Abstract
Background
One third of adults with cancer suffer from common mental disorders in addition to their malignant disease. However, it is unknown whether this proportion is the same in patients who have brain tumors and which factors modulate the risk for psychiatric comorbidity.
Methods
In a multicenter study, patients with high‐grade glioma at 13 neurooncology clinics were enrolled consecutively and interviewed with the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID) to diagnose common mental disorders. Predictors of psychiatric comorbidity were investigated using binary logistic regression.
Results
Six hundred ninety‐one patients were interviewed. The proportion of patients who had mental disorders was 31% (95% confidence interval [CI], 28%–35%). There was evidence for an association of psychiatric comorbidity with the following factors: younger age (odds ratio [OR], 1.9; 95% CI, 1.1–3.4; p = .04), stable disease versus complete remission (OR, 1.7; 95% CI, 1.1–2.8; p = .04), lower income (OR, 1.7; 95% CI, 1.0–2.8; p = .04), living alone (OR, 1.6; 95% CI, 1.0–2.6; p = .05), fatigue (OR, 1.6; 95% CI, 1.1–2.4; p = .03), and impaired cognitive functioning (OR, 2.3; 95% CI, 1.5–3.6; p < .01). There was no evidence for independent effects of gender, histology, affected lobe, time since diagnosis, or employment status.
Conclusions
Approximately one third of adult patients with high‐grade glioma may suffer from a clinically relevant common mental disorder, without notable disparity between the genders. In particular, clinicians should pay attention to possible comorbidities for cases in which patients exhibit compromised subjective cognitive function, are younger than 50 years, maintain a state of stable disease, or live alone.
Keywords: glioma, mental health, oncology, psycho‐oncology, structured clinical interview, Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID)
Short abstract
In a multicenter, cluster‐randomized clinical trial involving 691 adult patients with high‐grade glioma, the proportion with a comorbid psychiatric disorder was 31%. Comorbidity was more likely in younger patients and in those who had stable disease, were living alone, had lower income, had impaired subjective cognitive functioning, and had fatigue.
INTRODUCTION
Mental disorders are emotionally burdensome for patients and their caregivers. 1 , 2 , 3 , 4 , 5 This applies both when psychiatric disorders are the main disease and when they occur comorbid with a physical illness. 6 , 7
Various meta‐analyses 8 , 9 and studies worldwide 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 have demonstrated that approximately one third of all patients who have cancer suffer from common mental disorders in addition to their malignant disease, which is not surprising given the existential crisis a cancer diagnosis can cause. The term common mental disorders (or common mental health disorders) refers to a group of disorders typically encountered in community and primary care settings, comprising different types of depression and anxiety. Both cause marked emotional distress and interfere with daily life but usually do not affect insight as can occur in psychotic disorders. 20 , 21 Most people may be deeply disturbed to learn that their life span is expected to be shorter, their body functions altered, and their physical appearance changed. This is especially true when the brain is affected, which is a vital organ for all cognitive, emotional, and bodily processes. Therefore, malignant tumors of the brain could be a particular cause of psychological turbulence, leading to subclinical mental health problems becoming clinical. Patients who have brain tumors, especially those with high‐grade glioma, not only face the diagnosis itself but also suffer from various symptoms during their disease trajectory, including focal neurologic deficits, fatigue, seizures, and neurocognitive dysfunction. 22 , 23 , 24
Despite this, in most epidemiological studies investigating the frequency of common mental disorders among adult patients with cancer, brain tumors are not mentioned at all or are subsumed under other sites. 10 , 11 , 12 , 25 , 26 The few studies reporting prevalence numbers for adult patients with brain tumors using structured clinical interviews (the gold standard) to evaluate mental health have had small sample sizes 27 , 28 , 29 , 30 or assessed only one type of mental disorder, for example, only major depression or only substance use disorders. 31 , 32 , 33 Nevertheless, they provide valuable information about patients' mental health. Based on structured or semistructured clinical interviews or on manual review of electronic medical records, major depression was identified in 0%–28% of all patients, 27 , 28 , 29 , 30 , 32 , 34 and alcohol use disorder was identified in 0%–4%. 28 , 33 Other psychiatric diagnoses have only been ascertained using gold‐standard methods in small studies, with samples of n = 26, 29 n = 27, 28 and n = 35, 30 respectively. Hence, the following numbers are relatively uncertain: adjustment disorders were identified in 4%–11%, 28 , 29 , 30 generalized anxiety disorders in 0%–4%, 28 , 29 , 30 and acute stress disorders in 11%–19%, 29 , 30 whereas dysthymia and posttraumatic stress disorder (PTSD) were not identified in any of the patients in these studies. 28 , 29 , 30
The proportion of patients with any type of mental health disorder was 17%–38%. 28 , 29 , 30 Because these findings come from small and often monocentric studies and include a mixture of brain tumor diagnoses (e.g., meningioma, astrocytoma, glioblastoma, oligodendroglioma, medulloblastoma), it is important to obtain more precise prevalence estimates for a range of mental health diagnoses among patients with high‐grade glioma from multiple hospitals, while applying gold‐standard methods to ascertain mental health.
We were also interested in factors that are associated with an increased risk of common mental disorders in this group of patients. Previous research involving the general population, 21 patients with cancer, 14 , 25 , 35 and, in particular, patients diagnosed with brain tumors 28 , 31 , 34 suggested including the following variables as potential predictors of common mental disorders: gender (men are more likely to be diagnosed with alcohol dependence, women are more likely to be diagnosed with any psychiatric disorder), 14 , 17 , 21 , 28 , 36 younger age, 21 , 28 , 37 socioeconomic variables (unemployment, 14 , 35 , 36 low income, 35 , 37 living alone 15 , 36 , 37 ), reduced performance status, 25 , 31 time since diagnosis (although the direction of change over time is unclear), 34 decreased cognitive functioning, 34 and fatigue. 14 Regarding clinical variables (histology, grade, stage, affected lobe, laterality, and concurrent other somatic diseases), evidence to date is inconclusive, with conflicting results. 19 , 28 , 32 , 34 , 35 , 38 , 39 According to previous studies, antineoplastic treatment (extent of resection, radiotherapy, and chemotherapy) is not related to psychiatric comorbidity in patients with glioma. 28 , 34 , 40
Therefore, the objective of the current study was to investigate the prevalence of and potential risk factors for common mental disorders in adult patients with high‐grade glioma.
METHODS
Design and setting
To answer our research questions, we used baseline data from a multicenter, cluster‐randomized clinical trial (DRKS00018079) investigating the effect of two types of screening for psychosocial care needs on the subsequent use of such services among patients with high‐grade glioma. Participants were enrolled at 13 neurooncology clinics in Germany and were followed up for 3 months. Data collection commenced on September 27, 2019, and ended on April 14, 2023. This report was prepared according to the STROBE checklist (Strengthening the Reporting of Observational Studies in Epidemiology; University of Bern Institute of Social and Preventive Medicine). Ethical approval was granted by the responsible Ethics Review Board of the Medical Association of Rhineland‐Palatinate under 837.170‐17(11013).
Patients
In all centers, patients were included consecutively during their regular visits to the clinic. Eligible patients had to have a histologically confirmed diagnosis of high‐grade glioma, to be aged 18 years or older, and to be legally competent and able to consent. Patients were excluded when they did not provide written informed consent to participate in this study after being informed verbally and in writing about it.
Measures
Structured clinical interview
Common mental disorders were ascertained with the clinician version of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID), 41 which is considered to be a gold‐standard instrument for such purposes. 42 , 43 This instrument has shown an inter‐rater reliability from κ = 0.81 24 to κ = 0.95 44 in cancer studies and good criterion validity. 45 We used the validated German translation. 46 The interview operates on the principle that each disorder is first screened for with specific questions and, in the event of affirmative responses, further questions are posed until all criteria for a diagnosis are checked. In our study, the rule applied that, if it was unclear whether all criteria were fully met, no psychiatric diagnosis was given.
The interviews were scheduled after the patients had been enrolled and had talked to the physician once. The following parts of the SCID were used: major depressive episode, persistent depressive disorder, PTSD, generalized anxiety disorder, alcohol use disorder, and adjustment disorder. To keep the burden for participants as low as possible, only screening questions were used for panic disorder, agoraphobia, social anxiety disorder, specific phobia, social phobia, nonalcohol substance use disorder, and sleep disorders. This approach was chosen because we feared that participants would decline being interviewed if it became too time‐consuming or too burdensome, given the fragile state they might be in. The SCID took on average 17 minutes to complete in our study (ranging from 1 to 68 minutes).
Interviewers from each site participated in an initial full‐day training session conducted by a senior researcher from Mainz University (S.S.) who is a licensed psychotherapist and has extensive experience using the SCID in her studies. 14 , 18 , 42 , 47 This initial training was followed by monthly review sessions with all sites.
Clinical variables
For each participant, a fully trained medical doctor ascertained the histology and World Health Organization (WHO) glioma grade (oligodendroglioma central nervous system [CNS] WHO grade 3, astrocytoma CNS WHO grade 3, glioblastoma CNS WHO grade 4, oligoastrocytoma not otherwise specified WHO grade 3 48 ), the time since first diagnosis of glioma, the current stage of disease according to RANO (the Response Assessment in Neuro‐Oncology, with information about complete remission, partial remission, stable disease, or progressive disease), affected cerebral lobe(s) (frontal, parietal, temporal, or occipital), the status of the disease (primary vs. recurrent), and the Karnofsky performance score. 49
Somatic comorbidity was ascertained by trained study personnel after the SCID interview. The following diseases were assessed: other cancers, high blood pressure, diabetes, cardiovascular diseases, rheumatism, pain, diseases of the musculoskeletal system, diseases of the sensory organs, neurologic diseases, and other chronic or severe diseases. The exact name of the disease was also written down. When any of these conditions was present, it was considered to be a somatic comorbidity.
Demographic and socioeconomic variables
The study nurses documented the gender (man, woman, diverse) and age of the patients at the time of study entry. In addition, the participants completed a questionnaire in which they provided information about their employment status (employed, self‐employed, unemployed, retired, in training, or homemaker), household income, the number of adults and children living in the household, marital status, and cohabitation (with partner, changing partners, or without partner).
Subjective functional impairment and quality of life
Participants completed the European Organization for Research and Treatment of Cancer (EORTC) core quality‐of‐life instrument, the EORTC QLQ‐C30, 50 before the consultation with the physician. We used its cognitive functioning scale to determine the level of subjective cognitive impairment as well as its fatigue scale to measure aspects of quality of life known to be related to mental health. Cronbach α values of the scales were 0.75 and 0.86, respectively.
Statistical analysis
To explore possible selection bias, the age, gender, and tumor grade of participants and nonparticipants were compared. Subsequently, based on the threshold for clinical importance (TCI) developed by Giesinger and colleagues, 51 cognitive functioning and fatigue were dichotomized according whether an impairment was clinically relevant or not. The TCIs had been established based on patient interviews; they were asked to anchor the EORTC QLQ‐C30 score on whether they had limitations in their daily life, needed help, and had worries. The TCI for cognitive functioning is 75 (scores below this TCI indicate clinically important problems), and, for fatigue, it is 39 (scores above the TCI indicate clinically important problems).
The proportion of patients with common mental disorders among the entire sample and per predictor variable was calculated. Furthermore, the frequency of each mental disorder and the sum of all disorders were computed.
In univariate and multivariate binary logistic regression analyses, we investigated potential predictors of psychiatric comorbidity in general (at least one common mental disorder vs. none) and of each mental health disorder separately. All potential predictors were defined before beginning modeling based on the literature and the expertise of the involved clinicians. No deliberate selection procedure was used.
The same set of variables was used to examine associations with each mental health condition separately. This approach was not guided by specific hypotheses because, except for depression, not enough literature was available to define any. Therefore, we present no p values for these effect estimates and report only confidence intervals.
Multicollinearity was checked a priori using the variance inflation factor. The data analysis was performed using STATA (Stata Statistical Software, release 16; StataCorp LP).
RESULTS
Participant flow
During the study period, 1568 patients with glioma attended the clinics and were screened for this study. Of them, 1387 were eligible (for details, see Figure 1), 763 participated, 702 agreed to be interviewed, and 691 (50% of all eligible; 91% of all participants) could be interviewed with the SCID.
FIGURE 1.
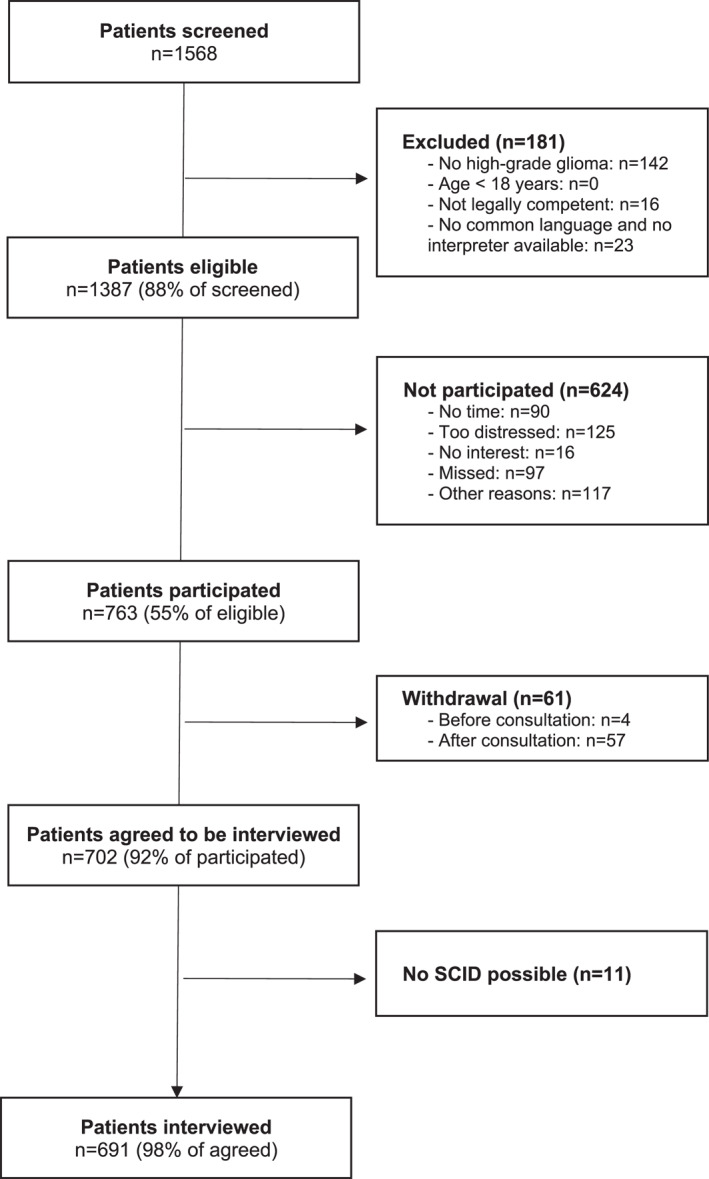
Patient flow through the study. SCID indicates the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders.
The eligible nonparticipants were on average 4 years older and were slightly more frequently diagnosed with glioblastoma (67% vs. 61%) than the participants. Regarding gender, there were no statistically significant differences between participants and nonparticipants.
Sample characteristics
The 691 interviewed patients were on average aged 52 years (range, 19–86 years), and 57% were men (Table 1). The largest group (n = 411; 59%) had been diagnosed with glioblastoma CNS WHO grade 4, followed by astrocytoma CNS WHO grade 3 (n = 173; 25%), and oligodendroglioma CNS WHO grade 3 (n = 89; 13%).
TABLE 1.
Characteristics of participating patients, n = 691.
| No. (%) | ||||
|---|---|---|---|---|
| Without CMD a | With CMD a | Total b | ||
| Total in sample | 474 (69) | 217 (31) | 691 (100) | |
| Gender | Man | 281 (71) | 115 (29) | 396 (57) |
| Woman | 192 (65) | 102 (35) | 294 (43) | |
| Diverse | 1 (100) | 0 (0) | 1 (0) | |
| Age, years | <50 | 180 (65) | 95 (35) | 275 (40) |
| 50–64 | 185 (68) | 87 (32) | 272 (39) | |
| ≥65 | 109 (76) | 35 (24) | 144 (21) | |
| Histology | Oligodendroglioma | 67 (75) | 22 (25) | 89 (13) |
| Oligoastrocytoma | 11 (73) | 4 (27) | 15 (2) | |
| Anaplastic astrocytoma | 108 (62) | 65 (38) | 173 (25) | |
| Glioblastoma | 285 (69) | 126 (31) | 411 (59) | |
| Other | 3 (100) | 0 (0) | 3 (0) | |
| RANO | Complete remission | 98 (74) | 34 (26) | 132 (19) |
| Partial remission | 53 (77) | 16 (23) | 69 (10) | |
| Stable disease | 252 (64) | 141 (36) | 393 (57) | |
| Progressive disease | 66 (72) | 26 (28) | 92 (13) | |
| Unknown | 5 (100) | 0 (0) | 5 (1) | |
| Status of disease | Primary | 330 (68) | 156 (32) | 486 (70) |
| Recurrent | 141 (71) | 59 (30) | 200 (29) | |
| Unknown | 3 (60) | 2 (40) | 5 (1) | |
| Lobe | Frontal | 171 (67) | 83 (33) | 254 (37) |
| Parietal | 72 (76) | 23 (24) | 95 (14) | |
| Temporal | 100 (65) | 54 (35) | 154 (22) | |
| Occipital | 21 (72) | 8 (28) | 29 (4) | |
| Other | 102 (69) | 46 (31) | 148 (21) | |
| Unknown | 8 (73) | 3 (27) | 11 (2) | |
| Laterality | Right | 212 (70) | 91 (30) | 303 (44) |
| Left | 240 (68) | 115 (32) | 355 (51) | |
| Both | 16 (67) | 8 (33) | 24 (3) | |
| Unknown | 6 (67) | 3 (33) | 9 (1) | |
| KPS | >70 | 403 (71) | 165 (29) | 568 (82) |
| ≤70 | 70 (58) | 51 (42) | 121 (18) | |
| Unknown | 1 (50) | 1 (50) | 2 (0) | |
| Time since first diagnosis, months | Mean ± SD | 39 ± 52 | 38 ± 51 | 39 ± 52 |
| Other diseases | No other somatic disease | 276 (69) | 122 (31) | 398 (58) |
| Comorbid somatic disease | 197 (67) | 95 (33) | 292 (42) | |
| Unknown | 1 (100) | 0 (0) | 1 (0) | |
| Employment | Unemployed/homemaker | 53 (64) | 30 (36) | 83 (12) |
| Employed/retired/in training | 384 (71) | 155 (29) | 539 (78) | |
| Unknown | 37 (54) | 32 (46) | 69 (10) | |
| Income pp/month | <€500 | 8 (67) | 4 (33) | 12 (2) |
| €500–€999 | 38 (62) | 23 (38) | 61 (9) | |
| €1000–€1499 | 56 (56) | 44 (44) | 100 (14) | |
| >€1500 | 264 (70) | 113 (30) | 377 (55) | |
| Unknown | 108 (77) | 33 (23) | 141 (20) | |
| Parenthood | Has no underage children | 324 (67) | 157 (33) | 481 (70) |
| Has underage children | 139 (73) | 52 (27) | 191 (28) | |
| Unknown | 11 (58) | 8 (42) | 19 (3) | |
| Lives alone | No | 405 (70) | 173 (30) | 578 (84) |
| Yes | 60 (59) | 42 (41) | 102 (15) | |
| Unknown | 9 (82) | 2 (18) | 11 (2) | |
| Fatigue | No important problems | 227 (78) | 65 (22) | 292 (42) |
| Important problems | 242 (62) | 150 (38) | 392 (57) | |
| Unknown | 5 (71) | 2 (29) | 7 (1) | |
| Cognitive functioning | No important problems | 208 (81) | 48 (19) | 256 (37) |
| Important problems | 264 (61) | 169 (39) | 433 (63) | |
| Unknown | 2 (100) | 0 (0) | 2 (0) | |
Note: Values are absolute numbers (percentages) unless stated otherwise. Diagnoses are according to the World Health Organization 2016 classification.
Abbreviations: CMD, common mental disorder; KPS, Karnofsky performance score; RANO, Response Assessment in Neuro‐Oncology; pp/month, per person per month; SD, standard deviation.
Row percentages.
Column percentages.
Frequency of mental disorders
Overall, 217 of the interviewed patients (31%; 95% CI, 28%–35%) were diagnosed with at least one common mental disorder. The number of diagnoses per patient ranged from zero to four. Among those with a mental health condition, the average number of diagnoses was 1.2 (mode, 1.0).
Major depressive episode was diagnosed in 40 participants (6%), persistent depressive disorder was diagnosed in 57 (8%), PTSD was diagnosed in 68 (10%), generalized anxiety disorder was diagnosed in 50 (7%), alcohol use disorder was diagnosed in eight (1%), and adjustment disorder was diagnosed in 29 (4%).
Another 104 patients were positively screened for other mental health conditions, resulting in an overall prevalence of 46%. Based on the screening questions, the proportion of the additional conditions in the entire sample were: panic disorder (n = 67; 10%), agoraphobia (n = 31; 5%), social phobia (n = 19; 3%), specific phobias (n = 43; 6%), insomnia (n = 88; 13%), hypersomnolence (n = 120; 17%), and nonalcohol substance use disorder (n = 4; 1%).
Predictors of psychiatric comorbidity
Because the mean variance inflation factor was 1.2, there was no indication that multicollinearity was a relevant problem. Therefore, all of the prespecified predictor variables were jointly modeled in the regression analysis. The results (Table 2) indicated that patients younger than 50 years were 1.9 times more likely to develop a common mental disorder than patients aged 65 years or older (95% CI, 1.1–3.4; p = .04). Patients with stable disease had 1.7 times the odds of being diagnosed with a mental disorder than patients in complete remission (95% CI, 1.1–2.8; p = .04). Those with an equivalent income of €1000–€1499 per month, compared with ≥€1500 per month, also had higher odds of being diagnosed with a mental disorder (OR, 1.7; 95% CI, 1.0–2.8; p = .04). Living alone was associated with increased odds of mental diseases (OR, 1.6; 95% CI, 1.0–2.6; p = .05). Finally, poor quality of life in terms of fatigue (OR, 1.6; 95% CI, 1.1–2.4; p = .03) and impaired self‐reported cognitive functioning (OR, 2.3; 95% CI, 1.5–3.6; p < .01) were also associated with a higher likelihood of mental disorders. There were only small differences between univariate and multivariate analyses, indicating that confounding played a minor role.
TABLE 2.
Predictors of comorbid mental disorders in patients with high‐grade glioma.
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | ||
| Gender | Woman | 1.3 | 0.9–1.8 | .11 | 1.2 | 0.9–1.8 | .25 |
| Age, years | <50 | 1.6 | 1.0–2.6 | .03 a | 1.9 | 1.1–3.4 | .04 a |
| 50–64 | 1.5 | 0.9–2.3 | .10 | 1.4 | 0.8–2.5 | .18 | |
| ≥65 | Base | Base | |||||
| Histology | Oligodendroglioma | 0.7 | 0.4–1.3 | .27 | 0.8 | 0.4–1.4 | .38 |
| Oligoastrocytoma | 0.8 | 0.3–2.6 | .74 | 1.2 | 0.3–4.1 | .82 | |
| Anaplastic astrocytoma | 1.4 | 0.9–2.0 | .10 | 1.4 | 0.9–2.3 | .16 | |
| Glioblastoma | Base | Base | |||||
| RANO | Complete remission | Base | Base | ||||
| Partial remission | 0.9 | 0.4–1.7 | .69 | 0.8 | 0.4–1.7 | .52 | |
| Stable disease | 1.6 | 1.0–2.5 | .03 a | 1.7 | 1.1–2.8 | .04 a | |
| Progressive disease | 1.1 | 0.6–2.1 | .68 | 1.0 | 0.5–2.1 | .93 | |
| Status of disease | Recurrent vs. primary | 0.9 | 0.6–1.3 | .46 | 0.8 | 0.5–1.3 | .34 |
| Lobe | Frontal vs. all other | 1.3 | 0.9–1.8 | .16 | 1.3 | 0.9–1.9 | .14 |
| KPS | ≤70 | 1.8 | 1.2–2.7 | .01 a | 1.6 | 1.0–2.6 | .06 a |
| Time since diagnosis, months | 1.0 | 1.0–1.0 | .85 | 1.0 | 1.0–1.0 | .94 | |
| Other diseases | Somatic comorbidity | 1.1 | 0.0–0.8 | .60 | 1.1 | 0.8–1.6 | .53 |
| Employment | Unemployed or homemaker | 1.4 | 0.9–2.3 | .17 | 1.1 | 0.6–1.8 | .84 |
| Income, € | <500 | 1.2 | 0.3–4.0 | .80 | 0.7 | 0.2–3.0 | .65 |
| 500–999 | 1.4 | 0.8–2.5 | .23 | 1.2 | 0.6–2.2 | .62 | |
| 1000–1499 | 1.8 | 1.2–2.9 | .01 a | 1.7 | 1.0–2.8 | .04 a | |
| >1500 | Base | Base | |||||
| Parenthood | Has underage children | 0.8 | 0.5–1.1 | .17 | 0.7 | 0.4–1.0 | .07 |
| Cohabitation | Lives alone | 1.6 | 1.1–2.5 | .03 a | 1.6 | 1.0–2.6 | .05 a |
| Self‐reported fatigue | Important problems | 2.2 | 1.5–3.1 | <.01 a | 1.6 | 1.1–2.4 | .03 a |
| Self‐reported cognitive functioning | Important problems | 2.8 | 1.9–4.0 | <.01 a | 2.3 | 1.5–3.6 | <.01 a |
Note: A new classification was introduced in 2021, but because our study started in 2019, most of the histologic diagnoses refer to the previous fourth classification. This is relevant because some astrocytomas are now classified as glioblastomas and some glioblastomas are classified as central nervous system astrocytomas, World Health Organization grade 4.
Abbreviations: CI, confidence interval; KPS, Karnofsky performance score; OR, odds ratio; RANO, Response Assessment in Neuro‐Oncology.
These values had ORs ≥1.5.
Predictors of specific mental health conditions
Several variables were specifically associated with certain diagnoses. A major depression episode was more likely in patients who had a Karnofsky performance score ≤70 (OR, 4.2; 95% CI, 1.8–9.9), severe fatigue (OR, 10.1; 95% CI, 2.2–46.7), and impaired self‐reported cognitive functioning (OR, 3.8; 95% CI, 1.1–13.8).
The odds of persistent depressive disorders were increased in patients who had anaplastic astrocytoma (OR, 2.9; 95% CI, 1.4–6.1), somatic comorbidity (OR, 2.0; 95% CI, 1.1–3.7), lower equivalent income (OR, 2.2; 95% CI, 1.0–4.7), and impaired self‐reported cognitive functioning (OR, 2.1; 95% CI, 1.0–4.4.).
There was an indication that PTSD was more frequent in patients younger than 50 years (OR, 4.3; 95% CI, 1.6–12.1) and less frequent in patients with recurrent disease (OR, 0.5; 95% CI, 0.2–1.0).
Generalized anxiety disorders are probably more common in women (OR, 1.8; 95% CI, 1.0–3.3), and adjustment disorders are more common in patients with lower income (OR, 2.7; 95% CI, 1.1.–7.0).
Because of the low number of diagnoses (n = 8), we did not calculate a separate regression model for alcohol use disorders.
DISCUSSION
In this multicenter study of adult patients with high‐grade glioma, common mental disorders were present in one third (31%) of the participants. This proportion is similar to the 38% reported by Goebel et al. 29 and higher than the 17% and 19% reported in our own previous work, 28 , 30 although the current study was much larger and thus able to obtain more precise estimates of the frequency of psychiatric comorbidity in patients with glioma. The 95% CI for this proportion spans from 28% to 35%. Because we did not conduct the complete SCID, and thereby did not assess all mental health conditions fully, the true proportion of common mental disorders might be even higher.
The most frequent diagnosis was PTSD at 10%. To put these numbers into context, in Germany, the 12‐month prevalence of PTSD was 2% in an interview‐based study with 5303 participants. 52 Depression, persistent depressive disorder, and major depression combined occurred in 14% of patients with glioma (in Germany, 9%), generalized anxiety disorder occurred in 7% (in Germany, 2%), alcohol use disorder occurred in 1% (in Germany, 2%), and adjustment disorder occurred in 4% (in Germany, not assessed).
Compared with other cancer groups, the proportion of patients with common mental disorders is similar. Meta‐analyses 8 , 9 and large, multicenter studies using gold‐standard diagnostic tools 11 , 12 , 14 , 19 , 30 consistently indicate that approximately 30% of all patients with cancer suffer from at least one common mental disorder.
In contrast to other studies, 34 our findings indicated a higher prevalence of mental disorders among young patients who have glioma compared with their older counterparts. A plausible rationale for this inconsistency may stem from the finding that a majority of the studies aggregated in the comprehensive review conducted by Rooney et al. predominantly used questionnaires for the detection of mental health issues, foregoing the use of thorough clinical interviews. However, neither of the interview‐based studies 31 , 32 indicated any association of age and comorbidity. Results from the general population in the United Kingdom 21 suggest that the proportion of common mental disorders increases up to the age of 50 years in women and 60 years in men, and then decreases. This is in line with our findings.
Another discrepancy in existing data is the missing association between the location of the lesion in the frontal lobe and psychiatric comorbidity in general or with any specific mental health disorder in particular. This is in contradiction to the findings of Wellisch et al., 32 who identified it as a strong predictor of depression. However, Rooney et al. 31 did not find that depression was more frequent in patients with an affected frontal lobe. This, in turn, is in line with our findings. Both Rooney et al. 31 as well as our observations revealed an association between poor physical and cognitive functioning and an increased likelihood of mental disorders, especially that of major depression.
Another risk factor for poor mental health that emerged from our study was low income—or, vice versa, poor mental health as a risk factor for low income. It is well known that financial hardship is related to various health conditions, 53 , 54 , 55 , 56 yet it is unfortunately often overlooked in clinical practice. One reason for this oversight could be that talking about money is taboo for some patients and possibly also for clinicians. Especially when there are financial problems, patients might feel ashamed and fear stigmatization. This calls for better communication about such topics and the involvement of social services in clinical care. Related to this, patients who live alone may also be at increased risk of mental disorders, particularly depression, because they lack the social support that could potentially mitigate social challenges and alleviate emotional distress. 15 , 36 , 37
Knowing the potential predictors of comorbid mental disorders in patients with high‐grade glioma may help to ascertain which patients should be offered specialized services, in addition to inquiring about their emotional well‐being and psychosocial support needs. 57 It should be noted that men and women in our study did not substantially differ regarding their mental health (with the exception of generalized anxiety disorder, which was more frequent in women, but the statistical certainty for this finding was rather low). This is important because men are known to be less often informed about psychosocial services although they have the same level of support needs as women. 14 , 36 , 58 Clinicians should look out for signs of mental health problems irrespective of gender and forward the patients to mental health specialists if needed. Especially men may need an active approach in this respect. 59 , 60
When interpreting the results of this study, the cross‐sectional design should be kept in mind, which precludes any possibility of causal inference. Another limitation is that the proportion of patients who were eligible but did not participate was relatively high at 45%. This raises the question whether our sample is representative for the population of patients with high‐grade gliomas. Based on cancer registry data, the distribution of gender (the proportion of men is 57% in our sample and 55% in the combined data set for Germany; www.krebsdaten.de, August 2, 2024) and of age is representative in our sample; this finding is backed by the comparison of participants versus nonparticipants. However, this does not preclude selection bias if nonparticipation is associated with mental health, which is not assessed in the registries and thus cannot be compared. Among the reasons for nonparticipation, being too distressed was the most frequent one. This indicates that our results are probably biased toward zero, which means that the proportion of patients with common mental disorders is likely even higher than what we observed in our data. Another limitation is that we were not able to obtain participants' family histories, which could be related to their mental health. Moreover, to keep the case report forms manageable, we had to reduce the number of variables; therefore, we did not document whether the patients were prescribed dexamethasone or anticonvulsant medication, which are clinically useful but could affect mental health as well. 31 Finally, the interviews were conducted by study nurses, not by psychiatrists or psychologists. Although the nurses had been carefully trained and supervised throughout the study, it is still possible that misclassifications in either direction occurred, leading to more imprecise effect estimates.
Furthermore, cognitive functioning was assessed with only two questions from a self‐report instrument. Although the internal consistency was high, it is still a limitation that we could not assess cognitive functioning in more detail. It is possible that patients with severe cognitive problems in particular would not even complete such a questionnaire. As for some mental disorders, for example, major depression, impaired cognition is a criterion of the disease itself (e.g., attention problems or an inability to decide), and our findings might be biased towards zero in this regard, too.
Another potential shortcoming is that we were unable to include isocitrate dehydrogenase (IDH) 61 mutation status in our analyses because of limited resources. Although no differences in mental health have been observed between patients who have a diagnosis of glioma with IDH mutation and glioma with wild‐type IDH to date, 62 other studies have found that IDH status is associated with age and fatigue. 63 Because both variables might be associated with the occurrence of common mental disorders, thereby establishing an indirect link between mutation status and mental health, it may be worthwhile exploring their relations in future studies.
An advantage of our methodology was the capacity to conduct gold‐standard clinical interviews with a high number of patients, resulting in well powered analyses, except for alcohol use disorders. For this disorder category, the prevalence within the sample was insufficient to permit reliable computations regarding clinical associations with other variables.
A strength of the interview‐based diagnostic approach is that the full clinical picture can be taken into account. If prevalence data are based on (often very short) self‐report measures, the sensitivity and specificity are limited. For example, the Hospital Anxiety and Depression Scale, a commonly used questionnaire to measure distress in patients who have cancer, exhibits a pooled sensitivity of 73% when screening for any ill mental health, according to a meta‐analysis that included 50 studies. 64 Similar results can be found for other self‐report instruments. 65 This means that about three quarter of the ill patients are correctly identified as ill—but one quarter are not. Conversely, the positive predictive value, i.e., the proportion of true positives among all positives, can be low, especially in community samples. 66
Self‐report measures usually assess mental health dimensionally. The frequency of mental health disorders is then estimated using certain thresholds. By this, subclinical ill health can be counted as a disorder, which usually leads to an overestimation of prevalent disorders. For example, the Beck Depression Inventory, a commonly used screening instrument for depression, identified depression in an average of 39% of patients with glioma, 34 which is much higher than the numbers found in our study (14%) or in other interview‐based studies (15%). 34
Another strength of our study is the large number of enrolled patients who also came from different hospitals. This enabled us to perform detailed analyses and yet the statistical certainty of the effect estimates is relatively high. Comparable studies to date have had significantly smaller sample sizes. 28 , 29 , 30
In summary, about one third of adult patients with high‐grade glioma may suffer from a clinically relevant common mental disorder, without notable disparity between men and women. In particular, clinicians should pay attention to possible comorbidities for cases in which patients endorse subjective cognitive complaints, are younger than 50 years, maintain a state of stable disease (compared with a complete remission), and live alone. To be better able to identify patients at increased risk and to provide adequate care, we advise clinicians to undergo comprehensive training in psycho‐oncology and seek supervision, particularly when treating patients who have complex psychiatric comorbidity. In addition, the implementation of routine screening for psychosocial distress is advisable and indeed is required in many national guidelines. 67 , 68 When doing so, one needs to ensure that the screening methods applied are adequate for patients with brain tumors to obtain valid results. 69 , 70 They may not be able to complete a self‐report questionnaire because of cognitive problems. Therefore, asking structured questions during the physician–patient consultation can be a viable alternative. 71 , 72 But clinicians should be aware that screening is only that—just screening. If scores are increased, patients must undergo further diagnostic procedures and potentially be forwarded to mental health specialists.
AUTHOR CONTRIBUTIONS
Susanne Singer: Conceptualization, methodology, formal analysis, supervision, funding acquisition, investigation, writing–original draft, writing–review and editing, validation, data curation, software, project administration, visualization, and resources. Melanie Schranz: Project administration, writing–review and editing, and data curation. Melina Hippler: Project administration, data curation, and writing–review and editing. Robert Kuchen: Writing–review and editing. Carolin Weiß Lucas: Writing–review and editing and resources. Juergen Meixensberger: Writing–review and editing and resources. Michael Karl Fehrenbach: Writing–review and editing and resources. Naureen Keric: Writing–review and editing and resources. Meike Mitsdoerffer: Writing–review and editing and resources. Jens Gempt: Writing–review and editing and resources. Jan Coburger: Writing–review and editing and resources. Almuth Friederike Kessler: Writing–review and editing and resources. Jens Wehinger: Writing–review and editing and resources. Martin Misch: Writing–review and editing and resources. Julia Onken: Writing–review and editing and resources. Marion Rapp: Writing–review and editing and resources. Martin Voß: Writing–review and editing and resources. Minou Nadji‐Ohl: Writing–review and editing and resources. Marcus Mehlitz: Writing–review and editing, and resources. Marcos Tatagiba: Supervision, writing–review and editing, and resources. Ghazaleh Tabatabai: Supervision, writing–review and editing, and resources. Mirjam Renovanz: Conceptualization, investigation, funding acquisition, writing–review and editing, project administration, supervision, and resources.
CONFLICT OF INTEREST STATEMENT
Susanne Singer reports grants/contracts from Lilly Deutschland; personal/consulting fees from Content Ed Net; and lecture fees from Lilly outside the submitted work. Martin Misch reports travel support and support for other professional activities from Novocure outside the submitted work. Mirjam Renovanz reports grants/contracts from Novocure outside the submitted work. The remaining authors disclosed no conflicts of interest.
ACKNOWLEDGMENTS
This study was funded by the Innovation Fund (Innovationsfonds) of the Federal Joint Committee (G‐BA) in Germany (Grant/Award No. 01VSF18011).
Open Access funding enabled and organized by Projekt DEAL.
Singer S, Schranz M, Hippler M, et al. Frequency and clinical associations of common mental disorders in adults with high‐grade glioma—a multicenter study. Cancer. 2025;e35653. doi: 10.1002/cncr.35653
DATA AVAILABILITY STATEMENT
Research data are stored in an institutional repository and will be shared upon reasonable request to the first and last authors.
REFERENCES
- 1. Das‐Munshi J, Stewart R, Ismail K, Bebbington PE, Jenkins R, Prince MJ. Diabetes, common mental disorders, and disability: findings from the UK National Psychiatric Morbidity Survey. Psychosom Med. 2007;69(6):543‐550. doi: 10.1097/psy.0b013e3180cc3062 [DOI] [PubMed] [Google Scholar]
- 2. Prince M, Patel V, Saxena S, et al. Global mental health 1—no health without mental health. Lancet. 2007;370(9590):859‐877. doi: 10.1016/s0140-6736(07)61238-0 [DOI] [PubMed] [Google Scholar]
- 3. Singer S, Sievers L, Linn LM, Janke J, Dietsch L, Negwer V. What is considered to be emotional suffering by psychotherapy patients and their therapists in Eastern versus Western Germany? A mixed‐methods study. Couns Psychother Res. 2021;21(2):448‐458. doi: 10.1002/capr.12345 [DOI] [Google Scholar]
- 4. Singer S, Sievers L, Scholz I, Taylor K, Blanck J, Maier L. Suicidal ideation and attempts in adults seeking outpatient psychodynamic psychotherapy. Clin Psychol Psychother. 2023;30(2):317‐334. doi: 10.1002/cpp.2797 [DOI] [PubMed] [Google Scholar]
- 5. Civitarese G. The Violence of Emotions. Routledge; 2013. [Google Scholar]
- 6. Bach A, Knauer K, Graf J, Schaeffeler N, Stengel A. Psychiatric comorbidities in cancer survivors across tumor subtypes: a systematic review. World J Psychiatry. 2022;12(4):623‐635. doi: 10.5498/wjp.v12.i4.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osazuwa‐Peters N, Simpson MC, Zhao LW, et al. Suicide risk among cancer survivors: head and neck versus other cancers. Cancer. 2018;124(20):4072‐4079. doi: 10.1002/cncr.31675 [DOI] [PubMed] [Google Scholar]
- 8. Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative‐care settings: a meta‐analysis of 94 interview‐based studies. Lancet Oncol. 2011;12(2):160‐174. doi: 10.1016/s1470-2045(11)70002-x [DOI] [PubMed] [Google Scholar]
- 9. Singer S, Das‐Munshi J, Brähler E. Prevalence of mental health conditions in cancer patients in acute care—a meta‐analysis. Ann Oncol. 2010;21(5):925‐930. doi: 10.1093/annonc/mdp515 [DOI] [PubMed] [Google Scholar]
- 10. Kadan‐Lottick NS, Vanderwerker LC, Block SD, Zhang BH, Prigerson HG. Psychiatric disorders and mental health service use in patients with advanced cancer—a report from the Coping With Cancer study. Cancer. 2005;104(12):2872‐2881. doi: 10.1002/cncr.21532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuhnt S, Brähler E, Faller H, et al. Twelve‐month and lifetime prevalence of mental disorders in cancer patients. Psychother Psychosom. 2016;85(5):289‐296. doi: 10.1159/000446991 [DOI] [PubMed] [Google Scholar]
- 12. Mehnert A, Brähler E, Faller H, et al. Four‐week prevalence of mental disorders in cancer patients across major tumor entities. J Clin Oncol. 2014;32(31):3540‐3546. doi: 10.1200/jco.2014.56.0086 [DOI] [PubMed] [Google Scholar]
- 13. Atesci FC, Baltalarli B, Oguzhanoglu NK, Karadag F, Ozdel O, Karagoz N. Psychiatric morbidity among cancer patients and awareness of illness. Support Care Cancer. 2004;12(3):161‐167. doi: 10.1007/s00520-003-0585-y [DOI] [PubMed] [Google Scholar]
- 14. Singer S, Szalai C, Briest S, et al. Co‐morbid mental health conditions in cancer patients at working age—prevalence, risk profiles, and care uptake. Psychooncology. 2013;22(10):2291‐2297. doi: 10.1002/pon.3282 [DOI] [PubMed] [Google Scholar]
- 15. Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751‐757. doi: 10.1001/jama.1983.03330300035030 [DOI] [PubMed] [Google Scholar]
- 16. Moncayo FLG, Requena GC, Perez FJ, Salamero M, Sanchez N, Sirgo A. Psychological adjustment and prevalence of psychiatric disorders in cancer patients. Med Clin (Barc). 2008;130(3):90‐92. doi: 10.1157/13115354 [DOI] [PubMed] [Google Scholar]
- 17. Nakasujja N, Musisi S, Walugembe J, Wallace D. Psychiatric disorders among the elderly on non‐psychiatric wards in an African setting. Int Psychogeriatr. 2007;19(4):691‐704. doi: 10.1017/s1041610207005418 [DOI] [PubMed] [Google Scholar]
- 18. Keszte J, Danker H, Dietz A, et al. Course of psychiatric comorbidity and utilization of mental health care after laryngeal cancer: a prospective cohort study. Eur Arch Otorhinolaryngol. 2017;274(3):1591‐1599. doi: 10.1007/s00405-016-4340-7 [DOI] [PubMed] [Google Scholar]
- 19. Singer S, Schwentner L, van Ewijk R, et al. The course of psychiatric co‐morbidity in patients with breast cancer—results from the prospective multi‐centre BRENDA II study. Psychooncology. 2016;25(5):590‐596. doi: 10.1002/pon.3978 [DOI] [PubMed] [Google Scholar]
- 20. National Collaborating Centre for Mental Health (UK) . Common Mental Health Disorders: Identification and Pathways to Care. National Institute for Health and Care Excellence (NICE) Clinical Guidelines. British Psychological Society; 2011. [PubMed] [Google Scholar]
- 21. Stansfeld S, Clark C, Bebbington P, King M, Jenkins R, Hinchliffe S. Chapter 2: Common mental disorders. In: McManus S, Bebbington P, Jenkins R, Brugha T, eds. Mental Health and Wellbeing in England: Adult Psychiatric Morbidity Survey 2014. NHS Digital; 2016:38‐68. Accessed October 25, 2023. https://www.researchgate.net/publication/309013865_Mental_health_and_wellbeing_in_England_Adult_Psychiatric_Morbidity_Survey_2014/link/57fe266608ae727564013231/download?_tp=eyJjb250ZXh0Ijp7ImZpcnN0UGFnZSI6InB1YmxpY2F0aW9uIiwicGFnZSI6InB1YmxpY2F0aW9uIn19 [Google Scholar]
- 22. Heinzel A, Mottaghy FM, Filss C, et al. The impact of brain lesions on health‐related quality of life in patients with WHO CNS grade 3 or 4 glioma: a lesion‐function and resting‐state fMRI analysis. J Neurooncol. 2023;161(3):643‐654. doi: 10.1007/s11060-023-04254-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piil K, Jakobsen J, Christensen KB, Juhler M, Jarden M. Health‐related quality of life in patients with high‐grade gliomas: a quantitative longitudinal study. J Neurooncol. 2015;124(2):185‐195. doi: 10.1007/s11060-015-1821-2 [DOI] [PubMed] [Google Scholar]
- 24. Dirven L, Aaronson NK, Heimans JJ, Taphoorn MJB. Health‐related quality of life in high‐grade glioma patients. Chin J Cancer. 2014;33(1):40‐45. doi: 10.5732/cjc.013.10214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akechi T, Okuyama T, Sugawara Y, Nakano T, Shima Y, Uchitomi Y. Major depression, adjustment disorders, and post‐traumatic stress disorder in terminally ill cancer patients: associated and predictive factors. J Clin Oncol. 2004;22(10):1957‐1965. doi: 10.1200/jco.2004.08.149 [DOI] [PubMed] [Google Scholar]
- 26. Leopold KA, Ahles TA, Walch S, et al. Prevalence of mood disorders and utility of the PRIME‐MD in patients undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 1998;42(5):1105‐1112. doi: 10.1016/s0360-3016(98)00346-0 [DOI] [PubMed] [Google Scholar]
- 27. Lichtenthal WG, Nilsson M, Zhang BH, et al. Do rates of mental disorders and existential distress among advanced stage cancer patients increase as death approaches? Psychooncology. 2009;18(1):50‐61. doi: 10.1002/pon.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singer S, Roick J, Danker H, et al. Psychiatric co‐morbidity, distress, and use of psycho‐social services in adult brain cancer patients—a prospective study. Acta Neurochir (Wien). 2018;160(6):1187‐1194. doi: 10.1007/s00701-018-3527-7 [DOI] [PubMed] [Google Scholar]
- 29. Goebel S, von Harscher M, Mehdorn HM. Comorbid mental disorders and psychosocial distress in patients with brain tumours and their spouses in the early treatment phase. Support Care Cancer. 2011;19(11):1797‐1805. doi: 10.1007/s00520-010-1021-8 [DOI] [PubMed] [Google Scholar]
- 30. Singer S, Bringmann H, Hauss J, et al. Prevalence of concomitant psychiatric disorders and the desire for psychosocial help in patients with malignant tumors in an acute hospital. Dtsch Med Wochenschr. 2007;132(40):2071‐2076. doi: 10.1055/s-2007-985643 [DOI] [PubMed] [Google Scholar]
- 31. Rooney AG, McNamara S, Mackinnon M, et al. Frequency, clinical associations, and longitudinal course of major depressive disorder in adults with cerebral glioma. J Clin Oncol. 2011;29(32):4307‐4312. doi: 10.1200/jco.2011.34.8466 [DOI] [PubMed] [Google Scholar]
- 32. Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients. Psychooncology. 2002;11(3):230‐238. doi: 10.1002/pon.562 [DOI] [PubMed] [Google Scholar]
- 33. Jimenez AE, Cicalese KV, Chakravarti S, et al. Substance use disorders are independently associated with hospital readmission among patients with brain tumors. World Neurosurg. 2022;166:E358‐E368. doi: 10.1016/j.wneu.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 34. Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011;103(1):61‐76. doi: 10.1093/jnci/djq458 [DOI] [PubMed] [Google Scholar]
- 35. Roick J, Danker H, Kersting A, et al. Predictors of psychiatric comorbidity in cancer patients at the time of discharge from the hospital. Soc Psychiatry Psychiatr Epidemiol. 2022;57(3):553‐561. doi: 10.1007/s00127-021-02138-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singer S, Sievers L, Scholz I, Blanck J, Taylor K, Maier L. Who seeks psychodynamic psychotherapy in community‐based practices? Patient characteristics examined in a large sample of applications for reimbursement of psychotherapy in Germany. Psychodyn Pract. 2023;29(2):117‐135. doi: 10.1080/14753634.2023.2182702 [DOI] [Google Scholar]
- 37. Schlegel RJ, Manning MA, Molix LA, Talley AE, Bettencourt BA. Predictors of depressive symptoms among breast cancer patients during the first year post diagnosis. Psychol Health. 2012;27(3):277‐293. doi: 10.1080/08870446.2011.559232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leonetti A, Puglisi G, Rossi M, et al. Factors influencing mood disorders and health related quality of life in adults with glioma: a longitudinal study. Front Oncol. 2021;11:662039. doi: 10.3389/fonc.2021.662039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hao AP, Huang JL, Xu X. Anxiety and depression in glioma patients: prevalence, risk factors, and their correlation with survival. Irish J Med Sci. 2021;190(3):1155‐1164. doi: 10.1007/s11845-020-02374-5 [DOI] [PubMed] [Google Scholar]
- 40. Khan F, Amatya B. Factors associated with long‐term functional outcomes, psychological sequelae and quality of life in persons after primary brain tumour. J Neurooncol. 2013;111(3):355‐366. doi: 10.1007/s11060-012-1024-z [DOI] [PubMed] [Google Scholar]
- 41. First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I), Clinician Version. American Psychiatric Press; 1997. [Google Scholar]
- 42. Wu Y, Levis B, Daray FM, et al. Comparison of the accuracy of the 7‐item HADS Depression subscale and 14‐item total HADS for screening for major depression: a systematic review and individual participant data meta‐analysis. Psychol Assess. 2023;35(2):95‐114. doi: 10.1037/pas0001181 [DOI] [PubMed] [Google Scholar]
- 43. van der Meer PB, Dirven L, Hertler C, et al. Depression and anxiety in glioma patients. Neurooncol Pract. 2023;10(4):335‐343. doi: 10.1093/nop/npad019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bringmann H, Singer S, Höckel M, Stolzenburg J‐U, Krauß O, Schwarz R. Prevalence and long‐term course of psychiatric disorders in cancer patients: a pilot study. Psychosoc Med. 2008;5:Doc03. [PMC free article] [PubMed] [Google Scholar]
- 45. Osorio FL, Loureiro SR, Hallak JEC, et al. Clinical validity and intrarater and test‐retest reliability of the Structured Clinical Interview for DSM‐5—Clinician Version (SCID‐5‐CV). Psychiatr Clin Neurosci. 2019;73(12):754‐760. doi: 10.1111/pcn.12931 [DOI] [PubMed] [Google Scholar]
- 46. Beesdo‐Baum K, Zaudig M, Wittchen H‐U. SCID‐5‐CV. Strukturiertes Klinisches Interview für DSM‐5(R)‐Störungen. Klinische Version. Hogrefe; 2019. [Google Scholar]
- 47. Singer S, Danker H, Roick J, et al. Effects of stepped psychooncological care on referral to psychosocial services and emotional well‐being in cancer patients: a cluster‐randomized phase III trial. Psychooncology. 2017;26(10):1657‐1683. doi: 10.1002/pon.4492 [DOI] [PubMed] [Google Scholar]
- 48. Renovanz M, Hippler M, Voß M, et al. Glioma patients in outpatient care—optimization of psychosocial care in neuro‐oncological patients (GLIOPT): study protocol of a multicenter cluster randomized controlled trial. Trials. 2020;21(1):434. doi: 10.1186/s13063-020-04321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949:191‐205. [Google Scholar]
- 50. Aaronson N, Ahmedzai S, Bergmann B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365‐376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 51. Giesinger JM, Loth FLC, Aaronson NK, et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ‐C30 in clinical practice and research. J Clin Epidemiol. 2020;118:1‐8. doi: 10.1016/j.jclinepi.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 52. Jacobi F, Höfler M, Strehle J, et al. Twelve‐months prevalence of mental disorders in the German Health Interview and Examination Survey for Adults—Mental Health Module (DEGS1‐MH): a methodological addendum and correction. Int J Methods Psychiatr Res. 2015;24(4):305‐313. doi: 10.1002/mpr.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rachet B, Quinn MJ, Cooper N, Coleman MP. Survival from cancer of the larynx in England and Wales up to 2001. Br J Cancer. 2008;99(suppl 1):S35‐S37. doi: 10.1038/sj.bjc.6604581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marmot M, Smith GD, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387‐1393. [DOI] [PubMed] [Google Scholar]
- 55. Dixit N, Crawford GB, Lemonde M, Rittenberg CN, Fernandez‐Ortega P. Left behind: cancer disparities in the developed world. Support Care Cancer. 2016;24(8):3261‐3264. doi: 10.1007/s00520-016-3192-4 [DOI] [PubMed] [Google Scholar]
- 56. Singer S, Bartels M, Briest S, et al. Socio‐economic disparities in long‐term cancer survival—10 year follow‐up with individual patient data. Support Care Cancer. 2017;25(5):1391‐1399. doi: 10.1007/s00520-016-3528-0 [DOI] [PubMed] [Google Scholar]
- 57. Carlson LE. Screening alone is not enough: the importance of appropriate triage, referral, and evidence‐based treatment of distress and common problems. J Clin Oncol. 2013;31(29):3616‐3617. doi: 10.1200/JCO.2013.51.4315 [DOI] [PubMed] [Google Scholar]
- 58. Bayram C, Fahridin S, Britt H. Men and mental health. Aust Fam Physician. 2009;38(3):91‐92. [PubMed] [Google Scholar]
- 59. Nascimento AF, Tondorf T, Rothschild SI, et al. Oncologist recommendation matters! Predictors of psycho‐oncological service uptake in oncology outpatients. Psychooncology. 2019;28(2):351‐357. doi: 10.1002/pon.4948 [DOI] [PubMed] [Google Scholar]
- 60. Singer S, Wünsch A, Ihrig A, et al. Men's access to outpatient psychosocial cancer counselling—a cluster‐randomized trial. Dtsch Arztebl Int. 2024;121(4):121‐127. doi: 10.3238/arztebl.m2024.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Han S, Liu Y, Cai SRJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580‐1589. doi: 10.1038/s41416-020-0814-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Buvarp D, Rydén I, Sunnerhagen KS, et al. Preoperative patient‐reported outcomes in suspected low‐grade glioma: markers of disease severity and correlations with molecular subtypes. J Clin Med. 2021;10(4):13‐645. doi: 10.3390/jcm10040645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vecchio TG, Rydén I, Ozanne A, et al. Global health status and fatigue score in isocitrate dehydrogenase‐mutant diffuse glioma grades 2 and 3: a longitudinal population‐based study from surgery to 12‐month follow‐up. Neurooncol Pract. 2024;11(3):347‐357. doi: 10.1093/nop/npae017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta‐analysis. J Affect Disord. 2010;126(3):335‐348. doi: 10.1016/j.jad.2010.01.067 [DOI] [PubMed] [Google Scholar]
- 65. Singer S, Danker H, Dietz A, et al. Screening for mental disorders in laryngeal cancer patients: a comparison of six methods. Psychooncology. 2008;17(3):280‐286. doi: 10.1002/pon.1229 [DOI] [PubMed] [Google Scholar]
- 66. Mitchell AJ. Clinical utility of screening for clinical depression and bipolar disorder. Curr Opin Psychiatry. 2012;25(1):24‐31. doi: 10.1097/YCO.0b013e32834de45b [DOI] [PubMed] [Google Scholar]
- 67. Grassi L, Watson M; IPOS Federation of Psycho‐Oncology Societies' co‐authors . Psychosocial care in cancer: an overview of psychosocial programmes and national cancer plans of countries within the International Federation of Psycho‐Oncology Societies. Psychooncology. 2012;21(10):1027‐1033. doi: 10.1002/pon.3154 [DOI] [PubMed] [Google Scholar]
- 68. Holland JG, Reznik I. Pathways for psychosocial care of cancer survivors. Cancer. 2005;104(11):2624‐2637. doi: 10.1002/cncr.21252 [DOI] [PubMed] [Google Scholar]
- 69. Renovanz M, Hickmann AK, Coburger J, et al. Assessing psychological and supportive care needs in glioma patients—feasibility study on the use of the Supportive Care Needs Survey Short Form (SCNS‐SF34‐G) and the Supportive Care Needs Survey Screening Tool (SCNS‐ST9) in clinical practice. Eur J Cancer Care. 2018;27(1):e12598. doi: 10.1111/ecc.12598 [DOI] [PubMed] [Google Scholar]
- 70. Lucas CW, Renovanz M, Jost J, Sabel M, Wiewrodt D, Rapp M. Assessment practice of patient‐centered outcomes in surgical neuro‐oncology: survey‐based recommendations for clinical routine. Front Oncol. 2021;11(13):702017. doi: 10.3389/fonc.2021.702017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Renovanz M, Kuchen R, Hippler M, et al. Abstract 518: Screening psychosocial care needs in patients with glioma by physician‐led conversation versus questionnaires: a cluster randomized controlled trial. Paper presented at: The 36th German Cancer Conference (Deutscher Krebkongress; DKK); February 21–24, 2024; Berlin, Germany. [Google Scholar]
- 72. Voß H, Scholz‐Kreisel P, Richter C, Ringel F, Singer S, Renovanz M. Development of screening questions for doctor‐patient consultation assessing the quality of life and psychosocial burden of glioma patients: an explorative study. Qual Life Res. 2021;30(5):1513‐1522. doi: 10.1007/s11136-021-02756-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon reasonable request to the first and last authors.


