Abstract
Background
Systemic light chain amyloidosis is a rare and debilitating disease, especially for which initially presented with digestive tract involvement. Myocardial amyloidosis is highly aggressive with generally poor prognosis and often resulted in missed diagnosis or misdiagnosis with routine examination tools. Multimodality imaging play an important role in diagnosing the amyloidosis effect on multiple organs. Chemoradiotherapy is the mainstay of treatment.
Case presentation
This article presents a rare case of systemic light chain amyloidosis, initially with gastrointestinal symptoms, in a 68-year-old male. He was hospitalized with diarrhea for one year and a half, dysphagia for 4 months, but he had no dyspnea. The transthoracic echocardiogram revealed myocardial hypertrophy of the left ventricle, the hypertrophic heart muscle echoed like "ground glass". The left ventricular ejection fraction (LVEF) detected by Simpson method was 51% and global longitudinal strain (GLS) was -9.00%. But cardiac magnetic resonance showed the patient without gadolinium delayed enhancement. The urinary protein series quantification and the serum free light chain levels were all increased. While the ratio of free κ and free λ was decreased. Hence, the abdominal fat biopsy of the patient was amyloidosis by electronic and immunoelectron microscopy. Organs involved include heart, kidneys, gastrointestinal tract and nervous system, stage III of mayo 2012 model. The patient was treated with Dara-BCD chemotherapy. This case underscores the diagnostic complexity, emphasizing the need for early identification given the grim prognosis associated with systemic AL amyloidosis requiring clinical data, detailed imaging, and histopathological insights. After discharge, the patient became better and followed up in the outpatient.
Conclusions
Systemic light chain amyloidosis can easily be missed diagnosis or misdiagnosis in its early stages, losing the opportunity for initiating earlier treatments to improve potential patient outcomes. Despite advancements in diagnostic biomarkers, this case highlights the potential for missed diagnosis with standard CMR imaging when gadolinium enhancement is negative. The utility of echocardiographic features such as reduced GLS and abnormal ECG findings emerges as critical in early identification of myocardial amyloidosis. The correct diagnosis of this case relied on the comprehensive utilization of multimodal imaging techniques including biopsy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04441-6.
Keywords: Multimodality imaging, Myocardial amyloidosis, Systemic, Light chain, Case report
Background
Systemic light chain (AL) amyloidosis is a rare and debilitating disease with an estimated incidence of 10–14 cases per million people in the United States [1]. The pathogenic immunoglobulin light chain in systemic AL amyloidosis is most often produced by a small clonal plasma cell population, and misfolded light chains aggregate and deposit in organs as amyloid fibrils, disrupting multiple organs’ normal function and leading to lethal complications [2].
Early mortality was 13.4% and did not improve, it remained high in patients with advanced cardiac disease, especially for stages III and IV [3]. Current treatments for AL amyloidosis target the plasma/B cell clone aiming to reduce the production of toxic light chains. Regimens based on bortezomib were the most frequently used as first-line therapy, only 6.2% of the patients received autologous stem cell transplant [4–7].
Case presentation
A 68-year-old male presented with diarrhea for one year and a half, dysphagia for 4 months was hospitalized in the gastroenterology department of our hospital. He had no history of hereditary diseases in the family and psychological disorders. Diarrhea began to occur 4–5 times per day after the third injection of Corona Virus Disease 2019 (COVID-19) vaccine, without nausea, abdominal pain, chills and fever. The diarrhea could not be controlled by antidiarrheal drugs (imodium). The patient developed symptoms of syncope, loss of consciousness, walking instability, and postural hypotension was diagnosed. However, he had no dyspnea.
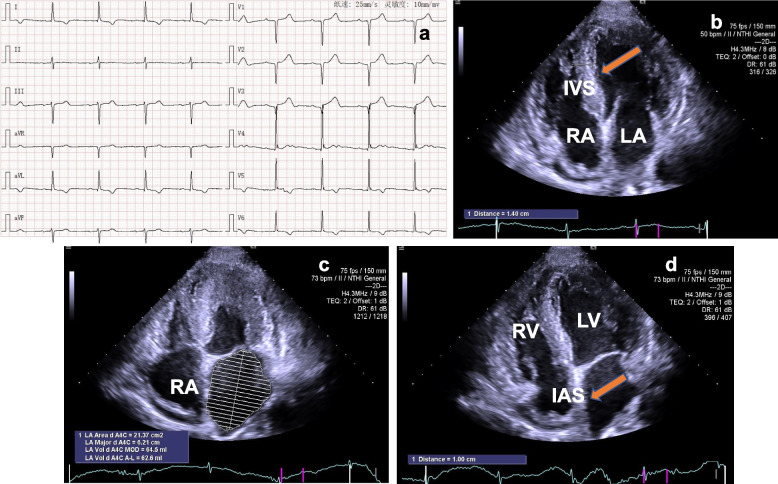
On admission, gastroenteroscopy examination showed chronic gastritis and enteritis. Initial 12-lead electrocardiogram showed sinus bradycardia, first degree atrioventricular block, V1, V2 and V3 displayed QS, T wave I, aVL, V5 and V6 inverted, V4 notched and presence of abnormal QRS axis and prolonged QTc (456 ms) (Fig. 1a). The levels of serum N-terminal proB-type natriuretic peptide (NT-proBNP) (1663.6 pg/mL) and cardiac troponin I (cTnI) (0.083 ng/mL) were elevated. 24-h urine protein (24hUP) was increased (3978.79 mg per day) and blood albumin was decreased (24.6 g/L). Then, he was diagnosed with hypoproteinemia.
Fig. 1.
Electrocardiogram and echocardiogram features. a ECG documented that the patient had sinus bradycardia, first degree atrioventricular block, and myocardial ischemia. b TTE demonstrated left and right ventricular myocardial hypertrophy, the myocardium echoed like "ground glass" (yellow arrow). c From apical four-chamber view, TTE detected the dilated left and right atrium. d TTE showed the thickened interatrial septum (yellow arrow) in apical four-chamber view. ECG: electrocardiogram; TTE: transthoracic echocardiography; LA: left atrium; RA: right atrium; IVS: interventricular septum; IAS: interatrial septum
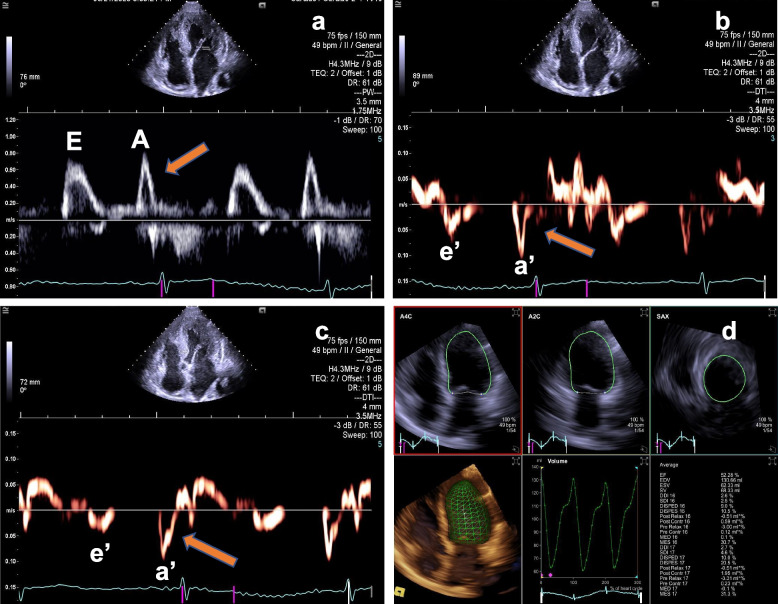
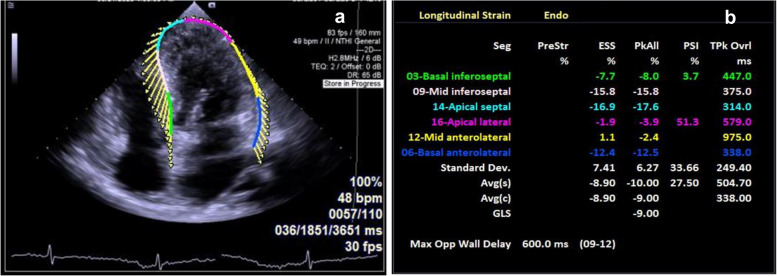
The transthoracic echocardiography (TTE) revealed myocardial hypertrophy of the left and right ventricle, the thickness of left ventricular wall was from 13.9 mm to 16 mm (Fig. 1b) and the lateral wall thickness of the right ventricle was 9.9 mm. The hypertrophic heart muscle echoed like "ground glass" (Fig. 1b) and the systolic activity of the left ventricular apical level was normal (Video 1). The left and right atrium slightly dilated, the volume was 62.6 ml and 45.9 ml, respectively, and the interatrial septum thickened with a thickness of 10 mm (Fig. 1c and 1d). At the same time, the patient also had a small amount of pericardial effusion (Video 2 and 3) and occasional premature beats. Color Doppler of transthoracic echocardiography revealed mild mitral and tricuspid regurgitation (video 4). The left ventricular ejection fraction (LVEF) detected by Simpson method was 51% and diastolic dysfunction was Grade I, E/e’ = 12.7 (Fig. 2a through c). Automatic volumetric left ventricular analysis (LVA) showed that LVEF was 52.28% (Fig. 2d; video 5). Strain tracking echocardiography of velocity vector imaging technique showed the patient with a global longitudinal strain (GLS) of −9.00% (Fig. 3a and b). We considered the patient with myocardial amyloidosis for the first time.
Fig. 2.
TTE and RT4D-TTE revealed the left ventricular ejection fraction and diastolic function. a Pulse wave Doppler showed that peak A velocity was higher than peak E of the mitral flow (yellow arrow). b Tissue Doppler Imaging (TDI) detected peak a’ velocity was higher than peak e’ of the mitral annular (yellow arrow). c Representative images of LV strain values, STE of VVI technique detected the patient with a GLS of −9.00%. d Automatic volumetric left ventricular analysis (LVA) showed that LV ejection fraction was 52.28%. RT4D-TTE: real-time 4-dimensional transthoracic echocardiography; TDI: Tissue Doppler Imaging
Fig. 3.
STE features of the heart. a, b Strain tracking echocardiography (STE) of velocity vector imaging (VVI) technique showed the patient with a GLS of −9.00%
Meanwhile, the entire process of NT-proBNP and cTnI exhibited dynamic changes in chronological order. The highest levels of serum NT-proBNP and cTnI were 4293 pg/mL and 0.083 ng/mL, respectively. The urinary protein series quantification was all increased: urinary immunoglobulin G 153.00 mg/L, urinary transferrin 69.90 mg/L, urinary microalbumin 1540.00 mg/L and A1-microglobulin 29.60 mg/L. While α2 macroglobulin was decreased (2.36 mg/L). The serum free light chain levels increased (Kappa 22.40 mg/L and Lambda 255.00 mg/L). The ratio of free κ and free λ was decreased (free κ/ free λ = 0.09). Meanwhile, 24-h urinary light chain were progressively increased (κ 118.94 mg/L and λ 141.55 mg/L). Immunofixation electrophoresis showed a series of M protein: albumin 48.4% was decreased, alpha 1 4.5% and gamma 21.3% were increased, while alpha 2 13.2% and beta 12.6% were normal. The pathological diagnosis of bone marrow was active proliferation of hematopoietic tissue.
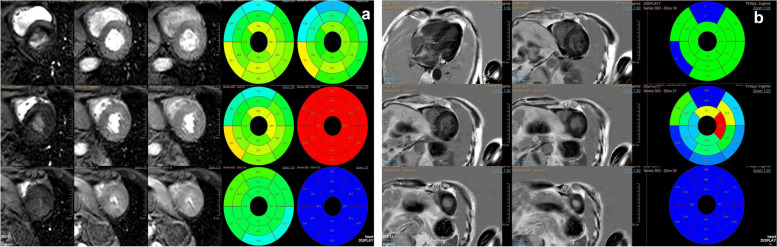
Cardiac magnetic resonance (CMR) showed homogeneous hypertrophy of the LV and the left ventricular systolic function was normal, a small amount of pericardial effusion, left ventricular myocardial perfusion and enhanced delayed scanning showed no significant abnormalities (Fig. 4a and b). In non-contrast CMR, the myocardial value was within the normal range. Therefore, the patient with negative late gadolinium enhancement was diagnosed. This brought us confusion.
Fig. 4.
CMR features of the patient. a CMR showed the left ventricular systolic function was normal, a small amount of pericardial effusion, left ventricular myocardial perfusion and enhanced delayed scanning showed no significant abnormalities. b Representative images of LGE images. The myocardial value was within the normal range and LGE was negative. CMR: cardiac magnetic resonance; LGE: late gadolinium enhancement
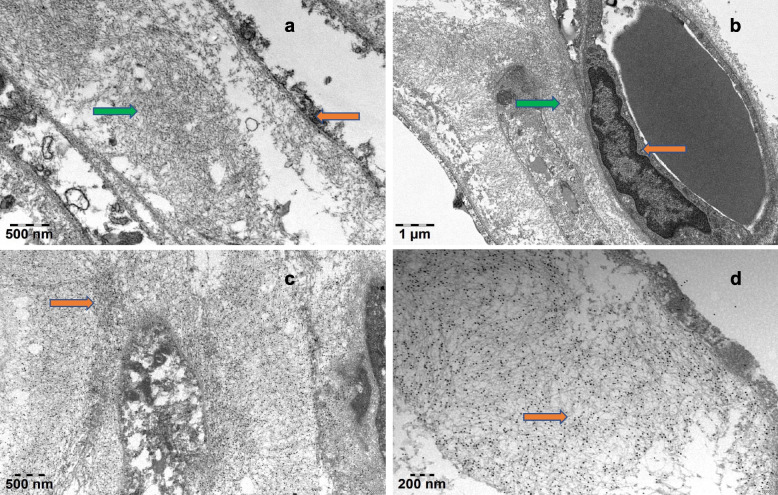
The patient was finally diagnosed with systemic AL amyloidosis by electronic microscopy (EM) and immunoelectron microscopy (IEM) (Fig. 5a through d). The abdominal fat biopsy of the patient was amyloidosis, EM showed the deposition of amyloid fibers between adipocytes or around the vessels. The filaments were unbranched, rigid, and disordered. IEM showed a large amount of colloidal gold particles attached to amyloid fibers. Organs involved include heart, kidneys, gastrointestinal tract and nervous system, stage III of mayo 2012 model. The acute management section started with diuretic therapy, control heart rate and manage blood pressure. Then, the patient accepted two courses of treatment, and the regimen was Dara-BCD chemotherapy. The treatments including 2.2 mg bortezomib, 300 mg cyclophosphamide, 960 mg Dara and 20 mg dexamethasone were pretreated per week, respectively. Two months later, his echocardiography showed the myocardial amyloidosis began to improve. The LVEF detected by Simpson method was 57%. Automatic volumetric left ventricular analysis showed that LVEF was 58%. Strain tracking echocardiography of velocity vector imaging technique showed the patient with a GLS of −13.00%. The levels of serum NT-proBNP (1441.31 pg/mL) and cTnI (0.046 ng/mL) were decreased. Lifestyle changes included dietary adjustments to limit fluid intake and manage sodium levels, as well as tailored exercise recommendations based on the patient's functional ability. The patient became better and followed up in the outpatient.
Fig. 5.
The pathological ultrastructure and electron microscopic examination confirmed the adipose tissue amyloidosis. a EM showed the fat cells (yellow arrow) and the deposition of amyloid fibers between adipocytes, that is, unbranched, rigid, and disordered filaments (green arrow). b EM showed the vascular endothelial cells (yellow arrow) and the deposition of amyloid fibers around the vessels (green arrow). Magnification 20000X, bar = 1.0um. c IEM showed lots of colloidal gold particles attached to amyloid fibers (black particles). Magnification 30000X, bar = 0.5um. d IEM showed a large amount of colloidal gold particles attached to amyloid fibers (black particles). Magnification 60000X, bar = 0.2um. EM: Electronic microscopy; IEM: Immunoelectron microscopy
Discussion and conclusions
Systemic AL amyloidosis is associated with non-specific symptoms and substantial heterogeneity in clinical presentation [8, 9]. It is usually not diagnosed and treated until irreversible organ damage has occurred because it requires a high degree of suspicion and specialized techniques [3–5, 10, 11]. Despite significant advances in diagnosis (introduction of serum free light chain measurement and the use of cardio-biomarkers) [12], timely diagnosis did not prompt, with half of the patients being diagnosed more than 5 months after the first symptoms. Over one-third of patients presenting with stage III disease were found and at an advanced cardiac stage.
This case underscored the diagnostic challenges in AL amyloidosis, particularly when symptoms were initially extra-cardiac. Echocardiographic features in this patient with myocardial amyloidosis were first diagnosed and finally he was proved with AL amyloidosis by the abdominal fat biopsy. In this case, the patient presented with gastrointestinal involvement, but without symptoms of heart failure such as dyspnea at the beginning. The severe cardiac amyloidosis of our diagnosis was based on echocardiographic features including thickening of left ventricular walls, echoed like "ground glass" in the myocardium, a small amount of pericardial effusion, bilateral atrial enlargement, and decreased GLS of left ventricle. Then, even if CMR imaging showed gadolinium delayed enhancement negative, we proved that LV strain values correlated significantly with the changes of EKG and GLS showed earlier impairment than serum biomarkers or LVEF. Multimodality imaging play an important role in diagnosing AL amyloidosis disease burden. Finally, amyloidosis involving multiple organs was verified by the abdominal fat biopsy, and stage III of mayo 2012 model was diagnosed.
At present, these cardiac biomarkers of NT-proBNP, cTnT, and the difference between the involved and uninvolved free light chain are often used to identify patients for optimal therapy selection and risk stratification during treatment for systemic amyloidosis in the Mayo staging system [13–17]. In this case, the entire process of NT-proBNP was showed exhibited dynamic changes in chronological order and the highest level of serum NT-proBNP was apparently increased, but less than 8500 pg/mL which was demonstrated by Vaxman, S. K.et al. [18]. The level of cTnI was from 0.083 ng/mL to 0.033 ng/mL before and after treatment while it was also exhibited dynamic changes. More recently Lilliness et al., [19] revised and validated the original Mayo staging to include BNP and cTnI cutoffs in place of NT-proBNP and cTnT offering centers without access to these latter markers, the ability to stage patients with systemic amyloidosis. Once patients develop AL cardiac amyloidosis, however, the clinical utility of these biomarkers is more uncertain. Nordlinger et al., [20] showed that BNP levels in those with cardiac amyloidosis did not discriminate between patients who had heart failure versus those who were asymptomatic. This indicates that BNP (a measure of wall stress) cannot distinguish between increased LV filling pressures and direct cardiac toxicity caused by extracellular amyloid deposits.
It was also reported that systemic AL amyloidosis with cardiac involvement was revealed in 50% of patients [21]. However, once symptomatic heart failure occurs, therapeutic options are limited, leading to a poor overall prognosis [22]. In addition, Kim D et al., [23] demonstrated that abnormal QRS axis and prolonged QTc (≥ 483 ms) showed significant incremental prognostic values in addition to revised Mayo stage. ECG score showed good associations with the mean absolute values of LV GLS and regional LS; and mean values of Log NT-proBNP differed significantly according to ECG score and showed an increasing trend as ECG score increased. In our case, ECG findings included presence of abnormal QRS axis and prolonged QTc (456 ms) were accompanied by the decrease in GLS of LV and the change in NT-proBNP, which were similar to the study of Kim D, et al. [23]. They found ECG scores consisting of presence of prolonged QTc (≥ 483 ms), and abnormal QRS axis showed good association with longitudinal LV dysfunction and NT-proBNP. Those parameters provided prognostic information regarding prognosis of AL amyloidosis with cardiac involvement.
As we all known, amyloid fibrils derived from antibody light chains are key pathogenic factors in systemic AL amyloidosis [2]. They were deposited in multiple organs, and one of the main risk agents of death was cardiac amyloid [4, 22]. Nevertheless, echocardiographic features of myocardial amyloidosis guiding clinical diagnosis had seldom been reported, especially for the negative result of CMR. The clinical symptoms such as diarrhoea, postural hypotension, damage of the nervous system and abnormal electrocardiogram findings which being inconsistent with myocardial hypertrophy presented the most valuable clues for diagnosis. Fat tissue biopsy is one of the requesting confirmations followed by risk stratification and disease staging.
AL amyloidosis should be distinguished with other restrictive cardiomyopathies, such as constrictive pericarditis, endomyocardial fibrosis (EMF), sarcoidosis, hemochromatosis and so on. TTE typically shows a thickened and calcified pericardium with diastolic ventricular collapse in the patient with constrictive pericarditis. CMR and CT scans can further delineate pericardial thickening and calcification. As for the patient with endomyocardial fibrosis (EMF), TTE shows thickened endocardial borders with chamber obliteration. Predominantly affects the right ventricle, leading to right heart failure. Cardiac catheterization may reveal elevated filling pressures with a characteristic "dip and plateau" pattern in the right ventricular pressure tracing. The diagnosis of sarcoidosis relies on tissue biopsy showing non-caseating granulomas. Additional tests such as chest X-ray, ECG, TTE, and CMR can support the diagnosis. The pathogenesis of hemochromatosis is excessive iron accumulation in tissues due to genetic or acquired disorders of iron metabolism. Diagnostic Tests including serum ferritin, transferrin saturation, and liver biopsy can provide a clear diagnosis. CMR imagings show iron deposition in different organs.
Once diagnosed, chemotherapy and/or autologous stem cell transplantation remains the standard of care and first-line treatment [21]. In this case, the patient responded well to chemotherapy with Dara-BCD, showing improvement in echocardiographic parameters and decreased biomarker levels after treatment. A 40-year longitudinal natural history study conducted by Staron A, et al. [24], revealed more effective management strategies for patients with advanced amyloid cardiomyopathy were still needed. It was reported that autologous stem cell transplant (ASCT) has improved the survival of AL amyloidosis [25] and other plasma cell-directed therapy such as immunomodulatory drugs (IMiDs), and proteasome inhibitors (PIs) has been used. Moreover, Gustine JN, et al. [26], found HDM/SCT-treated individuals had a highest 5-year overall survival rate, 50% patients received more than two lines of therapy, reflecting the potential long-term benefits of the treatment in AL amyloidosis. More accurate selection for those patients as different therapies became available.
Conclusions
In conclusion, systemic AL amyloidosis is prone to missed diagnosis or misdiagnosis in its early stages, losing the opportunity to initiate early treatment to improve the prognosis of potential patients. Despite advancements in diagnostic biomarkers, this case highlights the potential for missed diagnosis with standard CMR imaging when gadolinium enhancement is negative. The utility of echocardiographic features such as reduced GLS and abnormal ECG findings emerges as critical in early identification of myocardial amyloidosis. The successful diagnosis of our case depended on the comprehensive utilization of multiple imaging techniques including biopsy examinations.
Supplementary Information
Additional file 1: Video 1. The systolic activity of the left ventricular apical level was normal
Additional file 2: Video 2. From long axis view of left ventricle, the patient had a small amount of pericardial effusion
Additional file 3: Video 3. From apical three-chamber view of left ventricle, 2D-TTE image demonstrating small amount of pericardial effusion
Additional file 4: Video 4. Color Doppler of TTE revealed mild mitral and tricuspid regurgitation
Additional file 5: Video 5. Automatic volumetric left ventricular analysis (LVA) showed that LV ejection fraction was 52.28%
Acknowledgements
We appreciate all the doctors in Department of Hematology, Xinhua Hospital of Jiaotong University to treat this patient.
Abbreviations
- AL
Systemic light chain
- TTE
Transthoracic echocardiography
- LVEF
Left ventricular ejection fraction
- LV
Left ventricle
- NT-proBNP
N-terminal proB-type natriuretic peptide
- cTnT
Cardiac troponin T
- cTnI
Cardiac troponin I
- LVA
Left ventricular analysis
- GLS
Global longitudinal strain
- CMR
Cardiac magnetic resonance
- EM
Electronic microscopy
- IEM
Immunoelectron microscopy
Authors’ contributions
ZCL treated the patient. YY performed the echocardiography and was a major contributor in writing the manuscript. GYL performed the pathological examination. TW and YGL participated in the analysis and interpretation of the data. All authors read and approved the final manuscript.
Funding
This work was supported by grant 202240110 from the Shanghai Health and Family Planning Commission (Dr. Yi Yu) and by grant from Shanghai Chest Hospital affiliated with Shanghai Jiao Tong University School of Medicine (Dr. Yi Yu).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The present study was performed under clinical research protocols in accordance with the 1975 Declaration of Helsinki, and approved by the Ethics Committee of Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine (No. XHEC-D-2023–183).
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi Yu, Email: yuyichesthospital@163.com.
Yi-Gang Li, Email: liyigang@xinhuamed.com.cn.
References
- 1.Kyle RA, Larson DR, Kurtin PJ, Kumar S, Cerhan JR, Therneau TM, Rajkumar SV, Vachon CM, Dispenzieri A. Incidence of AL Amyloidosis in Olmsted County, Minnesota, 1990 through 2015. Mayo Clin Proc. 2019;94(3):465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamed RA, Bazarbachi AH, Bazarbachi A, Malard F, Harousseau JL, Mohty M. Comprehensive Review of AL amyloidosis: some practical recommendations. Blood Cancer. 2021;11(5):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palladini G, Schönland S, Merlini G, Milani P, Jaccard A, Bridoux F, Dimopoulos MA, Ravichandran S, Hegenbart U, Roeloffzen W, Cibeira MT, Agis H, Minnema MC, Bergantim R, Hájek R, João C, Leonidakis A, Cheliotis G, Sonneveld P, Kastritis E, Wechalekar A. The management of light chain (AL) amyloidosis in Europe: clinical characteristics, treatment patterns, and efficacy outcomes between 2004 and 2018. Blood Cancer J. 2023;13(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, Sanchorawala V, Gibbs S, Mollee P, Venner CP, Lu J, Schönland S, Gatt ME, Suzuki K, Kim K, Cibeira MT, Beksac M, Libby E, Valent J, Hungria V, Wong SW, Rosenzweig M, Bumma N, Huart A, Dimopoulos MA, Bhutani D, Waxman AJ, Goodman SA, Zonder JA, Lam S, Song K, Hansen T, Manier S, Roeloffzen W, Jamroziak K, Kwok F, Shimazaki C, Kim JS, Crusoe E, Ahmadi T, Tran N, Qin X, Vasey SY, Tromp B, Schecter JM, Weiss BM, Zhuang SH, Vermeulen J, Merlini G, Comenzo RL; ANDROMEDA Trial Investigators. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021; 385(1):46–58. [DOI] [PubMed]
- 5.Kastritis E, Leleu X, Arnulf B, Zamagni E, Cibeira MT, Kwok F, Mollee P, Hájek R, Moreau P, Jaccard A, Schönland SO, Filshie R, Nicolas-Virelizier E, Augustson B, Mateos MV, Wechalekar A, Hachulla E, Milani P, Dimopoulos MA, Fermand JP, Foli A, Gavriatopoulou M, Klersy C, Palumbo A, Sonneveld P, Johnsen HE, Merlini G, Palladini G. Bortezomib, melphalan, and dexamethasone for light-chain amyloidosis. J Clin Oncol. 2020;38(28):3252–60. [DOI] [PubMed] [Google Scholar]
- 6.Palladini G, Merlini G. How I treat AL amyloidosis. Blood. 2022;139(19):2918–30. [DOI] [PubMed] [Google Scholar]
- 7.Kastritis E, Dialoupi I, Gavriatopoulou M, Roussou M, Kanellias N, Fotiou D, Ntanasis-Stathopoulos I, Papadopoulou E, Ziogas DC, Stamatelopoulos K, Manios E, Ntalianis A, Eleutherakis-Papaiakovou E, Papanikolaou A, Migkou M, Papanota A-M, Gakiopoulou H, Psimenou E, Tselegkidi MI, Tsitsilonis O, Kostopoulos I, Terpos E, Dimopoulos MA. Primary treatment of light-chain amyloidosis with bortezomib, lenalidomide, and dexamethasone. Blood Adv. 2019;3(20):3002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641–54. [DOI] [PubMed] [Google Scholar]
- 9.Merlini G, Dispenzieri A, Sanchorawala V, Schönland SO, Palladini G, Hawkins PN, Gertz MA. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. [DOI] [PubMed] [Google Scholar]
- 10.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114(24):4957–9. [DOI] [PubMed] [Google Scholar]
- 11.Brambilla F, Lavatelli F, Di Silvestre D, Valentini V, Rossi R, Palladini G, Obici L, Verga L, Mauri P, Merlini G. Reliable typing of systemic amyloidoses through proteomic analysis of subcutaneous adipose tissue. Blood. 2012;119(8):1844–7. [DOI] [PubMed] [Google Scholar]
- 12.Radamaker L, Lin YH, Annamalai K, Huhn S, Hegenbart U, Schönland SO, Fritz G, Schmidt M, Fändrich M. Cryo-EM structure of a light chain-derived amyloid fibril from a patient with systemic AL amyloidosis. Nat Commun. 2019;10(1):1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Souza A, Brazauskas R, Dispenzieri A, Panepinto J, Flynn KE. Changes in patient-reported outcomes in light chain amyloidosis in the first year after diagnosis and relationship to NT-proBNP change. Blood Cancer J. 2021;11(2):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, McConnell JP, Litzow MR, Gastineau DA, Tefferi A, Inwards DJ, Micallef IN, Ansell SM, Porrata LF, Elliott MA, Hogan WJ, Rajkumar SV, Fonseca R, Greipp PR, Witzig TE, Lust JA, Zeldenrust SR, Snow DS, Hayman SR, McGregor CGA, Jaffe AS. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2004;104(6):1881–7. [DOI] [PubMed] [Google Scholar]
- 16.Rahman JE, Helou EF, Gelzer-Bell R, Thompson RE, Kuo C, Rodriguez ER, Hare JM, Baughman KL, Kasper EK. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43(3):410–5. [DOI] [PubMed] [Google Scholar]
- 17.Fotiou D, Theodorakakou F, Kastritis E. Biomarkers in AL Amyloidosis. Int J Mol Sci. 2021;22(20):10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaxman I, Kumar SK, Buadi F, Lacy MQ, Dingli D, Hwa Y, Fonder A, Hobbs M, Hayman S, Kourelis T, Warsame R, Muchtar E, Leung N, Kapoor P, Grogan M, Go R, Lin Y, Gonsalves W, Siddiqui M, Kyle RA, Rajkumar SV, Gertz MA, Dispenzieri A. Outcomes among newly diagnosed AL amyloidosis patients with a very high NT-proBNP: implications for trial design. Leukemia. 2021;35(12):3604–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lilleness B, Ruberg FL, Mussinelli R, Doros G, Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood. 2019;133(3):215–23. [DOI] [PubMed] [Google Scholar]
- 20.Nordlinger M, Magnani B, Skinner M, Falk RH. Is elevated plasma B-natriuretic peptide in amyloidosis simply a function of the presence of heart failure? Am J Cardiol. 2005;96(7):982–4. [DOI] [PubMed] [Google Scholar]
- 21.Tahir UA, Doros G, Kim JS, Connors LH, Seldin DC, Sam F. Predictors of Mortality in Light Chain Cardiac Amyloidosis with Heart Failure. Sci Rep. 2019;9(1):8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dispenzieri A, Kastritis E, Wechalekar AD, Schönland SO, Kim K, Sanchorawala V, Landau HJ, Kwok F, Suzuki K, Comenzo RL, Berg D, Liu G, Kumar A, Faller DV, Merlini G. A randomized phase 3 study of ixazomib-dexamethasone versus physician’s choice in relapsed or refractory AL amyloidosis. Leukemia. 2022;36(1):225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D, Lee GY, Choi JO, Kim K, Kim SJ, Jeon ES. Associations of electrocardiographic parameters with left ventricular longitudinal strain and prognosis in cardiac light chain amyloidosis. Sci Rep. 2019;9(1):7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staron A, Zheng L, Doros G, Connors LH, Mendelson LM, Joshi T, Sanchorawala V. Marked progress in AL amyloidosis survival: a 40-year longitudinal natural history study. Blood Cancer J. 2021;11(8):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidiqi MH, Aljama MA, Buadi FK, Warsame RM, Lacy MQ, Dispenzieri A, Dingli D, Gonsalves WI, Kumar S, Kapoor P, Kourelis T, Hogan WJ, Gertz MA. Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 2018;36(13):1323–9. [DOI] [PubMed] [Google Scholar]
- 26.Gustine JN, Staron A, Szalat RE, Mendelson LM, Joshi T, Ruberg FL, Siddiqi O, Gopal DM, Edwards CV, Havasi A, Kaku M, Lau KHV, Berk JL, Sloan JM, Sanchorawala V. Predictors of hematologic response and survival with stem cell transplantation in AL amyloidosis: a 25-year longitudinal study. Am J Hematol. 2022;97(9):1189–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Video 1. The systolic activity of the left ventricular apical level was normal
Additional file 2: Video 2. From long axis view of left ventricle, the patient had a small amount of pericardial effusion
Additional file 3: Video 3. From apical three-chamber view of left ventricle, 2D-TTE image demonstrating small amount of pericardial effusion
Additional file 4: Video 4. Color Doppler of TTE revealed mild mitral and tricuspid regurgitation
Additional file 5: Video 5. Automatic volumetric left ventricular analysis (LVA) showed that LV ejection fraction was 52.28%
Data Availability Statement
No datasets were generated or analysed during the current study.