Abstract
INTRODUCTION
Without disease‐modifying interventions, Medicare and Medicaid spending on Alzheimer's disease (AD) management is expected to reach 637 billion USD annually by 2050. The recent advent of promising AD therapies after decades of a near‐total failure rate in clinical trials suggests that more disease‐modifying therapies are on the horizon. In this review, we assess the late‐stage pipeline of disease‐modifying candidates for AD and offer a novel classification of intervention candidates by treatment paradigms—groups of candidates that share an underlying biological mechanism of action and general disease target.
METHODS
We extracted data from the National Library of Medicine clinical trials database regarding Phase 2 and 3 trials of disease‐modifying AD therapies. We categorized trials into eight unique treatment paradigms, which we defined by combinations of therapy (biologic, small molecule, cell and gene therapy, other) and target (amyloid, tau, other). We analyzed primary endpoints, eligibility criteria including clinical ratings of cognition, trial phase and length, and funding sources.
RESULTS
We identified 123 unique disease‐modifying intervention candidates in 175 late‐stage clinical trials. Biologic and small molecule drugs comprised 30% and 54% of trials, respectively. Eligibility criteria favored patients between the ages of 60 and 80 years with mild cognitive impairment. Including multi‐phase trials, 81% of studies were engaged in Phase 2 and 27% in Phase 3. Notably, within the Biologic–Amyloid paradigm, 64% of trials were engaged in Phase 3.
DISCUSSION
Current studies of disease‐modifying therapies for AD comprise a diverse set of approaches to treating the disease. However, effort is largely concentrated in a few treatment paradigms and a narrow patient population, causing varying rates of progress among treatment paradigms in the late‐stage clinical trial pipeline. Strategies may be warranted to accelerate successes in the most promising therapeutical paradigms and nurture growth within nascent areas lacking resources but not potential.
Highlights
An analysis of Alzheimer's disease trial treatment paradigms was conducted.
From April 2021 to March 2023, 175 trials of 123 unique candidates were reviewed.
Biologic and small molecule drugs comprised 30% and 54% of trials, respectively.
Eligibility criteria favored ages 60 through 80 with mild cognitive impairment.
Keywords: Alzheimer's disease, clinical trials, disease‐modifying therapy, pharmaceutical pipeline, pharmacoeconomics
1. INTRODUCTION
The biomedical community has spent decades attempting to discover disease‐modifying treatments for Alzheimer's disease (AD). Cumulative private expenditures on drug development for AD exceeded 42 billion USD between 1995 and 2021, with additional billions contributed from public sources, primarily the National Institutes of Health (NIH). Efforts to find disease‐modifying treatments are guided, in part, by the large and growing burden of AD. By 2050, without disease‐modifying interventions, Medicare and Medicaid spending on AD management is expected to increase to 637 billion USD annually, from 231 billion USD in 2024. 1 , 2
Until recently, success in discovering disease‐modifying treatments has been limited, as reflected in the near‐total failure rate for drug‐development attempts in this area. 3 The first possible disease‐modifying AD therapy, aducanumab, received accelerated U.S. Food and Drug Administration (FDA) approval in June 2021, but the drug was discontinued by its manufacturer in January 2024, after controversy over its approval and restricted coverage by Medicare. 4 The decision was likely hastened by the full FDA approval of lecanemab in July 2023, and the anticipation of approval of donanemab (approved July 2024), both of which demonstrated significant slowing in the progression of cognitive impairment in early AD. 5 , 6
The EU/US/Clinical Trials in Alzheimer's Disease (CTAD) Task Force has noted that the complexity of AD and the heterogeneity of clinical trials are impeding progress. 7 Although unable to address the complexity of AD, this work characterizes the therapy development pipeline, providing a framework for classifying potentially disease‐modifying treatments. Specifically, we assess the existing late‐stage pipeline of disease‐modifying candidates for AD and offer a novel classification of candidates by treatment paradigms—groups of candidates that share both an underlying biological mechanism of action and a general disease target–with the aim of organizing and characterizing the AD therapy pipeline for future research, policy, and clinical application.
2. MATERIALS AND METHODS
We extracted data on AD clinical trials from the United States National Library of Medicine (NLM) clinical trials database on April 10, 2021, and March 28, 2023. 8 All trials that listed AD as a condition were examined, and trials with interventions targeting AD progression were included. Our search strategy is described below.
2.1. Search strategy
We conducted a systematic review of late‐stage (Phase 2 and Phase 3) clinical trials of potentially disease‐modifying therapies for AD. We searched the NLM clinical trials database using the following search criteria: inclusion of the term “Alzheimer” in the study description and satisfaction of the following four filters (i) “Recruiting” or “Not yet recruiting” or “Active not recruiting”; (ii) “Enrolling by invitation”; (iii) “Interventional”; and (iv) “Phase 2” or “Phase 3”.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources (e.g., PubMed). Literature regarding the Alzheimer's disease therapeutic pipeline is appropriately cited. There is a sparsity of work providing detailed analysis of the late‐stage clinical trial pipeline of potentially disease‐modifying therapies.
Interpretation: We provide an organizational framework for the disease‐modifying therapy pipeline using distinct treatment paradigms based on therapeutic mechanism and disease target (e.g., amyloid, tau). We employ this framework to characterize emerging trends, including the dominance of amyloid‐targeting biologics, the ongoing challenges with advancing tau‐targeting therapies beyond Phase 2, and the investigative emphasis on populations between 60 and 80 years of age with mild cognitive impairment.
Future directions: Studies are needed to determine whether the multitude of outcome measures and eligibility criteria used by trialists is appropriate. Assessment of regulatory body (e.g., U.S. Food and Drug Administration [FDA]) guidance and future guidance needs is indicated, accounting for differences between treatment paradigms.
We evaluated identified studies to assess whether investigators were testing potentially disease‐modifying therapies, symptomatic therapies, or neither. Disease‐modifying treatments were identified as therapies that aimed to alter known biological characteristics of AD, such as amyloid plaques or neurofibrillary tau tangles. Symptomatic therapies were identified as treatments to improve symptoms experienced by patients, such as agitation and fatigue, without targeting biological progression of AD. Studies of symptomatic therapies, imaging studies, and behavioral interventions were removed from further review. The remaining studies were identified as trialing potentially disease‐modifying therapies and kept for data extraction.
2.2. Data extraction
For studies identified as testing potentially disease‐modifying therapies, we collected variables of interest from the NLM clinical trials database and distributed studies into treatment paradigms (described below). The following variables of interest were extracted: drug or therapeutic candidate, therapy type (biologic drug, small molecule drug, cell and gene therapy, other), therapy target category (amyloid, tau, other), primary outcome measures categorized by endpoint (e.g., Alzheimer's Disease Assessment Scale‐Cognitive Subscale [ADAS‐Cog], Mini‐Mental State Examination [MMSE], amyloid levels) and associated clinical domain (cognitive, behavioral, functional, biomarker, other), eligible ages for enrollment, eligible MMSE scores for enrollment, eligible Clinical Dementia Rating (CDR) scores for enrollment, trial phase, projected trial length, funding sources, and involvement of a study location based in the United States.
2.3. Data synthesis
We grouped therapies by the categories of therapy type and therapy target under a joint “treatment paradigm” variable. Treatment paradigms characterize groups of drug candidates with a similar mechanism of action and biological target. If a paradigm contained only a few drug candidates, we created a more inclusive paradigm that combined all target categories within a therapy type (specifically, we included a joint Cell and Gene Therapy paradigm and a joint Other paradigm). The following eight treatment paradigms were used for further analysis:
Biologic–Amyloid
Biologic–Tau
Biologic–Other
Small Molecule–Amyloid
Small Molecule–Tau
Small Molecule–Other
Cell and Gene Therapy
Other
3. RESULTS
Our first search yielded 195 results and our second search yielded 102 additional results, for a total of 297 clinical trials screened. After excluding trials that were not testing disease‐modifying treatment candidates, 175 trials remained. A breakdown of the trial‐exclusion process from the two search dates is shown in Figure 1. We show descriptive characteristics of these trials in Table 1, categorized by treatment paradigm.
FIGURE 1.
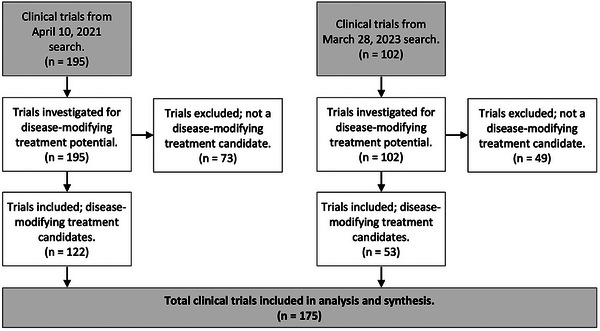
Flowchart of inclusion process for late‐stage clinical trials of potentially disease‐modifying therapies for Alzheimer's disease.
TABLE 1.
Descriptive characteristics by treatment paradigm for late‐stage clinical trials of potentially disease‐modifying therapies for Alzheimer's disease.
| Inclusion criteria | Trial phase | U.S. site included | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment paradigm | Count | Unique intervention candidates | Modal age range | MMSE, range (mode) | CDR, range (mode) | 1/2, n (%) | 2, n (%) | 2/3, n (%) | 3, n (%) | Yes, n (%) | No, n (%) |
| Biologic–Amyloid | 28 | 10 | 60–80 | 18–30 (27–28) | 0–2 (0.5) | 1 (4%) | 9 (32%) | 1 (4%) | 17 (61%) | 24 (86%) | 4 (14%) |
| Biologic–Tau | 10 | 9 | 60–75 | 16–30 (22–30) | 0–2 (0.5) | 2 (20%) | 7 (70%) | 1 (10%) | 0 (0%) | 9 (90%) | 1 (10%) |
| Biologic–Other | 14 | 10 | 65–80 | 10–30 (22–24) | 0.5–2 (0.5–1) | 2 (14%) | 11 (79%) | 0 (0%) | 1 (7%) | 10 (71%) | 2 (14%) |
| Small Molecule–Amyloid | 27 | 17 | 65–75 | 0–30 (22–24) | 0–2 (0.5) | 5 (19%) | 16 (59%) | 2 (7%) | 4 (15%) | 19 (70%) | 6 (22%) |
| Small Molecule–Tau | 9 | 7 | 65–80 | 12–30 (24) | 0.5–2 (0.5–1) | 3 (33%) | 5 (56%) | 0 (0%) | 1 (11%) | 7 (78%) | 2 (22%) |
| Small Molecule–Other | 58 | 49 | 65 | 0–30 (21–22) | 0–2 (1) | 4 (7%) | 36 (64%) | 9 (16%) | 8 (14%) | 36 (62%) | 18 (31%) |
| Cell and Gene Therapy | 8 | 3 | 55–70 | 10–30 (22–24) | 0.5–1 (0.5–1) | 5 (63%) | 3 (38%) | 0 (0%) | 0 (0%) | 3 (38%) | 4 (50%) |
| Other | 21 | 18 | 75–80 | 10–30 (24–26) | 0–3 (0.5) | 2 (10%) | 16 (76%) | 0 (0%) | 3 (14%) | 12 (57%) | 7 (33%) |
| Grand total | 175 | 123 | 65–70 | 0–30 (24) | 0–3 (0.5–1) | 24 (14%) | 104 (59%) | 13 (7%) | 34 (19%) | 120 (69%) | 44 (25%) |
Note: U.S. site included Yes/No; may not sum to 100% due to non‐reporting.
Abbreviations: CDR, Clinical Dementia Rating; MMSE, Mini‐Mental State Examination, U.S., United States.
3.1. Therapy targets and outcome measures
3.1.1. Therapy types and prominent targets
Of the four therapy types, biologic and small molecule drugs were most frequently explored, while cell and gene therapy and the remaining catch‐all other category, consisted of relatively few trials (Table 1). Biologic drugs comprised 52 of the 175 late‐stage clinical trials (30%) and 29 of the 123 unique intervention candidates (24%). Small molecule drugs comprised 94 of the 175 trials (54%) and 73 of the 123 unique candidates (56%).
Among both biologic and small molecule therapies, amyloid was the most frequent disease target, comprising 54% of biologic trials and 29% of small molecule trials. Another notable target was tau, comprising 19% of biologic trials and 10% of small molecule trials. The emphasis on targeting amyloid and tau with biologic and small molecule drugs is reflected in the treatment paradigms used for analysis.
3.1.2. Primary outcome measures
We identified the common primary outcome measures used within each treatment paradigm. Due to our focus on disease modification and late‐stage drug candidates, we did not classify adverse events or study them in detail.
A single trial may report one or multiple primary outcome measures. Across the eight paradigms, ADAS‐Cog (any version) was the most prevalent primary outcome measure in five paradigms and the most prevalent outcome measure overall (29% of trials). CDR and amyloid level were the second and third most prevalent outcome measures overall, used, respectively, in 15% and 13% of trials. A summary of the most common outcome measures by treatment paradigm is shown in Table 2.
TABLE 2.
Most common primary outcome measure by treatment paradigm for late‐stage clinical trials of potentially disease‐modifying therapies for Alzheimer's disease.
| Treatment Paradigms | Most common primary outcome measures | |
|---|---|---|
| 1st | 2nd | |
| Biologic–Amyloid | Amyloid (36%) | CDR (25%) |
| Biologic–Tau | ADAS‐Cog, ADCS‐ADL/iADL, tau (30%) | |
| Biologic–Other | ADAS‐Cog (29%) | CDR‐SB, amyloid (21%) |
| Small Molecule–Amyloid | ADAS‐Cog (37%) | CDR‐SB, ADCS‐CGIC, amyloid, PK/PD (15%) |
| Small Molecule–Tau | PK/PD (33%) | ADAS‐Cog, ADCS‐ADL/iADL (22%) |
| Small Molecule–Other | ADAS‐Cog (36%) | Homeostatic Biomarkers (19%) |
| Cell and gene therapy | Homeostatic biomarkers (25%) | — |
| Other | ADAS‐Cog (24%) | CDR‐SB (14%) |
| Grand total | ADAS‐Cog (29%) | CDR (15%) |
Note: Where primary outcome measures appear with equal frequency, multiple measures are listed. The Cell and Gene Therapy paradigm did not have other repeated primary outcome measures. CDR is listed when both CDR‐SB (Sum of Boxes) and CDR Global Score were used as measures within a paradigm; CDR‐SB is listed when only CDR‐SB was used.
Abbreviations: ADAS‐Cog, Alzheimer's Disease Assessment Scale—Cognitive Subscale; ADCS‐ADL/iADL, Alzheimer's Disease Cooperative Study—Activities of Daily Living/Instrumental Activities of Daily Living Scale; ADCS‐CGIC, Alzheimer's Disease Cooperative Study—Clinical Global Impression of Change; CDR, Clinical Dementia Rating; CDR‐SB, Clinical Dementia Rating—Sum of Boxes; PK/PD, pharmacokinetic or pharmacodynamic properties.
3.1.3. Clinical domains of primary outcome measures
Primary outcome measures were categorized into the following clinical domains: cognitive, behavioral, functional, and biomarker. Some measures test multiple domains and were treated as such (e.g., CDR is a composite measure of both cognitive and functional domains). Across paradigms, the greatest proportion of studies had outcome measures in the cognitive domain, although some paradigms also had high representation of functional and biomarker measures (Table 3). In addition, with the exception of the Small Molecule–Tau paradigm and the Cell and Gene Therapy paradigm, at least half of the studies in each paradigm used cognitive domain primary endpoints. In marked contrast, the behavioral domain was the least represented in all paradigms.
TABLE 3.
Proportion of studies with primary outcome measures in a particular clinical domain by treatment paradigm for late‐stage clinical trials of potentially disease‐modifying therapies for Alzheimer's disease.
| Treatment Paradigms | Count | Clinical domain | Adverse events | |||
|---|---|---|---|---|---|---|
| Cognitive | Behavioral | Functional | Biomarker | |||
| Biologic–Amyloid | 28 (100%) | 15 (54%) | 2 (7%) | 10 (36%) | 13 (46%) | 10 (36%) |
| Biologic–Tau | 10 (100%) | 5 (50%) | 2 (20%) | 5 (50%) | 4 (40%) | 5 (50%) |
| Biologic–Other | 14 (100%) | 9 (64%) | 0 (0%) | 3 (21%) | 4 (29%) | 3 (21%) |
| Small Molecule–Amyloid | 27 (100%) | 16 (59%) | 5 (19%) | 10 (37%) | 8 (30%) | 13 (48%) |
| Small Molecule–Tau | 9 (100%) | 2 (22%) | 0 (0%) | 2 (22%) | 2 (22%) | 5 (56%) |
| Small Molecule–Other | 58 (100%) | 32 (55%) | 5 (9%) | 11 (19%) | 21 (36%) | 16 (28%) |
| Cell and Gene Therapy | 8 (100%) | 3 (38%) | 2 (25%) | 2 (25%) | 3 (38%) | 4 (50%) |
| Other | 21 (100%) | 12 (57%) | 0 (0%) | 3 (14%) | 7 (33%) | 6 (29%) |
| Grand total | 175 (100%) | 94 (54%) | 16 (9%) | 46 (26%) | 62 (35%) | 62 (35%) |
3.1.4. Eligibility criteria
Figure 2 shows the distribution of eligibility criteria by treatment paradigm for age, MMSE scores, and CDR scores.
FIGURE 2.
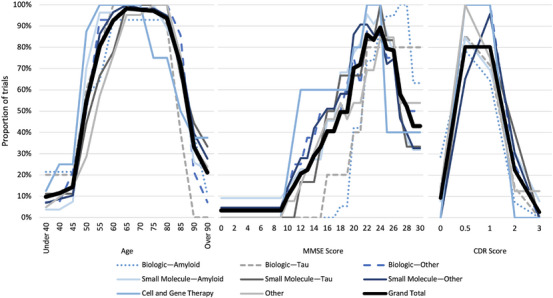
Eligibility criteria concerning patient ages, MMSE scores, and CDR scores by proportion of trials in each treatment paradigm. Trials that did not include a particular eligibility criterion (age, MMSE score, or CDR score) are excluded from the respective denominators used for the proportions presented. CDR, Clinical Dementia Rting; MMSE, Mini‐Mental State Examination.
3.1.5. Age eligibility criteria
Participants 60 through 80 years of age were eligible for inclusion in over 90% of trials. Furthermore, 54% of trials included ages as young as 50 years as eligible, whereas 33% of trials included ages as old as 90 years as eligible. It is important to note that the distribution of age eligibility did not vary markedly by paradigm.
3.1.6. MMSE and CDR score eligibility criteria
MMSE and CDR were frequently used as cognitive measures to assess participant eligibility. Overall, 69% of studies used MMSE score eligibility criteria. Scores 22 through 24 were eligible scores for enrollment in more than 80% of studies with MMSE score eligibility criteria. Scores 20 through 26 were eligible scores in at least 70% of such studies. For reference, MMSE scores of 25 through 30 are often considered within normal limits, and scores 20 through 24 are often considered mild cognitive impairment. This reflects an emphasis on enrolling patients with clinical symptoms but without severe disease. Comparing treatment paradigms, the Biologic–Amyloid paradigm had higher MMSE eligibility range endpoints, whereas the Cell and Gene Therapy paradigm had lower MMSE eligibility range endpoints.
Overall, 43% of trials contained CDR score eligibility criteria. In each paradigm, CDR scores of 0.5 and 1 were more prevalent, and represent very mild dementia and mild dementia, respectively. CDR scores of 0 (normal), 2 (moderate dementia), and 3 (severe dementia) were rare by comparison. Similar to MMSE score eligibility criteria, CDR score eligibility criteria emphasized enrolling patients with mild disease.
3.1.7. Clinical trials by phase, duration, funding sources, and location
Figure 3 shows the breakdown of trials by study phase, average phase duration, funding sources, and involvement of a U.S. trial site.
FIGURE 3.
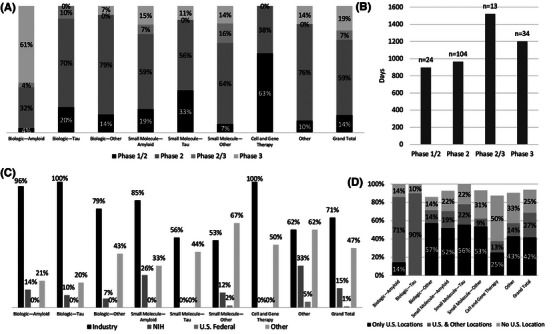
Characteristics of late‐stage clinical trials of potentially disease‐modifying therapies for Alzheimer's disease. (A) Clinical trial phases by treatment paradigm. (B) Average length of phases for clinical trials of potentially disease‐modifying therapies for Alzheimer's disease. (C) Funding sources by treatment paradigm. Funding sources are not mutually exclusive. (D) Involvement of a U.S. trial site by treatment paradigm. Not all studies listed locations and totals may not add up to 100%.
3.1.8. Trial phases
Of the 175 trials, 21% were engaged in multi‐phase trials; 14% were engaged in Phases 1/2 and 7% were engaged in Phases 2/3. In the following results, multi‐phase trials count toward both of their respective phases.
Overall, 81% of late‐stage trials were engaged in Phase 2, and 27% were engaged in Phase 3. This distribution varied across paradigms, but most reflected the high proportion of trials in Phase 2. The Biologic–Amyloid paradigm was an exception, with only 39% of trials engaged in Phase 2 and 64% engaged in Phase 3.
3.1.9. Duration of phases
Trial phases differed in average length with combined Phase 2/3 trials (1521 days = 4.2 years) exceeding the average length of Phase 2 trials (965 days = 2.6 years) or Phase 3 trials (1202 days = 3.3 years) in isolation. However, the duration of Phase 2/3 trials on average was 1.8 years shorter than the summative average lengths of Phase 2 and Phase 3 trials. Aggregate results are represented in Figure 3, and duration of phases with breakdown by paradigm are represented in Appendix Figure A.1.
3.1.10. Funding sources
Trials often had multiple funding sources. Overall, 71% of trials included funds from industry, 15% included funds from the NIH, 1% included funds from other U.S. federal government agencies, and 47% included funds from other sources.
The paradigms that most often included industry funding were Biologic–Amyloid (96%), Biologic–Tau (100%), Biologic–Other (79%), Small Molecule–Amyloid (85%), and Cell and Gene Therapy (100%). The NIH did not fund trials in the paradigms of Small Molecule–Tau or Cell and Gene Therapy.
3.1.11. Involvement of a U.S. trial site
The majority of trials reported site locations, and many took place in multiple countries. Overall, 69% of trials reported a U.S. location, 25% reported location data without any U.S. location, and 6% of trials did not report location data at the time of data extraction. Trials with exclusively U.S. locations comprised 42% of trials. Of trials with reported location data, at least one U.S. location was listed in 73%.
4. DISCUSSION
The candidates investigated in late‐stage clinical trials represent a wide range of potential treatments for AD. However, the high failure rate indicates that progress remains exceptionally difficult. 3 Here, we discuss emerging trends within the 175 captured late‐stage studies of potentially disease‐modifying therapies.
4.1. Amyloid as most frequent target
Amyloid was the most frequent target overall, with our results capturing 55 such biologic and small molecule trials. The emphasis on amyloid is prominent given that three Biologic–Amyloid therapies, all captured in this study, have been approved by the FDA: aducanumab in 2021, lecanemab in 2023, and donanemab in 2024—although aducanumab was withdrawn by its manufacturer after approval of lecanemab, which shares the same manufacturer. 9 , 10 The withdrawal of aducanumab was due to controversy surrounding its benefits, and in part to the approval of lecanemab. 4 Treatment effects reported for aducanumab, lecanemab, and donanemab include slowed decline of cognitive function, but not halted overall disease progression. 6 , 11
Other promising Biologic–Amyloid candidates have faced several trials. Eight trials captured in our analysis tested gantenerumab. Although gantenerumab has failed several trials, a modified version named trontinemab has been engineered to better cross the blood–brain barrier. 12 Crenezumab is another Biologic–Amyloid candidate that has generated interest, and the drug had reached a Phase 3 trial. 13 However, the trial was discontinued per interim analysis, and crenezumab is not being investigated in trials captured within the present study.
As more Biologic–Amyloid therapeutics are approved, it is important to investigate consequences on care delivery. Infusions are required for these drugs, and both drug administration and side effect management may result in high costs for monitoring patients. Some of the most prominent adverse effects are amyloid‐related imaging abnormalities related to edema (ARIA‐E) and microhemorrhage (ARIA‐H). These are relatively common and can change clinical management, sometimes pausing treatment. 14 Further research into best management is warranted.
4.2. Tau as a more challenging target
Accumulated neurofibrillary tau tangles are another notable target captured in our analysis. Compared with amyloid, fewer trials targeted tau, and none had advanced past Phase 2 at the time of data collection. In 2021, Eli Lilly disclosed discontinuation of zagotenemab (LY3303560), Abbvie stopped development of tilavonemab (ABBV‐8E12), and Biogen halted a trial of gosuranemab (BIIB092). 15 All companies acted due to failure to meet primary endpoints and significant adverse effects. Remaining tau‐targeting interventions in the pipeline include Ionis/Biogen's BIIB080, an injectable antisense oligonucleotide which acts upon ribonucleic acid to suppress tau production, and Janssen's JNJ‐63733657, an antibody that recognizes the tau microtubule‐binding region. Overall, targeting tau appears challenging given the lack of progression past Phase 2.
4.3. Biologic versus small molecule drugs
The majority of trials tested biologic or small molecule drugs, which comprised 30% and 54% of trials, respectively. Although more trials tested small molecules, a higher proportion of biologic drugs were engaged in Phase 3 (38% of biologic drugs, 25% of small molecule drugs). Among the paradigms, Biologic–Amyloid had the greatest number of Phase 3 trials (n = 18) and a near 100% rate of industry funding, possibly making it best poised for imminent breakthroughs.
4.4. Cell and gene therapy
Five of the eight studies in the Cell and Gene Therapy paradigm tested mesenchymal stem cell (MSC) therapies. Experimental AD models using MSCs have shown promising results, including enhancement of amyloid clearance and neuron survival. 16 Of the remaining studies in the paradigm, two assessed the antisense oligonucleotide BIIB080, and one assessed autologous regulatory T‐cell safety in the AD patient population. No studies had advanced past Phase 2 at the time of data collection.
4.5. The "Other" paradigm
The Other treatment paradigm was a catch‐all for studies that did not neatly fit the more specific paradigms involving biologic, small molecule, or cell and gene therapy mechanisms of action. Eight of the studies in the Other paradigm focused on diet modification, largely antioxidants. Oxidative stress from free radicals is a mechanism theorized to contribute to cognitive decline in AD, and antioxidants may be protective against this damage. 17 Five studies involved noninvasive brain stimulation, such as transcranial electromagnetic treatment. These non‐invasive interventions may slow decline in AD by disaggregating amyloid or tau, or by precipitating neuronal activity that enhances synaptic signaling. 18 , 19 Three studies investigated glucagon‐like peptide 1 (GLP‐1) agonists, which have recently received attention for their efficacy in weight loss and controlling type 2 diabetes mellitus. Proposed mechanisms for the possible benefit of GLP‐1 agonists in treating AD include protecting against oxidative damage, strengthening neuronal connections through promoting expression of synaptic proteins, and reducing amyloid and tau aggregation. 20 The proposed mechanisms for interventions in the Other paradigm are not well defined, but they are theorized to act on targets known to modulate neurological function.
4.6. Primary outcome measures and FDA guidance
In 2013, the FDA released guidance encouraging the use of both cognitive and functional outcome measures in AD clinical trials, suggesting a composite scale. 21 , 22 A 2018 revision used less‐specific language, generally encouraging broad endpoints. Previous analysis found that the guidance impacted outcome measure selection, with primary endpoints from multiple domains being employed in 27% of trials in the 5 years after the 2013 guidance, up from 15% during the 5 years prior. 21 Within studies captured in the present analysis, 35% included primary outcome measures from more than one domain. Notably, the Integrated Alzheimer's Disease Rating Scale (iADRS) cognitive‐functional composite scale, featured in the donanemab Phase 3 clinical trial, was used as a primary outcome measure in six of these trials. It appears that the FDA guidance is being practiced, but adherence remains low.
4.7. Funding priorities differ between sources
Priorities differed across funding sources, including between the NIH and industry, the two major funding sources. The paradigms in which at least 20% of trials received NIH funding were Small Molecule–Amyloid (26%) and Other (33%). The three paradigms with the greatest proportions of industry funding were Biologic–Amyloid (96%), Biologic–Tau (100%), and Cell and Gene Therapy (100%).
4.8. Limitations
Although we present a comprehensive view of the late‐stage clinical trial landscape of potentially disease‐modifying therapies in AD between April 10, 2021, and March 28, 2023, limitations to our analysis exist. We do not cover trials that were not active during this time frame. We believe this is appropriate given the relative recency of approved disease‐modifying therapies; however, there are trials not characterized here.
Another limitation is the difficulty in determining disease‐modifying potential. Although we used presiding opinions on which therapies have such potential and the intents of the trial investigators themselves, there is no standard to determine whether a therapy may be disease modifying, which is often the objective of the trials themselves to elucidate. In addition, the exact pathogenesis of AD remains unknown. Thus, it is possible that trials involving disease‐modifying therapies are not included, or that some will disagree with the inclusion of certain trials in the present analysis. We attempted to have broad inclusion of therapies that may be disease modifying, so the latter is more likely.
5. CONCLUSION
As both the burden of AD and the potential of disease‐modifying therapies increase, the value of understanding the landscape of potentially disease‐modifying therapies and characterizing emerging treatment candidates rises as well. In this work, we have organized candidates by mechanism of action (biologic, small molecule, cell and gene therapy, other) and general disease target (amyloid, tau, other), classifying potential disease‐modifying therapies by unique treatment paradigms.
Through analyzing the existing late‐stage pipeline of potentially disease‐modifying candidates, important trends emerge. Among 175 trials, there were 123 unique intervention candidates. This is consistent with other recent reviews of the AD drug‐development pipeline that demonstrated considerable and increasing investment in potentially disease‐modifying therapies. 23 , 24 Among therapy targets, amyloid was predominant, comprising 30% of the trials captured. Also prominent was tau, but there has been more difficulty advancing these trials past Phase 2. Considering therapy mechanisms, small molecule drug trials were the most numerous, although a higher proportion of biologic drugs were engaged in Phase 3. Furthermore, industry funding backs a higher proportion of late‐stage biologic studies compared to small molecule studies.
This review of the late‐stage AD pipeline informs understanding of trial structure and expectations about when results can be obtained. Trials focus largely on participants between 60 and 80 years of age, with about two‐thirds of trials including a U.S. clinical site. Among trials with MMSE or CDR score eligibility criteria, the eligible ranges most often include possible or mild cognitive impairment, demonstrating emphasis on preventing cognitive decline early in the disease course. In measuring trial length and excluding multi‐phase designs (e.g., Phase 1/2 and Phase 2/3 trials), Phase 2 trials averaged 2.6 years in predicted length and Phase 3 trials averaged 3.3 years. With 128 of the captured trials in Phase 1/2 or 2, and with 47 trials in Phase 2/3 or 3, there is hope for multiple breakthroughs in the next decade.
Although disease‐modifying therapies for AD constitute a diverse set of approaches, efforts and progress are largely concentrated in a few treatment paradigms and a narrow patient population. The AD research, clinical, and policy community should recognize that the late‐stage clinical trial pipeline is progressing at varying paces in the disparate paradigms, and craft strategies to accelerate the success of the most promising paradigms while nurturing the growth of nascent areas lacking resources but not potential. Assuming the imminent approval of new AD therapies, it is important to anticipate the associated consequences on care delivery and costs. As new disease‐modifying therapies emerge and trends evolve, future research may draw on the concept of treatment paradigms to better understand key differences in trial characteristics and their implications on pipeline and portfolio planning by manufacturers, funding and investment by private and public sources, and regulatory actions by national authorities.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
This study did not involve human subjects; consent processes were not applicable.
Supporting information
Supporting information
ICMJE Disclosure Form
ACKNOWLEDGMENTS
We are grateful for constructive comments provided by Bryan Tysinger and Darius Lakdawalla, affiliated with the University of Southern California. This work was supported by the National Institutes of Health (Award R01AG062277); and project nr. LX22NPO5107 (MEYS), financed by EU – Next Generation EU. Funders had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Hlávka JP, Kinoshita AT, Jeyasingh D, Huang C, Mirsafian L, Jacobson M. Emerging Alzheimer's disease treatment paradigms: A late‐stage clinical trial review. Alzheimer's Dement. 2024;10:e70022. 10.1002/trc2.70022
REFERENCES
- 1. Alzheimer's Association . Costs of Alzheimer's to Medicare and Medicaid. Alzheimer's Impact Movement. Published 2024. Accessed November 25, 2024. https://portal‐legacy.alzimpact.org/media/serve/id/62509c7a54845
- 2. 2023 Alzheimer's disease facts and figures 2023. Alzheimers Dementia. 2023;19:1598‐1695. doi: 10.1002/alz.13016 [DOI] [PubMed] [Google Scholar]
- 3. Cummings JL, Goldman DP, Simmons‐Stern NR, Ponton E. The costs of developing treatments for Alzheimer's disease: a retrospective exploration. Alzheimers Dement. 2022;18:469‐477. doi: 10.1002/alz.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biogen . Biogen to Realign Resources for Alzheimer's Disease Franchise. Published 2024. Accessed November 25, 2024. https://investors.biogen.com/news‐releases/news‐release‐details/biogen‐realign‐resources‐alzheimers‐disease‐franchise
- 5. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9‐21. doi: 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
- 6. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER‐ALZ 2 randomized clinical trial. JAMA. 2023;330:512‐527. doi: 10.1001/jama.2023.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aisen PS, Bateman RJ, Carrillo M, et al. Platform trials to expedite drug development in Alzheimer's disease: a report from the EU/US CTAD task force. J Prev Alzheimers Dis. 2021;8:306‐312. doi: 10.14283/jpad.2021.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Library of Medicine . About ClinicalTrials.gov | ClinicalTrials.gov. Published 2024. Accessed November 25, 2024. https://www.clinicaltrials.gov/about‐site/about‐ctg
- 9. Food and Drug Administration . FDA Grants Accelerated Approval for Alzheimer's Drug. FDA; 2021. https://www.fda.gov/news‐events/press‐announcements/fda‐grants‐accelerated‐approval‐alzheimers‐drug [Google Scholar]
- 10. Food and Drug Administration . FDA Grants Accelerated Approval for Alzheimer's Disease Treatment. FDA; 2023. https://www.fda.gov/news‐events/press‐announcements/fda‐grants‐accelerated‐approval‐alzheimers‐disease‐treatment [Google Scholar]
- 11. Eli Lilly and Co . Lilly's donanemab significantly slowed cognitive and functional decline in phase 3 study of early Alzheimer's disease. Eli Lilly and Company; 2023. https://investor.lilly.com/news‐releases/news‐release‐details/lillys‐donanemab‐significantly‐slowed‐cognitive‐and‐functional [Google Scholar]
- 12. Alzforum . Trontinemab. Published 2023. Accessed November 25, 2024. https://www.alzforum.org/therapeutics/trontinemab
- 13. Hoffmann‐La Roche . A Phase III, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, efficacy and safety study of Crenezumab in patients with prodromal to mild Alzheimer's disease. clinicaltrials.gov; 2020. [Google Scholar]
- 14. Filippi M, Cecchetti G, Spinelli EG, Vezzulli P, Falini A, Agosta F. Amyloid‐related imaging abnormalities and β‐amyloid‐targeting antibodies: a systematic review. JAMA Neurol. 2022;79:291‐304. doi: 10.1001/jamaneurol.2021.5205 [DOI] [PubMed] [Google Scholar]
- 15. Cummings JL, Gonzalez MI, Pritchard MC, May PC, Toledo‐Sherman LM, Harris GA. The therapeutic landscape of tauopathies: challenges and prospects. Alzheimers Res Ther. 2023;15:168. doi: 10.1186/s13195-023-01321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chakari‐Khiavi F, Dolati S, Chakari‐Khiavi A, et al. Prospects for the application of mesenchymal stem cells in Alzheimer's disease treatment. Life Sci. 2019;231:116564. doi: 10.1016/j.lfs.2019.116564 [DOI] [PubMed] [Google Scholar]
- 17. Knight E, Geetha T, Broderick TL, Babu JR. The role of dietary antioxidants and their potential mechanisms in Alzheimer's disease treatment. Metabolites. 2023;13:438. doi: 10.3390/metabo13030438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weiler M, Stieger KC, Long JM, Rapp PR. Transcranial magnetic stimulation in Alzheimer's disease: are we ready? eNeuro. 2020;7. doi: 10.1523/ENEURO.0235-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arendash G, Cao C, Abulaban H, et al. A clinical trial of transcranial electromagnetic treatment in Alzheimer's disease: cognitive enhancement and associated changes in cerebrospinal fluid, blood, and brain imaging. J Alzheimers Dis. 2019;71(1):57‐82. doi: 10.3233/JAD-190367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reich N, Hölscher C. The neuroprotective effects of glucagon‐like peptide 1 in Alzheimer's and Parkinson's disease: an in‐depth review. Front Neurosci. 2022;16:970925. doi: 10.3389/fnins.2022.970925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu JC, Hlávka JP, Joe E, Richmond FJ, Lakdawalla DN. Impact of non‐binding FDA guidances on primary endpoint selection in Alzheimer's disease trials. Alzheimers Dement. 2022;8:e12280. doi: 10.1002/trc2.12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Food and Drug Administration . Early Alzheimer's disease: developing drugs for treatment, Draft guidance. FDA; 2024. [Google Scholar]
- 23. Cummings J, Lee G, Nahed P, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimers Dement Transl Res Clin Interv. 2022;8:e12295. doi: 10.1002/trc2.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang LK, Kuan YC, Lin HW, Hu CJ. Clinical trials of new drugs for Alzheimer disease: a 2020‐2023 update. J Biomed Sci. 2023;30:83. doi: 10.1186/s12929-023-00976-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
ICMJE Disclosure Form


