Abstract
Introduction
Hippocampal hyperactivity is a hallmark of prodromal Alzheimer's disease (AD) that predicts progression in patients with amnestic mild cognitive impairment (aMCI). AGB101 is an extended‐release formulation of levetiracetam in the dose range previously demonstrated to normalize hippocampal activity and improve cognitive performance in aMCI. The HOPE4MCI study was a 78‐week trial to assess the progression of MCI due to AD. As reported in Mohs et al., the decline in the Clinical Dementia Rating Sum of Boxes score (CDR‐SB) was reduced by 40% in apolipoprotein E (APOE) ε4 non‐carriers over the 78‐week duration of the study with a negligible effect in carriers. Here we report an exploratory analysis of the effects of AGB101 on neuroimaging and biomarker measures in the 44 APOE ε4 non‐carriers who completed the 78‐week protocol.
Methods
Structural magnetic resonance imaging scans obtained at baseline and after 78 weeks were analyzed using the Automated Segmentation of Hippocampal Subfields software providing volume measures of key structures of the medial temporal lobe relevant to AD progression. Blood samples collected at 78 weeks in the study were analyzed for plasma biomarkers.
Results
Treatment with AGB101 significantly reduced atrophy of the left entorhinal cortex (ERC) compared to placebo. This reduction in atrophy was correlated with less decline in the CDR‐SB score over 78 weeks and with lower neurofilament light chain (NfL), a marker of neurodegeneration.
Discussion
The HOPE4MCI study showed that APOE ε4 non‐carriers treated with AGB101 demonstrated a substantially more favorable treatment effect compared to carriers. Here we report that treatment with AGB101 in non‐carriers of APOE ε4 significantly reduced atrophy of the left ERC over 78 weeks. That reduction in atrophy was closely coupled with the change in CDR‐SB and with plasma NfL indicative of neurodegeneration in the brain. These exploratory analyses are consistent with a reduction in neurodegeneration in APOE ε4 non‐carriers treated with AGB101 before a clinical diagnosis of dementia.
Highlights
AGB101 slows entorhinal cortex (ERC) atrophy in apolipoprotein E (APOE) ε4 non‐carriers with mild cognitive impairment (MCI) due to Alzheimer's disease (AD).
Slowing ERC atrophy by AGB101 is associated with less Clinical Dementia Rating Sum of Boxes decline.
Slowing ERC atrophy by AGB101 is associated with lower neurofilament light chain.
AGB101 treatment reduces neurodegeneration in APOE ε4 non‐carriers with MCI due to AD.
Keywords: AGB101, Alzheimer's disease, clinical trial, entorhinal cortex atrophy, levetiracetam, mild cognitive impairment
1. INTRODUCTION
It is well established that heightened neural activity localized to the medial temporal lobe (MTL) and hippocampal formation specifically occurs in the early prodromal phases of Alzheimer's disease (AD). 1 In early observational studies hippocampal hyperactivity in participants with mild cognitive impairment (MCI), before a diagnosis of dementia, was interpreted as a potential compensatory condition to preserve memory function. 2 , 3 However, a series of clinical studies, informed by extensive preclinical research, 4 , 5 demonstrated that normalization of hippocampal hyperactivity in patients with MCI could be achieved with low doses of levetiracetam, an efficacy that was lost at higher dosing in the range typically used for the treatment of seizures. 6 , 7 The experimental evidence provided in those studies showed that reducing hippocampal hyperactivity improved memory performance in the participants.
Based on both preclinical and clinical research, the HOPE4MCI clinical trial (NCT03486938) sponsored by Agenebio, Inc., was conducted to test the hypothesis that the occurrence of aberrant hyperactivity in the condition of MCI due to AD plays a role in the progression of disease in prodromal AD before a clinical diagnosis of dementia. The study enrolled 164 participants with MCI due to AD confirmed by a positive amyloid positron emission tomography (PET) scan in a 78‐week trial using a once‐a‐day dose of AGB101, a formulation of levetiracetam in the dose range previously demonstrated to normalize hippocampal activity and improve cognitive performance in amnestic MCI. 6 , 7 As reported in Mohs et al., decline on the Clinical Dementia Rating Sum of Boxes (CDR‐SB) score was reduced by 40% in a prespecified analysis of apolipoprotein E (APOE) ε4 non‐carriers over the 78‐week duration of the study, although this effect was not significant given the small sample size of the study. APOE ε4 carriers showed a negligible effect of treatment in the study. 8
Here we report the results of an analysis of the longitudinal brain imaging data and blood plasma biomarker data acquired in the HOPE4MCI study for non‐carriers of APOE ε4 (results for the APOE ε4 carriers are provided in in Table S1 and Figure S1 in supporting information). Structural magnetic resonance imaging (MRI) scans obtained in the study at baseline and at the week 78 end‐of‐trial visit were analyzed for volumetric change over time. Analysis of the structural MRI scans focused on the MTL, particularly the entorhinal cortex (ERC), which has been shown to undergo volume loss in cross‐sectional analyses comparing participants with prodromal AD to healthy age‐matched controls 9 , 10 , 11 and is the site of some of the earliest pathological changes in AD with the accumulation of early tau pathology localized to a subregion of the ERC referred to as the transentorhinal cortex. 12 Recent studies have used advances in image resolution to show that subregions of the ERC, particularly the lateral ERC including the transentorhinal cortex, provide greater neuroanatomical specificity and increased sensitivity in assessing progression in patients with amnestic MCI. 13 , 14 , 15 , 16 , 17 Therefore, the current study used an analysis of the ERC and adjacent subregions to assess structural changes over 78 weeks in APOE ε4 non‐carrier participants with MCI due to AD who completed participation in the HOPE4MCI study. Blood samples collected at the week 78 visit were analyzed for blood plasma biomarkers of AD including the amyloid beta (Aβ)42/40 ratio reflecting amyloid deposition, phosphorylated tau (p‐tau) 181 as a measure of tau accumulation, and neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) as general measures of neurodegeneration. 18 Results show a statistically significant reduction of left ERC atrophy and association with corresponding biomarkers of neurodegeneration at the end of the 78‐week protocol consistent with reduced degeneration and gliosis in APOE ε4 non‐carriers with MCI due to AD treated with AGB101.
2. METHODS
2.1. Study design
The HOPE4MCI study, a randomized double blind clinical trial of AGB101, enrolled participants with MCI due to AD across 29 trial sites in the United States and Canada. Complete details of the study design, inclusion and exclusion criteria, and study endpoints are provided in Mohs et al. 8 Briefly, participants were between 55 and 85 years old and met criteria for MCI due to AD according to National Institute on Aging–Alzheimer's Association criteria 19 including a CDR global score of 0.5, objective evidence of lower memory performance based on the delayed recall portion of the International Shopping List Test, and a positive amyloid PET scan. Enrolled participants were randomized using a 1:1 ratio to AGB101 or placebo treatments completing eight study visits over 78 weeks. The CDR‐SB was used as the primary endpoint of the study. Analysis of the primary endpoint did not show an overall effect of AGB101 on progression determined as change from baseline to 78 weeks on the CDR‐SB. However, a prespecified subgroup analysis showed that progression on the CDR‐SB score was reduced by 40% in APOE ε4 non‐carriers over the 78‐week duration of the study with a negligible treatment response in APOE ε4 carriers, although this effect was not significant due to the subgroup sample size. 8 Neuroimaging data obtained at baseline and the end of study visit and blood plasma obtained at the end of study visit from APOE ε4 non‐carriers were examined in the current study. The study was designed by AgeneBio in collaboration with academic co‐investigators at Johns Hopkins University and funded by the National Institutes of Health (R01AG048349, R56AG055416, and RO1AG061091). The prespecified statistical analysis plan and full reporting of adverse events for the study are provided in the Supplemental Materials for Mohs et al. 8
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using traditional (e.g., PubMed) sources. Significant literature supports the use of low‐dose levetiracetam as a novel therapeutic, especially in the mild cognitive impairment (MCI) phase of Alzheimer's disease (AD) when hippocampal hyperactivity is most pronounced and forecasts a decline.
Interpretation: The HOPE4MCI study showed that apolipoprotein E (APOE) ε4 non‐carriers treated with AGB101 demonstrated a substantially more favorable treatment effect compared to carriers. In this study, an exploratory analysis examined the effects of AGB101 on neuroimaging and biomarker measures in the APOE ε4 non‐carriers who completed the 78‐week protocol.
Future Directions: Results show a statistically significant reduction of entorhinal cortex atrophy and association with biomarkers of neurodegeneration consistent with reduced degeneration in APOE ε4 non‐carriers with MCI due to AD treated with AGB101. These results support the view that further testing of AGB101 in patients with MCI due to AD who are non‐carriers of APOE ε4 is warranted.
2.2. MRI data acquisition and analysis
MRI scans were obtained for all participants enrolled in the study at the screening, 52‐week, and 78‐week study visits. Site neuroimaging facilities were trained on relevant study procedures by the central imaging laboratory (Clario, Inc.) and completed site qualification and assessment of scanner performance for the duration of the study using American College of Radiology phantoms. All sites used 3T scanners (Philips, Siemens, or General Electric). Collected scans included a 3D T1 consisting of a sagittal magnetization‐prepared rapid gradient echo (Philips), sagittal 3D turbo field echo (Siemens), or a 3D fast spoiled gradient‐recalled sequence (General Electric) with a 1 × 1 mm2 in‐plane resolution, and 1.2 mm slice thickness. Two 3D T1 scans were collected during each session to increase the likelihood of obtaining good‐quality data. Upon receipt, each scan was reviewed by Clario, Inc. for quality assurance ensuring uniform signal‐to‐noise ratios, gray and white matter contrast, intensity uniformity, and geometric accuracy across sites and across time.
To optimize a volumetric analysis of the ERC, the structural MRI scans in the current report were analyzed using the Automated Segmentation of Hippocampal Subfields (ASHS) software, which provides measures for the ERC; Brodmann area 35 (BA35) which largely overlaps with what some studies have called transentorhinal cortex; and Brodmann area 36, which largely encompasses the adjacent perirhinal cortex. 20 , 21 , 22 The ASHS software incorporates a segmentation algorithm specifically designed for T1‐weighted MR images, denoted as ASHS‐T1, implementing a series of steps to achieve automatic segmentation. Initially, the target MRI scan is up‐sampled to a resolution of 0.5 × 0.5 × 1.0 mm 2 using a non–local‐mean super‐resolution algorithm. 23 Then, symmetric greedy diffeomorphic registration within the Advanced Normalization Tools software 24 is used to warp each segmentation atlas to the target MRI scan. A joint label fusion algorithm integrates anatomical labels from the warped atlases, yielding a consensus segmentation. This fusion process assigns spatially varying weights to each atlas based on patch‐level similarity to the target image, while considering potential redundancy among the atlases. 25 Additionally, a corrective learning algorithm corrects systematic segmentation biases using classifiers trained from leave‐one‐out segmentation of the atlas images. 26 Bootstrapping is applied, leveraging the results of multi‐atlas segmentation to improve the matching between the atlas and target images. All images were manually inspected to ensure quality and accurate segmentation post‐processing. Volumetric assessments, measured in cubic millimeters, were separately analyzed for each hemisphere.
2.3. Blood plasma collection and analysis
Blood samples were obtained for all participants in the study at the week 78 end‐of‐study visit. Sites were trained on relevant sample collection, processing, and shipping procedures by the central laboratory (Eurofins, Inc.). Plasma samples were analyzed by Labcorp/Monogram Bioscience using the Quanterix Simoa Neuro 4‐plex E multiplex assay consisting of amyloid Abeta1‐42, amyloid Abeta1‐40, GFAP, and NfL, and the Quanterix Simoa p‐Tau181 v.2 assay. These assays were analytically validated as research‐use‐only/exploratory assays, which include Clinical Laboratory Improvement Amendment‐like parameters such as precision, accuracy, reproducibility sensitivity, sample stability, sample interference, and parallelism/dilutional linearity. The assays were performed side by side on two instruments, with one assay per instrument, and from the same sample aliquot and thaw.
2.4. Statistical analyses
Statistical analyses were conducted using SPSS (version 29), considering P < 0.05 significant. Descriptive statistics were used to analyze demographic variables. The comparison of sex and race distributions between groups was executed using a chi‐square test. Independent samples t tests were used to assess differences in age and neuropsychological test scores between groups. Pearson correlations were used to examine the associations between volume change over time and change in the CDR‐SB score over 78 weeks, and blood plasma biomarkers obtained at the week 78 end‐of‐study visit.
3. RESULTS
3.1. Participants
As reported in Mohs et al. and shown in Figure 1, participants with MCI due to AD who were non‐carriers of APOE ε4 treated with AGB101 showed a non‐significant 40% reduction in the 78‐week CDR‐SB change score compared to placebo. 8 This group included 44 participants with MCI due to AD who completed the 78‐week study of which 18 were randomized to AGB101 treatment and 26 were randomized to placebo. Baseline demographics and clinical characteristics were similar across the study arms and were not significantly different between the two groups at randomization (Table 1). Blood samples collected at the week 78 end‐of‐study visit were available from a subsample of 17 participants in the AGB101 condition and 16 participants in the placebo condition. Participants in the AGB101 treatment group had an average blood level of AGB101 of 2.37 (standard deviation ± 1.95) ug/mL while participants in the placebo group had non‐detectable blood levels of AGB101 at the week 78 end‐of‐study visit.
FIGURE 1.
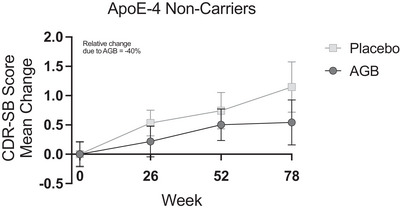
Treatment with AGB101 provides a reduction in the 78‐week CDR‐SB change score compared to the placebo. The graph shows the progression of CDR‐SB scores over the 78‐week treatment period for both the placebo and AGB101 groups for participants with MCI due to AD who are APOE ε4 non‐carriers and completed the study. The intent‐to‐treat analysis for all APOE ε4 non‐carriers enrolled in the study also indicated a non‐significant 40% reduction in the AGB101 treated compared to the placebo group. Values are mean ± SEM. From Mohs et al., 2024. AD, Alzheimer's disease; APOE, apolipoprotein E; CDR‐SB, Clinical Dementia Rating Sum of Boxes score; MCI, mild cognitive impairment; SEM, standard error of the mean
TABLE 1.
Demographics and baseline characteristics.
| MCI due to AD | ||
|---|---|---|
|
AGB101 (N = 18) |
Placebo (N = 26) |
|
| Age at screening (years) | 68.94 (7.0) | 70.04 (8.0) |
| Sex, n (%) | ||
| Male | 11 (61.1) | 10 (38.5) |
| Female | 7 (38.9) | 16 (61.5) |
| Race, n (%) | ||
| White | 17 (94.4) | 24 (92.3) |
| Black or African American | 1 (5.6) | 2 (7.7) |
| American Indian or Alaska Native | 0 (0) | 0 (0) |
| CDR | ||
| Sum of Boxes | 2.39 (1) | 2.71 (1) |
| Global Score | 0.5 (0.1) | 0.5 (0.1) |
| MMSE Total Score | 26.2 (2) | 25.5 (2.2) |
| FAQ Total Score | 7.8 (7.4) | 6.6 (4.8) |
| BPS‐O | 0.3 (0.3) | 0.3 (0.2) |
| ISLT | ||
| Immediate Recall | 17.2 (4.7) | 14.8 (3.7) |
| Delayed Recall | 2.9 (1.1) | 2.8 (1.3) |
Notes: Values are mean ± standard deviation. Participants with MCI due to AD assigned to the AGB101 condition did not differ from those assigned to the placebo condition on any measure listed (all P > 0.05).
Abbreviations: AD, Alzheimer's disease; BPS‐O, Behavioral Pattern Separation Task – Objects; CDR, Clinical Dementia Rating; FAQ, Functional Activities Questionnaire; ISLT, International Shopping List Test; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination.
3.2. Structural imaging results
Subregion volumes in ERC and adjacent areas did not show a significant difference between study participants assigned to the AGB101 condition and those assigned to the placebo condition at randomization (left and right ERC [P > 0.30], left and right BA35 [P > 0.29], or left and right BA36 [P > 0.95], Table 2).
TABLE 2.
Volumes at baseline and change over 78 weeks.
| Baseline volume (mm3) mean (SD) a |
Week78 volume (mm3) mean (SD) |
Percent volume change | |
|---|---|---|---|
| ERC | |||
| Left ERC | |||
| Placebo | 489.80 (122.41) | 461.87 (129.87) | −6.19 (6.60) |
| AGB101 | 526.46 (96.99) | 518.81 (116.11) | −1.88 (7.32)* |
| Right ERC | |||
| Placebo | 512.54 (123.87) | 488.54 (125.50) | −4.59 (7.88) |
| AGB101 | 537.20 (97.92) | 509.90 (117.12) | −5.65 (7.72) |
| BA35 | |||
| Left BA35 | |||
| Placebo | 517.24 (150.80) | 495.03 (145.57) | −4.01 (5.99) |
| AGB101 | 555.35 (80.52) | 534.24 (87.77) | −4.00 (4.44) |
| Right BA35 | |||
| Placebo | 561.45 (110.04) | 538.16 (126.05) | −4.76 (6.06) |
| AGB101 | 540.47 (92.30) | 538.79 (118.99) | −0.79 (9.34)** |
| BA36 | |||
| Left BA36 | |||
| Placebo | 1785.82 (468.30) | 1696.61 (462.65) | −5.03 (5.10) |
| AGB101 | 1781.55 (283.95) | 1727.88 (300.58) | −3.07 (6.70) |
| Right BA36 | |||
| Placebo | 1724.47 (483.44) | 1646.26 (486.81) | −4.85 (5.75) |
| AGB101 | 1716.22 (340.92) | 1646.91 (315.93) | −3.71 (6.90) |
Notes: Values are mean ± SD in mm3.
Participants with MCI due to AD assigned to the AGB101 condition did not differ from those assigned to the placebo condition in the baseline volume of the regions of interest (all P > 0.05).
Significant difference between treatment arms (P < 0.05).
Trend toward the significant difference between treatment arms (P < 0.1).
Abbreviations: AD, Alzheimer's disease; BA35, Brodmann area 35; BA36, Brodmann area 36; ERC, entorhinal cortex; MCI, mild cognitive impairment; SD, standard deviation.
As shown in Figure 2, treatment with AGB101 in APOE ε4 non‐carriers with MCI due to AD significantly reduced atrophy of the left ERC and showed a trend toward significantly reduced atrophy in the right BA35 compared to placebo. At the week 78 visit, participants treated with placebo showed significantly reduced volume compared to baseline across ERC and neighboring cortical regions (all P < 0.01, see Table 2) confirming significant progression of cortical volume loss consistent with a diagnosis of MCI due to AD. However, APOE ε4 non‐carrier participants with MCI due to AD who were treated with AGB101 over 78 weeks showed significantly less volume loss in the left ERC (t[42] = −2.04, P < 0.05) and a trend toward significantly less volume loss in the right BA35 (t[42] = −1.71, P = 0.09) compared to the placebo‐treated participants (Table 2).
FIGURE 2.
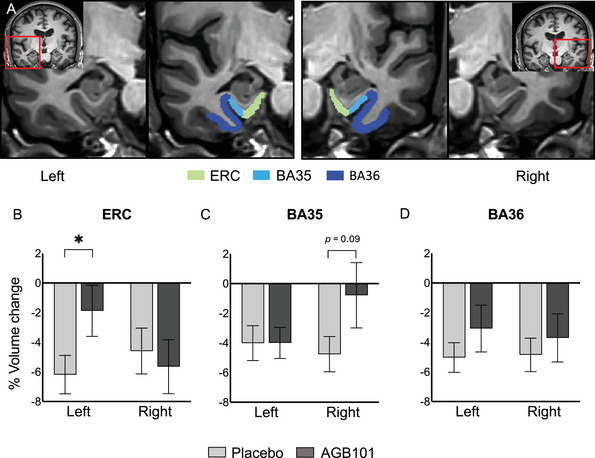
Treatment with AGB101 reduces ERC atrophy over 78 weeks in patients with MCI due to AD who are non‐carriers of APOE ε4. A, Anatomical location of regions of interest with segmentation of the left and right MTL provided by the ASHS software including the ERC; BA35, which largely overlaps with what some studies have called the transentorhinal cortex; and BA36, which largely encompasses the adjacent perirhinal cortex. Participants with MCI due to AD who are non‐carriers of the APOE ε4 allelic variation who were treated with AGB101 showed a significant reduction in atrophy in the left ERC (B), a trend toward reduced atrophy in right BA35 (C), and no treatment effect in BA36 (D) compared to participants who were treated with placebo. Values represent mean ± SEM, * p < 0.05. AD, Alzheimer's disease; APOE, apolipoprotein E; ASHS, Automated Segmentation of Hippocampal Subfields; BA, Brodmann area; ERC, entorhinal cortex; MCI, mild cognitive impairment; MTL, medial temporal lobe; SEM, standard error of the mean
The change in volume in the left ERC over 78 weeks showed a significant correlation with the change in CDR‐SB score over 78 weeks (r = −0.51, P < 0.001; Figure 3), such that reduced cortical volume loss was associated with reduced clinical/cognitive worsening indicated by higher CDR‐SB scores. In addition, the measures obtained from blood plasma at the end of the trial showed a significant correlation between left ERC volume change over 78 weeks and reduced NfL plasma levels (r = −0.38, P < 0.05) and GFAP plasma levels (r −0.40, P < 0.05), which are considered biomarkers of neurodegeneration. No significant correlations were observed with the Aβ42/40 ratio, or with p‐tau181 (all r < 0.26, P > 0.14). The change in volume in the right ERC over 78 weeks also showed a significant correlation with the change in CDR‐SB score over 78 weeks (r = −0.36, P < 0.05) and with NfL plasma levels ( −0.46, P < 0.05) but not with GFAP plasma levels (r −0.19, P = 0.30).
FIGURE 3.
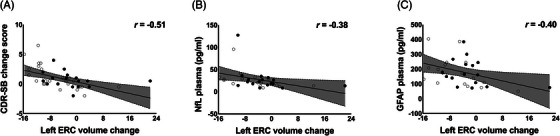
ERC volume change over 78 weeks of treatment in participants with MCI due to AD who are non‐carriers of APOE ε4 is associated with clinical cognitive change and blood plasma markers of neurodegeneration. The change in left ERC volume over 78‐week treatment showed a significant correlation with (A) change in the CDR‐SB score over 78 weeks (placebo: r = −0.69, P < 0.001; AGB101: r = −0.41, P = 0.12; overall: r = −0.51, P < 0.001), (B) NfL blood plasma levels at the end of study visit (placebo: r = −0.44, P = 0.10; AGB101: r = −0.47, P = 0.07; overall: −0.38, P < 0.05), and (C) GFAP blood plasma levels at the end of study visit (placebo: r = −0.39, P = 0.15; AGB101: r = −0.10, P = 0.72; overall: r = −0.40, P < 0.05). Open circles identify participants in the placebo condition and closed circles identify participants in the AGB101 condition. AD, Alzheimer's disease; APOE, apolipoprotein E; CDR‐SB, Clinical Dementia Rating Sum of Boxes score; ERC, entorhinal cortex; GFAP, glial fibrillary acidic protein; MCI, mild cognitive impairment; NfL, neurofilament light chain
As evident in Figure 3, some outliers were identified with scores either exceeding a univariate outlier test cutoff of ± two standard deviations from the mean percent change score for left ERC volume or for exceeding the critical cutoff for the Mahalanobis distance test for multivariate outliers examining the relationship between left ERC volume change, CDR‐SB change, NfL plasma, or GFAP (χ2 [2] = 5.99). Removing these outliers did not substantially alter the observed pattern of results. In fact, after removal of these cases, the change in volume in the left ERC over 78 weeks showed a stronger significant correlation with the change in CDR‐SB score over 78 weeks (r = −0.62, P < 0.001; three outliers), as did the change in volume in the left ERC with NfL plasma levels (r = −0.44, P = 0.02; four outliers). The correlation between change in volume in the left ERC over 78 weeks and GFAP plasma levels was reduced and no longer significant (r = –0.27, P = 0.14; two outliers).
4. DISCUSSION
The HOPE4MCI study reported that reduction of hippocampal overactivity appears to confer benefit to patients with MCI due to AD showing progression on the CDR‐SB score was reduced by a non‐significant but 40% effect in APOE ε4 non‐carriers over the 78‐week duration of the study. Here we report an exploratory analysis of the effects of AGB101 on neuroimaging and biomarker measures in those APOE ε4 non‐carriers who completed the 78‐week protocol. Participants with MCI due to AD assigned to the placebo condition did not differ in any of the baseline demographic and clinical characteristics from those assigned to the AGB101 treatment condition. Furthermore, the volume of the regions of interest in the MTL did not differ at randomization between participants assigned to the placebo and AGB101 treatment conditions. An analysis of the change in volume over 78 weeks in the placebo condition showed significant volume loss in all regions examined ranging from ≈ 4% to 6% loss. The observed loss in volume reflects progression consistent with a diagnosis of MCI due to AD and is consistent with previously reported rates of volume loss of the ERC in this population. 13 , 14 , 15 Compared to placebo, participants with MCI due to AD treated with AGB101 showed significantly less volume loss over 78 weeks in the left ERC with ≈ 2% volume change over this period with AGB101 treatment. In addition, treatment with AGB101 showed a trend toward a significant decrease in volume loss in the right BA35, sometimes referred to as transentorhinal cortex, with < 1% volume loss over 78 weeks in this region.
The change in volume over 78 weeks across participants showed a significant correlation with the change in CDR‐SB score over the same period such that less entorhinal volume loss was robustly associated with less decline on the CDR‐SB. An analysis of blood samples collected at the end of the trial additionally showed a robust correlation between the change in ERC volume and NfL as well as GFAP such that those with reduced volume loss also showed lower plasma levels of NfL and GFAP consistent with a reduction in neurodegeneration. Together these findings strongly suggest that targeting hippocampal hyperactivity by AGB101 provides both clinical–cognitive benefits to participants and slows the progression of neuronal loss in the ERC in the early stages of the disease.
AGB101, an extended‐release low‐dose formulation of levetiracetam, exerts its clinical effect by binding to the SV2A receptor, a membrane protein that regulates neurotransmitter release. 27 It is co‐localized with the amyloid precursor protein, suggesting it is involved in amyloid processing 28 and is likely involved in processing of tau protein as well with extensive preclinical data indicating that hippocampal overactivity is a driver of neurodegeneration and the spread of tau pathology 28 providing a potential mechanism for its effects on progression and neurodegeneration in early AD. In previous studies, treatment with low‐dose levetiracetam of patients with AD dementia showed encouraging results with some broad cognitive benefit in the executive domain in these patients. 29 However, in the MCI phase of the disease, treatment with low‐dose levetiracetam not only reduced hippocampal hyperactivity but also normalized hypoactivation in the ERC, likely through restoration of the inhibitory–excitatory balance in this network, 6 , 7 , 30 which is thought to benefit both the function and structure of this network including atrophy although additional studies in this area are needed. Brain atrophy in this region is a hallmark feature of the MCI phase of AD 1 and as such a clear target for interventions aiming to alter the disease trajectory. Lecanemab 31 and donanemab 32 have both been shown to slow disease progression on clinical–cognitive measures in early AD, with the efficacy of lecanemab in APOE ε4 non‐carrier participants showing a similar 40% decrease in progression on the CDR‐SB score observed with AGB101 (note that participants in those trials included both MCI and early dementia AD patients). Treatment effects on cortical atrophy have not been reported for lecanemab while an analysis of brain volume under donanemab treatment did not consider topography of earliest degeneration 33 and blood biomarkers show a more consistent effect on GFAP than NfL. 34
The findings reported here are limited by the relatively small number of APOE ε4 non‐carriers in the study. However, results show a statistically significant reduction of ERC atrophy and association with corresponding biomarkers of neurodegeneration at the end of the 78‐week protocol consistent with reduced degeneration and gliosis in APOE ε4 non‐carriers with MCI due to AD treated with AGB101. Although additional studies are needed to elucidate the limited benefit of AGB101 treatment in APOE ε4 carriers, its potentially substantial effect in APOE ε4 non‐carriers together with the limited side effects in this population, holds significant promise for this approach in MCI due to AD.
CONFLICT OF INTEREST STATEMENT
M.G. is the founder of AgeneBio. M.G. and A.B. are inventors on Johns Hopkins University intellectual property licensed exclusively to AgeneBio. M.G. owns company stock, which is subject to certain restrictions under university policy. A.B. consults for the company. M.G. and A.B.’s role in the current study complied with the conflict of interest policies of the Johns Hopkins School of Medicine. N.R. and R.M. have nothing to disclose. R.M. is VP of Clinical Research at AgeneBio. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants in this study provided written informed consent.
Supporting information
Supporting Information
Supporting information
ACKNOWLEDGMENTS
We thank all the patients, their families, and caregivers who participated in the HOPE4MCI trial as well as the site staff, raters, and site investigators; members of the data and safety monitoring board; and vendor partners including Worldwide Clinical Trials, Clinical Inc, Cogstate, Eurofins, Suvoda, Clario, Alcemi, and Labcorp/Monogram Bioscience. The authors would also like to thank Ken Payie at KGP‐Biotech for the production and manufacture of the extended‐release medication, Kevin Arauz at Worldwide Clinical Trials for project management, and Carrie L. Speck at Johns Hopkins University for project coordination. This work was supported by NIH grants R01AG061091 to RM, R56AG055416 to RM, R01AG048349 to MG and MSA, and the Phyllis F. Albstein Fund.
Bakker A, Rani N, Mohs R, Gallagher M. The HOPE4MCI study: AGB101 treatment slows progression of entorhinal cortex atrophy in APOE ε4 non‐carriers with mild cognitive impairment due to Alzheimer's disease. Alzheimer's Dement. 2024;e70004. 10.1002/trc2.70004
REFERENCES
- 1. Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends Neurosci. 2011;34:430‐442. doi: 10.1016/j.tins.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bakker A, Krauss GL, Albert MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohs R, Bakker A, Rosenzweig‐Lipson S, et al. The HOPE4MCI study: A randomized double‐blind assessment of AGB101 for the treatment of MCI due to AD. Alzheimers Dement. 2024;10:e12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bobinski M, de Leon MJ, Convit A, et al. MRI of entorhinal cortex in mild's disease. Lancet. 1999;353:38‐40. [DOI] [PubMed] [Google Scholar]
- 10. Kantarci K, Jack CR. Neuroimaging in Alzheimer disease: an evidence‐based review. Neuroimaging Clin N Am. 2003;13:197‐209. doi: 10.1016/S1052-5149(03)00025-X [DOI] [PubMed] [Google Scholar]
- 11. Killiany RJ, Gomez‐Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimers disease. Ann Neurol. 2000;47:430‐439. [PubMed] [Google Scholar]
- 12. Braak H, Braak E. Staging of Alzheimer's disease‐related neurofibrillary changes. Neurobiol Aging. 1995;16:271‐284 [DOI] [PubMed] [Google Scholar]
- 13. Tward DJ, Sicat CS, Brown T, et al. Entorhinal and transentorhinal atrophy in mild cognitive impairment using longitudinal diffeomorphometry. Alzheimers Dement. 2017;9:41‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kulason S, Tward DJ, Brown T, et al. Cortical thickness atrophy in the transentorhinal cortex in mild cognitive impairment. Neuroimage Clin. 2019;21:101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulason S, Xu E, Tward DJ, et al. Entorhinal and transentorhinal atrophy in preclinical Alzheimer's disease. Front Neurosci. 2020;14:804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tran TT, Speck CL, Gallagher M, Bakker A. Lateral entorhinal cortex dysfunction in amnestic mild cognitive impairment. Neurobiol Aging. 2022;112:151‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rani N, Alm KH, Corona‐Long CA, et al. Tau accumulation in Brodmann areas 35 and 36 is associated with individual differences in cognition in non‐demented older adults. Front Aging Neurosci. 2023;15:1272946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giangrande C, Delatour V, Andreasson U, Blennow K, Gobom J, Zetterberg H. Harmonization and standardization of biofluid‐based biomarker measurements for AT(N) classification in Alzheimer's disease. Alzheimers Dement. 2023;15(3):e12465. doi: 10.1002/dad2.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie L, Wisse LEM, Pluta J, et al. Automated segmentation of medial temporal lobe subregions on in vivo T1‐weighted MRI in early stages of Alzheimer's disease. Hum Brain Mapp. 2019;40:3431‐3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yushkevich PA, Yang Gao, Gerig G. ITK‐SNAP: An interactive tool for semi‐automatic segmentation of multi‐modality biomedical images. in 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2016:3342‐3345. (IEEE; ). doi: 10.1109/EMBC.2016.7591443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yushkevich PA, Pluta JB, Wang H, et al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp. 2015;36:258‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manjón JV, Coupé P, Buades A, Fonov V, Louis Collins D, Robles M. Non‐local MRI upsampling. Med Image Anal. 2010;14:784‐792. [DOI] [PubMed] [Google Scholar]
- 24. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross‐correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Suh JW, Das SR, Pluta JB, Craige C, Yushkevich PA. Multi‐atlas segmentation with joint label fusion. IEEE Trans Pattern Anal Mach Intell. 2013;35:611‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang H, Das SR, Suh JW, et al. A learning‐based wrapper method to correct systematic errors in automatic image segmentation: consistently improved performance in the hippocampus, cortex and brain segmentation. Neuroimage. 2011;55;968‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci. 2004;101(26):9861‐9866. https://www.pnas.orgcgidoi10.1073pnas.0308208101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kong Y, Huang L, Li W, et al. The synaptic vesicle protein 2A interacts with key pathogenic factors in Alzheimer's disease: implications for treatment. Front Cell Dev Biol. 2021;9:609908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vossel K, Ranasinghe KG, Beagle AJ, et al. Effect of levetiracetam on cognition in patients with Alzheimer disease with and without epileptiform activity: a randomized clinical trial. JAMA Neurol. 2021;78:1345‐1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanchez PE, Zhu L, Verret L, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc Natl Acad Sci. 2012;109:E2895‐E2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9‐21. [DOI] [PubMed] [Google Scholar]
- 32. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER‐ALZ 2 randomized clinical trial. JAMA. 2023;330:512‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in Early Alzheimer's disease. N Engl J Med. 2021;384:1691‐1704. [DOI] [PubMed] [Google Scholar]
- 34. Pontecorvo MJ, Lu M, Burnham SC, et al. Association of Donanemab Treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER‐ALZ randomized clinical trial. JAMA Neurol. 2022;79:1250‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting information


