ABSTRACT
Significant tricuspid regurgitation (TR) is seen as a relevant contributor of cardiac morbidity and mortality. Transcatheter tricuspid valve replacement (TTVR) is a novel technique to treat this condition. We present the case of an 82‐year‐old lady who was admitted for recurrent right heart decompensation despite having undergone treatment with tricuspid edge‐to‐edge repair (TEER). The patient underwent transfemoral implantation of a 48 mm EVOQUE valve. Since the implanted Clip was not in the central part of the valve, it was pushed toward the valvular anulus by the expanded prothesis. Echocardiography showed a good result with no residual TR. Options for residual TR after T‐TEER are very limited. TTVR might be suitable in selected patients with non‐central Clip placement.
Keywords: EVOQUE, transcatheter tricuspid valve replacement, Triclip
1. Introduction
Transcatheter tricuspid valve replacement (TTVR) using the EVOQUE system is a novel, recent technique that allows effective treatment of tricuspid regurgitation (TR). Significant TR is seen as a relevant contributor of cardiac morbidity and mortality [1]. The single arm “TRISCEND” study and the randomized trial "TRISCEND II" showed safety, feasibility and effectiveness of this new system [2, 3]. However, little is known about TTVR in patients who have formerly been treated with tricuspid edge‐to‐edge repair (TEER) and demonstrate unsatisfactory results.
2. Case Report
An 82‐year‐old female presented to our clinic's emergency department with shortness of breath on small exertion and severe weight gain over the course of the last week. Other than severe bilateral peripheral edema, physical examination was unremarkable. The patient's past medical history included paroxysmal atrial fibrillation, coronary artery disease, chronic kidney disease, heart failure with preserved ejection fraction, postcapillary pulmonary hypertension and massive TR. She had been discharged 6 weeks prior after undergoing T‐TEER for recurrent right heart decompensation. However, the procedure had to be aborted after implantation of the first device, an XTW Triclip in the anterior‐septal commissure (Abbott Laboratories, Green Oaks, Illinois), due to poor interventional echocardiographic image quality especially in the transgastric views. Under optimized medical therapy which included loop diuretics, her condition improved so that she had been transferred to a rehabilitation clinic.
At hospital readmission, laboratory workup showed mildly elevated creatinine levels of 2.2 mg/dL (with a known baseline creatinine of around 1.8 mg/dL) and mildly elevated CRP levels of 5.9 mg/dL. Transthoracic echocardiography revealed persistent massive TR (Figure 1), chest X‐ray was unremarkable. To shed light on tricuspid valvular pathology, transesophageal echocardiography was carried out. This demonstrated massive TR due to a central gap despite treatment with an XTW Triclip in the anterior‐septal commissure. TR etiology was likely secondary to right atrial enlargement in atrial fibrillation and mild pulmonary hypertension due to left ventricular diastolic dysfunction. This confirmed our diagnoses of recurrent right heart decompensation due to TR.
Figure 1.
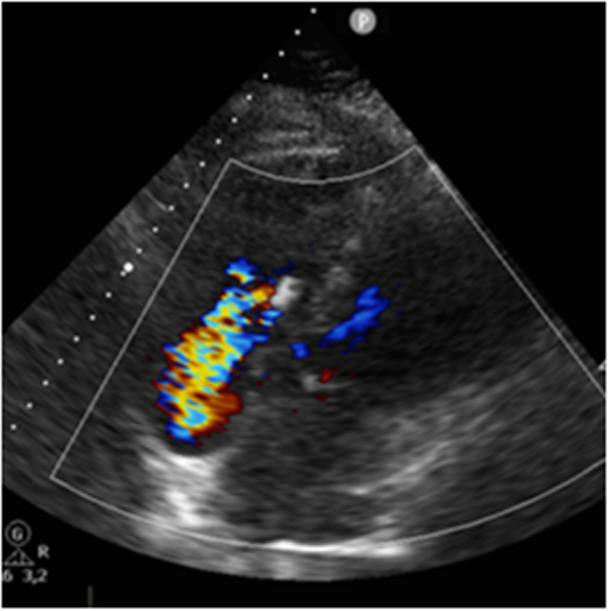
Echocardiography showing massive tricuspid regurgitation due to a persisting central gap after implantation of an XTW Triclip. [Color figure can be viewed at wileyonlinelibrary.com]
Initially, the patient was admitted to the ward for recompensation with intravenous diuretic treatment. After heart team discussion, she was evaluated for TTVR. Sizing CT revealed suitability for implantation of a 48 mm EVOQUE valve (Edwards Lifesciences, Irvine, California) (Figure 2).
Figure 2.
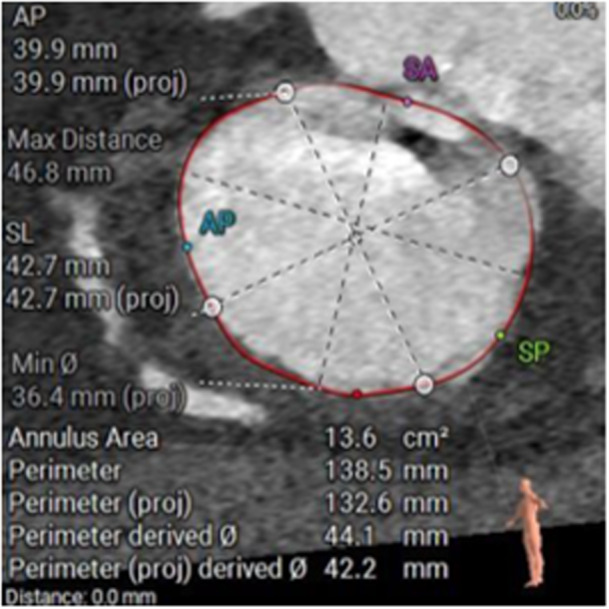
CT screening demonstrating suitability for implantation of a 48 mm EVOQUE tricuspid valve prothesis. [Color figure can be viewed at wileyonlinelibrary.com]
Through a transfemoral venous access, the 28‐F EVOQUE system was advanced across the tricuspid valve along a “Safari” guidewire (Boston Scientific Corporation, Marlborough, Massachusetts). Even though echocardiographic image quality was still limited, the valve was expanded without any complications under echocardiographic and fluoroscopic guidance (Figure 3 and 4, Video S1). The mean gradient was 2 mmHg and the functional result was excellent (Figure 5 and 6, Videos S2/3). Since the implanted Clip was not in the central part of the valve, advancement of the delivery system and expansion of the EVOQUE device was not impaired and the Clip was pushed toward the valvular anulus caged by the anchors of the valve prosthesis. Right atrial v‐wave was reduced from 25 to 10 mmHg (Figure 7).
Figure 3.
Multiplane reconstruction (MPR) transesophageal echocardiographic imaging of the EVOQUE system being expanded underneath the tricuspid valve with the XTW Triclip in the anteroseptal commissure (asterisk). [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
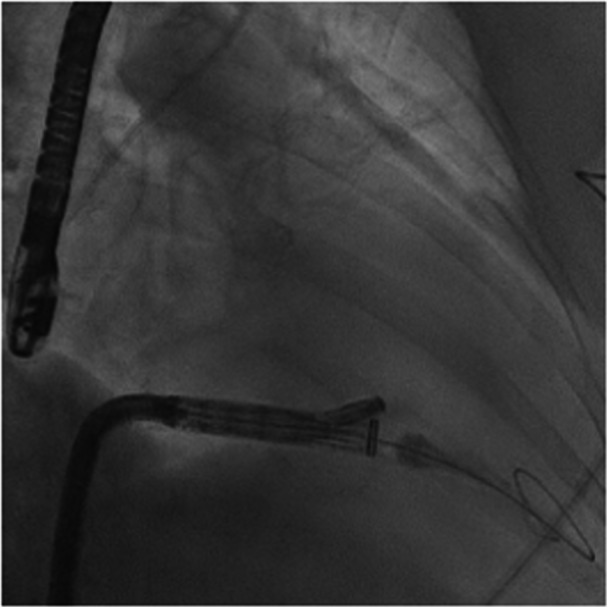
Fluoroscopic imaging of the EVOQUE system being advanced across the tricuspid valve. [Color figure can be viewed at wileyonlinelibrary.com]
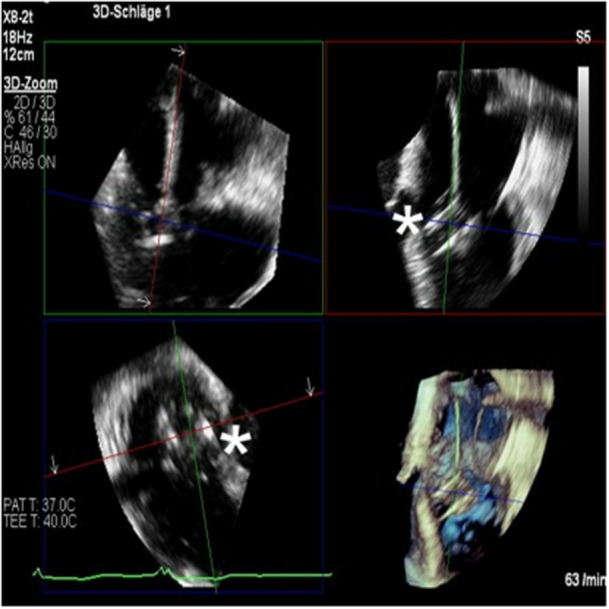
Figure 5.
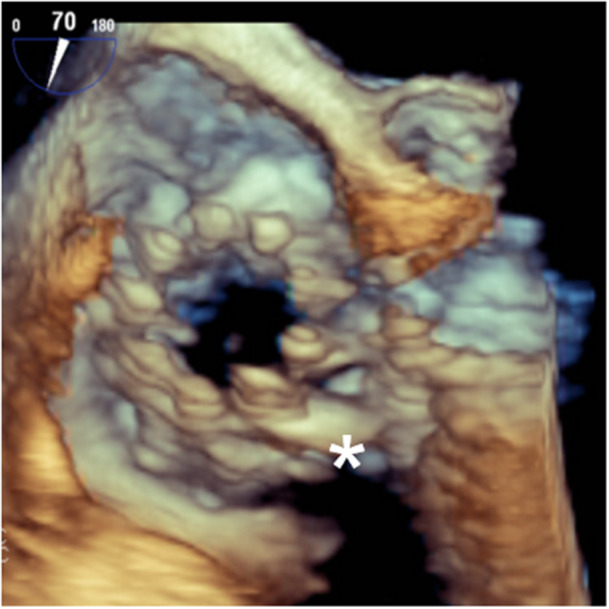
3D‐ Transesophageal echocardiographic image of the implanted valve. The XTW Triclip is being pushed toward the valvular anulus (asterisk). [Color figure can be viewed at wileyonlinelibrary.com]
Figure 6.
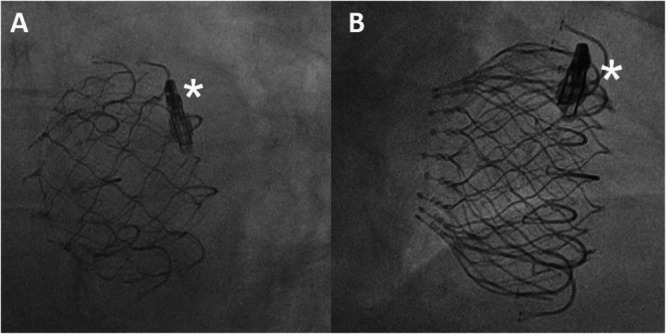
Fluoroscopic image of the implanted valve in an LAO (A) and RAO (B) projection. The XTW Triclip is being pushed toward the valvular anulus (asterisk).
Figure 7.
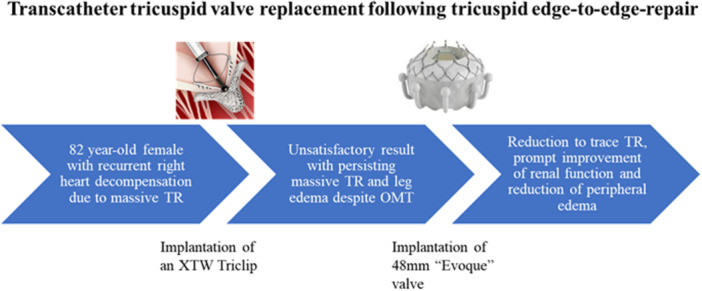
Visual summary of the case report. [Color figure can be viewed at wileyonlinelibrary.com]
Kidney function improved immediately and the patient was extubated the next day. Complications like paravalvular leakage or AV‐nodal block were not observed. Short term follow‐up echocardiography demonstrated good valvular function and no residual TR. After postinterventional monitoring she was discharged into a rehabilitation clinic and was scheduled for echocardiographic follow‐up. The patient presented 1 month later to our emergency department due to sepsis secondary to urinary tract infection but recovered after antibiotic treatment. Echocardiography during this admission also showed good valvular function with no residual TR.
3. Discussion
The novelty of this case lies in the fact that the patient was already treated with an XTW Triclip which was attached to the anterior and septal leaflet. Edge‐to‐edge procedures are well established and carried out more and more often [1]. Unfortunately, experiences of transcatheter options after failed TEER are sparse so far and stem from cases with single leaflet detachment (SLD). This is fundamentally different to our case since the placed device is only attached to a single leaflet and does not exert any stress on the valvular apparatus anymore. In a case published by Wild et al. Clip detachment of the septal leaflet caused laceration and a large coaptation gap [2]. This made placement of a second device in their case virtually impossible. We opted against Re‐TEER because of echocardiographic shadowing in the septal region caused by lipomatous hypertrophy of the interatrial septum as well as the first XTW Triclip. Unfortunately, this shadowing occurred in the region were a second Clip would have been placed, making reliable leaflet grasping impossible. However, we have to state that intracardiac echocardiography (ICE) has been demonstrated to guide T‐TEER under challenging conditions and might have presented an additional image modality [3, 4].
Especially in elderly patients with a high surgical risk this means reliance on medical therapy alone. In many cases, even optimal diuretic therapy is not able to prevent recurrent cardiac decompensation and hospital admissions. Several interventional options have been developed for patients in this setting. Case reports have demonstrated successful Percutaneous Circumferential Annuloplasty in the setting of secondary TR using a semirigid ring [5]. Another approach is heterotopic bicaval stenting which allows indirect treatment of severe TR without directly affecting valvular anatomy. A single arm multicenter study of this procedure showed promising first results [6].
In selected cases, TTVR might also be a suitable option. Despite being a novel technique, the randomized TRISCEND II study demonstrated improvement in quality of life compared to medical therapy alone [7]. However, procedural success is strongly depended on valvular anatomy and Clip placement. In our case, the XTW Triclip was placed in the commissure between the anterior and septal leaflet. This allowed the EVOQUE device to expand freely and push the Triclip toward the commissure. This would not have been possible if the Clip was placed in a more central position. Shadowing in the septal region which prevented Re‐TEER made valvular placement challenging. Fortunately, we were able to visualize enough anchors of the prothesis to ensure safe placement.
AV nodal block is a known complication of the EVOQUE system and occurred in 11% of the TRISCEND collective [8]. This is likely due to physical strain on the heart's conduction system by the valve. We cannot exclude that this risk is increased if a device like a Triclip is being pushed toward the valvular anulus after transvalvular expansion of the EVOQUE system. The anatomical location of the XTW Triclip in the anteroseptal commissure might be especially associated with an increased risk for AV nodal block due to its proximity to the heart's conduction system. This highlights the necessity for careful postinterventional monitoring in experienced centers and might even warrant extended ambulatory monitoring with Holter systems.
Another common complication after TTVR is bleeding. The TRISCEND study showed a bleeding rate of roughly 15% over 1 year which is likely due to the study cohort consisting mostly of elderly patients with multiple comorbidities [8]. As in our case, the vast majority of patients treated with TTVR had an indication for life long anticoagulation before the procedure. Despite several risk factors for bleeding (HAS‐BLED score of 3), our patient tolerated her previous anticoagulation with Apixaban 2.5 mg BD well. However, the previously implanted XTW Triclip might possess prothrombotic properties. Unfortunately, randomized data regarding optimal antithrombotic therapy following T‐TEER is lacking but similarly to the TRICSEND cohort, most individuals had an indication for life long anticoagulation in the first place [9]. Because of the high bleeding risk, we pragmatically opted against addition of platelet inhibition or a switch to a vitamin K antagonist (VKA) and recommended continuation of anticoagulation with Apixaban 2.5 mg BD.
4. Conclusion
This case demonstrates the feasibility of TTVR using the novel EVOQUE system in patients with residual TR after T‐TEER without central Clip placement.
Consent
Written patient consent for publication of images and patient related data was acquired.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Video 1: Periinterventional multiplane reconstruction 3D echocardiography during release of the EVOQUE valve.
Video 2: Multiplane reconstruction 3D echocardiography after successful transcatheter tricuspid valve replacement.
Video 3: Periinterventional multiplane reconstruction 3D echocardiography before ventricular valve expansion.
Acknowledgments
Open Access funding enabled and organized by Projekt DEAL.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Nickenig G., Weber M., Lurz P., et al., “Transcatheter Edge‐To‐Edge Repair for Reduction of Tricuspid Regurgitation: 6‐Month Outcomes of the TRILUMINATE Single‐Arm Study,” The Lancet 394 (2019): 2002–2011. [DOI] [PubMed] [Google Scholar]
- 2. Wild M. G., Zahr F., Näbauer M., Massberg S., and Hausleiter J., “Transfemoral Transcatheter Tricuspid Valve Replacement After Failed Leaflet Repair,” EuroIntervention 17 (2021): e1022–e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamid N., Aman E., Bae R., et al., “3D Navigation and Intraprocedural Intracardiac Echocardiography Imaging for Tricuspid Transcatheter Edge‐to‐Edge Repair,” JACC: Cardiovascular Imaging 17 (2024): 441–447. [DOI] [PubMed] [Google Scholar]
- 4. Singh G. D., Aman E., Pham T., Rogers J. H., Atsina K., and Smith T. W. R., “vICE‐Guided T‐TEER in a Patient Without TEE Windows,” JACC: Cardiovascular Interventions 15 (2022): e245–e247. [DOI] [PubMed] [Google Scholar]
- 5. Reddy V. Y., Abbo A., Ruiz C. E., et al., “First‐in‐Human Percutaneous Circumferential Annuloplasty for Secondary Tricuspid Regurgitation,” JACC: Case Reports 2 (2020): 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Estévez‐Loureiro R., Sánchez‐Recalde A., Amat‐Santos I. J., et al., “6‐Month Outcomes of the TricValve System in Patients With Tricuspid Regurgitation,” JACC: Cardiovascular Interventions 15 (2022): 1366–1377. [DOI] [PubMed] [Google Scholar]
- 7. Arnold S. V., Hahn R. T., Thourani V. H., et al., “Quality of Life After Transcatheter Tricuspid Valve Replacement: 1‐Year Results From TRISCEND II Pivotal Trial,” Journal of the American College of Cardiology 24 (2024): 735–1097. [DOI] [PubMed] [Google Scholar]
- 8. Kodali S., Hahn R. T., George I., et al., “Transfemoral Tricuspid Valve Replacement in Patients With Tricuspid Regurgitation: TRISCEND Study 30‐Day Results,” JACC Cardiovascular Interventions 15 (2022): 471–480. [DOI] [PubMed] [Google Scholar]
- 9. Maznyczka A. and Pilgrim T., “Antithrombotic Treatment After Transcatheter Valve Interventions: Current Status and Future Directions,” Clinical Therapeutics 46 (2024): 122–133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Periinterventional multiplane reconstruction 3D echocardiography during release of the EVOQUE valve.
Video 2: Multiplane reconstruction 3D echocardiography after successful transcatheter tricuspid valve replacement.
Video 3: Periinterventional multiplane reconstruction 3D echocardiography before ventricular valve expansion.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


