brown JE, Royle K-L, Gregory W, et al. Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): an open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Oncol 2023; 24: 213–227. [DOI] [PubMed] [Google Scholar]
- 4. Choueiri TK, Powles T, Peltola K, et al. Belzutifan versus everolimus for advanced renal-cell carcinoma. N Engl J Med 2024; 391: 710–721. [DOI] [PubMed] [Google Scholar]
- 5. Rini BI, Battle D, Figlin RA, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer 2019; 7: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beirat AF, Menakuru SR, Khan I, et al. Pathological complete response of metastatic clear cell renal carcinoma with pembrolizumab and axitinib: a case report and review of literature. Case Rep Oncol 2023; 16: 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimizu K, Tamada S, Matsuoka Y, et al. Pathologic complete response with pembrolizumab plus axitinib in metastatic renal cell carcinoma. Int Cancer Conf J 2022; 11: 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Plimack ER, Powles T, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib as first-line treatment of advanced renal cell carcinoma: 43-month follow-up of the phase 3 KEYNOTE-426 study. Eur Urol 2023; 84: 449–454. [DOI] [PubMed] [Google Scholar]
- 9. Aweys H, Lewis D, Sheriff M, et al. Renal cell cancer—insights in drug resistance mechanisms. Anticancer Res 2023; 43: 4781–4792. [DOI] [PubMed] [Google Scholar]
- 10. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021; 384: 1289–1300. [DOI] [PubMed] [Google Scholar]
- 11. Bergamini M, Dalla Volta A, Valcamonico F, et al. Pathological complete response to pembrolizumab plus axitinib combination following serious immune-related adverse events in an advanced renal cell carcinoma patient with a history of rheumatoid arthritis. Case Rep Oncol 2024; 17: 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tucker MD, Beckermann KE, Gordetsky JB, et al. Complete pathologic responses with immunotherapy in metastatic renal cell carcinoma: case reports. Front Oncol 2020; 10: 609235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meagher MF, Minervini A, Mir MC, et al. Does the timing of cytoreductive nephrectomy impact outcomes? Analysis of REMARCC registry data for patients receiving tyrosine kinase inhibitor versus immune checkpoint inhibitor therapy. Eur Urol Open Sci 2024; 63: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bell H, Cotta BH, Salami SS, et al. “PROBE”ing the role of cytoreductive nephrectomy in advanced renal cancer. Kidney Cancer J 2022; 6: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iisager L, Ahrenfeldt J, Donskov F, et al. Multicenter randomized trial of deferred cytoreductive nephrectomy in synchronous metastatic renal cell carcinoma receiving checkpoint inhibitors: the NORDIC-SUN-trial. BMC Cancer 2024; 24: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abel EJ, Carrasco A, Karam J, et al. Positive vascular wall margins have minimal impact on cancer outcomes in patients with non-metastatic renal cell carcinoma (RCC) with tumour thrombus. BJU Int 2014; 114: 667–673. [DOI] [PubMed] [Google Scholar]
- 17. Hatcher PA, Anderson EE, Paulson DF, et al. Surgical management and prognosis of renal cell carcinoma invading the vena cava. J Urol 1991; 145: 20–23. [DOI] [PubMed] [Google Scholar]
- 18. Xie L, Hong G, Nabavizadeh R, et al. Outcomes in patients with renal cell carcinoma undergoing inferior vena cava ligation without reconstruction versus thrombectomy: a retrospective, case controlled study. J Urol 2021; 205: 383–391. [DOI] [PubMed] [Google Scholar]