Abstract
Fresh meat is highly perishable, presenting challenges in spoilage mitigation and waste reduction globally. Despite the efforts, foodborne outbreaks from meat consumption persist. Biopreservation offers a natural solution to extend shelf life by managing microbial communities. However, challenges include the effective diffusion of bacteriocins through the meat matrix and the potential inhibition of starter cultures by bacteriocins targeting closely related lactic acid bacteria (LAB). LAB, predominant in meat, produce bacteriocins – small, stable peptides with broad antimicrobial properties effective across varying pH and temperature conditions. This review highlights the recent advances in the optimization of bacteriocin use, considering its structure and mode of action. Moreover, the strengths and weaknesses of different techniques for bacteriocin screening, including novel bioengineering methods, are described. Finally, we discuss the advantages and limitations of the modes of application of bacteriocins toward the preservation of fresh, cured, and novel meat products.
Key words: antimicrobial peptides, biopreservation, meat, lactic acid bacteria
Introduction
Meat is a source of high-quality proteins, minerals, and vitamins (Geiker et al., 2021). However, it is especially prone to spoilage, as it undergoes microbial (e.g., microbiological spoilage), chemical (e.g., autolytic enzymatic reactions), and physical (e.g., slime and liquid formation) deterioration; in fact, estimates show that as much as 23% of the annual production in the meat sector is lost and wasted (Luong et al., 2020; Odeyemi et al., 2020; Karwowska et al., 2021).
Regarding the safety of meats, recent cases (2016-2021) of foodborne outbreaks in the European Union have been linked to the consumption of contaminated meats, for instance, with Salmonella spp. (n=289), Clostridium perfringens (n=102), Staphylococcus aureus toxins (n=34), and Listeria monocytogenes (n=17), resulting in a total of 1363 hospitalizations and economic losses of up to US$90 billion annually (Scharff, 2020; EFSA, 2023). Research on meat preservation considers not only the extension of the product’s organoleptic features but also its microbiological safety (European Commission, 2005 - EC No. 2073/2005). In the domain of biopreservation, natural or controlled microbial communities, and their antibacterial products are used as an approach for controlling microbial growth. An integral component of the initial microbial community of meat is lactic acid bacteria (LAB), which rapidly develops under chill-stored, post-processed, and vacuum-packed/modified atmosphere conditions (Nauman et al., 2022). LAB are classified as Gram-positive, catalase-negative, anaerobic, with a varied morphology (rods or cocci), and play a crucial role in fermentation. Moreover, LAB can be used as biocontrol agents by the generation of metabolites with antimicrobial properties against pathogens, including organic acids (e.g., lactic acid, acetic acid), short-chain fatty acids, proteases/peptidases, and bacteriocins (Ibrahim et al., 2021). This review highlights the recent advances in the optimization of bacteriocin use, considering the bacteriocin’s structure and mode of action. Moreover, the strengths and weaknesses of different techniques for bacteriocin screening, including novel bioengineering methods, are described.
Finally, we discuss the advantages and limitations of different bacteriocins’ modes of application toward the preservation of fresh, cured, and novel meat products.
General overview
Bacteriocins are small peptides synthesized in the ribosome and can be categorized into four groups, according to their size, structure, and function: class I or lantibiotics (<5 kDa), class II (<10 kDa), class III or bacteriolysins/non-lytic (>30 kDa), and class IV (reclassified as bacteriolysins) (Simons et al., 2020; Barcenilla et al., 2023). These peptides play a crucial role in the competition for colonization sites and are able to influence the dynamics of the microbiome (Umu et al., 2017). For instance, bacteriocinogenic LAB inhibit target bacteria by interacting with the negatively charged cell membrane, a process mediated by their cationic and amphiphilic motifs (Lei et al., 2019; Heilbronner et al., 2021).
Mode of action
The bioactivity of bacteriocins can be either of a narrow spectrum (if the inhibition is exclusive to species that are closely related) or a broad spectrum, which is the case for lantibiotics (Woraprayote et al., 2016); in addition to presenting either bacteriostatic (inhibition) or bactericidal (killing) effects (Negash and Tsehai, 2020). The general mode of action of these antimicrobial peptides is given by their specialized functional domains: substrate binding site, translocation, and catalytic site (Gillor et al., 2008). The binding domain attaches to specific receptors on bacterial membranes, the translocation domain interacts with specific proteins integral to the cell membrane, and the effector domain executes lethal action: DNA degradation and/or induction of pores in the membrane (Davidson et al., 2008).
Nisin
Lantibiotics act by blocking the lipid II cycle (Figure 1) (Hsu et al., 2004), preventing correct cell wall synthesis, and inducing pore formation by interacting with the outer membrane (Diep et al., 2009). Nisin (grey) is composed of 34 amino acids and has a positive charge (+4), which allows it to interact with the anionic lipid II that constitutes the peptidoglycan layer in the bacterial cell membrane. The peptidoglycan is formed by a chain composed of N-acetylglucosamine (green), N-acetyl muramic acid (MurNAc; red), a pentapeptide (not shown), two pyrophosphate molecules (blue), and a prenyl chain (black).
The lipid II mediated pathway of nisin starts with the recognition of lipid II MurNAc and isoprene units. The dehydrobutyrine and α-aminobutyric acid of nisin then establish a hydrogen bond with the pyrophosphate molecules of lipid II. The assembly of nisin occurs without a canonical secondary structure, where two lanthionine rings fold to form a cage-like structure with the nisin backbone amides and the lipid II pyrophosphate, with a molar ratio of 8:4 for nisin and lipid II, respectively. Finally, the cage-like structure induces pore formation, resulting in disruption of the cell membrane.
Pediocin
On the other hand, class II or non-lantibiotic bacteriocins (Figure 2), divided into four subclasses, utilize the mannose phosphotransferase system (man-PTS) to permeabilize the membrane, disrupt proton motive force, and deplete adenosine triphosphate pools (Diep et al., 2007). Class IIb bacteriocins activity depends on two-component, α and β subunits, which fold into α-helical structures and insert themselves into target bacterial membranes to alter their permeability, resulting in ion leakage and cell death (NissenMeyer et al., 2011; Proutière et al., 2023).
Pediocin PA-1 is composed (Figure 2) (Zhu et al., 2022) of a hydrophilic and cationic N-terminal (brown) which consists of a three-stranded-β-sheet (containing the pediocin-box) linked by disulfide bridges (black). The region between N- and C-terminal is a flexible region composed of Asp/Asn in residue 17. The C-terminal is hydrophobic and is organized in an α-helix (blue), coupled with a hairpin-like tail (light pink).
The N-terminal β-sheet of pediocin PA-1 links with the extracellular man-PTS core domain (red). Normally, this transporter protein composed of a v-motif (green) and a core domain (red) switches from position depending on the transport of mannose. The change in conformation occurs by an elevator movement.
The binding occurs with the effector domain of pediocin PA-1 (pediocin-box and positively charged Lysn II and His 12) and the core domain of the man-PTS (Val 7, Cys 9, Cys 14, Tyr 3) establishing a linkage that blocks the elevator movement of man-PTS, with a molar ratio of pediocin PA-1 and man-PTS at 3:3. The hairpin-like tail of pediocin makes pi-stacking interactions with Trp 18 (white) of man-PTS and stabilizes the pediocin-manPTS structure, leading to disruption of the cell membrane (Zhu et al., 2022).
Screening of bacteriocins
The screening of bacteriocins can be divided into three stages: search for the presence of bacteriocin-encoding genes, evaluation of bacteriocin expression, and assessment of the bacteriocin ntimicrobial activity. For cultivable bacteria, the search for a bacteriocinogenic LAB strain begins with the isolation of autochthonous strains from the meat matrix. Then, the bacteriocinencoding genes can be amplified by polymerase chain reaction (PCR), and their expression is evaluated in real-time using realtime quantitative PCR (RT-qPCR). After purification, the bacteriocin’s antimicrobial activity can be assessed by antagonism tests (Figure 3) against indicator microorganisms.
Figure 1.
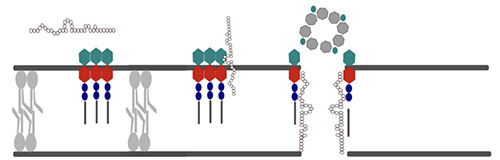
Nisin mode of action.
Figure 2.
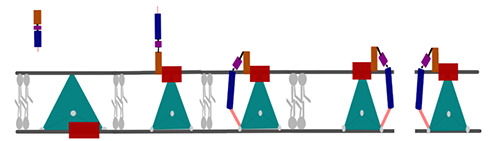
Pediocin-PA-1 mode of action.
For instance, the in vitro assessment of bacteriocins or bacteriocin-like substances entails methodologies such as agar well diffusion (Bungenstock et al., 2020), agar spot (Moraes et al., 2010; Selman et al., 2021), turbidimetric (Yang et al., 2018; Piazentin et al., 2022), enzyme-linked immunosorbent assay (Martínez et al., 2000; Surati, 2020) and RT-qPCR (Dortu et al., 2009; Balutis, 2014; Wan et al., 2015). Moreover, the bacteriocin does not have an antimicrobial effect on the producer strain. These mechanisms are constituted of self-immunity proteins that competitively antagonize the putative bacteriocin receptors by anchoring the membrane surface (antagonism), being embedded in the membrane (repulsion), or producing metalloproteases that degrade the bacteriocin (Deegan et al., 2006; Bastos et al., 2015).
Culture-based methods for assessing antimicrobial activity mostly relying on outdated protocols persist in being the most used (Balouiri et al., 2016), despite recent advancements in genomics, namely the whole genome sequencing (WGS), RNA sequencing (RNA-seq), and PCR-based techniques. While agar-based tests offer cost-effectiveness and simplicity, particularly at an initial sample screening, they lack the depth necessary to fully explore a strain’s bacteriocinogenic potential. Moreover, utilizing different agar-based tests within a single study may introduce intra-study variability and errors due to differing experimental conditions and subjective interpretation of results (Hossain, 2024).
In the case of unculturable microbes, the use of metagenomics tools is utilized since it is estimated that 99% of microorganisms are not possible to culture in isolation (Ayrapetyan and Oliver, 2016). The DNA of the food sample is extracted, followed by library preparation and then sequencing of all of the DNA present in the sample. This sequencing data enables the visualization of the microbial community composition within the food matrix, providing insights into the relationship between meat preservation and 16S rRNA diversity analysis as the microbiota continually changes during storage (Dorn-In et al., 2024).
Shotgun metagenomics enables the discovery of the microbial profile of samples, allowing for the detection of spoilage and pathogenic organisms, as well as differentiation at the strain level (Srinivas et al., 2022). This method can be used to monitor the supply chain for agents of concern, providing valuable insights that incentivize food manufacturers to invest in preventative control measures (Imanian et al., 2022).
The development of new in silico techniques has made it possible to analyze bacteriocins in a high-throughput manner (Nedyalkova et al., 2024). In contrast with classical assays for antimicrobial activity determination, the current methods for predicting bacteriocin gene clusters are high-speed and can be automated. BAGEL is a web mining tool that uses whole-genome sequence data to analyze the technological potential of bacterial strains (van Heel et al., 2018).
Mining genomes with automated software (Wosinska et al., 2022; Sowers et al., 2023) for the identification of bacteriocins reveal gene loci that can be functional or not. The data generated from this type of analysis has the potential to be exploited by bioengineering, including de novo design of novel bacteriocins (Deo et al., 2022; Kordi et al., 2024).
The recent increase in RNA-seq data, which describes the presence and quantity of RNA in a biological sample, demonstrates that RNA-seq can be used to follow the survival of target bacteria in the presence of the bacteriocin, by measurement of the expression of genes in food systems. Moreover, it is useful for analyzing the influence displayed by various environmental conditions on gene expression and fine-tuning them to achieve optimal conditions (Yang et al., 2024).
Representative genomes on the NCBI platform have been used in comparative genomics to predict peptide expression and secretion by the bacteria (Marques et al., 2023). These tools in combination with molecular dynamics analysis allow automated assessment of the binding mechanism of action performed by bacteriocins (Walsh, 2017; Leslie et al., 2021; Rodrigues Blanco et al., 2022;). The computational tools include docking software and three-dimensional structure modeling of the putative peptides (Frederix et al., 2018; Nain et al., 2020; Xin et al., 2020; Das et al., 2021; Palmer et al., 2021; Krishnamoorthi et al., 2022; Wang et al., 2023).
The development of these novel methodologies enables the fast discovery of structural and functional characteristics of specific amino acid residues, which can be associated with binding sites in the bacterial membrane (Bindu and Lakshmidevi, 2021; Chen et al., 2022; Marques et al., 2023; Oftedal, 2023). By understanding the mode of action of bacteriocins, given the varied nature of their structures, strategies to enhance and potentiate the bacteriocin’s antimicrobial activity can be further developed (Amarh et al., 2023). Lantibiotics, for instance, are subjected to post-translational processing (Figure 4) (Hsu et al., 2004), featuring compact structures with modified amino acids (e.g., lanthionine bridges, β-methyllanthionine, and dehydrated amino acids) and thioether ring structures. In the case of nisin, its structure comprises a globular chain consisting of lanthionine and dehydrated serine residues. These elements undergo post-translational modifications and proteolytic cleavage during the peptide processing phase, contributing to the unique structure of nisin.
Figure 3.
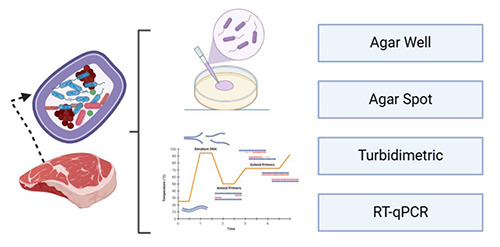
Diagram for different in vitro methods for bacteriocin activity screening from foodstuffs.
Lactocin S, however, is prone to oxidation due to the sulfide bonds in its α and β rings, which results in its inactivation; therefore, the synthesis of this bacteriocin occurs under anaerobic conditions, which could be improved by replacement of the sulfide bonds with hydrocarbon chains in analogs of lactocin S leading to more oxidative stability (Ross et al., 2012; Tsukano et al., 2024).
Lantibiotics active sites include the amino acid residues such as the catalytic site and the substrate binding site, with the F(ND)L(DEN)(LVI) motif (Figure 5) being conserved across different bacteriocins from this class. There is a gap in the literature on the correlation between the conserved motifs of bacteriocins and their mode of action considering their 3D structure and its influence on the bacteriocin’s activity. Figure 5 depicts predicted 3D structures produced by the α Fold algorithm and visualized with Chimera X (Jumper et al., 2021; Varadi et al., 2022; Meng et al., 2023;), N- and C-terminus indicated in the figure, region highlighted in red indicates the conserved region FNDLV, the conserved region of the three peptides appear differently across the space on the 3D structure format.
Class IIa bacteriocins are characterized by a conserved sequence at the N-terminal (YGNGV), also known as pediocin-box (Figure 6) (Waterhouse et al., 2009), a sequence related to strong anti-listerial activity first described in pediocin PA-1 (Cui et al., 2012). Class IIb is composed of two short chains. Class IIc are circular bacteriocins that lack the leader peptide sequence and are dependent on the general secretion pathway (sec) for transportation across the cytoplasmic membrane (Perez et al., 2014; Choi et al., 2023). Class IId bacteriocins are typically constituted of NGY residues at the N- terminus and central YxVTK motifs (Yoo et al., 2023); this class covers the remaining single-peptide and non-pediocin-like bacteriocins (Iwatani et al., 2011).
Class II (pediocin-like) suffers cleavage of a leader peptide (Figure 6) (Waterhouse et al., 2009) in the N- terminus in order to turn into a mature bacteriocin, which can be seen from the representation of class IIa bacteriocins isolated from meat aligned by the CLUSTAL W algorithm (Thompson et al., 1994; Drider et al., 2006; Lee and Kim, 2011). Sequences were fetched from UniProt. The pediocin-box is highlighted in a black square, hydrophobic (blue), positive charge (red), polar (green) glycine (orange), prolines (yellow), and aromatic (cyan) residues.
3D primary structures of class IIa bacteriocins (Figure 7), generated using the α fold algorithm and visualized with Chimera X, show that the conserved region YGNGV exhibits distinct spatial arrangements within the 3D structures of the different class IIa bacteriocins. This variation suggests that although the sequence is conserved, the spatial positioning and orientation of this region can differ significantly from one peptide to another (Figure 7). Such differences in the three-dimensional conformation could influence how these bacteriocins interact with their target receptors, potentially affecting their antimicrobial activity and specificity (Jumper et al., 2021; Varadi et al., 2022; Meng et al., 2023).
Bacteriocins exhibit robust resistance to high temperatures and low pH due to their specific amino acid composition, a high number of disulfide bridges, and ion pairs (Szilágyi and Závodszky, 2000). The solubility of these peptides increases at low pH due to a net charge change that facilitates greater diffusion through bacterial membranes (Yu et al., 2023). Lantibiotics, in particular, demonstrate strong resistance under extreme conditions. Additionally, owing to their proteinaceous nature, bacteriocins are susceptible to proteolytic enzymes—such as pancreatin complex, trypsin, and chymotrypsin—found in the gastrointestinal tract (Aljohani et al., 2023). The characteristic nature of bacteriocins can be determined by testing their sensitivity to an array of proteolytic enzymes, producing a pattern of protease sensitivity (Bromberg et al., 2004).
Figure 4.
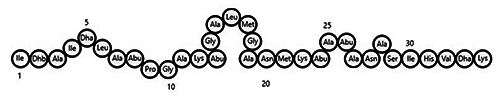
The amino acid composition of nisin.
Figure 5.
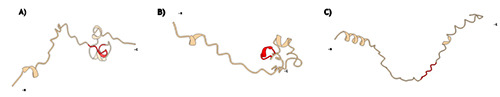
Lantibiotics three-dimensional primary structures. A) Nisin A (P13068); B) nisin Z (P29559); C) lactocin S (P23826).
WGS has made it possible to identify conserved open reading frames and understand the organization of gene loci encoding the bacteriocin and its immunity genes, in addition, it allowed to predict the promoter and terminator sequence of the peptide from the DNA data by predicting the RNA-polymerase binding motif, which can be useful for improving expression of bacteriocin encoding genes (Ruiz Puentes et al., 2022).
Promotion of bacteriocin synthesis can be obtained by constitutive expression of genes or by regulating gene expression as a response to the metabolite production from competing strains (González and Keshavan, 2006; Ng and Bassler, 2009). The bacteriocin synthesis gene clusters have been found to be located in the chromosome and in mobile elements such as plasmids and/or transposons (Achemchem et al., 2005; Lahiri et al., 2022). These clusters encode genes for the expression of the bacteriocin itself, enzymes, and self-immunity regulators of bacteriocin production and are organized in operons and/or regulons that undergo rapid evolution and are susceptible to high rates of horizontal transfer and spontaneous loss (Mørtvedt and Nes, 1990; Noda et al., 2018; Almeida-Santos et al., 2021).
A series of databases have been developed specifically for information on bacteriocins, for instance, the open-access database BACTIBASE (http://bactibase.hammamilab.org) with information on bacteriocins based on published literature extracted from PubMed (Hammami et al., 2010). LABiocin (https://labiocin.univlille.fr/) is a database of LAB bacteriocins containing 517 entries extracted from literature searches on Scopus, PubMed/Medline, and ScienceDirect with articles published up until 2017 (Kassaa et al., 2019). These databases provide valuable information on structure, amino acid sequence, gene sequence, purification, and physicochemical characteristics of bacteriocins; homology search is an additional feature comprised in these databases which allows for sequence alignment using algorithms such as BLAST (McGinnis and Madden, 2004).
Figure 6.
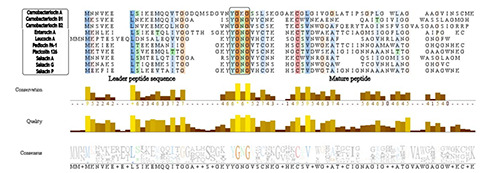
Representation of class IIa bacteriocins isolated from meat aligned by the CLUSTAL W algorithm and visualized in Jalview.
Figure 7.
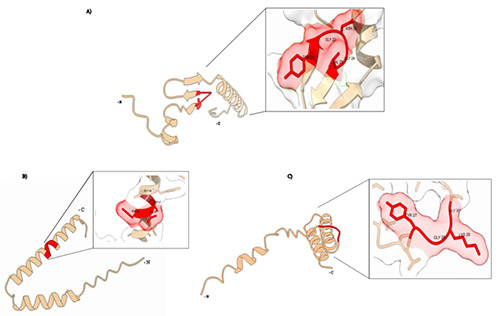
3D primary structures of class IIa bacteriocins. A) Pediocin PA-1 (P29430); B) divergicin 750 (Q46597); C) carnobacteriocin A (P38578).
Meat application
Gram-positive bacteria, particularly LAB, are the most studied source of bacteriocins from the meat environment (da Costa et al., 2019). The bacteriocins isolated from LAB in meat and meat products belong to different species, for instance, Lactobacillus sakei from vacuum-packed lamb meat (Holck et al., 1994), Carnobacterium piscicola from spoiled meat (Jack et al., 1996), Leuconostoc gelidum from processed packaged meat (Hastings et al., 1991), Leuconostoc carnosum from packaged meat (Raimondi et al., 2021), Enterococcus faecium from dry fermented sausages (Casaus et al., 1997), Carnobacterium divergens from vacuumpacked meat (Zhang et al., 2019) and Carnobacterium maltaromicus from vacuum-packed chilled meat (Quadri et al., 1994).
In the last 30 years, bacteriocins have been screened and applied in meat to control microbial decay and spoilage, acting as natural inhibitors and extending the shelf life of meat products (da Costa et al., 2019). However, there are still challenges associated with this approach, including the variability in peptide function depending on the nature of the meat matrix – especially in more fibrous matrices, effective inhibition of target microorganisms, resistance development, and compatibility with surrounding LAB (Sionek et al., 2024).
Regarding the nature of the meat matrix, challenges arise when applying bacteriocins in meat products primarily due to the hydrophobic nature of the meat and its instability at neutral pH, its interaction with phospholipids derived from meat products and other emulsifiers that make it difficult to distribute and solubilize the bacteriocin at pH values higher than 6.0. For instance, in fresh meat, three glutathione molecules are able to conjugate with one nisin molecule resulting in activity loss. However, it is possible to regulate the amount of free sulfhydryl groups present in the matrix, such as with the process of cooking the meat which reduces the free sulfhydryl groups and prevents the formation of the nisin-glutathione complex (Rose et al., 2002).
Strains resistant to a specific class of bacteriocins express immunity genes that confer protection against antimicrobial peptides from different classes, for instance, nisin resistant strain of L. monocytogenes has been reported to show cross-resistance to pediocin PA-1 and leuconocin S (Crandall and Montville, 1998; Darbandi et al., 2021).
To overcome the resistance development related to bacteriocins in food systems, it is advisable to apply a combined strategy of peptides in multi-hurdle strategies, for instance, in combination with additives, pH and atmosphere control, recipe modifications with spices and condiments, natural extracts and essential oils as ingredients (Kaur et al., 2013; Soltani et al., 2021).
When classifying bacteriocins isolated from fresh and processed meat products, two main classes based on biochemical structure emerge: lantibiotics and pediocin-like peptides (Woraprayote et al., 2016). There are different methods regarding their practical application as biocontrol agents (Figure 8) and each comes with its set of advantages and disadvantages; for instance, crude preparations are tasteless, colorless, and odorless, however, their activity may be limited by a narrow spectrum reach, limited diffusion in solid matrices, and cross-resistance generation (Morata, 2015; Urso et al., 2006). In the next section, we describe the types of bacteriocin applications regarding meat preservation.
Mixed starters
The use of LAB as inoculum is the most commonly used mode of application for preservation of foodstuffs. For meat preservation, bacteriocins can be applied as an inoculum of pure or mixed cultures (Baillo et al., 2023), as a crude bacteriocin preparation (Xin et al., 2023), and as a purified or semi-purified formulation (da Costa et al., 2019).
A selection of two autochthonous LAB strains (6.3 log CFU/g) isolated from spontaneously fermented Spanish sausages (salchichón) – Lactiplantibacillus paraplantarum BPF2 (producer of leucocin K) and P. acidilactici ST6 (producer of pediocin PA-1) were used as starter and reduced levels of rancidity in aroma and taste and improved the intensity and the persistence of the sausage’s characteristic flavor (García-López et al., 2023).
Figure 8.
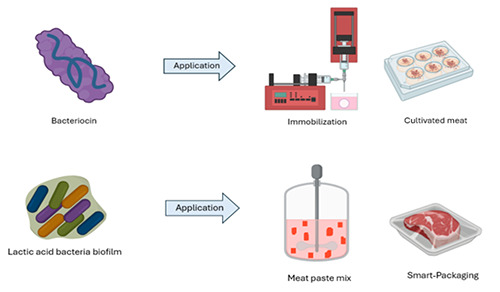
Diagram of lactic acid bacteria biofilm and bacteriocin for different kinds of applications in the processing and conservation of meat and meat products.
Commercial bacteriocin products such as Nisaplin© (Aplin and Barrett Ltd.) and Bactoferm© (Chr. Hansen AS) often consist of a mixture of crude preparations and organic acids in bioactive powder form. These products offer a mix of lyophilized starters for use in meat processing industries, such as for the production of salami, pepperoni, dry and cured meats, which are frequently used combined in multi-hurdle strategies with curing, drying and smoking preservative methods (Soltani et al., 2021).
Traditional Iberian cold-smoked fermented sausages underwent testing to assess the antilisterial effect of Bactoferm F-LC©. While Bactoferm F-LC© exhibited bacteriostatic activity at 10ºC, L. sakei CTC494 showed a more pronounced and rapid inhibition of Listeria. This resulted in a significant reduction of the pathogen by 2 log counts (Ortiz et al., 2014).
The application of mixed starters is beneficial because it combines strains that shorten fermentation time with those that enhance the meat’s organoleptic properties. Additionally, strains that produce antimicrobial metabolites can be included to combat hazardous and pathogenic bacteria, thereby increasing the food’s microbial shelf life.
However, compatibility between strains remains a significant issue, as the antimicrobial metabolites produced by biocontrol strains can be detrimental to the survival of strains promoting fermentation. Therefore, the choice of strains must consider their competitiveness with surrounding bacteria, the expression levels of bacteriocin synthesis, and their compatibility with the fermenting culture (Zacharof and Lovitt, 2012).
Pure culture
A novel multi-hurdle strategy was developed to extend the shelf life of the Portuguese fermented sausage alheira, combining mild high-pressure processing (300 MPa, 5 min at 10ºC), 0.1% (v/w) Pediococcus acidilactici (producer of pediocin PA-1), and 0.1% (v/w) phage Listex. This approach displayed no significant differences in color, texture, or lipid peroxidation between unprocessed and minimally processed samples (Komora et al., 2023). In this study, homogenization was manually performed by gently massaging the sample for approximately 3 minutes, which did not impact the texture. Although the acidifying capacity of LAB can increase the firmness of sausages, the selected LAB in this case was not a strong acidifier, which may explain why the texture remained unchanged.
In contrast, The E. lactis Q1, producer of enterocin P, added as a pure culture (107 CFU/g) on raw beef reduced L. monocytogenes counts by 6 log units after 1 week of cold storage and improved the sensorial characteristics such as color, odor, and appearance, especially since Enterococci are good acidifiers, particularly in meat products (Ben Braïek et al., 2020). When comparing mixed and pure cultures, mixed cultures offer greater advantages due to their diverse LAB, which can be fine-tuned for better product quality. Moreover, while pure LAB cultures are effective in reducing pathogen growth in meats, their efficacy is enhanced when used alongside other hurdle strategies.
Crude preparation
The application of the peptide (1280 AU/g) onto the surface of ham resulted in a 1.74 log reduction of L. monocytogenes counts (p<0.05); therefore, potentially increasing the ham shelf life to 1 month in refrigerated storage (4ºC). Incorporating the peptide in the meat paste produced an inhibition of background spoilage bacteria resulting in significantly lower counts (p<0.01). However, L. monocytogenes strains were reported to develop resistance to plantaricin UG1 in subsequent generations (Enan, 2006).
A combination of lactocin and high hydrostatic pressure treatments increased L. monocytogenes cell death in chilled vacuumpacked pork loin slices (Dallagnol et al., 2017) by a 6 log CFU/g reduction. By applying 200 AU/mL of the pure bacteriocinogenic culture in the salami batter, L. monocytogenes was reduced by 2 log CFU/g (de Souza Barbosa et al., 2015).
Pediocin PA-1 reduced the counts of L. monocytogenes inoculated in raw chicken meat from 5 log CFU/g to 3.8 log CFU/g when stored at 4 ºC for 1 month, however, re-growth was observed after this period, which could be due to the actions of proteases derived from the meat (Kiran and Osmanagaoglu, 2014).
Choosing between a pure culture of bacteriocin-producing bacteria and a crude bacteriocin preparation involves a nuanced assessment of their respective advantages and disadvantages. A pure culture offers the benefit of continuous bacteriocin production, potentially increasing antimicrobial activity over time and reducing initial preparation costs. However, using live bacteria can lead to compatibility issues, where different strains may inhibit each other’s growth and compete for nutrients, resulting in inconsistent efficacy. Additionally, regulatory concerns arise with live cultures, particularly in food applications, due to potential health risks and spoilage.
In contrast, a crude bacteriocin preparation provides predictable and controlled antimicrobial effects, as the activity of the bacteriocins is well-characterized and targeted against specific bacteria. This method bypasses compatibility issues and is generally more acceptable from a regulatory perspective. However, the process of purifying bacteriocins is labor-intensive and costly, with challenges in achieving effective concentrations across different food matrices and target microorganisms. Moreover, maintaining the stability of crude bacteriocin preparations requires careful handling and storage. Ultimately, the choice depends on the specific application, balancing cost, regulatory considerations, and the need for precise antimicrobial activity.
Encapsulation
Challenges with the bacteriocin adsorption in the matrix can be addressed by using immobilized preparations, such as encapsulation on gel coatings, films, silica particles, or liposomes (Gálvez et al., 2007). Encapsulation of bacteriocins involves incorporating these antimicrobial peptides into a protective matrix or carrier to enhance their stability, control their release, and improve their effectiveness in various applications.
The application of encapsulated bacteriocins combined with citrus extract, and thyme essential oil led to a synergistic antimicrobial activity for meatballs protection against L. monocytogenes and all tested LAB; however, there was no effect observed on the inhibition of S. Typhimurium (Sarmast et al., 2023). Moreover, the anti-listerial activity of cell-adsorbed bacteriocins combined with oregano essential oil had a synergistic effect on the reduction of L. monocytogenes counts and delayed the growth rebound by 2 weeks in pork meat during storage at 4 ºC (Ghalfi et al., 2007).
Smart packaging represents an advanced approach to packaging design, integrating technology to augment the functionality, safety, and user experience of packaged products. A recent advancement in this domain is the incorporation of bacteriocins into packaging materials, utilizing natural antimicrobial agents to enhance food safety and preservation. Typically, this method employs crude bacteriocin preparations rather than inoculum. Nevertheless, significant gaps remain in the current field, particularly concerning the varying characteristics of different matrices for the establishment of effective smart packaging solutions.
Edible films
For instance, whey protein-based edible films, enriched with the cell-free supernatant of Lactobacillus sakei strains were applied in beef, resulting in a decrease from 3.5 log + 0.2 CFU/g to 0.3 + 0.1 log CFU/g of Escherichia coli counts after 36 h of refrigerated storage. Moreover, sensory evaluation of grilled beef wrapped with the antimicrobial films demonstrated no significant differences in flavor and color as assessed by the panelists, the overall acceptability was high (Beristain-Bauza et al., 2017).
Polythylene-based films
A plantaricin solution applied in an active package made with polyvinylidene chloride film in pork fresh meat inhibited L. monocytogenes growth by 1.4 log CFU/g after 7 days of cold storage (Xie et al., 2018). Plantaricin BM-1 solution was used to soak polyethylene-based films applied in meat artificially inoculated with L. monocytogenes and exerted antimicrobial activity that inhibited the pathogen growth during storage for 120 days at 25ºC (Zhang et al., 2017).
Curvaticin 32Y produced by L. curvatus 32Y isolated from dry sausages has been shown to reduce viable counts of L. monocytogenes by 1 log when applied in a polythene film by soaking, spraycoating as a preservative for artificially inoculated pork steaks (Mauriello et al., 2004). Moreover, in bioactive packaging made of sawdust particles and poly lactic acid biocomposite film, the adsorption of pediocin PA1-AcH enhanced raw sliced pork meat protection against L. monocytogenes, with counts reduced by ~2 log units after storage at 4ºC for 14 days (Woraprayote et al., 2013).
Cellophane coating
Nisin has been shown to have greater antimicrobial potential in meat preparation when used in combination with organic acids and salts; for instance, nisin reduced the total aerobic bacteria counts in 0.1 log CFU/g from veal meats when applied in a cellophane coating packaging and extended the shelf life of chopped meat under refrigerated storage (Guerra et al., 2005).
Alginate matrix
As a component in antimicrobial packaging, nisin had an inhibitory effect against microbial decay and extended the shelf life of refrigerated chicken meat up to 15 days more than the control when applied and incorporated in an alginate matrix (Carrión et al., 2023). A packaging composed of alginate films and containing immobilized viable enterocin-producing E. faecium Smr18 reduced S. Typhimurium counts by 3 log CFU/g in chicken meat after 34 days at cold storage (Rashid et al., 2023).
Cultivated meat
Meat grown from animal cells in a laboratory setting is called ‘cultivated meat’ (CM). CM appears as a new solution to several safety issues with livestock farming such as the zoonotic transfer of viruses and infection through human consumption (Ramani et al., 2021). The development of CM is dependent on the retrieval of animal cells by biopsy, creation of a bank of cells, growth, and differentiation by reprogramming stem cells into skeletal muscle cells, harvesting the cells, and processing them into tissues.
CM meat can become contaminated by bacteria, fungi, and viruses, which are managed by the addition of antibiotics during cell growth, as well as by the addition of cryoprotectants during cell storage. Moreover, the risk for contamination during further downstream processing is expected to be similar to the case of traditional meat products (Broucke et al., 2023).
CM production systems are considered to be more sustainable and safer in comparison to conventional meat production systems, but they may have a completely different risk profile; such a risk coming from antibiotic application in vitro to promote the growth of cells still consists of a gap in the field and much attention would require to be paid to the safety of added substrates and other compounds of the culture medium to the human health.
As it will be easier to keep control of pathogenic contamination in cultured meat production, CM is associated with fewer risks with respect to microbial contamination. The application of LAB as a starter for CM production is expected to influence the final product similarly than it already affects the meat that incorporates starter cultures, having a positive effect on organoleptic features and on microbial safety (Kolodkin-Gal et al., 2024).
Conclusions and future perspectives
Fresh meat is particularly susceptible to spoilage, posing a significant challenge in reducing losses and waste associated with meat and its products on a global scale. Furthermore, despite concerted efforts, foodborne outbreaks linked to the consumption of meat products persist as an unresolved issue.
In this context, biopreservation emerges as a natural alternative for extending the shelf life of meat products by managing their inherent microbial communities; however, proper diffusion of the bacteriocin through the meat matrix remains a challenge, in addition to the inhibition by the bacteriocins against closely related LAB, which could cause loss of starter culture effectiveness.
LAB are the dominant group present in meat and produce a variety of metabolites with antimicrobial effects. Bacteriocins are secondary metabolites produced by LAB with antimicrobial properties, these small peptides are stable in extreme temperatures and pH. While culture-based methods remain prevalent in assessing antimicrobial activity due to their cost-effectiveness and simplicity, they often rely on outdated protocols that may not fully explore the bacteriocinogenic potential of strains. Recent advancements in genomics, such as WGS, RNA-seq, and PCR-based techniques, offer more comprehensive insights into antimicrobial mechanisms. Moreover, computational tools, including docking software and three-dimensional structure modeling, now enable automated assessment of bacteriocin binding mechanisms. This advanced understanding of bacteriocin structures and their modes of action holds promise for developing strategies to enhance and optimize their antimicrobial efficacy in practical applications.
The development for higher efficiency of bacteriocin diffusion from packaging surfaces or application sites into meat, considers multiple factors, including meat texture and the thoroughness of mixing between the bacteriocin and the meat, which significantly influence homogeneous distribution within the meat matrix. The choice of application method is influenced by factors such as the complexity of the food matrix, scalability, and cost-effectiveness of production. Looking forward, advancements in 3D printing and CM could profit from integrating LAB and bacteriocins to improve their safety and functionality. Continued research into bacteriocins is crucial for advancing meat safety and expanding functional meat product offerings in the future.
Funding Statement
Funding: the research was funded by the Foundation for Science and Technology (FCT, Portugal) financial support through national funds FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2021) and through funding of the PAS-AGRO-PAS (PRIMA/0014/2022).
Availability of data and materials
Data and materials are available from the corresponding author upon request.
References
- Achemchem F, Martínez-Bueno M, Abrini J, Valdivia E, Maqueda M, 2005. Enterococcus faecium F58, a bacteriocinogenic strain naturally occurring in Jben, a soft, farmhouse goat’s cheese made in Morocco. J Appl Microbiol 99:141-50. [DOI] [PubMed] [Google Scholar]
- Aljohani AB, Al-Hejin AM, Shori AB, 2023. Bacteriocins as promising antimicrobial peptides, definition, classification, and their potential applications in cheeses. Food Sci Technol 43:e118021. [Google Scholar]
- Almeida-Santos AC, Novais C, Peixe L, Freitas AR, 2021. Enterococcus spp. as a producer and target of bacteriocins: a double-edged sword in the antimicrobial resistance crisis context. Antibiotics 10:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarh MA, Laryea MK, Borquaye LS, 2023. De novo peptides as potential antimicrobial agents. Heliyon 9:e19641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayrapetyan M, Oliver JD, 2016. The viable but non-culturable state and its relevance in food safety. Curr Opin Food Sci 8:127-33. [Google Scholar]
- Baillo AA, Cisneros L, Villena J, Vignolo G, Fadda S, 2023. Bioprotective lactic acid bacteria and lactic acid as a sustainable strategy to combat Escherichia coli O157:H7 in meat. Foods 12:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK, 2016. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balutis AM, 2014. Quantification of bacteriocin gene expression in Carnobacterium maltaromaticum ATCC PTA-5313. Available from: https://era.library.ualberta.ca/items/294af8ad-8bbe4ae5-95dd-680c22caf4df. [Google Scholar]
- Barcenilla C, Puente A, Cobo-Díaz JF, Alexa EA, Garcia-Gutierrez E, O’Connor PM, Cotter PD, González-Raurich M, López M, Prieto M, Álvarez-Ordóñez A, 2023. Selection of lactic acid bacteria as biopreservation agents and optimization of their mode of application for the control of Listeria monocytogenes in ready-to-eat cooked meat products. Int J Food Microbiol 403:110341. [DOI] [PubMed] [Google Scholar]
- Bastos MCF, Coelho MLV, Santos OCS, 2015. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiology 161:683-700. [DOI] [PubMed] [Google Scholar]
- Ben Braïek O, Smaoui S, Ennouri K, Ben Ayed R, Hani K, Mastouri M, Ghrairi T, 2020. In situ Listeria monocytogenes biocontrol and sensory attributes enhancement in raw beef meat by Enterococcus lactis. J Food Process Preserv 44:e14633. [Google Scholar]
- Beristain-Bauza SC, Mani-López E, Palou E, López-Malo A, 2017. Antimicrobial activity of whey protein films supplemented with Lactobacillus sakei cell-free supernatant on fresh beef. Food Microbiol 62:207-11. [DOI] [PubMed] [Google Scholar]
- Bindu A, Lakshmidevi N, 2021. In vitro and in silico approach for characterization of antimicrobial peptides from potential probiotic cultures against Staphylococcus aureus and Escherichia coli. World J Microbiol Biotechnol 37:172. [DOI] [PubMed] [Google Scholar]
- Bromberg R, Moreno I, Zaganini CL, Delboni RR, de Oliveira J, 2004. Isolation of bacteriocin-producing lactic acid bacteria from meat and meat products and its spectrum of inhibitory activity. Braz J Microbiol 35:137-44. [Google Scholar]
- Broucke K, Van Pamel E, Van Coillie E, Herman L, Van Royen G, 2023. Cultured meat and challenges ahead: a review on nutritional, technofunctional and sensorial properties, safety and legislation. Meat Sci 195:109006. [DOI] [PubMed] [Google Scholar]
- Bungenstock L, Abdulmawjood A, Reich F, 2020. Evaluation of antibacterial properties of lactic acid bacteria from traditionally and industrially produced fermented sausages from Germany. PLoS One 15:e0230345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaus P, Nilsen T, Cintas LM, Nes IF, Hernández PE, Holo H, 1997. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology 143:2287-94. [DOI] [PubMed] [Google Scholar]
- Carrión MG, Corripio MAR, Contreras JVH, Marrón MR, Olán GM, Cázares ASH, 2023. Optimization and characterization of taro starch, nisin, and sodium alginate-based biodegradable films: antimicrobial effect in chicken meat. Poul Sci 102:103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ma L, Dai H, Fu Y, Wang H, Zhang Y, 2022. Advances in rational protein engineering toward functional architectures and their applications in food science. J Agric Food Chem 70:4522-33. [DOI] [PubMed] [Google Scholar]
- Choi GH, Holzapfel WH, Todorov SD, 2023. Diversity of the bacteriocins, their classification and potential applications in combat of antibiotic resistant and clinically relevant pathogens. Crit Rev Microbiol 49:578-97. [DOI] [PubMed] [Google Scholar]
- Crandall AD, Montville TJ, 1998. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl Environ Microbiol 64:231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Zhang C, Wang Y, Shi J, Zhang L, Ding Z, Qu X, Cui H, 2012. Class IIa bacteriocins: diversity and new developments. Int J Mol Sci 13:16668-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa RJ, Voloski FLS, Mondadori RG, Duval EH, Fiorentini ÂM, 2019. Preservation of meat products with bacteriocins produced by lactic acid bacteria isolated from meat. J Food Qual 2019:e4726510. [Google Scholar]
- Dallagnol AM, Barrio Y, Cap M, Szerman N, Castellano P, Vaudagna SR, Vignolo G, 2017. Listeria inactivation by the combination of high hydrostatic pressure and lactocin AL705 on cured-cooked pork loin slices. Food Bioprocess Technol 10:1824-33. [Google Scholar]
- Darbandi A, Asadi A, Mahdizade Ari M, Ohadi E, Talebi M, Halaj Zadeh M, Darb Emamie A, Ghanavati R, Kakanj M, 2021. Bacteriocins: properties and potential use as antimicrobials. J Clin Lab Anal 36:e24093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Sercu T, Wadhawan K, Padhi I, Gehrmann S, Cipcigan F, Chenthamarakshan V, Strobelt H, Dos Santos C, Chen PY, Yang YY, Tan JPK, Hedrick J, Crain J, Mojsilovic A, 2021. Accelerated antimicrobial discovery via deep generative models and molecular dynamics simulations. Nat Biomed Eng 5:613-23. [DOI] [PubMed] [Google Scholar]
- Davidson AL, Dassa E, Orelle C, Chen J, 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev 72:317-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Barbosa M, Todorov SD, Ivanova I, Chobert JM, Haertlé T, de Melo Franco BDG, 2015. Improving safety of salami by application of bacteriocins produced by an autochthonous Lactobacillus curvatus isolate. Food Microbiol 46:254-62. [DOI] [PubMed] [Google Scholar]
- Deegan LH, Cotter PD, Hill C, Ross P, 2006. Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int Dairy J 16:1058-71. [Google Scholar]
- Deo S, Turton KL, Kainth T, Kumar A, Wieden HJ, 2022. Strategies for improving antimicrobial peptide production. Biotechnol Adv 59:107968. [DOI] [PubMed] [Google Scholar]
- Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF, 2007. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc Natl Acad Sci U S A 104:2384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep DB, Straume D, Kjos M, Torres C, Nes IF, 2009. An overview of the mosaic bacteriocin pln loci from Lactobacillus plantarum. Peptides 30:1562-74. [DOI] [PubMed] [Google Scholar]
- Dorn-In S, Mang S, Cosentino RO, Schwaiger K, 2024. Changes in the microbiota from fresh to spoiled meat, determined by culture and 16S rRNA analysis. J Food Prot 87:100212. [DOI] [PubMed] [Google Scholar]
- Dortu C, Fickers P, Franz CMAP, Ndagano D, Huch M, Holzapfel WH, Joris B, Thonart P, 2009. Characterisation of an antilisterial bacteriocin produced by Lactobacillus sakei CWBI-B1365 isolated from raw poultry meat and determination of factors controlling its production. Probiotics Antimicrobial Proteins 1:75-84. [DOI] [PubMed] [Google Scholar]
- Drider D, Fimland G, Héchard Y, McMullen LM, Prévost H, 2006. The continuing story of class IIa bacteriocins. Microbiol Mol Biol Rev 70:564-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ECDC, 2023. The European Union one health 2022 zoonoses report. EFSA J 21:e8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enan G, 2006. Nature and phenotypic characterization of plantaricin UG1 resistance in Listeria monocytogenes LMG 10470. J Food Agric Environ 4:105-8. [Google Scholar]
- European Commission, 2005. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. In: Official Journal, L338/1, 22/12/2005. [Google Scholar]
- Frederix PWJM, Patmanidis I, Marrink SJ, 2018. Molecular simulations of self-assembling bio-inspired supramolecular systems and their connection to experiments. Chem Soc Rev 47:3470-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez A, Abriouel H, López RL, Omar NB, 2007. Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol 120:51-70. [DOI] [PubMed] [Google Scholar]
- García-López JD, Barbieri F, Baños A, Madero JMG, Gardini F, Montanari C, Tabanelli G, 2023. Use of two autochthonous bacteriocinogenic strains as starter cultures in the production of salchichónes, a type of Spanish fermented sausages. Curr Res Food Sci 7:100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiker NRW, Bertram HC, Mejborn H, Dragsted LO, Kristensen L, Carrascal JR, Bügel S, Astrup A, 2021. Meat and human health—current knowledge and research gaps. Foods 10:1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalfi H, Benkerroum N, Doguiet DDK, Bensaid M, Thonart P, 2007. Effectiveness of cell adsorbed bacteriocin produced by Lactobacillus curvatus CWBI B28 and selected essential oils to control Listeria monocytogenes in pork meat during cold storage. Lett Appl Microbiol 44:268-73. [DOI] [PubMed] [Google Scholar]
- Gillor O, Etzion A, Riley MA, 2008. The dual role of bacteriocins as anti- and probiotics. Appl Microbiol Biotechnol 81:591-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JE, Keshavan ND, 2006. Messing with bacterial quorum sensing. Microbiol Mol Biol Rev 70:859-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra NP, Macías CL, Agrasar AT, Castro LP, 2005. Development of a bioactive packaging cellophane using Nisaplin as biopreservative agent. Lett Appl Microbiol 40:106-10. [DOI] [PubMed] [Google Scholar]
- Hammami R, Zouhir A, Le Lay C, Ben Hamida J, Fliss I, 2010. BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiol 10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings JW, Sailer M, Johnson K, Roy KL, Vederas JC, Stiles ME, 1991. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J Bacteriol 173:7491-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner S, Krismer B, Brötz-Oesterhelt H, Peschel A, 2021. The microbiome-shaping roles of bacteriocins. Nat Rev Microbiol 19:726-39. [DOI] [PubMed] [Google Scholar]
- Holck AL, Axelsson L, Hühne K, Kröckel L, 1994. Purification and cloning of sakacin 674, a bacteriocin from Lactobacillus sake Lb674. FEMS Microbiol Lett 115:143-9. [DOI] [PubMed] [Google Scholar]
- Hossain TJ, 2024. Methods for screening and evaluation of antimicrobial activity: a review of protocols, advantages, and limitations. Eur J Microbiol Immunol 14:97-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu STD, Breukink E, Tischenko E, Lutters MAG, de Kruijff B, Kaptein R, Bonvin AMJJ, van Nuland NAJ, 2004. The nisin– lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol 11:963-7. [DOI] [PubMed] [Google Scholar]
- Ibrahim SA, Ayivi RD, Zimmerman T, Siddiqui SA, Altemimi AB, Fidan H, Esatbeyoglu T, Bakhshayesh RV, 2021. Lactic acid bacteria as antimicrobial agents: food safety and microbial food spoilage prevention. Foods 10:3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanian B, Donaghy J, Jackson T, Gummalla S, Ganesan B, Baker RC, Henderson M, Butler EK, Hong Y, Ring B, Thorp C, Khaksar R, Samadpour M, Lawless KA, MacLaren-Lee I, Carleton HA, Tian R, Zhang W, Wan J, 2022. The power, potential, benefits, and challenges of implementing high-throughput sequencing in food safety systems. Npj Sci Food 6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatani S, Zendo T, Sonomoto K, 2011. Class IId or linear and non-pediocin-like bacteriocins. In: Drider D, Rebuffat S. (eds.), Prokaryotic antimicrobial peptides: from genes to applications (pp. 237–252). Springer, New York, USA. [Google Scholar]
- Jack RW, Wan J, Gordon J, Harmark K, Davidson BE, Hillier AJ, Wettenhall RE, Hickey MW, Coventry MJ, 1996. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl Environ Microbiol 62:2897-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D, 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596:583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwowska M, Łaba S, Szczepański K, 2021. Food loss and waste in meat sector—why the consumption stage generates the most losses? Sustainability 13:6227. [Google Scholar]
- Kassaa IA, Rafei R, Moukhtar M, Zaylaa M, Gharsallaoui A, Asehraou A, Omari KE, Shahin A, Hamze M, Chihib NE, 2019. LABiocin database: a new database designed specifically for lactic acid bacteria bacteriocins. Int J Antimicrob Agents 54:771-9. [DOI] [PubMed] [Google Scholar]
- Kaur G, Singh TP, Malik RK, 2013. Antibacterial efficacy of Nisin, Pediocin 34 and Enterocin FH99 against Listeria monocytogenes and cross resistance of its bacteriocin resistant variants to common food preservatives. Braz J Microbiol 44:63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran F, Osmanagaoglu O, 2014. Inhibition of Listeria monocytogenes in chicken meat by pediocin AcH/PA-1 produced by Pediococcus pentosaceus OZF. Agro Food Industry Hi-Tech, 25:66-9. [Google Scholar]
- Kolodkin-Gal I, Dash O, Rak R, 2024. Probiotic cultivated meat: bacterial-based scaffolds and products to improve cultivated meat. Trends Biotechnol 42:269-81. [DOI] [PubMed] [Google Scholar]
- Komora N, Maciel C, Isidro J, Pinto CA, Fortunato G, Saraiva JMA, Teixeira P, 2023. The impact of HPP-assisted biocontrol approach on the bacterial communities’ dynamics and quality parameters of a fermented meat sausage model. Biology 12:1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordi M, Talkhounche PG, Vahedi H, Farrokhi N, Tabarzad M, 2024. Heterologous production of antimicrobial peptides: notes to consider. Protein J 129-58. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthi R, Srinivash M, Mahalingam PU, Malaikozhundan B, Suganya P, Gurushankar K, 2022. Antimicrobial, anti-biofilm, antioxidant and cytotoxic effects of bacteriocin by Lactococcus lactis strain CH3 isolated from fermented dairy products—an in vitro and in silico approach. Int J Biol Macromol 220:291-306. [DOI] [PubMed] [Google Scholar]
- Lahiri D, Nag M, Dutta B, Sarkar T, Pati S, Basu D, Abdul Kari Z, Wei LS, Smaoui S, Wen Goh K, Ray RR, 2022. Bacteriocin: a natural approach for food safety and food security. Front Bioeng Biotechnol 10:1005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim H Y, 2011. Lantibiotics, class I bacteriocins from the genus Bacillus. J Microbiol Biotechnol 21:229-35. [PubMed] [Google Scholar]
- Lei J, Sun L, Huang S, Zhu C, Li P, He J, Mackey V, Coy DH, He Q, 2019. The antimicrobial peptides and their potential clinical applications. Am J Transl Res 11:3919-31. [PMC free article] [PubMed] [Google Scholar]
- Leslie VA, Alarjani KM, Malaisamy A, Balasubramanian B, 2021. Bacteriocin producing microbes with bactericidal activity against multidrug resistant pathogens. J Infect Public Health 14:1802-9. [DOI] [PubMed] [Google Scholar]
- Luong NDM, Coroller L, Zagorec M, Membré JM, Guillou S, 2020. Spoilage of chilled fresh meat products during storage: a quantitative analysis of literature data. Microorganisms 8:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques PH, Jaiswal AK, de Almeida FA, Pinto UM, Ferreira-Machado AB, Tiwari S, de Castro Soares S, Paiva AD, 2023. Lactic acid bacteria secreted proteins as potential Listeria monocytogenes quorum sensing inhibitors. Mol Divers doi: 10.1007/s11030-023-10722-7. [DOI] [PubMed] [Google Scholar]
- Martínez JM, Martínez MI, Herranz C, Suárez AM, Cintas LM, Fernández MF, Rodríguez JM, Hernández PE, 2000. Use of genetic and immunological probes for pediocin PA-1 gene detection and quantification of bacteriocin production in Pediococcus acidilactici strains of meat origin. Food Agric Immunol 12:299-310. [Google Scholar]
- Mauriello G, Ercolini D, La Storia A, Casaburi A, Villani F, 2004. Development of polythene films for food packaging activated with an antilisterial bacteriocin from Lactobacillus curvatus 32Y. J Appl Microbiol 97:314-22. [DOI] [PubMed] [Google Scholar]
- McGinnis S, Madden TL, 2004. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res 32:W20-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng EC, Goddard TD, Pettersen EF, Couch GS, Pearson ZJ, Morris JH, Ferrin TE, 2023. UCSF ChimeraX: tools for structure building and analysis. Protein Sci 32:e4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes PM, Perin LM, Tassinari Ortolani MB, Yamazi AK, Viçosa GN, Nero LA, 2010. Protocols for the isolation and detection of lactic acid bacteria with bacteriocinogenic potential. LWT -Food Sci Technol 43:1320-4. [Google Scholar]
- Morata A, 2015. Nuevas Tecnologías de Conservación de Alimentos 2010 2Ed-Resumen. Available from: https://doi.org/10.13140/RG.2.1.4187.6641. [Google Scholar]
- Mørtvedt CI, Nes IF, 1990. Plasmid-associated bacteriocin production by a Lactobacillus sake strain. Microbiology 136:1601-7. [Google Scholar]
- Nain Z, Adhikari UK, Abdulla F, Hossain N, Barman NC, Mansur FJ, Azakami H, Karim MM, 2020. Computational prediction of active sites and ligands in different AHL quorum quenching lactonases and acylases. J Biosci 45:26. [PubMed] [Google Scholar]
- Nedyalkova M, Paluch AS, Potes Vecini D, Lattuada M, 2024. Progress and future of the computational design of antimicrobial peptides (AMPs): bio-inspired functional molecules. Digital Discovery 3:9-22. [Google Scholar]
- Negash AW, Tsehai BA, 2020. Current applications of bacteriocin. Int J Microbiol 2020:4374891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauman K, Jaspal MH, Asghar B, Manzoor A, Akhtar KH, Ali U, Ali S, Nasir J, Sohaib M, Badar IH, 2022. Effect of different packaging atmosphere on microbiological shelf life, physicochemical attributes, and sensory characteristics of chilled poultry fillets. Food Sci Anim Resour 42:153-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WL, Bassler BL, 2009. Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen-Meyer J, Oppegård C, Rogne P, Haugen HS, Kristiansen PE, 2011. The two-peptide (class-iib) bacteriocins: genetics, biosynthesis, structure, and mode of action. In: Drider D, Rebuffat S. (eds.), Prokaryotic antimicrobial peptides: from genes to applications.. Springer, New York, USA; pp 197-212. [Google Scholar]
- Noda M, Miyauchi R, Danshiitsoodol N, Matoba Y, Kumagai T, Sugiyama M, 2018. Expression of genes involved in bacteriocin production and self-resistance in lactobacillus brevis 174A is mediated by two regulatory proteins. Appl Environ Microbiol 84:e02707-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeyemi OA, Alegbeleye OO, Strateva M, Stratev D, 2020. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr Rev Food Sci Food Saf 19:311-31. [DOI] [PubMed] [Google Scholar]
- Oftedal TF, 2023. Bacteriocins: from discovery to characterization and applications. Available from: https://nmbu.brage.unit.no/ nmbu-xmlui/handle/11250/ 3098644. [Google Scholar]
- Ortiz S, López V, Garriga M, Martínez-Suárez JV, 2014. Antilisterial effect of two bioprotective cultures in a model system of Iberian chorizo fermentation. Int J Food Sci Technol 49:753-8. [Google Scholar]
- Palmer N, Maasch JRMA, Torres MDT, de la Fuente-Nunez C, 2021. Molecular dynamics for antimicrobial peptide discovery. Infect Immun 89:e00703-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RH, Zendo T, Sonomoto K, 2014. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb Cell Fact 13:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazentin ACM, Mendonça CMN, Vallejo M, Mussatto SI, de Souza Oliveira RP, 2022. Bacteriocin-like inhibitory substances production by Enterococcus faecium 135 in co-culture with Ligilactobacillus salivarius and Limosilactobacillus reuteri. Braz J Microbiol 53:131-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proutière A, du Merle L, Garcia-Lopez M, Léger C, Voegele A, Chenal A, Harrington A, Tal-Gan Y, Cokelaer T, Trieu-Cuot P, Dramsi S, 2023. Gallocin A, an atypical two-peptide bacteriocin with intramolecular disulfide bonds required for activity. Microbiol Spectr 11:e0508522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri LE, Sailer M, Roy KL, Vederas JC, Stiles ME, 1994. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J Biol Chem 269:12204-11. [PubMed] [Google Scholar]
- Raimondi S, Spampinato G, Candeliere F, Amaretti A, Brun P, Castagliuolo I, Rossi M, 2021. Phenotypic traits and immunomodulatory properties of leuconostoc carnosum isolated from meat products. Front Microbiol 12:730827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani S, Ko D, Kim B, Cho C, Kim W, Jo C, Lee CK, Kang J, Hur S, Park S, 2021. Technical requirements for cultured meat production: a review. J Anim Sci Technol 63:681-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid M, Sharma S, Kaur A, Kaur A, Kaur S, 2023. Biopreservative efficacy of Enterococcus faecium-immobilised film and its enterocin against Salmonella enterica. AMB Express 13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues Blanco I, Luduverio Pizauro LJ, dos Anjos Almeida JV, Nóbrega Mendonça CM, de Mello Varani A, Pinheiro de Souza Oliveira R, 2022. Pan-genomic and comparative analysis of Pediococcus pentosaceus focused on the in silico assessment of pediocin-like bacteriocins. Comput Struct Biotechnol J 20:5595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NL, Palcic MM, Sporns P, McMullen LM, 2002. Nisin: a novel substrate for glutathione S-transferase isolated from fresh beef. J Food Sci 67:2288-93. [Google Scholar]
- Ross AC, McKinnie SMK, Vederas JC, 2012. The synthesis of active and stable diaminopimelate analogues of the lantibiotic peptide lactocin S. J Am Chem Soc 134:2008-11. [DOI] [PubMed] [Google Scholar]
- Ruiz Puentes P, Henao MC, Cifuentes J, Muñoz-Camargo C, Reyes LH, Cruz JC, Arbeláez P, 2022. Rational discovery of antimicrobial peptides by means of artificial intelligence. Membranes 12:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmast E, Foudjing GGD, Salmieri S, Lacroix M, 2023. Application of combined essential oils and bacteriocins encapsulated in gelatin for bio-preservation of meatballs. J Food Saf 43:e13080. [Google Scholar]
- Scharff RL, 2020. Food attribution and economic cost estimates for meat- and poultry-related illnesses. J Food Prot 83:959-67. [DOI] [PubMed] [Google Scholar]
- Selman HM, Mahdi AA, Rofaei NAE, Mutwali EM, Selman HM, Mahdi AA, Rofaei NAE, Mutwali EM, 2021. Antibacterial activity of the bacteriocins producing- lactic acid bacteria isolated from some processed meat products against selected indicator bacterial strains. World J Adv Res Rev 12:640-5. [Google Scholar]
- Simons A, Alhanout K, Duval RE, 2020. Bacteriocins, antimicrobial peptides from bacterial origin: overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 8:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionek B, Szydłowska A, Trząskowska M, Kołożyn-Krajewska D, 2024. The impact of physicochemical conditions on lactic acid bacteria survival in food products. Fermentation 10:298. [Google Scholar]
- Srinivas M, O’Sullivan O, Cotter PD, van Sinderen D, Kenny JG, 2022. The application of metagenomics to study microbial communities and develop desirable traits in fermented foods. Foods 11:3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani S, Hammami R, Cotter PD, Rebuffat S, Said LB, Gaudreau H, Bédard F, Biron E, Drider D, Fliss I, 2021. Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol Rev 45:fuaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers A, Wang G, Xing M, Li B, 2023. Advances in antimicrobial peptide discovery via machine learning and delivery via nanotechnology. Microorganisms 11:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surati S, 2020. Bacteriocin, antimicrobial as a new natural food preservative: its potential and challenges. ERUDITIO Indonesia J Food Drug Saf 1:63-82. [Google Scholar]
- Szilágyi A, Závodszky P, 2000. Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: results of a comprehensive survey. Structure 8:493-504. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ, 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukano C, Uchino A, Irie K, 2024. Synthesis and applications of symmetric amino acid derivatives. Org Biomol Chem 22:411-28. [DOI] [PubMed] [Google Scholar]
- Umu ÖCO, Rudi K, Diep DB, 2017. Modulation of the gut micro-biota by prebiotic fibres and bacteriocins. Microb Ecol Health Dis 28:1348886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso R, Rantsiou K, Cantoni C, Comi G, Cocolin L, 2006. Technological characterization of a bacteriocin-producing Lactobacillus sakei and its use in fermented sausages production. Int J Food Microbiol 110:232-9. [DOI] [PubMed] [Google Scholar]
- van Heel AJ, de Jong A, Song C, Viel JH, Kok J, Kuipers OP, 2018. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res 46:W278-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, Žídek A, Green T, Tunyasuvunakool K, Petersen S, Jumper J, Clancy E, Green R, Vora A, Lutfi M, Figurnov M, Cowie A, Hobbs N, Kohli P, Kleywegt G, Birney E, Hassabis D, Velankar S, 2022. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res 50:D439-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, 2017. An in silico analysis of bacteriocin production in the human microbiota. PhD Thesis, University College Cork. [Google Scholar]
- Wan X, Saris PEJ, Takala TM, 2015. Genetic characterization and expression of leucocin B, a class IId bacteriocin from Leuconostoc carnosum 4010. Res Microbiol 166:494-503. [DOI] [PubMed] [Google Scholar]
- Wang D, Cui F, Ren L, Li J, Li T, 2023. Quorum-quenching enzymes: promising bioresources and their opportunities and challenges as alternative bacteriostatic agents in food industry. Compr Rev Food Sci Food Saf 22:1104-27. [DOI] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ, 2009. Jalview version 2 - a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woraprayote W, Kingcha Y, Amonphanpokin P, Kruenate J, Zendo T, Sonomoto K, Benjakul S, Visessanguan W, 2013. Anti-listeria activity of poly(lactic acid)/sawdust particle biocomposite film impregnated with pediocin PA-1/AcH and its use in raw sliced pork. Int J Food Microbiol 167:229-35. [DOI] [PubMed] [Google Scholar]
- Woraprayote W, Malila Y, Sorapukdee S, Swetwiwathana A, Benjakul S, Visessanguan W, 2016. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci 120:118-32. [DOI] [PubMed] [Google Scholar]
- Wosinska L, Walsh CJ, O’Connor PM, Lawton EM, Cotter PD, Guinane CM, O’Sullivan O, 2022. In vitro and in silico based approaches to identify potential novel bacteriocins from the athlete gut microbiome of an elite athlete cohort. Microorganisms 10:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhang M, Gao X, Shao Y, Liu H, Jin J, Yang W, Zhang H, 2018. Development and antimicrobial application of plantaricin BM 1 incorporating a PVDC film on fresh pork meat during cold storage. J Appl Microbiol 125:1108-16. [DOI] [PubMed] [Google Scholar]
- Xin B, Liu H, Zheng J, Xie C, Gao Y, Dai D, Peng D, Ruan L, Chen H, Sun M, 2020. In silico analysis highlights the diversity and novelty of circular bacteriocins in sequenced microbial genomes. mSystems 5:00047-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin WG, Wu G, Ying JP, Xiang YZ, Jiang YH, Deng XY, Lin LB, Zhang QL, 2023. Antibacterial activity and mechanism of action of bacteriocin LFX01 against Staphylococcus aureus and Escherichia coli and its application on pork model. Meat Sci 196:109045. [DOI] [PubMed] [Google Scholar]
- Yang E, Fan L, Yan J, Jiang Y, Doucette C, Fillmore S, Walker B, 2018. Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Express 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Peng Z, He M, Li Z, Fu G, Li S, Zhang J, 2024. Screening, probiotic properties, and inhibition mechanism of a Lactobacillus antagonistic to Listeria monocytogenes. Sci Total Environ 906:167587.167587. [DOI] [PubMed] [Google Scholar]
- Yoo JM, Song JH, Vasquez R, Hwang IC, Lee JS, Kang DK, 2023. Characterization of novel amylase-sensitive, anti-listerial class IId bacteriocin, agilicin C7 produced by Ligilactobacillus agilis C7. Food Sci Animal Resour 43:625-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Guo J, Liu Y, Xue X, Wang X, Wei L, Ma J, 2023. potential impact of combined inhibition by bacteriocins and chemical substances of foodborne pathogenic and spoilage bacteria: a review. Foods 12:3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharof MP, Lovitt RW, 2012. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Procedia 2:50-6. [Google Scholar]
- Zhang M, Gao X, Zhang H, Liu H, Jin J, Yang W, Xie Y, 2017. Development and antilisterial activity of PE-based biological preservative films incorporating plantaricin BM-1. FEMS Microbiol Lett 364. doi: 10.1093/femsle/fnw283. [DOI] [PubMed] [Google Scholar]
- Zhang P, Gänzle M, Yang X, 2019. Complementary antibacterial effects of bacteriocins and organic acids as revealed by comparative analysis of Carnobacterium spp. from meat. Appl Environ Microbiol 85:e01227-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zeng J, Wang C, Wang J, 2022. Structural basis of pore formation in the mannose phosphotransferase system by pediocin PA-1. Appl Environ Microbiol 88:e0199221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are available from the corresponding author upon request.


